-
PDF
- Split View
-
Views
-
Cite
Cite
Cinzia Crescenzi, Elisa Silvetti, Fabiana Romeo, Annamaria Martino, Edoardo Bressi, Germana Panattoni, Matteo Stefanini, Alessandra Stazi, Maria Ludovica Danza, Marco Rebecchi, Stefano Canestrelli, Elisa Fedele, Oreste Lanza, Chiara Lanzillo, Armando Fusco, Paolo Golia, Ermenegildo De Ruvo, Leonardo Calò, The electrocardiogram in non-ischaemic-dilated cardiomyopathy, European Heart Journal Supplements, Volume 25, Issue Supplement_C, May 2023, Pages C179–C184, https://doi.org/10.1093/eurheartjsupp/suad043
Close - Share Icon Share
Abstract
This article summarizes the main electrocardiogram (ECG) findings in dilated cardiomyopathy (DCM) patients. Recent reports are described in the great ‘pot’ of DCM peculiar ECG patterns that are typical of specific forms of DCM. Patients with late gadolinium enhancement on CMR, who are at greatest arrhythmic risk, have also distinctive ECG features. Future studies in large DCM populations should evaluate the diagnostic and prognostic value of the ECG.
Introduction
The term non-ischaemic dilated cardiomyopathy (DCM) refers to a large spectrum of genetical or acquired conditions characterized by left ventricular (LV) or biventricular dilatation and systolic dysfunction in the absence of either pressure or volume overload or coronary artery disease sufficient to explain the dysfunction.1 Considering that the phenotype may not meet standard disease criteria at the time of disease manifestation and can change during the follow-up, a new term ‘hypokinetic non-DCM’ has recently been proposed.
Although the prognosis of DCM has improved substantially during the last decades, sudden cardiac death (SCD) occurs in up to 12% of patients with DCM and may be the initial manifestation in previously asymptomatic individuals. The so-called ‘arrhythmogenic DCM’ phenotype, in overlaps with the current concept of arrhythmogenic cardiomyopathy (AC), can occur in up to one-third of DCM patients.1–3
The arrhythmic stratification in this population remains extremely challenging. Recent data highlighted the role of myocardial fibrosis detected by cardiac magnetic resonance (CMR) and the emerging knowledge of the genotype–phenotype correlations for diagnosing and treating high-risk subsets of patients with DCM. In this broad spectrum of advanced technologies, the electrocardiogram (ECG) represents a simple, easily accessible, and useful tool for clinicians. Although ECG abnormalities were generally depicted as non-specific, a careful ECG analysis may in fact provide information about aetiological definition (clues of genotype-phenotype correlation, so-called ‘red flags’) and prognostic stratification of subjects with DCM. This article summarizes the main ECG aspects to be analysed for the proper management of DCM patients.
Conduction disturbances
Conduction system diseases are common findings in patients with DCM.
The literature has shown that first-degree atrio-ventricular (AV) block was present in about 10–20% of cases of DCM4 but advanced AV block has also been reported in this population. These ECG features, especially if present in young patients, can occur before LV dysfunction and should raise suspicion of a genetic aetiology (lamin A/C, SCN5A, variants in the emerin gene). Furthermore, AV block is one of the risk factors to consider for ICD implantation in patients with LMNA mutation according to new guidelines.5
Right bundle branch block (RBBB) is an uncommon finding in DCM subjects (2–6%) but is one of the prevalent ECG features in patients with sarcoidosis or neuromuscular disease (dystrophin gene).
Left bundle branch block (LBBB) may be found in about one-third of DCM patients and may precede the development of structural changes in the heart. True LBBB should be diagnosed if QRS duration is ≥140 ms (130 ms in women), there is a QS or rS pattern in V1–V2 and mid-QRS notching or slurring in ≥2 of leads V1, V2, V5, V6, I, aVL. Interestingly, as pointed out by some reports,6–8 an LBBB with a prominent R-wave in V1 was observed in patients with septal fibrosis. Grigoratos et al.9 in a cohort of 196 patients with non-ischaemic cardiomyopathy submitted to CMR, observed that patients with left bundle disease (left anterior hemiblock or complete LBBB) were associated with a higher LV septal scar burden on CMR compared to patients with normal intra-ventricular conduction.
