-
PDF
- Split View
-
Views
-
Cite
Cite
Marina M Demidova, Fredrik Holmqvist, David Erlinge, Pyotr G Platonov, Ventricular arrhythmias during ST-segment elevation myocardial infarction and arrhythmic complications during recurrent ischaemic events, European Heart Journal, Volume 45, Issue 5, 1 February 2024, Pages 393–395, https://doi.org/10.1093/eurheartj/ehad740
Close - Share Icon Share
Introduction
Early malignant ventricular arrhythmias (VAs) during the course of ST-elevation myocardial infarction (STEMI) markedly contribute to in-hospital mortality, yet have no influence on long-term prognosis,1,2 as the cause of arrhythmia is believed to be reversed after revascularization.3 It is unknown why some patients develop VA during ischaemia, while others in a seemingly similar situation do not. Despite a number of proposed risk factors associated with a higher risk of VA in STEMI, predicting malignant VA in an individual patient remains challenging. In this study, we aimed to test the hypothesis that VA during STEMI indicates an increased susceptibility to ischaemia-induced VA and is therefore associated with a higher risk of VA during recurrent ischaemic episodes.
Methods
Consecutive STEMI patients discharged alive after primary percutaneous coronary intervention (PCI) during 2007–10 were included in the study and followed up until 31 December 2017. The Swedish Web System for Enhancement and Development of Evidence-based care in Heart disease Evaluated According to Recommended Therapies (SWEDEHEART) registry was used to assess demographic, clinical, and angiography characteristics. Patients who underwent cardiopulmonary resuscitation (CPR) or defibrillation for malignant early VA (haemodynamically unstable ventricular tachycardia or fibrillation) during the first 48 h of STEMI were identified using SWEDEHEART. All instances of CPR and VA were verified in the medical records.
SWEDEHEART was used to identify repeated admissions to the cardiac intensive care unit (ICU) during follow-up. Medical records were accessed to verify acute coronary syndrome (ACS) as the cause of readmission.
To account for patients who had ischaemia-induced out-of-hospital sudden cardiac death (SCD) and were therefore not included in SWEDEHEART, survival status and the cause of death were retrieved from the Swedish National Cause of Death Registry. Sudden cardiac death was deemed ischaemia induced if a patient had either of the ICD-10 codes, I46, I46.1, or I46.9 (sudden death), and either of the codes I20, I21, or I25 (ischaemic heart disease) as the primary cause of death. All suspected ischaemic SCD cases were verified, and an arrhythmia mechanism (shockable vs. non-shockable rhythm) was identified using medical records and death certificates. Information regarding the use of implantable cardioverter defibrillators (ICDs) was obtained from the Swedish ICD and Pacemaker Registry.
The association between VA during index STEMI and VA during repeated ACS (re-ACS) was tested using logistic regression analysis adjusted for age, gender, and reduced left ventricular ejection fraction (LVEF <30%) at discharge in all re-ACS patients and in the subgroup of patients who did not have myocardial infarction before index STEMI.
Student’s t-test or the Mann–Whitney test was used for continuous variables, and Fisher’s exact test was used for categorical variables. All tests were two-sided, and P-values <.05 were considered statistically significant. SPSS 28.0 package (SPSS Inc., Chicago, IL, USA) was used for analysis.
The study was approved by the Regional Ethics Committee in Lund, Sweden (# 2010/585).
Results
The study cohort comprised 2148 patients discharged after primary PCI for STEMI (age 66 ± 12 years, 70% males, 15% with prior myocardial infarction, 35% smokers, 7% with LVEF <30% at discharge, and 30% with inferior STEMI). Early VA was observed in 151 patients. During follow-up, 159 patients were admitted to the ICU with re-ACS. Eight more patients developed fatal ischaemia-related out-of-hospital cardiac arrest and were included in the re-ACS group.
In total, 167 patients had re-ACS (62 STEMI, 50 non-STEMI, 47 unstable angina, and 8 ischaemia-related SCD), of whom 163 (98%) were fully revascularized at index STEMI. The median time from index STEMI to re-ACS was 198 (25–1373) days. Two of the 55 patients who received ICD during follow-up developed re-ACS without early VA.
