-
PDF
- Split View
-
Views
-
Cite
Cite
Christine Landlinger, Marianne G. Pouwer, Claudia Juno, José W.A. van der Hoorn, Elsbet J. Pieterman, J. Wouter Jukema, Guenther Staffler, Hans M.G. Princen, Gergana Galabova, The AT04A vaccine against proprotein convertase subtilisin/kexin type 9 reduces total cholesterol, vascular inflammation, and atherosclerosis in APOE*3Leiden.CETP mice, European Heart Journal, Volume 38, Issue 32, 21 August 2017, Pages 2499–2507, https://doi.org/10.1093/eurheartj/ehx260
Close - Share Icon Share
Abstract
Proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as a promising therapeutic target for the treatment of hypercholesterolaemia and atherosclerosis. PCSK9 binds to the low density lipoprotein receptor and enhances its degradation, which leads to the reduced clearance of low density lipoprotein cholesterol (LDLc) and a higher risk of atherosclerosis. In this study, the AT04A anti-PCSK9 vaccine was evaluated for its therapeutic potential in ameliorating or even preventing coronary heart disease in the atherogenic APOE*3Leiden.CETP mouse model.
Control and AT04A vaccine-treated mice were fed western-type diet for 18 weeks. Antibody titres, plasma lipids, and inflammatory markers were monitored by ELISA, FPLC, and multiplexed immunoassay, respectively. The progression of atherosclerosis was evaluated by histological analysis of serial cross-sections from the aortic sinus. The AT04A vaccine induced high and persistent antibody levels against PCSK9, causing a significant reduction in plasma total cholesterol (−53%, P < 0.001) and LDLc compared with controls. Plasma inflammatory markers such as serum amyloid A (SAA), macrophage inflammatory protein-1β (MIP-1β/CCL4), macrophage-derived chemokine (MDC/CCL22), cytokine stem cell factor (SCF), and vascular endothelial growth factor A (VEGF-A) were significantly diminished in AT04A-treated mice. As a consequence, treatment with the AT04A vaccine resulted in a decrease in atherosclerotic lesion area (−64%, P = 0.004) and aortic inflammation as well as in more lesion-free aortic segments (+119%, P = 0.026), compared with control.
AT04A vaccine induces an effective immune response against PCSK9 in APOE*3Leiden.CETP mice, leading to a significant reduction of plasma lipids, systemic and vascular inflammation, and atherosclerotic lesions in the aorta.
Translational perspective
The present study is the first intervention study in a well-established, translational mouse model for hyperlipidaemia and atherosclerosis showing that active immunization against PCSK9 reduces atherosclerosis development as well as systemic and vascular inflammation. Anti-PCSK9 AT04A vaccine may therefore represent an effective and economic approach for the treatment and even prevention of cardiovascular pathologies.
Introduction
Elevated circulating low density lipoprotein cholesterol (LDLc) is one of the major risk factors positively correlated with premature development of cardiovascular disease (CVD).1 The main pathway of LDLc clearance from the blood circulation involves the LDLR on hepatocytes.2 , 3 The serine protease PCSK9, mainly synthesized in the liver, binds to the LDLR and enhances its degradation, thereby modulating cholesterol levels of circulating apoB-containing lipoproteins (i.e., VLDL and LDL).4 , 5 By inhibiting PCSK9, LDLR expression and its activity are increased, leading to plasma VLDL- and LDL-cholesterol lowering.
