-
PDF
- Split View
-
Views
-
Cite
Cite
Luciano A Sposato, Gregory Y H Lip, Karl Georg Haeusler, Atrial fibrillation first detected after stroke: is timing and detection intensity relevant for stroke risk?, European Heart Journal, Volume 45, Issue 5, 1 February 2024, Pages 396–398, https://doi.org/10.1093/eurheartj/ehad744
Close - Share Icon Share
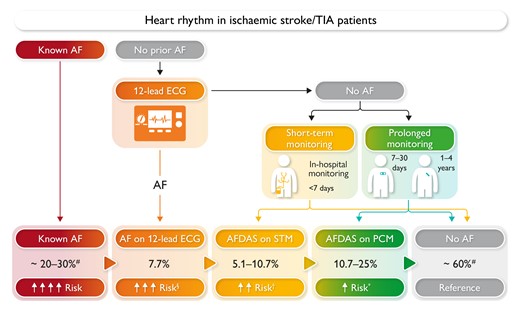
Proportions represent the incidence of atrial fibrillation diagnostic yields with a sequential increase in the intensity of cardiac rhythm monitoring based on Sposato et al..6 Risk: recurrent ischaemic stroke risk. §Five-fold higher than prolonged cardiac monitoring–detected atrial fibrillation. †More data are needed to characterize the stroke risk of atrial fibrillation found on 24–48 h Holter. *Five-fold lower than 12-lead electrocardiogram-diagnosed atrial fibrillation. AF, atrial fibrillation; AFDAS, atrial fibrillation detected after stroke; ECG, electrocardiogram; PCM, prolonged cardiac monitoring; STM, short-term monitoring.
Introduction
Atrial fibrillation (AF) is responsible for up to one-third of all strokes worldwide, and AF-related ischaemic strokes are more frequently fatal or disabling than in non-AF patients.1 The proliferation of novel devices and their technical improvements have resulted in a steady increase in the use of prolonged cardiac monitoring (PCM) for detecting AF post-stroke in those with no history of AF. The rationale behind PCM post-stroke is that finding AF would warrant oral anticoagulation with the aim of preventing recurrent strokes, although evidence from randomized controlled trials is lacking for this patient population. Prolonged cardiac monitoring increases AF detection rates.2,3 However, randomized controlled trials have not demonstrated a benefit of PCM for secondary stroke prevention, either by using implantable loop recording (ILR) or by repetitive Holter electrocardiograms (ECGs),4 as the effect of oral anticoagulation may be minor or absent.
Current knowledge about AF embolic risk stratification is based on 12-lead ECG-detected AF. However, stroke risk in PCM-detected AF differs from clinically known AF diagnosed on a 12-lead ECG.5 Prolonged cardiac monitoring–detected AF is defined as any AF found on prolonged external monitoring. Atrial fibrillation first detected in stroke patients on PCM is generally paroxysmal and has a lower AF burden,4 lower prevalence of risk factors and cardiovascular comorbidities,5 milder structural heart disease (e.g. smaller left atrium or atrial substrate),5 and lower stroke recurrence risk5 than clinical AF diagnosed before stroke onset, which is generally detected on a 12-lead ECG or short-term Holter ECG. Indeed, a recent meta-analysis has shown that the risk of stroke recurrence in patients with AF first detected after stroke is 26% lower than in those with known AF.5
Classification of heart rhythm in stroke patients
Based on the distinctive characteristics of AF first detected after ischaemic stroke or transient ischaemic attack (TIA), patients can be conceptually classified into three categories: No AF, AF clinically known before stroke or TIA occurrence (known AF), and AF first detected after stroke or TIA (AFDAS) (Graphical Abstract).4,6 Of note, a baseline ECG is a standard-of-care test in the post-ischaemic stroke diagnostic workup and can newly detect AF in 8% of patients without clinically known AF.7 Although 12-lead ECG-detected AF represents a new diagnosis post-stroke, it is not included under the category of AFDAS because it is most probably a high-burden AF, entailing a higher risk of stroke recurrence and systemic embolism.4,8 AFDAS includes all types of AF detected on PCM or during short-term cardiac monitoring in stroke patients. Prolonged cardiac monitoring ranges from 7 days of Holter monitoring (prescribed monitoring duration regardless of the net monitoring potentially affected by signal noise or earlier discontinuation of monitoring) to several years of ILR.6 A total of 7 days of monitoring is the shortest prescribed duration resulting in an increase in AF detection compared with standard of care.2 Short-term monitoring is defined as <7 days (using Holter monitoring) and in-hospital telemetry monitoring6 (Graphical Abstract).
