-
PDF
- Split View
-
Views
-
Cite
Cite
Julien Dreyfus, Fernando Juarez-Casso, Alessandra Sala, Manuel Carnero-Alcazar, Andrea Eixerés-Esteve, Yohann Bohbot, Baptiste Bazire, Michele Flagiello, Elisabeth Riant, Yannick Mbaki, Jacques Tomasi, Thomas Senage, Kenza Rahmouni El Idrissi, Augustin Coisne, Damien Eyharts, Fabien Doguet, Florence Viau, Florian Eggenspieler, Samuel Heuts, Peyman Sardari Nia, Gregor Heitzinger, Xavier Galloo, Nina Ajmone Marsan, Giovanni Benfari, Luigi Badano, Denisa Muraru, Francesco Maisano, Yan Topilsky, Hector Michelena, Maurice Enriquez-Sarano, Jeroen Bax, Philipp Bartko, Christine Selton-Suty, Gilbert Habib, Yoan Lavie-Badie, Thomas Modine, Vincent Chan, Thierry Le Tourneau, Erwan Donal, Pascal Lim, Costin Radu, Jordan Bernick, George A Wells, Christophe Tribouilloy, Bernard Iung, Jean-François Obadia, Michele De Bonis, Juan Crestanello, David Messika-Zeitoun, on behalf of the TRIGISTRY Investigators, Benefit of isolated surgical valve repair or replacement for functional tricuspid regurgitation and long-term outcomes stratified by the TRI-SCORE, European Heart Journal, Volume 45, Issue 42, 7 November 2024, Pages 4512–4522, https://doi.org/10.1093/eurheartj/ehae578
Close - Share Icon Share
Abstract
Severe tricuspid regurgitation is associated with increased mortality rates, but benefit of its correction and ideal timing are not clearly determined. This study aimed to identify patient subsets who might benefit from the surgery.
In TRIGISTRY, an international cohort study of consecutive patients with severe isolated functional tricuspid regurgitation (33 centres, 10 countries), survival rates up to 10 years were compared between patients who underwent isolated tricuspid valve surgery (repair or replacement) and those conservatively managed, overall and according to TRI-SCORE category (low: ≤3, intermediate: 4–5, and high: ≥6).
One thousand and two hundred seventeen were managed conservatively, and 551 underwent isolated tricuspid valve surgery (200 repairs and 351 replacements). TRI-SCORE distribution was 33% low, 32% intermediate, and 35% high. At 10 years, survival rates were similar between surgical and conservative management [41% vs. 36%; hazard ratio (HR) .97; 95% confidence interval (CI) .88–1.08, P = .57]. Surgery improved survival compared with conservative management in the low TRI-SCORE category (72% vs. 44%; HR .27; 95% CI .20–.37, P < .0001), but not in the intermediate (36% vs. 37%; HR 1.17; 95%CI .98–1.40, P = .09) or high categories (20% vs. 24%; HR 1.06; 95% CI .91–1.25, P = .45). Both repair and replacement improved survival in the low TRI-SCORE category (84% and 61% vs. 44%; HR .11; 95% CI .06–.19, P < .0001, and HR .65; 95% CI .47–.90, P = .009). Repair showed benefit in the intermediate category (59% vs. 37%; HR .49; 95% CI .35–.68, P < .0001) while replacement was possibly harmful (25% vs. 37%; HR 1.43; 95% CI 1.18–1.72, P = .0002).
Higher survival rates were observed with repair than replacement and benefit of intervention declined as TRI-SCORE increased with no benefit of any type of surgery in the high TRI-SCORE category. These results emphasize the importance of timely intervention and patient selection to achieve the best outcomes and the need for randomized controlled trials.
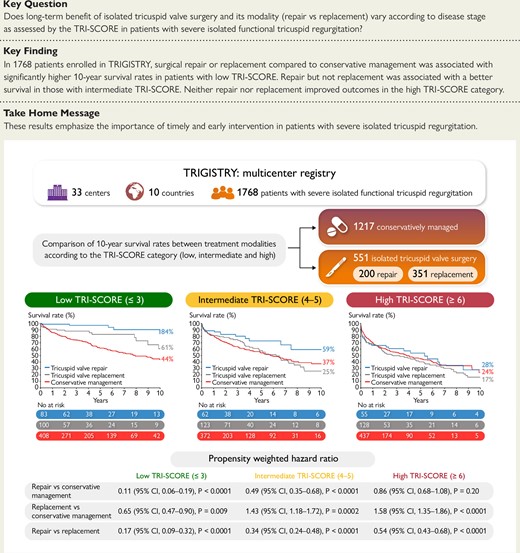
Comparison of the 10-year survival rates between surgical repair, surgical replacement, and conservative management according to the TRI-SCORE category in patients with severe isolated functional tricuspid regurgitation shows a benefit of both surgical modalities in the low TRI-SCORE category, a benefit of repair only in the intermediate TRI-SCORE category, and no benefit from either repair or replacement in the high TRI-SCORE category.
See the editorial comment for this article ‘Tricuspid valve trilogy: between risks and timing for surgery’, by N. Bonaros et al., https://doi.org/10.1093/eurheartj/ehae562.
Introduction
Tricuspid regurgitation (TR) is an important public health problem. Tricuspid regurgitation prevalence is high and expected to further increase as the population ages, and TR is associated with increased risk of mortality and morbidity.1–5 North American and European Societies of Cardiology/Cardiac Surgery Societies recommend performing isolated tricuspid valve (TV) surgery in patients with isolated severe TR who are symptomatic or who present with right ventricular (RV) dilatation in the absence of severe RV dysfunction.6,7 However, despite the poor outcomes associated with severe TR and recommendations to perform an isolated TV surgery, most patients remain conservatively managed with diuretics to relieve symptoms, while surgical correction for severe isolated TR is seldom performed even in the recent era.8–10 The reluctance to refer patients for isolated TV surgery stems from the high post-operative mortality rate observed in most series and the uncertainties regarding the benefit of TR correction.11,12
One key element driving the outcome after isolated TV surgery is TR disease stage and the timing of the intervention.13–18 The TRI-SCORE, based on eight parameters reflecting the severity of the clinical presentation as well as RV, kidney, and liver consequences, accurately predicts in-hospital mortality after isolated TV surgery (see Supplementary data online, Figure S1).19,20 Its prognostic value has been further extended to transcatheter and conservatively managed cohorts not only for predicting in-hospital mortality but also mid-term outcome.16–18,21–24 In TRIGISTRY, a large multicentre international registry of patients with severe isolated functional TR, we have previously shown a potential benefit of isolated TV surgery compared with conservative management in selected patient subsets but only up to 2 years, and potential impact of type of surgery was not evaluated.24 In the present study, we aimed to compare long-term survival rates of isolated TV surgery and conservative management according to TR clinical stage as assessed using the TRI-SCORE and the impact of the type of surgical intervention—repair vs. replacement.