The current hypothesis relating to the role of cholesterol in CVD is that the effect of lowering LDLc on CVD is independent of the means by which LDLc is lowered.6 The most commonly-used drugs to treat hypercholesterolaemia are 3-hydroxy-3-methyl-glutaryl-CoA reductase inhibitors, known as statins. Statins in general have been shown to reduce LDLc by 30–60%, providing an estimated 25–30% reduction in CVD.7 However, many patients are unable to achieve their optimal lipid levels despite intensive statin therapy,8 , 9 and high doses of statins may increase the incidence and severity of multiple adverse events such as myopathy and hepatotoxicity.10 Moreover, statins do not only induce a beneficial increase in LDLR, but also increase PCSK9, which leads to LDLR degradation, thus causing a negative feedback response that attenuates the statins’ lipid effects.11
The most advanced alternative approach for LDLc lowering is the inhibition of PCSK9 action by monoclonal antibodies (mAbs). The novel anti-PCSK9 antibodies alirocumab and evolocumab, which were recently granted FDA approval, are safe and achieved a 50–70% reduction in LDLc when used either as monotherapy or in combination with a statin.12 , 13 They are approved as second line treatment in patients at risk who are unable to reach the LDLc goal despite maximally tolerated statin therapy or for patients who are statin-intolerant.14 , 15 Preclinical efficacy studies with the PCSK9 mAb alirocumab were performed in the APOE*3Leiden.CETP mouse model. These double-transgenic mice represent a valuable model for the preclinical evaluation of interventions on atherosclerosis development, because of its humanized lipoprotein metabolism. The diet-induced development of atherosclerosis in these mice has a pro-inflammatory plaque phenotype, and shows responsiveness to all lipid-modulating interventions that are being used in the clinic.16–18 In this model, alirocumab was able to decrease plasma lipids and atherosclerosis development, and in combination with atorvastatin the beneficial effects of PCSK9 mAb treatment were enhanced.18 , 19 However, mAbs show relatively short in vivo half-lives, thus the long-term efficacy of mAb therapy is accompanied by frequent application and high costs. We developed an active immunization against the body’s own PCSK9 for a widely-applicable and more cost-effective long-term LDLc cholesterol management.20 This so-called AT04A vaccine was tested for its efficacy in APOE*3Leiden.CETP transgenic mice. AT04A was able to induce a high immune response against PCSK9 without any side effects; leading to a significant reduction of plasma lipids over the whole intervention period. Consequently, a reduction of systemic and vascular inflammation and atherosclerotic lesions in the aorta at the end of the intervention period was observed.
Materials and methods
Animals
Female APOE*3Leiden.CETP transgenic mice on a C57BL/6 background (7–9 weeks of age) were used. The number of animals per group was calculated using a probability of 0.05. Based on our experience from previous studies, we expected to have a variance of 15% (sigma 40%) in plasma lipids and a minimal effect of treatment of 30%, resulting in 15 animals per group.
Animal experiments were approved by the Animal Experiment Committee of The Netherlands Organization of Applied Scientific Research TNO under registration number 3655.
Treatment and analyses
Mice were immunized five times subcutaneously either with AT04A or a control vaccine. Four weeks after prime immunization (W0) normal chow was switched to WTD 0.1% (w/w) cholesterol in order to induce atherosclerosis (see Supplementary material online, Figure S I). Blood was withdrawn at the time points W−4, W0, W4, W8, W12, W16, and W18 and used for the determination of the titre and total cholesterol levels, the lipoprotein profile, and the plasma PCSK9 concentration. After W18 the atherosclerotic lesion area and severity in the aortic root of each individual mouse were assessed. The plaque composition, the number of monocytes adhering to the endothelium, ICAM-1 expression, and NLRP3 expression in macrophages were analysed.
Detailed information about materials and methods are provided in the Supplementary material online.
Statistical and correlation analysis
All values were evaluated for homoscedasticity and normality assumption using both Kolmogorov–Smirnov and Shapiro–Wilk normality tests. For the comparison of two groups, the unpaired two-tailed Student’s t-test was used, followed by the Mann–Whitney correction for non-parametric data as indicated in the respective figure legend. A Spearman’s rank-order correlation was used to determine the relation between two parameters. The software used for statistical analyses were IBM SPSS Statistics version 18. The P-values ≤ 0.05 was considered statistically significant.
Results
AT04A induces a strong and persistent immune response and decreases plasma PCSK9 levels
APOE*3Leiden.CETP mice were used as a model for atherosclerosis with a pro-inflammatory plaque phenotype that can be induced upon high fat and cholesterol-containing diet.