Brief epidemiology of atrial fibrillation in stroke patients
Atrial fibrillation screening can utilize a range of technologies from simple monitoring strategies to implantation of an ILR, aligned with ‘looking harder, looking longer, and looking in more sophisticated ways’ to increase the detection of AF, almost independently of the presumed stroke aetiology and similar to non-stroke patients without or with cardiovascular risk factors.9 The prevalence of known AF in population-based studies ranges between 20% and 30% in stroke patients, although it varies in relation to the age of study cohorts.4 The incidence of AFDAS depends on AF episode definition, the duration and the quality of the ECG analysis of post-stroke PCM, ranging from ∼6% for 5 days of Holter monitoring2 to up to 30% after using an ILR for 3 years (Graphical Abstract).3 Although we have initially proposed that the early initiation of cardiac monitoring is associated with higher AF detection yield,7 a subsequent meta-analysis did not find a significant association between these variables.10
Differences between 12-lead electrocardiogram-detected and prolonged cardiac monitoring–detected atrial fibrillation after an ischaemic stroke
The Differences in ECG- Versus Prolonged Cardiac Monitoring-Detected Atrial Fibrillation in STROKE Patients (DELIMIT-AF STROKE) study compared the time to the first recurrent ischaemic stroke (primary endpoint) in 218 ischaemic stroke or TIA patients with 12-lead ECG-detected AF and in 148 stroke patients with PCM-detected (14 days of Holter monitoring) AF from the London Ontario Stroke Registry.8 The median follow-up was 1.7 years. Both groups had an anticoagulation rate of >80%. On Cox proportional hazard models accounting for the competing risk of death and adjusted for oral anticoagulation and other variables known to influence stroke risk, 12-lead ECG-detected AF was associated with a 5-fold higher risk of ischaemic stroke recurrence than PCM-detected AF (adjusted hazard ratio 5.1, 95% confidence interval 1.1–23). At baseline, patients with 12-lead ECG-detected AF had a lower left ventricular ejection fraction, a larger left atrial volume index, and higher high-sensitivity troponin T levels. These differences resemble those observed between AFDAS and known AF,5 suggesting that 12-lead ECG-detected AF should be considered to have a similar stroke risk to known AF.4 The median duration of PCM-detected AF was 5.2 h [interquartile range (IQR), 0.3–33 h], and the median AF burden was 2.2% (IQR, 0.1–12). Overall, 70% of patients had a total duration of AF lasting ≤24 h, 52% lasted <5.5 h, and 19% lasted <6 min. The results of the DELIMIT-AF STROKE study support that (i) a considerable proportion of AFDAS is low burden; (ii) AFDAS occurs in patients with healthier cardiovascular profiles; and (iii) AFDAS is associated with a lower risk of stroke than 12-lead ECG-detected AF.