Methods
Study design
TRIGISTRY (ClinicalTrials.gov, NCT05825898) is an international multicentre registry across 10 countries (Austria, Canada, France, Germany, Israel, Italy, Netherlands, Spain, Switzerland, and the USA) and 33 centres retrospectively gathering consecutive adult patients with severe isolated functional TR on native valve who were either conservatively managed or underwent isolated TV surgery or transcatheter TV repair. Inclusion and exclusion criteria have been previously reported.24 Functional TR was defined by structurally normal TV. Tricuspid regurgitation severity was assessed using an integrative multiparametric approach.25,26 Patients with moderate or greater concomitant left-sided valvular heart disease and patients who underwent aortic or mitral valve intervention (either surgical or transcatheter) within 3 months prior to the TV intervention were excluded. In the surgical group, only patients who underwent isolated TV surgery (repair or replacement) were included (i.e. no concomitant intervention such as mitral or aortic valve surgery, or coronary artery bypass graft). In the present study, we excluded patients who underwent transcatheter TV intervention (Figure 1) due to the limited follow-up duration in this subset. Analyses were performed by the Cardiovascular Research Methods Center at the University of Ottawa Heart Institute. The list of participating centres and investigators is provided in the Supplementary data online, Appendix S1. The study was conducted in accordance with local institutional policies and was approved by each local institutional review board.
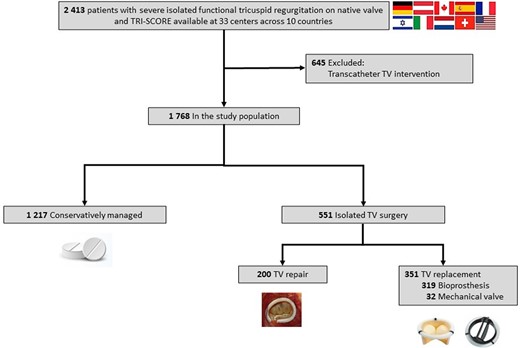
Participant flow in the study. TRIGISTRY includes 2413 patients with severe isolated functional tricuspid regurgitation on native valve collected across 33 centres and 10 countries. We excluded 645 who underwent a transcatheter tricuspid valve intervention. Our study population comprised 1768 patients: 1217 were conservatively managed and 551 underwent an isolated tricuspid valve surgery (surgical tricuspid valve repair in 200 and a replacement in 351)
Baseline characteristics and primary endpoint
Clinical, laboratory, echocardiographic, and outcome information was collected locally by each centre and centralized in a unique depository anonymized database at the University of Ottawa Heart Institute. The TRI-SCORE ranges from 0 to 12 points, and the population was divided into three subsets based on previously validated TRI-SCORE risk categories and predicted post-operative mortality (low risk: ≤3 points, intermediate risk: 4–5 points, and high risk: ≥6 points) (see Supplementary data online, Figure S1).19,20,24 The primary endpoint was survival up to 10 years.
Statistical analysis
Variables were expressed as mean ± standard deviation or median (25th–75th percentile) for continuous variables and number of patients (percentage) for categorical variables. Comparisons of baseline characteristics between the treatment groups were performed using analysis of variance, χ2, Student’s t-test, or non-parametric Wilcoxon test as appropriate. Time zero was defined as the time of the first echocardiography showing significant TR for the medical population and the time of surgery for the surgical population. None of the patients conservatively managed underwent TV intervention. To compare survival rates between conservative management and surgery, inverse probability of treatment weighting was used to minimize baseline differences between treatment groups. The weights were obtained by taking the inverse of the propensity scores, representing the probability of receiving a treatment conditional on observed covariates. A logistic regression model was used to estimate the propensity scores for each individual, with the treatment groups as a dependent variable and the baseline covariates as independent variables. Variables were selected a priori by the clinical investigators and included comorbidities and TRI-SCORE parameters. Standardized differences for each variable before and after weighting were calculated with a difference < .10 indicating a good balance. Hazard ratios (HR) and 95% confidence intervals (CIs) were calculated using the conservative management group as reference. Survival rates were presented using the Kaplan–Meier method overall and in each TRI-SCORE risk category. Proportionality was assessed using a treatment by logtime interaction term in the proportional hazard models where time to death at 10 years was the outcome. Analyses were performed using SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Study population
We enrolled 1768 adult patients with severe isolated functional TR on native valve: 1217 (69%) patients were conservatively managed and 551 (31%) underwent isolated TV surgery (Figure 1). Before weighting, baseline characteristics of the conservative management and surgical groups differed significantly (Table 1; Supplementary data online, Table S1). Patients in the surgical group were younger and more frequently female, with more prior left heart valve interventions; exhibited greater symptomatic severity [New York Heart Association (NYHA) functional class III–IV, right-sided heart failure signs, and higher daily doses of diuretics]; and were more frequently in atrial fibrillation and with more RV dilatation, but had lower incidence of diabetes, coronary artery disease, left ventricular and RV dysfunction, and lower systolic pulmonary artery pressure. There was no significant difference in the permanent pacemaker rate. TRI-SCORE distribution was similar in both groups, with each risk score category accounting for approximately one-third of the population. The proportional hazards assumption was checked, and no violation was detected. P-value for interaction across models varies from .07 to .95. After propensity weighting, variables were well balanced (standardized deviation ≤ .1).