A peptide (AFFITOPE®) which was previously designed to mimic the N-terminal epitope of the mature human and homolog mouse PCSK9 protein (153aa-692aa) was formulated into the AT04A vaccine.20 The mice were immunized with AT04A and control vaccine five times in bi-weekly intervals, and an 18-week western type diet (WTD) was started 4 weeks after prime immunization, which refers to time point W0 (Figure 1A and Supplementary material online, Figure S I). Titre analyses over time revealed that AT04A was able to induce a specific and long-lasting immune response against PCSK9, which reached a maximum mean titre of 1/12 243 (ODmax/2) 12 weeks after prime immunization, which corresponds to W8 of WTD. The immune response to PCSK9 highly varies between individual mice and no statistically significant differences in titres of W4–W18 were found (Figure 1A).
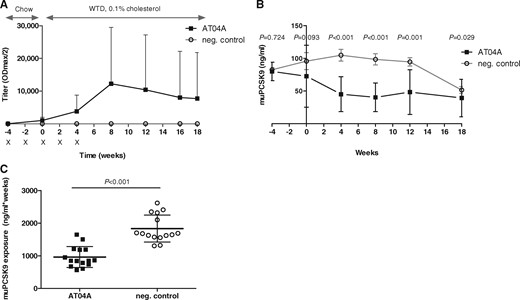
AT04A immunization induces a high and long-lasting immune response, and decreases plasma muPCSK9 levels. Antibody titres were assessed at different time points throughout the study. Crosses indicate the time points of the bi-weekly immunizations of control and AT04A vaccine (A). The muPCSK9 plasma concentration was determined at different time points throughout the study and compared to control (B–C). Data are presented as group means ± SD (n = 15). To compare AT04A and control treated group, the unpaired two-tailed Student’s t-test was used, followed by the Mann–Whitney correction for non-parametric data.
As expected, control immunized mice did not show any immune response against PCSK9 (Figure 1A).
As evidence for a direct interaction between the induced anti-PCSK9 antibodies and the target protein, the plasma concentration of muPCSK9 in AT04A-treated vs. control immunized mice was determined over time (Figure 1B and C). At the immunization start (W−4) the PCSK9 level in AT4A and control treated group was comparable. However, in W4, W8, W12, and W18 AT04A vaccine treated mice showed a highly significant decrease in PCSK9 concentration of 57, 59, 49, and 24%, respectively (P < 0.001, P < 0.001, P = 0.001, and P = 0.029, respectively) (Figure 1B) demonstrating a consistent and long-lasting effectiveness of the AT04A vaccine (Figure 1C). Due to unknown reasons, the plasma PCSK9 level of control treated mice dropped in W18, however, the difference between AT04A and control treated mice remained significant (Figure 1B).
AT04A decreases plasma TC and non-HDLc levels in APOE*3Leiden. CETP mice
Four weeks after the cholesterol-containing WTD was initiated, control immunized APOE*3Leiden.CETP mice showed a strong increase of plasma TC level from 3.4 mM (W0) to 15.5 mM (W4), which remained elevated until W18 (12.1 mM) (Figure 2A). In contrast, in the AT04A immunized mice a sustained reduction of plasma TC from W0 (2.8 mM, P = 0.002) to W18 (7.8 mM, P = 0.002) was observed (Figure 2A). Thus, cholesterol exposure over the whole time period of atherosclerosis inducing WTD (W0–W18) was decreased by 53% (P < 0.001) in AT04A vs. control-treated mice (Figure 2B).
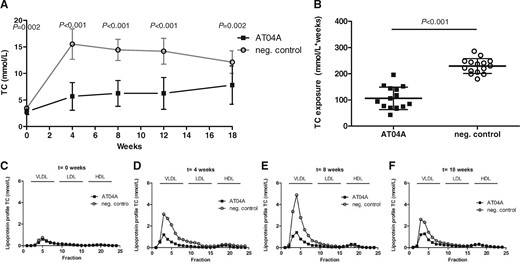
AT04A immunization decreases plasma TC and non-HDLc. Plasma cholesterol levels were measured at various time points throughout the study (A) and TC exposure (mmol/L*weeks) was calculated (B). Data are presented as group means ± SD (n = 15). To compare AT04A and control treated group, the unpaired two-tailed Student’s t-test was used, followed by the Mann–Whitney correction for non-parametric data. Plasma was pooled per group and the distribution of cholesterol over the individual lipoproteins was determined after separation by FPLC (C–F).