The occurrence of LBBB over time is associated with an adverse prognostic value,10 but also a less prolonged QRS duration (110–120 ms) has been shown to be predictive for a worse outcome in patients with congestive heart failure.11,12
Amiya et al.13 were able to show that a QRS duration greater than 120 ms was a significant predictor for cardiac death or hospitalization in 78 patients with non-ischaemic DCM.
Left anterior fascicular block (LAFB) and non-specific intra-ventricular conduction delay (NICD) are often found in this pathology, even if are not specific ECG signs. Left posterior fascicular block (LPFB) is rarely found in the general population. When present, it may be the expression of an underlying fibrotic remodelling that could injure posterior radiation of the left bundle branch, leading to LPFB or different degrees of left intra-ventricular conduction disturbances in DCM arrhythmogenic sub-types [Figure 1(A–C)].14
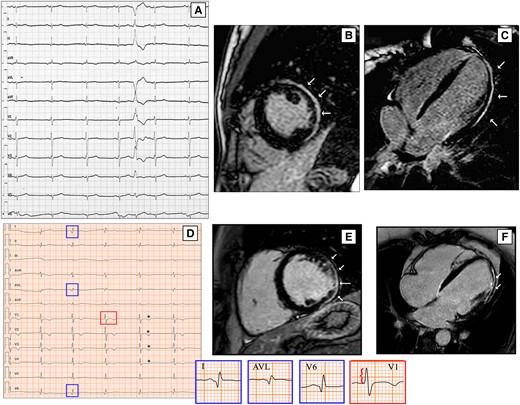
ECG findings of two DCM patients with LGE on CMR. ECG performed in 21-year-old woman shows low QRS voltages in V4–V6, LPFB, with AQRS ≈ + 120° and a premature ventricular beat with RBBB morphology and right axis deviation suggesting an origin in the LV lateral wall (A). On CMR, LGE involves the sub-epicardial lateral wall (B and C, white arrows). A 41-year-old man with a history of ventricular arrhythmias and ventricular dysfunction shows on ECG pathological lateral q waves (D, blue boxes), tall R wave in V1 (red box), and anterior T wave inversion (asterisks); his CMR reveals biventricular dilation and a stria LGE pattern with sub-epicardial distribution, mainly involving the LV lateral wall (E and F, white arrows). CMR, cardiac magnetic resonance; DCM, dilated cardiomyopathy; LGE, late gadolinium enhancement; LPFB, left posterior fascicular block; LV, left ventricular; RBBB, right bundle branch block.
Depolarization abnormalities
Left ventricular hypertrophy (LVH) has been observed in patients with DCM.4 Interestingly, Merlo et al.15 noted as LVH is a protective factor and may be the expression of a prognostic benefit deriving from an increased LV mass due to a preserved contractile reserve.
Low electrocardiographic QRS voltages (LQRSV) are classically defined as QRS complexes <0.5 mV in all peripheral leads and may be the electrocardiographic expression loss of vital myocardium and its replacement by fibrotic tissue. LQRSV were in fact frequently reported in non-ischaemic LV scar (NILVS)16 and in AC patients, particularly in those with LV involvement by significant fibro-fatty myocardial replacement.17 Therefore, low QRS voltages are not rarely found and can be related to arrhythmogenic forms. Merlo et al.15 observed that a lower R wave in lead II had a bad prognostic impact in patients with DCM.
Low QRS voltages have also been described in the precordial leads15,18,19 with an unfavourable prognostic significance in DCM. Oloriz et al.19 found a strict relationship between r in V3 ≤ 0.3 mV and QRS <0.6 mV in inferior leads and the presence of fibrosis in LV antero-septal and infero-lateral regions, respectively. Te Rijdt et al.20 noted that both the presence of low voltages and inverted lateral T-waves were associated with LV late gadolinium enhancement (LGE) on CMR in PLN p.Arg14del mutation carriers and found that LV-LGE is independently associated with the occurrence of malignant VA.
