
Cover image
Percutaneous treatment of severe transvalvular and paravalvular regurgitation in a failing surgical aortic valve prosthesis due to recurrent endocarditis
Ronald K. Binder1*, Fabian Nietlispach1, Tomas Holubec2, and Francesco Maisano2
Department of Cardiology, University Heart Center; and Department of Cardiovascular Surgery, University Hospital Zürich, Rämistrasse 100, Zürich 8091, Switzerland
*Corresponding author. Tel: +41 44 255 17 64, Fax: +41 44 255 44 01, Email: [email protected]
Cardiovascular flashlight
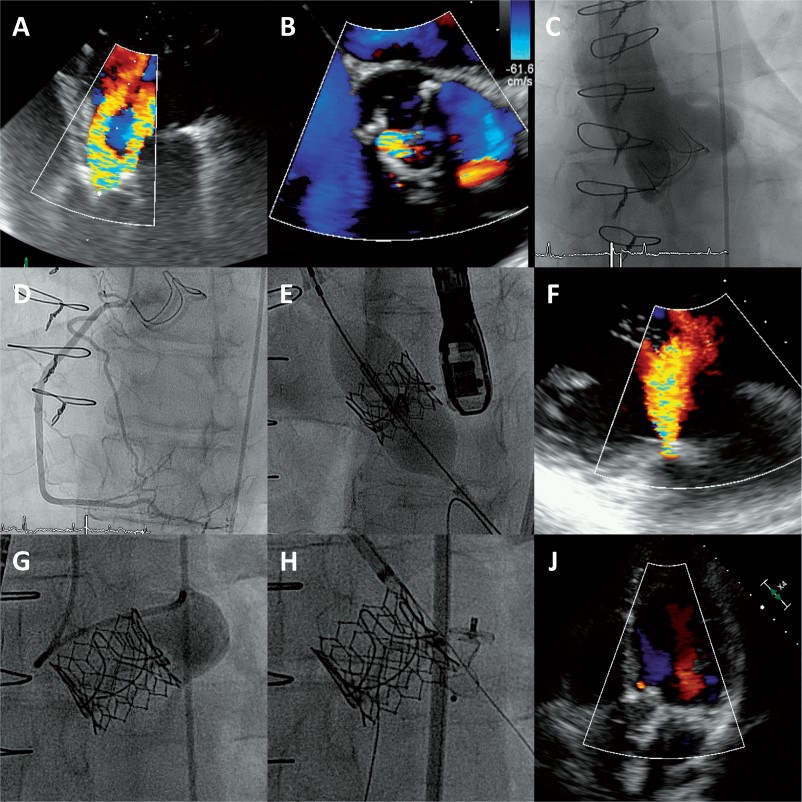
A 45-year-old male intravenous drug user known for human immunodeficiency virus infection and chronic hepatitis-C was admitted due to shortness of breath. Seven months before this admission he had undergone urgent surgical aortic valve replacement with a bioprosthesis (Edwards Perimount Magna 23 mm; Edwards Lifesciences, Irvine, California, USA) due to destructive Serratia marcescens endocarditis. Echocardiography showed new severe transvalvular and paravalvular aortic regurgitation (Figure 1A and B; Supplementary material online, Video S1). Intravenous antibiotics were administered for presumed recurrent prosthetic valve endocarditis. Further on during the therapeutic window of 3 months without antibiotic treatment the patient stayed free of signs of infective endocarditis, but was admitted again with cardiac decompensation. Reoperation of the bioprosthesis vs. transcatheter valve-in-valve implantation with percutaneous paravalvular leak closure was discussed in the heart team. Due to his comorbidities, the recent open-heart surgery and the continuous intravenous drug abuse, it was decided to pursue a percutaneous approach. After assessment of the aortic root (Figure 1C; Supplementary material online, Video S2) and confirming the absence of coronary artery disease (Figure 1D) transfemoral transcatheter aortic valve-in-valve implantation was performed with a balloon-expandable bioprosthesis (Edwards SAPIEN 3, 23 mm; Edwards Lifesciences, Irvine, California, USA; Figure 1E; Supplementary material online, Video S3). Intraprocedural echocardiography (Figure 1F) and angiography (Figure 1G) confirmed the clearing of transvalvular regurgitation but the persistence of severe paravalvular regurgitation (Supplementary material online, Video S4). Subsequently, the paravalvular leak in the left coronary sinus was occluded with an Amplatzer Vascular Plug III 5 x 10 mm (St. Jude Medical, Minessonta, USA; Figure 1H; Supplementary material online, Video S5). Symptoms of heart failure resolved and the post-procedural echocardiography showed no transvalvular and only minimal paravalvular regurgitation (Supplementary material online, Video S6). Two months later, the patient reported normal exercise capacity, no shortness of breath and there were no signs of recurrent endocarditis.
Figure 1 (A) Apical five chamber view with severe prosthetic valve regurgitation showing two jets; (B) Short axis of the surgical bioprosthesis with transvalvular regurgitation between the right and the non-coronary cusp (7 o’clock) and paravalvular regurgitation in the left coronary sinus (4 o’clock); (C) Aortic root angiogram; (D) Right coronary artery; (E) Valve-in-valve implantation of a balloon expandable transcatheter aortic valve prosthesis; (F) Persistent paravalvular regurgitation; (G) Left sinus angiogram with paravalvular leak; (H) Amplatzer vascular plug implantation in the paravalvular leak at the left coronary cusp; (J) Apical four chamber view showing no transvalvular and only minimal paravalvular regurgitation.
Infective endocarditis is considered a contraindication to transcatheter aortic valve implantation and to percutaneous paravalvular leak closure. However, this case illustrates that in selected patients transcatheter valve therapy may be considered after an adequate antibiotic course and therapeutic window. Paravalvular regurgitation has to be meticulously discerned from transvalvular regurgitation but their coexistence does not preclude a successful percutaneous treatment approach.
Supplementary material is available at European Heart Journal online.
Conflict of interest: R.K.B is proctor for Boston Scientific and consultant for Edwards Lifesciences. F.N is consultant for Edwards Lifesciences, Medtronic, St. Jude Medical and Direct Flow Medical. F.M is a consultant for Abbott Vascular, St. Jude Medical, ValtechCardio, and Medtronic; receives royalties from Edwards Lifesciences; and is a cofounder of 4Tech.