The TC lipoprotein profile of pooled plasmas per group was determined by FPLC (Figure 2C–F). Plasma levels of HDLc remained unaffected by AT04A vaccination, however the levels of non-HDLc (VLDLc and LDLc) were clearly reduced (Figure 2F–H), indicating that the anti-PCSK9 vaccine may be a powerful therapeutic approach for long-term non-HDLc/LDLc management. A strong positive correlation between TC and plasma PCSK9 concentration was found (r s = 0.75; P < 0.001) suggesting a specific and effective targeting of PCSK9 by AT04A vaccine.
AT04A reduces number, size, and severity of atherosclerotic lesions in the aorta
In order to assess the effect of AT04A on atherosclerotic development upon 18 weeks of WTD, the aortic root was isolated and the lesions as well as the lesion severity were determined. AT04A vaccine-treated mice showed a significantly reduced lesion area (−64%, P = 0.004; Figure 3A) and number of lesions per cross section (−35%, P = 0.037; Figure 3B), compared with controls. Moreover, in AT04A-vaccinated mice a significant higher percentage of lesion-free area per section (+119%, P = 0.026) was detected (Figure 3C). The lesion severity was categorized according to the American Heart Association guidelines.21 Although the relative amount of mild lesions (type I–III) was similarly distributed between AT04A and control immunized mice, the more severe lesions (type IV–V) were decreased in AT04A treated mice (−64%, P = 0.051) (Figure 3C). Representative images of the differently categorized atherosclerotic lesions in the aortic root of AT04A and control immunized mice are depicted in Figure 3D. A strong positive correlation between the atherosclerotic lesion area and plasma TC as well as plasma PCSK9 levels was observed (r s = 0.75, P < 0.001 and r s = 0.6, P = 0.001, respectively).
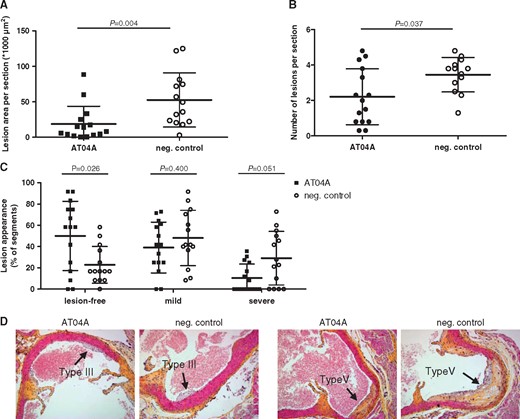
AT04A immunization decreases lesion size and severity. After 18 weeks of treatment, lesion area (A), number of lesions (B), and lesion severity (C) were determined per cross-section in the aortic sinus. Due to a technical error one mouse of the control group was excluded from analysis. Lesion severity was classified as mild (type I–III) or severe (type IV–V). Representative images of haematoxylin-phloxine-saffron-stained type III and V lesions in a cross-section (D). A Mann–Whitney U test was used for statistical analysis. Data are presented as group means ± SD (n = 14–15).
AT04A immunization reduces necrotic core content
The effects of AT04A vaccination on the plaque composition of severe lesions (type IV–V) were assessed by immunohistochemistry. The necrotic core areas including cholesterol clefts and lesion macrophages were quantified as pro-inflammatory factors, and smooth muscle cells (SMCs) in the fibrotic cap and collagen area as fortifying factors (Figure 4A). Representative images are shown in Figure 4B. All data are expressed as percentages of total plaque areas. In AT04A-treated mice the necrotic core area was 1.9 vs. 8.1% in the control-treated group, representing a reduction of 77% (Figure 4A) (P = 0.001). Macrophage, SMC, and collagen content were not significantly altered by AT04A treatment. Despite the decrease in necrotic core content, the plaque stability index was not changed, and was 2.3 in control and 3.0 in AT04A treated mice.