Baseline characteristics of the medical and surgical groups, overall and according to the type of surgery
| Characteristics . | Medical (n = 1217) . | Surgical (n = 551) . | P-value surgical vs. medical . | Standardized difference not weighted . | Standardized difference weighted . | Surgical repair (n = 200) . | Surgical replacement (n = 351) . | P-value repair vs. replacement . |
|---|---|---|---|---|---|---|---|---|
| Clinical | ||||||||
| Age, years | 71 ± 13 | 68 ± 11 | <.0001 | .2736 | .0571 | 68 ± 11 | 68 ± 11 | .93 |
| Age ≥ 70 years, no. (%)a | 769 (63) | 283 (51) | <.0001 | 102 (51) | 181 (52) | .90 | ||
| Female sex, no. (%) | 535 (44) | 348 (63) | <.0001 | −.3732 | .0557 | 128 (64) | 220 (63) | .76 |
| Male sex, no. (%) | 682 (56) | 203 (37) | 72 (36) | 131 (37) | ||||
| Diabetes mellitus, no. (%) | 339 (28) | 118 (21) | .0042 | −.1447 | −.1020 | 38 (19) | 80 (23) | .30 |
| Chronic lung disease, no. (%) | 241 (20) | 90 (16) | .083 | −.0893 | .0206 | 26 (13) | 64 (18) | .11 |
| Coronary artery disease, no. (%) | 529 (43) | 132 (24) | <.0001 | −.4129 | .0083 | 44 (22) | 88 (25) | .42 |
| Permanent pacemaker, no./total no. (%) | 319 (26) | 145/544 (27) | .85 | 36/198 (18) | 109/346 (32) | .0007 | ||
| Prior left heart valve intervention, no. (%) | 157 (13) | 257 (47) | <.0001 | .7550 | −.0176 | 74 (37) | 183 (52) | .0006 |
| New York Heart Association functional class III–IV, no. (%)a | 637 (52) | 366 (66) | <.0001 | .2855 | .0502 | 120 (60) | 246 (70) | .016 |
| Right-sided heart failure signs, no. (%)a | 660 (54) | 364 (66) | <.0001 | .2908 | −.0271 | 116 (58) | 248 (71) | .0026 |
| Daily dose of loop diuretics, mg | 40 [32–80] | 60 [40–120] | .0013 | 60 [40–120] | 60 [40–60] | .78 | ||
| Daily dose of loop diuretics ≥ 125 mg, no. (%)a | 145 (12) | 129 (23) | <.0001 | .3047 | .0117 | 47 (24) | 82 (23) | .97 |
| Atrial fibrillation, no./total no. (%) | 685/1204 (57) | 367 (67) | <.0001 | .1546 | .0021 | 139 (70) | 228 (65) | .28 |
| Laboratory | ||||||||
| Haemoglobin, g/dL | 12.1 ± 2.2 | 12.2 ± 1.9 | .33 | −.0537 | .0564 | 12.6 ± 1.9 | 12 ± 2 | .0018 |
| Creatinine, µmol/L | 129 ± 90 | 112 ± 63 | <.0001 | .2009 | .0091 | 107 ± 49 | 115 ± 70 | .14 |
| Glomerular filtration rate, mL/min | 67 ± 39 | 64 ± 29 | .23 | 67 ± 30 | 63 ± 28 | .19 | ||
| Glomerular filtration rate < 30 mL/min, no. (%)a | 148 (12) | 37 (7) | .0005 | −.1863 | .0134 | 13 (7) | 24 (7) | .88 |
| Elevated total bilirubin, no. (%)a | 373 (31) | 169 (31) | .99 | −.0302 | .0746 | 52 (26) | 117 (33) | .073 |
| Echocardiographic | ||||||||
| Left ventricle ejection fraction, % | 43 ± 15 | 57 ± 10 | <.0001 | 58 ± 9 | 57 ± 10 | .45 | ||
| Left ventricle ejection fraction < 60%, no. (%)a | 992 (82) | 265 (48) | <.0001 | −.7340 | .0242 | 90 (45) | 175 (50) | .27 |
| Tricuspid annulus diameter, mm | 43 ± 8 | 47 ± 7 | <.0001 | 45 ± 6 | 48 ± 8 | .008 | ||
| Moderate/severe right ventricular dilatation, no./total no. (%) | 594/1217 (49) | 442 (80) | <.0001 | 150 (75) | 292 (83) | .020 | ||
| Moderate/severe right ventricular dysfunction, no. (%)a | 657 (54) | 173 (31) | <.0001 | −.4498 | .1020 | 56 (28) | 117 (33) | .19 |
| Systolic pulmonary artery pressure, mmHg | 54 ± 19 | 42 ± 11 | <.0001 | 42 ± 12 | 42 ± 10 | .80 | ||
| TRI-SCORE | 5 [3–6] | 4 [3–6] | .13 | 4 [2–6] | 5 [3–6] | .0026 | ||
| ≤3, no. (%) | 408 (34) | 183 (33) | 83 (42) | 100 (28) | ||||
| 4–5, no. (%) | 372 (31) | 185 (34) | 62 (31) | 123 (35) | ||||
| ≥6, no. (%) | 437 (36) | 183 (33) | 55 (28) | 128 (36) |
| Characteristics . | Medical (n = 1217) . | Surgical (n = 551) . | P-value surgical vs. medical . | Standardized difference not weighted . | Standardized difference weighted . | Surgical repair (n = 200) . | Surgical replacement (n = 351) . | P-value repair vs. replacement . |
|---|---|---|---|---|---|---|---|---|
| Clinical | ||||||||
| Age, years | 71 ± 13 | 68 ± 11 | <.0001 | .2736 | .0571 | 68 ± 11 | 68 ± 11 | .93 |
| Age ≥ 70 years, no. (%)a | 769 (63) | 283 (51) | <.0001 | 102 (51) | 181 (52) | .90 | ||
| Female sex, no. (%) | 535 (44) | 348 (63) | <.0001 | −.3732 | .0557 | 128 (64) | 220 (63) | .76 |
| Male sex, no. (%) | 682 (56) | 203 (37) | 72 (36) | 131 (37) | ||||
| Diabetes mellitus, no. (%) | 339 (28) | 118 (21) | .0042 | −.1447 | −.1020 | 38 (19) | 80 (23) | .30 |
| Chronic lung disease, no. (%) | 241 (20) | 90 (16) | .083 | −.0893 | .0206 | 26 (13) | 64 (18) | .11 |
| Coronary artery disease, no. (%) | 529 (43) | 132 (24) | <.0001 | −.4129 | .0083 | 44 (22) | 88 (25) | .42 |
| Permanent pacemaker, no./total no. (%) | 319 (26) | 145/544 (27) | .85 | 36/198 (18) | 109/346 (32) | .0007 | ||