Zorzi et al.21 showed that LQRSV was an uncommon ECG finding in a large population of athletes with different ethnicities (prevalence ranging from 0.2% to 1.1%) if compared with cardiomyopathy patients affected by AC or NILVS. Finally, LQRSV in limb leads are one of the five components of a new score (Madrid Genotype Score), developed to predict a positive genetic test result in patients with non-ischaemic DCM or isolated LV systolic dysfunction.22
Q waves have been described in DCM in the anterior, lateral, and inferior leads.15,19,23–25 Wilensky et al.24 found abnormal Q waves in 36% of DCM patients. Instead, Tzou et al.26 noted pathologic Q waves in only 3 of 43 patients. In our population of 159 DCM patients, pathological Q waves were found in 24 patients (15%) and were much more frequent in patients with LGE [25% vs. 5%, P = 0.0003; Figure 1(D–F)] (Unpublished data).
The areas of fibrosis are not only expressed by the q waves but also by fragmented QRS (fQRS). QRS fragmentation can be explained by significant scarring of the myocardium that causes non-homogenous activation due to regional conduction slowing or block. Some studies have identified fQRS as a predictor of arrhythmic event and SCD in DCM.27,28 Pei et al. showed that the presence of J wave or fQRS in the inferior leads was an independent predictor for a higher risk of SCD in CHF patients with ischaemic and non-ischaemic aetiology.29 Oloriz et al.19 described the presence of fQRS in the inferior leads specifically in the group of patients with an infero-lateral scar compared to an antero-septal scar. Also, Basaran et al.30 recently found that in most DCM patients with LGE, fQRS segments were concordant with the fibrotic myocardial segments. But the data are not always consistent and therefore the utility of fQRS in risk stratification in DCM is not clear. In a prospective investigation of a group of patients with ischaemic and non-ischaemic LV dysfunction, fQRS was not associated with a higher risk of either all-cause or arrhythmic mortality.31 Ahn et al.32 reported that fQRS was not correlated with LGE even if they found a significant poor prognosis in patients with DCM and fQRS. Moreover, the fQRS is frequently observed in apparently healthy individuals. Terho et al.33 noted inferior fQRS in 15.6%, anterior in 2.9%, and lateral in 0.5% in a general population sample of over 10 000 middle-aged subjects without a known cardiac disease.
We frequently found fQRS in our DCM population (26%), even if in our patients the presence of fQRS was not specific for LGE presence (Unpublished data). We can speculate that probably, fQRS could be due to other causes in the patients without LGE, such as interstitial fibrosis, different geometry of LV, and anisotropic conduction.
Repolarization abnormalities
Repolarization abnormalities are commonly observed in DCM.4,15
T wave inversion (TWI) in lateral and infero-lateral leads can identify specific patients with arrhythmogenic DCM forms.3,4,15 In a large cohort of DCM patients, Merlo et al.15 brought out the unfavourable prognostic role of anterolateral TWI, probably due to an overlapping phenotype with AC with LV involvement.
Pei et al.29 observed that the presence of J wave or fQRS in the inferior leads predicted a higher risk of SCD in DCM. We can hypothesize that in some cases the presence of notches on the descending branch of the QRS may have been classified as fQRS and not as early repolarization. However, as recently pointed by Haissaguerre et al.34 through mapping data, the early repolarization pattern in the infero-lateral leads can be an expression of both abnormal early repolarization and delayed depolarization.
Prolonged QTc interval was a strong, independent predictor for mortality in patients with heart failure and elevated B-type natriuretic peptide.35
QT variability at prolonged monitoring has been shown to be of potential use in SCD risk stratification in DCM.36
Atrial fibrillation
Atrial fibrillation (AF) is observed in about 3–25% patients with DCM4 and its occurrence during follow-up is an unfavourable prognostic marker, probably due to structural disease progression.37 Of note, the early onset of AF at a young age may suggest an underlying genetic aetiology [Figure 2(A)]. Loss-of-function variants in TTN gene are the most commonly associated variants in early onset AF; LMNA mutation carriers show also a high prevalence of atrial arrhythmia.