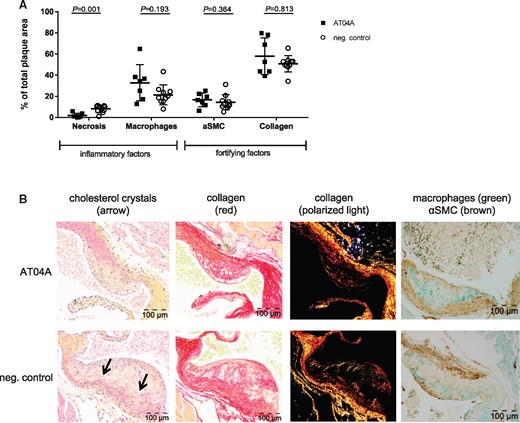
AT04A immunization reduces necrotic content and cholesterol crystals in plaques. Necrotic and macrophage content as unstable factors, and αSMCs and collagen as stable factors, were determined in the severe (type IV–V) lesions, and expressed as percentage of total plaque area. 10 mice in the control and 7 in vaccine group had severe lesions and were included in this analysis (A). Representative images of HPS staining, Sirius red staining for collagen, collagen expression under polarized light and double immunostaining with α-actin for SMCs (brown) and MAC-3 for macrophages (green) (B). The arrows depict necrotic areas, including cholesterol clefts. A Mann–Whitney U test was used for statistical analysis. Data are presented as group means ± SD (n = 7–10).
AT04A reduces vascular inflammation
Since atherosclerosis is not only a lipid-driven disease, but also a chronic low-grade inflammatory disease of the vessel wall, the number of monocytes adhering to the activated endothelium, the expression of the adhesion molecule ICAM-1 in the endothelium as well as the expression of the caspase-1-activating inflammasome protein NLRP3 in macrophages was determined in the diseased aortic root area. In the control group 2.3 monocytes per cross-section were counted, compared with 1.4 in the AT04A-treated group (−38%, P = 0.014) (Figure 5A). The reduction in monocyte adherence was reinforced by a similar significant decrease in ICAM-1 expression in endothelial cells upon AT04A vaccination (−37%, P = 0.018) (Figure 5B and C). Since NLRP3 is predominantly expressed by myeloid cells, we determined the amount of NLRP3 expression in the macrophage area and a decrease by 68% in AT04A compared with control treated mice was observed (0.3 ± 0.2 vs. 0.9 ± 0.8, P = 0.06) (Figure 5D and E). Together, these findings suggest that AT04A immunization results in a reduction of activated endothelial cells and pro-inflammatory macrophages.
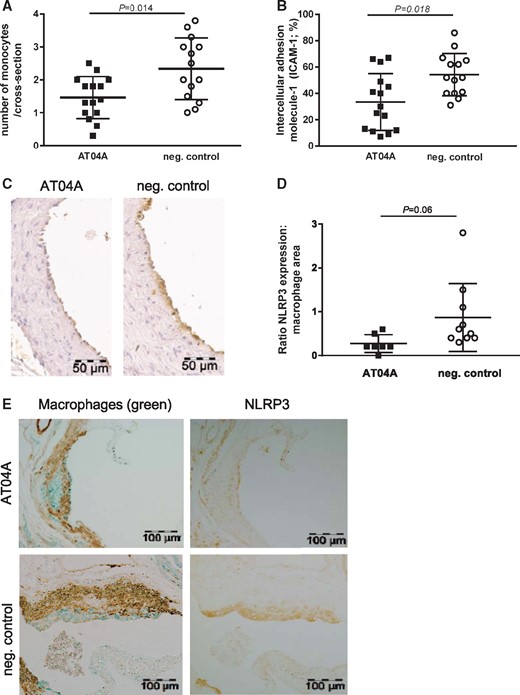
AT04A immunization reduces the number of monocytes adhering to the endothelium, ICAM-1 expression and NLRP3 expression in macrophages. (A) The number of monocytes was counted per cross-section after staining with AIA 31240 and (B) endothelial ICAM-1 was determined as percentage of the endothelial surface in the cross sections and (C) a representative image is shown. (D) NLRP3 expression was determined in the severe (type IV–V) lesions after immune staining with anti-NLRP3 antibodies and the amount of NLRP3 expression in the macrophage area was calculated. (E) Photographs of atherosclerotic plaques stained with aSMC (brown), macrophages (green) and NLRP3 (brown). A Mann–Whitney U test was used for statistical analysis. Data are presented as means ± SD (n = 14–15 in A–B, n = 7–10 in D).