| Prior left heart valve intervention, no. (%) | 157 (13) | 257 (47) | <.0001 | .7550 | −.0176 | 74 (37) | 183 (52) | .0006 |
| New York Heart Association functional class III–IV, no. (%)a | 637 (52) | 366 (66) | <.0001 | .2855 | .0502 | 120 (60) | 246 (70) | .016 |
| Right-sided heart failure signs, no. (%)a | 660 (54) | 364 (66) | <.0001 | .2908 | −.0271 | 116 (58) | 248 (71) | .0026 |
| Daily dose of loop diuretics, mg | 40 [32–80] | 60 [40–120] | .0013 | 60 [40–120] | 60 [40–60] | .78 | ||
| Daily dose of loop diuretics ≥ 125 mg, no. (%)a | 145 (12) | 129 (23) | <.0001 | .3047 | .0117 | 47 (24) | 82 (23) | .97 |
| Atrial fibrillation, no./total no. (%) | 685/1204 (57) | 367 (67) | <.0001 | .1546 | .0021 | 139 (70) | 228 (65) | .28 |
| Laboratory | ||||||||
| Haemoglobin, g/dL | 12.1 ± 2.2 | 12.2 ± 1.9 | .33 | −.0537 | .0564 | 12.6 ± 1.9 | 12 ± 2 | .0018 |
| Creatinine, µmol/L | 129 ± 90 | 112 ± 63 | <.0001 | .2009 | .0091 | 107 ± 49 | 115 ± 70 | .14 |
| Glomerular filtration rate, mL/min | 67 ± 39 | 64 ± 29 | .23 | 67 ± 30 | 63 ± 28 | .19 | ||
| Glomerular filtration rate < 30 mL/min, no. (%)a | 148 (12) | 37 (7) | .0005 | −.1863 | .0134 | 13 (7) | 24 (7) | .88 |
| Elevated total bilirubin, no. (%)a | 373 (31) | 169 (31) | .99 | −.0302 | .0746 | 52 (26) | 117 (33) | .073 |
| Echocardiographic | ||||||||
| Left ventricle ejection fraction, % | 43 ± 15 | 57 ± 10 | <.0001 | 58 ± 9 | 57 ± 10 | .45 | ||
| Left ventricle ejection fraction < 60%, no. (%)a | 992 (82) | 265 (48) | <.0001 | −.7340 | .0242 | 90 (45) | 175 (50) | .27 |
| Tricuspid annulus diameter, mm | 43 ± 8 | 47 ± 7 | <.0001 | 45 ± 6 | 48 ± 8 | .008 | ||
| Moderate/severe right ventricular dilatation, no./total no. (%) | 594/1217 (49) | 442 (80) | <.0001 | 150 (75) | 292 (83) | .020 | ||
| Moderate/severe right ventricular dysfunction, no. (%)a | 657 (54) | 173 (31) | <.0001 | −.4498 | .1020 | 56 (28) | 117 (33) | .19 |
| Systolic pulmonary artery pressure, mmHg | 54 ± 19 | 42 ± 11 | <.0001 | 42 ± 12 | 42 ± 10 | .80 | ||
| TRI-SCORE | 5 [3–6] | 4 [3–6] | .13 | 4 [2–6] | 5 [3–6] | .0026 | ||
| ≤3, no. (%) | 408 (34) | 183 (33) | 83 (42) | 100 (28) | ||||
| 4–5, no. (%) | 372 (31) | 185 (34) | 62 (31) | 123 (35) | ||||
| ≥6, no. (%) | 437 (36) | 183 (33) | 55 (28) | 128 (36) |
Values are number of patients (percentage), mean ± standard deviation, or median [inter-quartiles].
aThe eight parameters included in the TRI-SCORE.
Baseline characteristics of the medical and surgical groups, overall and according to the type of surgery
| Characteristics . | Medical (n = 1217) . | Surgical (n = 551) . | P-value surgical vs. medical . | Standardized difference not weighted . | Standardized difference weighted . | Surgical repair (n = 200) . | Surgical replacement (n = 351) . | P-value repair vs. replacement . |
|---|---|---|---|---|---|---|---|---|
| Clinical | ||||||||
| Age, years | 71 ± 13 | 68 ± 11 | <.0001 | .2736 | .0571 | 68 ± 11 | 68 ± 11 | .93 |
| Age ≥ 70 years, no. (%)a | 769 (63) | 283 (51) | <.0001 | 102 (51) | 181 (52) | .90 | ||
| Female sex, no. (%) | 535 (44) | 348 (63) | <.0001 | −.3732 | .0557 | 128 (64) | 220 (63) | .76 |
| Male sex, no. (%) | 682 (56) | 203 (37) | 72 (36) | 131 (37) | ||||
| Diabetes mellitus, no. (%) | 339 (28) | 118 (21) | .0042 | −.1447 | −.1020 | 38 (19) | 80 (23) | .30 |
| Chronic lung disease, no. (%) | 241 (20) | 90 (16) | .083 | −.0893 | .0206 | 26 (13) | 64 (18) | .11 |
| Coronary artery disease, no. (%) | 529 (43) | 132 (24) | <.0001 | −.4129 | .0083 | 44 (22) | 88 (25) | .42 |
| Permanent pacemaker, no./total no. (%) | 319 (26) | 145/544 (27) | .85 | 36/198 (18) | 109/346 (32) | .0007 | ||
| Prior left heart valve intervention, no. (%) | 157 (13) | 257 (47) | <.0001 | .7550 | −.0176 | 74 (37) | 183 (52) | .0006 |
| New York Heart Association functional class III–IV, no. (%)a | 637 (52) | 366 (66) | <.0001 | .2855 | .0502 | 120 (60) | 246 (70) | .016 |
| Right-sided heart failure signs, no. (%)a | 660 (54) | 364 (66) | <.0001 | .2908 | −.0271 | 116 (58) | 248 (71) | .0026 |
| Daily dose of loop diuretics, mg | 40 [32–80] | 60 [40–120] | .0013 | 60 [40–120] | 60 [40–60] | .78 | ||
| Daily dose of loop diuretics ≥ 125 mg, no. (%)a | 145 (12) | 129 (23) | <.0001 | .3047 | .0117 | 47 (24) | 82 (23) | .97 |
| Atrial fibrillation, no./total no. (%) | 685/1204 (57) | 367 (67) | <.0001 | .1546 | .0021 | 139 (70) | 228 (65) | .28 |
| Laboratory | ||||||||
| Haemoglobin, g/dL | 12.1 ± 2.2 | 12.2 ± 1.9 | .33 | −.0537 | .0564 | 12.6 ± 1.9 | 12 ± 2 | .0018 |
| Creatinine, µmol/L | 129 ± 90 | 112 ± 63 | <.0001 | .2009 | .0091 | 107 ± 49 | 115 ± 70 | .14 |
| Glomerular filtration rate, mL/min | 67 ± 39 | 64 ± 29 | .23 | 67 ± 30 | 63 ± 28 | .19 | ||