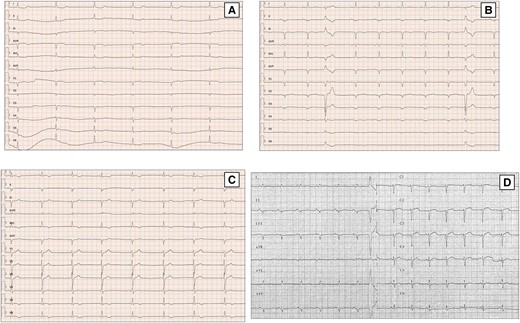
ECG findings of DCM patients with arrhythmic genotypes. First ECG evidence of asymptomatic slow atrial fibrillation in 48 years-old female with a familiar history of SCD carrying likely pathogenic mutation in LMNA gene (A). Basal ECG of a 33 years-old man with a missense mutation in LMNA gene (p.Arg190Trp) displays signs of septal remodelling, such as pathological Q waves in leads V1–V4, poor R-wave progression (R wave <3 mm) in leads V1–V3 and QRS fragmentation. Extremely low QRS voltages in precordial leads, diffuse flat T waves, premature ventricular beats with LBBB, and inferior axis morphology are also present (B). A 51-year-old man with FLNC mutation and familiar history of DCM shows on ECG negative TWI in infero-lateral leads (C). ECG performed in 61-year-old man with a PLN p.Arg14del mutation. The ECG shows very low QRS voltages in limb leads, flat T waves, and poor R wave progression in precordial leads (D). DCM, dilated cardiomyopathy; FLNC, filamin C; LBBB, left bundle branch block; LMNA, lamin A/C; LV, left ventricular; PLN, phospholamban; SCD, sudden cardiac death; TWI, T wave inversion.
Yoneda et al.38 in a prospective cohort study of 1293 participants diagnosed with AF before 66 years of age and who underwent whole genome sequencing, showed that the likelihood of a disease-associated variant was highest in participants with AF diagnosed before the age of 30 years. Disease-associated variants were more frequent in genes associated with inherited cardiomyopathy syndromes (TTN, MYH7, MYH6, LMNA), particularly DCM.
The multiple faces of dilated cardiomyopathy: electrocardiogram findings
In recent years, different sub-types of pathologies have been better characterized in the great ‘pot’ of DCM, ranging from various acquired cardiomyopathy to genetic forms. Thus, recent reports described ECG patterns that are typical of specific forms of DCM. In LMNA mutation carriers, LGE on CMR typically involves the mid-basal ventricular septum and basal LV wall. Accordingly, these patients show specific ECG signs of septal (leads V1–V3) remodelling (pathological Q waves, septal fragmentation, poor R-wave progression; Figure 2(B)), AV block, or LBBB.4,23
The ECG in DCM related to Duchenne’s muscular dystrophy and Becker’s muscular dystrophy manifests abnormal Q waves in leads I, aVL, and V6 or in leads II, III, and aVF, associated with high-voltage R waves in leads V1 and V2, which is determined by a transmural fibrosis in the LV posterolateral region.25
Desmosomal genes very often involve the LV, in some cases with a phenotype of DCM.39 These patients often present low voltages, fragmented QRS, and repolarization abnormalities in inferior and lateral leads.4
The ECG pattern described in filamin C gene (FLNC) variants is characterized by repolarization abnormalities, especially TWI in the precordial or infero-lateral leads [Figure 2(C)], and in about a quarter of cases by low voltages in the limb leads.3
Haghighi et al. found in patients with phospholamban variants low QRS complex potentials and decreased R-wave amplitude in precordial leads [Figure 2(D)].40
Conclusions
The ECG is a valid diagnostic tool that can allow an early identification of specific forms of DCM, referring patients to subsequent diagnostic steps (echocardiogram, CMR, genetic analysis) and proper management.
Of note, the ECG has demonstrated peculiar characteristics in some forms of cardiomyopathy with an arrhythmic phenotype.
Moreover, patients with LGE on CMR, who are at greatest arrhythmic risk, have distinctive ECG features. Future studies in large DCM populations should evaluate the value of the ECG from a prognostic point of view and its correlation with the evolution of fibrosis, not only determined with the LGE but also with the T1 mapping to the CMR.
Funding
None declared.
Data availability
No new data were generated or analysed in support of this research.
References
Author notes
Conflict of interest: None declared.