AT04A decreases inflammatory biomarkers
In order to assess system inflammation upon high-fat diet-induced atherosclerosis in APOE*3Leiden.CETP mice, a panel of plasma inflammatory markers was analysed. The liver-derived inflammation marker serum amyloid A (SAA), reported to be elevated in diet-induced atherosclerosis in mice,22 showed a significant reduction in anti-PCSK9 AT04A treated mice at W4 (−21%; P = 0.007) and W8 (−28%; P < 0.001) relative to the negative control, but was not significantly affected later in the study (see Supplementary material online, Figure S II). Myriad RBM’s mouse inflammation multi-analyte profile represents a comprehensive and quantitative analysis of multiple inflammatory biomarkers and pathways including cytokines, chemokines, and growth factors. In plasma obtained 18 weeks after the start of WTD four markers, namely macrophage inflammatory protein-1β (MIP-1β/CCL4), macrophage-derived chemokine (MDC/CCL22), the cytokine stem cell factor (SCF), and vascular endothelial growth factor A (VEGF-A), showed a significant reduction in AT04A compared with control-immunized mice whereas the decreases in SCF was most pronounced (see Supplementary material online, Table S I). Furthermore, the macrophage colony stimulating factor 1 (M-CSF-1) and monocyte chemotactic protein 5 (MCP-5/CCL12) tended to be decreased (see Supplementary material online, Table S I). A Spearman's test showed a strong, positive correlation between TC and SCF (r s = 0.65; P < 0.001) and VEGF-A (r s = 0.58; P = 0.001), but not for TC and MIP-1β/CCL4 (r s = 0.02; P = 0.93) and MDC/CCL22 (r s = 0.26; P = 0.18).
Safety aspects
No effects on viability, body weight, or food intake were found in the AT04A-compared with control-immunized mice (see Supplementary material online, Table S II). Liver weight, which is considered as a sensitive indicator for toxicity, was not affected by AT04A vaccine-treated mice (see Supplementary material online, Table S II). Moreover, plasma pooled per group showed no apparent differences in aspartate transaminase (AST) and alanine transaminase (ALT) values as markers of hepatocellular damage (see Supplementary material online, Table S II). Other safety aspects such as absence of target-specific T-cell response and absence of cross-reactivity of induced antibodies with other endogenous proteins have been previously reported by Galabova et al. 20
Discussion
The present study shows that active immunization against PCSK9 with the so-called AT04A peptide-based vaccine elicits antibodies that effectively bind and remove PCSK9 from the circulation and reduce circulating TC, (V)LDLc, TG, and biomarkers of inflammation, which is accompanied by reduction of vascular inflammation and atherosclerotic lesions and plaques in the aortas of a mouse model of atherosclerosis.