Clinical and research implications, knowledge gaps
From the clinician’s point of view, it seems relevant to distinguish whether AFDAS is causal, stroke-induced, or just a bystander, as a competing stroke aetiology is found in ∼15% of ischaemic stroke AF patients.1 At present, AFDAS is regarded as a risk factor for thromboembolism irrespective of the mode of detection, although emerging evidence suggests that a more personalized approach to secondary prevention may be possible in the future by identifying specific risk phenotypes based on the interplay between AF burden (e.g. number of AF episodes, duration of the longest AF episode, or the percentage of time spent in AF during a defined period) and other markers of embolic risk. Currently, oral anticoagulation is recommended for stroke prevention in all patients with any AF episode ≥30 s after ischaemic stroke or TIA.11 However, recent evidence suggests that not all patients with AFDAS may benefit from anticoagulation and that a more personalized AFDAS-specific approach to risk stratification may be warranted. In this regard, AFDAS may sometimes be a transient phenomenon. Atrial fibrillation detected after stroke persistence and recurrence rates are unclear, as previous ILR-based studies and randomized controlled trials did not address this topic. The level of AF burden in AFDAS patients that substantially increases the risk of recurrent stroke is unknown and should be addressed in future ILR-based trials. Whether there are parallels in this respect to pacemaker/ILR studies such as TRENDS (NCT00279981), ASSERT (NCT00256152), NOAH-AFNET 6 (NCT02618577), and ARTESiA (NCT01938248), which did not primarily include stroke patients, can be assumed from the authors’ point of view, but cannot be regarded as certain. Of note, ASSERT identified a higher stroke risk with a 24 h AF duration. As such, the risk of stroke recurrence in stroke patients with AFDAS lasting <24 h remains unknown. The placebo-controlled NOAH-AFNET 6 trial showed that oral anticoagulation using edoxaban did not reduce the incidence of a composite of cardiovascular death, stroke, or systemic embolism compared with placebo, or aspirin if indicated for another reason, but led to a higher incidence of a composite of death or major bleeding in (primarily non-stroke) patients with atrial high rate episodes detected by implantable devices.12 There were no differences between edoxaban and placebo with regard to the secondary outcome of ischaemic stroke either. Only 18.2% of the cohort was found to have ECG-diagnosed AF.
A randomized clinical trial comparing direct oral anticoagulants vs. anti-platelet agents in patients with a total duration of ILR-detected AFDAS episodes <24 h with normal atrial size may be justified. However, some patients and physicians may be reluctant to participate, despite the fact that there is no evidence from a randomized controlled trial regarding recurrent stroke risk reduction by oral anticoagulation. In line with this, the Sweet spoT for cArdiac Rhythm monitorinG After sTrokE (STARGATE) pilot clinical trial funded by three Canadian agencies is applying ILRs to all stroke patients and randomizing them 1:1 to a disclosure group in which all AFDAS episodes lasting >6 min are communicated to the treating team and a non-disclosure group in which participants are notified only if the AFDAS episode lasts >24 h (NCT05717504).
Another knowledge gap is whether rhythm control therapy in AFDAS patients (or patients with AF first diagnosed within the last 12 months) may lead to a reduction in the risk of recurrent stroke, as this was not the case in the numerically comparatively small stroke cohort in the EAST-AFNET 4 trial.13 Finally, attention should be paid to cognitive dysfunction in AFDAS patients during follow-up, as demonstrated for primarily ECG-detected or short-term Holter-detected AF in the Ontario Stroke Registry.14 Whether 12-lead ECG- or PCM-detected AF is of similar importance for cognitive decline remains to be established in future trials.
Declarations
Disclosure of Interest
L.A.S. reports speaker/consulting honoraria from Boehringer Ingelheim, Pfizer, Bayer, and Gore as well as research grants from Boehringer Ingelheim, Medtronic and Bayer. L.A.S. is member of the Editorial Board: Neurology, Stroke, and JAHA; co-editor: Neurocardiology section of Stroke; associate editor: JAHA. G.Y.H.L. reports consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Daiichi Sankyo, Anthos; no fees are received personally. G.Y.H.L. is co-principal investigator of the AFFIRMO project on multimorbidity in AF, which has received funding from the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 899871). K.G.H. reports speaker’s honoraria, consulting fees, lecture honoraria, and/or study grants from Abbott, Amarin, Alexion, AstraZeneca, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Portola, Premier Research, Sanofi, SUN Pharma, and W.L. Gore and Associates.