| Glomerular filtration rate < 30 mL/min, no. (%)a | 148 (12) | 37 (7) | .0005 | −.1863 | .0134 | 13 (7) | 24 (7) | .88 |
| Elevated total bilirubin, no. (%)a | 373 (31) | 169 (31) | .99 | −.0302 | .0746 | 52 (26) | 117 (33) | .073 |
| Echocardiographic | ||||||||
| Left ventricle ejection fraction, % | 43 ± 15 | 57 ± 10 | <.0001 | 58 ± 9 | 57 ± 10 | .45 | ||
| Left ventricle ejection fraction < 60%, no. (%)a | 992 (82) | 265 (48) | <.0001 | −.7340 | .0242 | 90 (45) | 175 (50) | .27 |
| Tricuspid annulus diameter, mm | 43 ± 8 | 47 ± 7 | <.0001 | 45 ± 6 | 48 ± 8 | .008 | ||
| Moderate/severe right ventricular dilatation, no./total no. (%) | 594/1217 (49) | 442 (80) | <.0001 | 150 (75) | 292 (83) | .020 | ||
| Moderate/severe right ventricular dysfunction, no. (%)a | 657 (54) | 173 (31) | <.0001 | −.4498 | .1020 | 56 (28) | 117 (33) | .19 |
| Systolic pulmonary artery pressure, mmHg | 54 ± 19 | 42 ± 11 | <.0001 | 42 ± 12 | 42 ± 10 | .80 | ||
| TRI-SCORE | 5 [3–6] | 4 [3–6] | .13 | 4 [2–6] | 5 [3–6] | .0026 | ||
| ≤3, no. (%) | 408 (34) | 183 (33) | 83 (42) | 100 (28) | ||||
| 4–5, no. (%) | 372 (31) | 185 (34) | 62 (31) | 123 (35) | ||||
| ≥6, no. (%) | 437 (36) | 183 (33) | 55 (28) | 128 (36) |
| Characteristics . | Medical (n = 1217) . | Surgical (n = 551) . | P-value surgical vs. medical . | Standardized difference not weighted . | Standardized difference weighted . | Surgical repair (n = 200) . | Surgical replacement (n = 351) . | P-value repair vs. replacement . |
|---|---|---|---|---|---|---|---|---|
| Clinical | ||||||||
| Age, years | 71 ± 13 | 68 ± 11 | <.0001 | .2736 | .0571 | 68 ± 11 | 68 ± 11 | .93 |
| Age ≥ 70 years, no. (%)a | 769 (63) | 283 (51) | <.0001 | 102 (51) | 181 (52) | .90 | ||
| Female sex, no. (%) | 535 (44) | 348 (63) | <.0001 | −.3732 | .0557 | 128 (64) | 220 (63) | .76 |
| Male sex, no. (%) | 682 (56) | 203 (37) | 72 (36) | 131 (37) | ||||
| Diabetes mellitus, no. (%) | 339 (28) | 118 (21) | .0042 | −.1447 | −.1020 | 38 (19) | 80 (23) | .30 |
| Chronic lung disease, no. (%) | 241 (20) | 90 (16) | .083 | −.0893 | .0206 | 26 (13) | 64 (18) | .11 |
| Coronary artery disease, no. (%) | 529 (43) | 132 (24) | <.0001 | −.4129 | .0083 | 44 (22) | 88 (25) | .42 |
| Permanent pacemaker, no./total no. (%) | 319 (26) | 145/544 (27) | .85 | 36/198 (18) | 109/346 (32) | .0007 | ||
| Prior left heart valve intervention, no. (%) | 157 (13) | 257 (47) | <.0001 | .7550 | −.0176 | 74 (37) | 183 (52) | .0006 |
| New York Heart Association functional class III–IV, no. (%)a | 637 (52) | 366 (66) | <.0001 | .2855 | .0502 | 120 (60) | 246 (70) | .016 |
| Right-sided heart failure signs, no. (%)a | 660 (54) | 364 (66) | <.0001 | .2908 | −.0271 | 116 (58) | 248 (71) | .0026 |
| Daily dose of loop diuretics, mg | 40 [32–80] | 60 [40–120] | .0013 | 60 [40–120] | 60 [40–60] | .78 | ||
| Daily dose of loop diuretics ≥ 125 mg, no. (%)a | 145 (12) | 129 (23) | <.0001 | .3047 | .0117 | 47 (24) | 82 (23) | .97 |
| Atrial fibrillation, no./total no. (%) | 685/1204 (57) | 367 (67) | <.0001 | .1546 | .0021 | 139 (70) | 228 (65) | .28 |
| Laboratory | ||||||||
| Haemoglobin, g/dL | 12.1 ± 2.2 | 12.2 ± 1.9 | .33 | −.0537 | .0564 | 12.6 ± 1.9 | 12 ± 2 | .0018 |
| Creatinine, µmol/L | 129 ± 90 | 112 ± 63 | <.0001 | .2009 | .0091 | 107 ± 49 | 115 ± 70 | .14 |
| Glomerular filtration rate, mL/min | 67 ± 39 | 64 ± 29 | .23 | 67 ± 30 | 63 ± 28 | .19 | ||
| Glomerular filtration rate < 30 mL/min, no. (%)a | 148 (12) | 37 (7) | .0005 | −.1863 | .0134 | 13 (7) | 24 (7) | .88 |
| Elevated total bilirubin, no. (%)a | 373 (31) | 169 (31) | .99 | −.0302 | .0746 | 52 (26) | 117 (33) | .073 |
| Echocardiographic | ||||||||
| Left ventricle ejection fraction, % | 43 ± 15 | 57 ± 10 | <.0001 | 58 ± 9 | 57 ± 10 | .45 | ||
| Left ventricle ejection fraction < 60%, no. (%)a | 992 (82) | 265 (48) | <.0001 | −.7340 | .0242 | 90 (45) | 175 (50) | .27 |
| Tricuspid annulus diameter, mm | 43 ± 8 | 47 ± 7 | <.0001 | 45 ± 6 | 48 ± 8 | .008 | ||
| Moderate/severe right ventricular dilatation, no./total no. (%) | 594/1217 (49) | 442 (80) | <.0001 | 150 (75) | 292 (83) | .020 | ||
| Moderate/severe right ventricular dysfunction, no. (%)a | 657 (54) | 173 (31) | <.0001 | −.4498 | .1020 | 56 (28) | 117 (33) | .19 |
| Systolic pulmonary artery pressure, mmHg | 54 ± 19 | 42 ± 11 | <.0001 | 42 ± 12 | 42 ± 10 | .80 | ||
| TRI-SCORE | 5 [3–6] | 4 [3–6] | .13 | 4 [2–6] | 5 [3–6] | .0026 | ||
| ≤3, no. (%) | 408 (34) | 183 (33) | 83 (42) | 100 (28) | ||||
| 4–5, no. (%) | 372 (31) | 185 (34) | 62 (31) | 123 (35) | ||||
| ≥6, no. (%) | 437 (36) | 183 (33) | 55 (28) | 128 (36) |
Values are number of patients (percentage), mean ± standard deviation, or median [inter-quartiles].