PCSK9 plays a fundamental role in LDL metabolism through the binding and degradation of LDLR. With respect to LDLc as one of the major risk factors positively correlated with premature development of CVD,1 PCSK9 inhibition is supposed to reduce atherosclerotic events. Recently, the human PCSK9 mAbs evolocumab and alirocumab reached FDA approval and were recommended in the 2016 ESC/EAS Guidelines14 as second line treatment for high LDLc for adults whose cholesterol is not adequately controlled by diet or statin treatment. However, whereas both mAbs reduced the incidence of cardiovascular events in a post-hoc exploratory analysis, trial results whether anti-PCSK9 mAb therapy actually reduces atherosclerotic burden and CVD events is still under investigation.12 , 13
The main limitation for mAb therapies with broader applications certainly is that their use for chronic disorders is cost-prohibitive. Moreover, it has been reported that a substantial proportion of patients undergoing a sustained treatment with mAbs lose responsiveness over time due to the induction of anti-drug antibodies.23 Thus, an appealing new alternative to mAb-based therapies is the active immunization against self-antigens involved in chronical disorders.24 , 25
Previously, we showed that our AFFITOPE®-based anti-PCSK9 active immunization approach is able to induce a strong humoral immune response against PCSK9, which persisted for up to one year in vaccinated mice.20 Furthermore, TC concentration was reduced by up to 30% and LDLc up to 50% in anti-PCSK9 immunized compared with control mice. Immunization against PCSK9 has also been tested by others using different approaches.26 In the first approach, human recombinant PCSK9, along with a DNA oligonucleotide as an adjuvant, was tested and found to induce an approximately 40% reduction of LDLc in mice.27 Crossey et al. 28 designed a human PCSK9 vaccine where PCSK9 peptides are displayed at high valency on the surface of a bacteriophage virus-like particle, resulting in a ∼55% reduction of TC relative to controls
However, the present study is the first to show that active immunization against PCSK9 does not only reduce TC and (V)LDLc, but also has an impact on systemic and vascular inflammation and atherosclerotic development in a mouse model of diet-induced atherosclerosis. Vaccination with AT04A, which led to a reduction of TC exposure by 53%, had a strong impact on atherosclerotic development. The AT04A vaccine was able to induce a specific immune response against PCSK9. In contrast to previously reported studies where an anti-PCSK9 peptide vaccine20 as well as monoclonal anti-PCSK9 antibodies19 rather stabilized the target protein and increased the murine plasma PCSK9 level, AT04A-treated APOE*3Leiden.CETP mice showed a reduction of the target protein exposure during the study by 48%. Already after the second immunization with AT04A vaccine the induced anti-PCSK9 antibody concentration was sufficient to significantly decrease TC in APOE*3Leiden.CETP mice on normal chow (W0). Four weeks after the onset of WTD the anti-PCSK9 titre in AT04A-vaccinated mice rose to approximately 1/4000, corresponding to an antibody concentration of ∼200 µg/mL, and resulted in a TC decrease of 63% compared with control (P < 0.001). Although the mean anti-PCSK9 titre reached its maximum in week 8, no additional TC lowering was found at this timepoint, indicating a plateau effect after 4 weeks with maximally retained efficacy during the study. Due to this sustained TC reduction, anti-PCSK9 vaccine-treated mice showed significantly less lesion areas (−64%, P = 0.004) and severity (−64%, P = 0.051) in the aortic root compared with control. Consistent with our data, Kühnast et al. 18 reported a dose-dependent decrease in atherosclerosis development in APOE*3Leiden.CETP mice treated with the human PCSK9 monoclonal antibody alirocumab. Notably, the AT04A vaccination was applied as a stand-alone therapy and thus, future evaluations are required to test whether these effects are still ensured on a statin background treatment.
For many years, atherosclerosis was considered to be mainly a lipid-driven disease caused by the continuous accumulation of cholesterol in the arterial intima. However, it is increasingly recognized that atherosclerosis is predominately a chronic low-grade inflammatory disease of the vessel wall with an interplay of humoral, cellular, and locally produced pro-inflammatory factors.29 , 30 One of the initial stages of atherosclerotic disease is endothelial cell activation and the recruitment of inflammatory cells to the vessel wall.29 , 30 Multi-analyte profiling of inflammatory markers showed a reduction of several cytokines with chemotactic activity for monocytes by AT04A vaccine (see Supplementary material online, Table S I) which is in line with the reduced expression of endothelial ICAM-1 and adherence of monocytes to the vessel wall. The decreased levels of M-CSF-1 and VEGF-A, which activate endothelial cells and stimulate monocyte/macrophage migration, may have additionally contributed to the reduction in lesion number and size.