A total of 11 patients (6.6%) had VA during re-ACS. Patients who had VA during index STEMI had a greater incidence of VA during re-ACS (33.3% vs. 3.9%, P < .001). Ventricular arrhythmia at baseline was independently associated with VA during re-ACS [adjusted odds ratio (ORadj) 8.22, 95% confidence interval (CI) 1.78–38.05; P = .007]; the association remained significant in the subgroup of patients without a history of myocardial infarction (n = 117; ORadj 12.06, 95% CI 2.26–64.39; P = .004). Ventricular arrhythmia during re-ACS was not associated with either a history of myocardial infarction prior to the index STEMI, infarct localization, the completeness of revascularization at index STEMI, or therapy at discharge.
Discussion
Patients with early VA in STEMI were eight times more likely to have VA at re-ACS during follow-up than patients without VA in index STEMI (Figure 1). Ventricular arrhythmia during re-ACS was not associated with other comorbidities, medical therapy, or angiographic characteristics at the time of re-ACS.
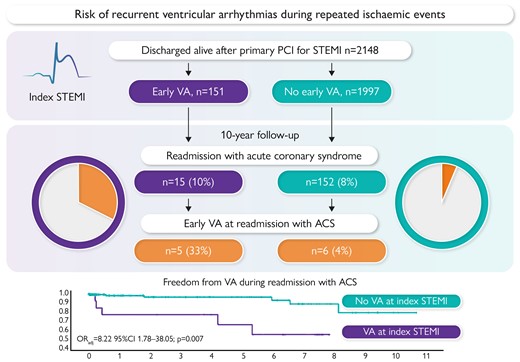
Risk of recurrent ventricular arrhythmias during repeated ischaemic events.
Our findings suggest that some individuals are predisposed to malignant VA and SCD related to acute ischaemia, although the cause of this susceptibility remains unclear. Recent studies reported an increased risk of VA and SCD in the setting of ACS in patients with a family history of SCD,4,5 thus supporting a possible genetic predisposition. Identifying the genetic factors that make the heart vulnerable to ischaemia-induced arrhythmias is difficult because of the wide range of possible pathophysiological pathways, such as those related to atherothrombosis, cardiac ion channels,6,7 gap junctions,8,9 and extra-cardiac mechanisms responsible for neural regulation and control.10
Our study cannot clarify what genetic factors underlie the occurrence of ischaemia-related malignant VA. Based on our observational data, we can speculate that the small group of patients who are defibrillated during the early hours of STEMI and who are at a high risk of recurrent ischaemia may have a higher risk of recurrent VA, and may therefore benefit from ICD therapy. More research is needed to test this hypothesis; however, our data indicate that post-STEMI SCD risk stratification should include the presence, type, and timing of VA, as well as the likelihood of recurrent ischaemia.
Limitations
While the study patients were prospectively followed up, the cohort emerged from a retrospective registry-based study. Post-mortem examinations were not routinely performed. The observed low rate of re-ACS and the number of VA during re-ACS limit an accurate estimation of the risk of VA in recurrent ischaemia.
Conclusion
Despite a generally benign long-term prognosis, STEMI patients with early VA remain at risk of recurrent arrhythmic complications in case of repeated ischaemic events. Our data support the need for an individualized risk assessment and secondary prevention strategies for patients at high risk of ischaemia recurrence.
Declarations
Disclosure of Interest
All authors declare no disclosure of interest for this contribution.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Funding
This study was supported by the Swedish Heart Lung Foundation (#20180222 to M.M.D. and #20200674 to P.G.P.), Donation funds at the Skane University Hospital (grant #96336, P.G.P.) and Governmental funding of clinical research by the Swedish National Health Service (ALF, grant #46702, P.G.P.).
Ethical Approval
The study was approved by the Regional Ethics Committee in Lund, Sweden (# 2010/585, 29 November 2010).
Pre-registered Clinical Trial Number
Not applicable.