aThe eight parameters included in the TRI-SCORE.
Long-term outcome according to TRI-SCORE category
Follow-up was available in 98% of patients [mean: 3.4 ± 3.1 years and median: 2.4 years (.70–5.67) overall; mean: 3.3 ± 3.1 years and median: 2.2 years (.68–5.57) in the conservative management group; and mean: 3.6 ± 3.2 years and median: 2.8 years (.71–5.72) in the surgical group). Median and mean follow-up significantly decreased as TRI-SCORE category increased [overall: mean: 4.4 ± 3.3 years and median: 3.8 years (1.41–7.12); mean: 3.4 ± 3.0 years and median: 2.5 years (.78–5.66); and mean: 2.5 ± 2.7 years and median: 1.5 years (.30–3.84), in the low, intermediate, and high TRI-SCORE categories, respectively, P < .0001] reflecting the impact of the severity of the clinical presentation and its impact on mortality.
In-hospital mortality
Overall, the mean in-hospital mortality rate after isolated TV surgery was 9.6% (n = 53). This rate significantly increased with the TRI-SCORE category (2.7%, 9.2%, and 16.9% in the low, intermediate, and high TRI-SCORE categories, respectively, P < .0001).
Overall
During follow-up, there were 529 deaths in the conservative management group and 187 in the surgical group (including in-hospital deaths). At 10 years, the inverse propensity weighted survival rate was not different between the two treatment groups (41% vs. 36% in the surgical vs. conservative management group; HR .97; 95% CI .88–1.08, P = .57) (Figure 2A).
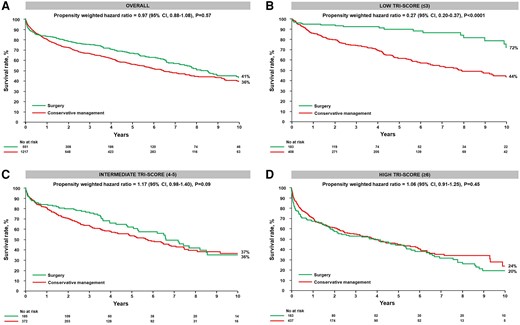
Survival rate according to medical or surgical treatment and TRI-SCORE category. The figure displays the Kaplan–Meier survival curves of the conservative management and surgical groups at 10 years: (A) overall and (B) in the low TRI-SCORE (≤3 points), (C) intermediate TRI-SCORE (4–5 points), and (D) high TRI-SCORE (≥6 points) categories
Low TRI-SCORE
The inverse propensity weighted survival rate at 10 years was higher in the surgical group than in the conservative management group (72% vs. 44%; adjusted HR .27; 95% CI .20–.37, P < .0001) (Figure 2B).
Intermediate TRI-SCORE
At 10 years, the inverse propensity weighted survival rates were not different between groups (36% and 37% in the surgical vs. conservative management group; HR 1.17; 95% CI .98–1.40, P = .09) (Figure 2C).
High TRI-SCORE
The inverse propensity weighted survival rates at 10 years were also not different between groups in the high TRI-SCORE subset (20% and 24% in the surgical vs. conservative management group; HR 1.06; 95% CI .91–1.25, P = .45) (Figure 2D).
Impact of the type of intervention
Among the 551 patients who underwent isolated TV surgery, TV repair was performed in 200 patients (36%) and a TV replacement in 351 patients (64%), of whom 91% received a bioprosthetic valve. Patients in the TV replacement group had more prior left heart valve interventions and permanent pacemakers compared with patients in the TV repair group. They also exhibited greater symptomatic severity (NYHA functional class III–IV and right-sided heart failure signs), more RV dilatation, and higher TRI-SCORE categories. On the contrary, there was no significant difference in age, sex, presence of diabetes, coronary artery disease, chronic lung disease, daily doses of diuretics, rate of atrial fibrillation, liver and kidney function, left ventricular function, and RV function, as well as in pulmonary artery pressures between the two groups (Table 1).
In-hospital mortality
Overall, the in-hospital mortality rate was not different between repair and replacement (8.5% and 10.3%, respectively, P = .50). These rates significantly increased with the TRI-SCORE category both in the repair and in the replacement groups (1.2%, 9.7%, and 18.1% in the repair group and 4%, 8.9%, and 16.4% in the replacement group in the low, intermediate, and high TRI-SCORE categories, P = .002 and P < .0001, respectively).
Overall
At 10 years, the inverse propensity weighted survival rate was higher in the repair group than in the conservative management group (61% vs. 36%; adjusted HR .46; 95% CI .39–.55, P < .0001) while it was lower in the replacement group (30% vs. 36%; adjusted HR 1.45; 95% CI 1.35–1.69, P < .0001) (Figure 3A). As a result, the inverse propensity weighted survival rate was higher in the repair group compared with the replacement group (HR .31; 95% CI .26–.37, P < .0001).