Interestingly, despite the reduced monocyte adherence, the macrophage content was not affected by AT04A immunization. A potential explanation is that the amount of macrophages is similar but that the macrophages in the control group, are more activated and overloaded with cholesterol which may result in damage and necrosis. The necrotic core is a major characteristic of advanced atherosclerotic lesions and plays an important role in both their progression to a hazardous state and their vulnerability.31 To test this hypothesis, we measured the necrotic core and NLRP3 as marker of macrophage activation. Morphological analysis indicated that vaccination with AT04A strongly decreased the necrotic core content, including cholesterol clefts, by 77%. Overload of lipid material in the vessel wall leads to apoptosis and necrosis of foam cells,32 and to deposition of cholesterol crystals,33 , 34 which stimulate caspase-1-activating NLRP3 inflammasomes and aggravate the inflammatory response.33 , 35 Indeed NLRP3 expression in the macrophage area was three-fold increased in the control mice as compared to the AT04-treated mice, which substantiates this hypothesis. Collectively, these data indicate that immunization with AT04A diminishes the formation of a necrotic core and macrophage inflammation, which may have contributed to the reduced inflammatory response in the vessel wall and attenuation of atherosclerotic development.
Little is known about the pro-inflammatory role of PCSK9. Inflammatory cytokines were measured in the plasma from septic shock patients carrying either a PCSK9 LOF allele or a PCSK9 GOF allele. Significantly lower plasma cytokine concentrations in patients carrying a LOF allele were found.36 These results mirrored the data obtained from PCSK9 knockout mice, which displayed decreases in inflammatory cytokine production in response to LPS.36 It was also reported that PCSK9 of macrophage origin directly promotes lesion inflammation in mice, independently of systemic lipid changes. PCSK9 accumulated in the artery wall induced infiltration of pro-inflammatory Ly6Chi monocytes into the atherosclerotic lesion, indicating a local effect of human PCSK9 on atherosclerotic lesion composition.37 Thus, lowering of plasma PCSK9 levels per se may also have direct local effects on vascular inflammation and atherosclerotic plaque formation. Unfortunately, we were not able to examine the local effects of PCSK9 in aortic plaques due to known technical limitations.38
The current challenge for the prevention of atherosclerotic events is certainly the side-effect free lowering of lifetime LDLc exposure, thus preventing chronic low-grade inflammatory disease of the vessel walls. The association between the change in relative risk for CHD and the absolute change in LDLc levels over lifetime due to genetic variation has recently been emphasized.39
The AT04A anti-PCSK9 vaccine would be an ideal therapeutic agent to fulfill these requirements for long-term LDLc management, because of its sustained efficacy and cost-effective application, accompanied by anti-inflammatory effects. AT04A is currently being tested in a phase I clinical trial.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
We thank O. Otava for statistical analyses and J. Hutchins for correcting the scientific English.
Conflict of interest: AT04A is a product invented and developed by AFFiRiS which is currently under trial. C.L., C.J., G.S. and G.G. are employees of AFFiRiS. J.W.A.v.d.H., E.J.P. and H.M.G.P. are employees of TNO during the conduct of this work. TNO performed this study partially as fee-for-service work funded by AFFiRiS. J.W.J. received research grants from and was speaker on (CME-accredited) meetings sponsored by Amgen, Astellas, Astra-Zeneca, Biotronik, Boston Scientific, Daiichi Sankyo, Lilly, Genzyme, Medtronic, Merck-Schering-Plough, Pfizer, Orbus Neich, Novartis, Roche, Servier, Sanofi-Aventis, the Netherlands Heart Foundation, the Interuniversity Cardiology Institute of the Netherlands, and the European Community Framework KP7 Program.
Footnotes
See page 2508 for the editorial comment on this article (doi: 10.1093/eurheartj/ehx302)
References
Author notes
Christine Landlinger, Marianne G. Pouwer, Hans M.G. Princen and Gergana Galabova authors contributed equally to this work.



