
Cover image
The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice
Christine Landlinger1†, Marianne G. Pouwer2,3†, Claudia Juno1, José W.A. van der Hoorn3, Elsbet J. Pieterman3, J.Wouter Jukema2, Guenther Staffler1*, Hans M.G. Princen3†, and Gergana Galabova1,
1AFFiRiS AG, Karl-Farkas-Gasse 22, Vienna 1030, Austria; 2Department of Cardiology, LUMC, Albinusdreef 2, 2333 ZA Leiden, The Netherlands; and 3The Netherlands Organization of Applied Scientific Research (TNO)—Metabolic Health Research, Gaubius Laboratory, Zernikedreef 9, 2333 CK, PO Box 2215, 2301CE, Leiden, The Netherlands
See page 2508 for the editorial comment on this article (doi: 10.1093/eurheartj/ehx302)
* Corresponding author. Tel: +49 (0) 176 30585019, Fax: +34 615 319370, Email: [email protected]
* Corresponding author. Tel: +43 1 7981575300, Fax: +43 1 7981575311, Email: [email protected]
† These authors contributed equally to this work.
Aims Proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as a promising therapeutic target for the treatment of hypercholesterolaemia and atherosclerosis. PCSK9 binds to the low density lipoprotein receptor and enhances its degradation, which leads to the reduced clearance of low density lipoprotein cholesterol (LDLc) and a higher risk of atherosclerosis. In this study, the AT04A anti-PCSK9 vaccine was evaluated for its therapeutic potential in ameliorating or even preventing coronary heart disease in the atherogenic APOE*3Leiden.CETP mouse model.
Methods and results Control and AT04A vaccine-treated mice were fed western-type diet for 18 weeks. Antibody titres, plasma lipids, and inflammatory markers were monitored by ELISA, FPLC, and multiplexed immunoassay, respectively. The progression of atherosclerosis was evaluated by histological analysis of serial cross-sections from the aortic sinus. The AT04A vaccine induced high and persistent antibody levels against PCSK9, causing a significant reduction in plasma total cholesterol (−53%, P < 0.001) and LDLc compared with controls. Plasma inflammatory markers such as serum amyloid A (SAA), macrophage inflammatory protein-1β (MIP-1β/CCL4), macrophage-derived chemokine (MDC/CCL22), cytokine stem cell factor (SCF), and vascular endothelial growth factor A (VEGF-A) were significantly diminished in AT04A-treated mice. As a consequence, treatment with the AT04A vaccine resulted in a decrease in atherosclerotic lesion area (−64%, P = 0.004) and aortic inflammation as well as in more lesion-free aortic segments (+119%, P = 0.026), compared with control.
Conclusions AT04A vaccine induces an effective immune response against PCSK9 in APOE*3Leiden.CETP mice, leading to a significant reduction of plasma lipids, systemic and vascular inflammation, and atherosclerotic lesions in the aorta.
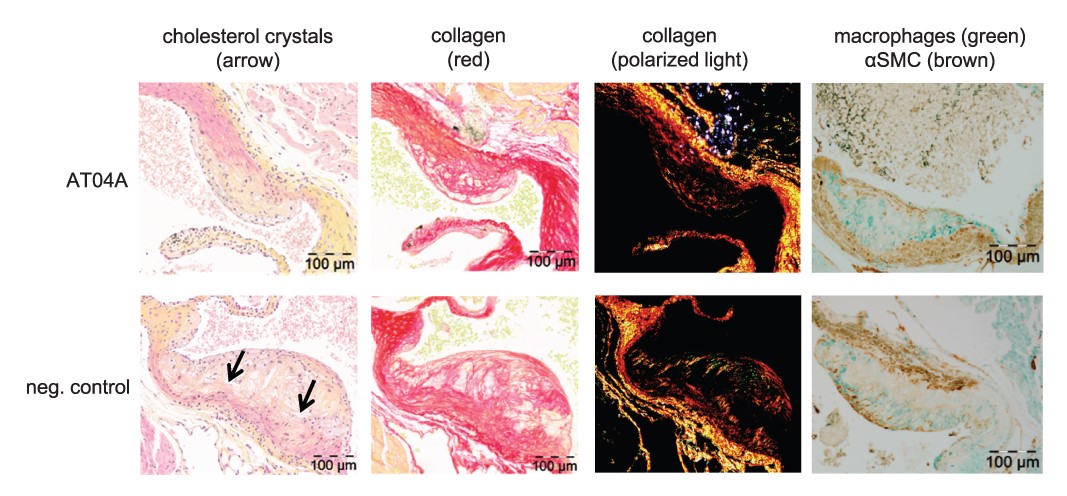
Figure 4 AT04A immunization reduces necrotic content and cholesterol crystals in plaques. Necrotic and macrophage content as unstable factors, and αSMCs and collagen as stable factors, were determined in the severe (type IV–V) lesions, and expressed as percentage of total plaque area. 10 mice in the control and 7 in vaccine group had severe lesions and were included in this analysis (A). Representative images of HPS staining, Sirius red staining for collagen, collagen expression under polarized light and double immunostaining with α-actin for SMCs (brown) and MAC-3 for macrophages (green) (B). The arrows depict necrotic areas, including cholesterol clefts. A Mann–Whitney U test was used for statistical analysis. Data are presented as group means ± SD (n = 7–10).