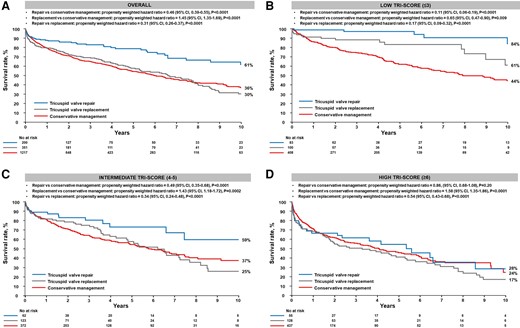
Survival rate according to the type of surgical intervention and TRI-SCORE category. The figure displays Kaplan–Meier survival curves of the conservative management, the surgical tricuspid valve repair, and the surgical tricuspid valve replacement groups at 10 years: (A) overall and (B) in the low TRI-SCORE (≤3 points), (C) intermediate TRI-SCORE (4–5 points), and (D) high TRI-SCORE (≥6 points) categories
Low TRI-SCORE
The inverse propensity weighted survival rates at 10 years were higher in both the repair and replacement groups compared with the conservative management group (84% and 61% vs. 44%, respectively; HR .11; 95% CI .06–.19, P < .0001, and HR .65; 95% CI .47–.90, P = .009, respectively). The inverse propensity weighted survival rate was also higher in the repair group compared with the replacement group (HR .17; 95% CI .09–.32, P < .0001) (Figure 3B).
Intermediate TRI-SCORE
At 10 years, compared with the conservative management group, the inverse propensity weighted survival rates were higher in the repair group but lower in the replacement group (59% and 25% vs. 37%, respectively; HR .49; 95% CI .35–.68, P < .0001, and HR 1.43; 95% CI 1.18–1.72, P = .0002, respectively) (Figure 3C). The inverse propensity weighted survival rate was also higher in the repair compared with the replacement group (HR .34; 95% CI .24–.48, P < .0001).
High TRI-SCORE
The inverse propensity weighted survival rates at 10 years were similar between the repair and conservative management groups (28% vs. 24%; adjusted HR .86; 95% CI .68–1.08, P = .20) while the replacement group exhibited a lower survival than the conservative management groups (17% vs. 24%; HR 1.58; 95% CI 1.35–1.86, P < .0001). Survival was also higher in the repair than in the replacement group (28% vs. 17%; HR .54, 95% CI .43–.68, P < .0001) (Figure 3D).
Discussion
In this large multicentre international registry of patients with severe functional TR, we evaluated the long-term benefit of isolated TV surgery compared with conservative management according to TR disease stage as assessed by the TRI-SCORE. At 10 years, there was no benefit of surgery compared with conservative management overall. However, surgery was associated with higher survival rates in the low TRI-SCORE category but not in the intermediate and high TRI-SCORE categories. Survival rates were higher in the repair than in the replacement groups. Compared with conservative management, both repair and replacement showed a beneficial impact on survival in the low TRI-SCORE category, while only surgical repair was associated with better survival in the intermediate TRI-SCORE category. No benefit of either surgical repair or replacement was observed in the high TRI-SCORE category (Structured Graphical Abstract).
In addition to the difficulties of evaluating symptoms in patients with TR and the challenges of assessing TR severity, reluctance to perform an isolated TV surgery relies on two contradictory beliefs, on the one side that TR is a benign condition and on the other side that isolated TV surgery is associated with a high—prohibitive—mortality. Therefore, patients suffering from TR are usually conservatively managed for a long period of time until an intervention is considered at an advanced disease stage and likely too late. However, both premises are erroneous. Evidence is accumulating that TR presence and severity are associated with an increasing risk of mortality. In a large patient-level database of almost half million patients, TR was associated with a significant increased mortality risk that increased with TR severity reaching up to 50% at 5 years in patients with severe TR. Critically, the increased mortality risk was observed in all subgroups irrespective of age, sex, comorbidities, and associated conditions.4 Nevertheless, causality remains to be demonstrated, and TRILUMINATE, the only published randomized controlled trial, failed to demonstrate a benefit of transcatheter correction of TR over conservative management on survival—and admission for heart failure—at 1 year.27 This gap fuels the undertreatment of TR patients. On the other hand, the 10% in-hospital mortality rate reported in most surgical series hides important disparities with mortality rates ranging from 2% to more than 50% in selected subsets. Clinical presentation is the main driver of outcome, and excellent outcomes can be achieved if surgery is performed early in the course of the disease while an intervention is likely futile at the other end of the spectrum.14,15
Recently, the TRI-SCORE has been developed to predict in-hospital mortality after isolated TV surgery. The TRI-SCORE is specific to TR and based on eight easy to ascertain clinical, biological, and echocardiographic parameters (age, NYHA functional class, right-sided heart failure signs, daily dose of furosemide, glomerular filtration rate, total bilirubin level, left ventricular ejection fraction, and RV function). Its accuracy has been further confirmed and externally validated in other surgical series including in the Asian population.16–18 Interestingly, the TRI-SCORE also predicts mid-term outcome of TR patients referred for a transcatheter intervention as well as the outcome of conservatively managed patients.21–24 As shown in Supplementary data online, Table S1, the TRI-SCORE reflects the consequences of TR, including the severity of symptoms such as NYHA functional class III/IV and signs of right-sided heart failure, along with significant secondary organ dysfunction, including reduced glomerular filtration rate and elevated bilirubin level. As such, it is associated with more advanced disease. Therefore, the TRI-SCORE is ideally suited to assess the benefit of interventions in relatively homogeneous population based on disease stage.
We have previously shown a benefit of isolated TV surgery on survival in patients with low TRI-SCORE (0–3) but only at 2 years.24 In the present study, we evaluated the benefit of isolated TV surgery compared with conservative management up to 10 years. In the low TRI-SCORE category, we observed an absolute 28% higher survival rate in the surgical group compared with the conservatively managed group while no benefit was observed in the intermediate and high TRI-SCORE subsets. To the best of our knowledge, such long-term follow-up has never been reported before. Importantly, there was no survival rate difference between surgery and conservative management in the overall population possibly explaining the absence of benefit of TV intervention observed in prior series including in TRILUMINATE.27 In addition, we were able to further refine this analysis and to evaluate the benefit of the type of surgery—TV repair vs. replacement (which accounted for approximately 2/3 of the interventions). Overall, repair was associated with better survival rates than replacement in all TRI-SCORE categories. In the low TRI-SCORE category, both types of surgery were associated with higher survival rates than conservative management (+40% and +17% at 10 years in the surgical repair and replacement group, respectively). In the intermediate group, repair but not replacement was associated with better survival than conservative management while in the high TRI-SCORE category, neither repair nor replacement seemed to provide any benefit on survival. It is worth noting that compared with conservative management, replacement was even harmful in intermediate and high TRI-SCORE categories. Critically, we are certainly not implying that repair is intrinsically superior to replacement in patients with TR but that patients who underwent a repair seemed to derive a better survival than those who underwent a replacement, which is consistent with a recent meta-analysis.28 Indeed, although the TRI-SCORE enabled us to account for TR disease stage and consequences, we were not able to consider or adjust for anatomical characteristics or TR grade (severe–massive–torrential) which likely differed between patients who underwent a repair or a replacement and explain the high rate of replacement in TRIGISTRY compared with the high repair rate when the tricuspid surgery is performed at the time of left-sided heart valve surgery. However, these results carry significant clinical implications when considering an isolated TV surgery, and the heart team assists patients in making informed decisions. In patients with low TRI-SCORE, an isolated TV surgery could be considered irrespective of anatomical considerations while in those with intermediate TRI-SCORE, a more aggressive approach might be pursued only when the likelihood of a successful repair is high. In contrast, isolated TV surgery is likely futile or even detrimental in patients with high TRI-SCORE. Critically, these results should not be extrapolated to transcatheter interventions. In contrast to surgical repair, transcatheter TV repair is associated with a high rate of moderate/severe residual TR (between 30% and 50%)27,29 which negatively impacts the outcome.30 Conversely, transcatheter TV replacement usually eliminates TR. Future studies are needed to clarify the respective role of surgical and transcatheter TV repair and replacement, but our study clearly highlights the importance of performing a TV intervention at an early disease stage as defined by clinical (TRI-SCORE) and by anatomical characteristics (enabling TV repair) to achieve optimal outcomes.
Several limitations of the present study deserve comments. First, there is inherent bias associated with its retrospective design. However, our population was derived from consecutive collection of TR patients at each centre. Second, our study was observational, and there were significant differences between groups. Although there was no crossover between groups, to account for differences between treatment groups, we used inverse propensity weight matching with relatively well-balanced groups and compared outcomes between treatment modalities according to disease stage as assessed by the TRI-SCORE. Nevertheless, we cannot exclude confounders, and future randomized controlled trials are needed to confirm these results. Third, we only evaluated survival, and potential benefit of each treatment modality on heart failure could not be assessed. Fourth, the rate of residual TR post-TV repair (or replacement) was very low (3%), but the rate of TR recurrence and its impact on outcome could not be assessed and deserve further study. Fifth, the TRI-SCORE was used as a surrogate for the severity of the clinical presentation. Longitudinal prospective studies are needed to formally demonstrate that the TRI-SCORE reflects clinical stage and TR disease duration. Sixth, there were differences in treatment modalities between men and women. The impact of sex and its interaction with the TRI-SCORE in relation to the benefits of surgery warrant dedicated analysis. Finally, as previously discussed, there were likely significant differences between patients who underwent a TV repair and those who underwent a TV replacement that we could not account for. Therefore, superiority of repair over replacement (and its potential harm) cannot be derived from this study. Nevertheless, we show a better outcome of patients with severe functional TR who underwent a repair compared with those who underwent a replacement based on local assessment performed by each team. Randomized controlled trials enrolling patients suitable for both treatment modalities are needed to establish the superiority of one modality vs. the other.
Conclusion
In this large multicentre international registry, we showed that compared with conservative management, curative surgical intervention, either repair or replacement, significantly improved 10-year survival in patients with low TRI-SCORE, while repair but not replacement was associated with a better survival in those with intermediate TRI-SCORE and neither of them in the high TRI-SCORE category. Our results emphasize the importance of timely intervention and patient selection to achieve the best outcomes. Future randomized controlled trials are clearly needed to confirm these results and especially the role of the TRI-SCORE in guiding the treatment strategy.
Acknowledgements
We thank Dr George Wells and Jordan Bernick for their help with the statistical analysis.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
N.-A.M. received speaker fees from GE Healthcare, Philips Ultrasound, Abbott Vascular, and Omron. L.B. received consulting or speaker fees from Edwards Lifesciences, GE Healthcare, and Philips Medical Systems. P.B. received speaker fees from Abbott Vascular. M.-C.A. received consulting fees from Edwards Lifesciences and for lectures and proctoring from Abbott Vascular and AtriCure. J.B. received lecture fees from Abbott and Edwards Lifesciences. A.C. received speaker fees from Abbott Vascular and GE Healthcare. J.C. received consulting fees from Medtronic. J.D. received consulting fees from Abbott. Y.L.-B. received speaker or proctoring fees from Abbott Vascular, GE Healthcare, and Philips Healthcare. T.-L.T. received speaker fees from GE Healthcare and Bayer. F.M. received grant and/or research institutional support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific Corporation, NVT, Terumo, and Venus; consulting fees and honoraria (personal and institutional) from Abbott, Medtronic, Edwards Lifesciences, Xeltis, Cardiovalve, Occlufit, Simulands, Mtex, Venus, and Squadra; and royalty income/IP rights from Edwards Lifesciences and is shareholder (including share options) of CardioGard, Cardiovalve, Magenta, SwissVortex, Transseptal Solutions, 4Tech, and Perifect. D.-M.Z. received research grants from Edwards. T.M. received speaker or consulting fees from Abbott, Edwards Lifesciences, Medtronic, MicroPort, and GE. J.F.-O. received consulting or speaker fees from Abbott, Delacroix Chevalier, and Medtronic. P.S.N. received consulting fees from NeoChord, Edwards Lifesciences, Medtronic, Abbott, and Simurghy. C.T. received speaker fees from Novartis and Sanofi. All other authors have no relationship to declare.
Data Availability
Data sharing with qualified researchers may be considered after submission of a proposal to Doctor Julien Dreyfus.
Funding
None declared.
Ethical Approval
The study was conducted in accordance with local institutional policies and was approved by each local institutional review board.
Pre-registered Clinical Trial Number
TRIGISTRY: ClinicalTrials.gov, NCT05825898.



