-
Views
-
Cite
Cite
Helmut Raphael Lieder, Umut Paket, Andreas Skyschally, Andreas D Rink, Theodor Baars, Markus Neuhäuser, Petra Kleinbongard, Gerd Heusch, Vago-splenic signal transduction of cardioprotection in humans, European Heart Journal, Volume 45, Issue 34, 7 September 2024, Pages 3164–3177, https://doi.org/10.1093/eurheartj/ehae250
Close - Share Icon Share
Abstract
The spleen serves as an important relay organ that releases cardioprotective factor(s) upon vagal activation during remote ischaemic conditioning (RIC) in rats and pigs. The translation of these findings to humans was attempted.
Remote ischaemic conditioning or electrical auricular tragus stimulation (ATS) were performed in 10 healthy young volunteers, 10 volunteers with splenectomy, and 20 matched controls. Venous blood samples were taken before and after RIC/ATS or placebo, and a plasma dialysate was infused into isolated perfused rat hearts subjected to global ischaemia/reperfusion.
Neither left nor right RIC or ATS altered heart rate and heart rate variability in the study cohorts. With the plasma dialysate prepared before RIC or ATS, respectively, infarct size (% ventricular mass) in the recipient rat heart was 36 ± 6% (left RIC), 34 ± 3% (right RIC) or 31 ± 5% (left ATS), 35 ± 5% (right ATS), and decreased with the plasma dialysate from healthy volunteers after RIC or ATS to 20 ± 4% (left RIC), 23 ± 6% (right RIC) or to 19 ± 4% (left ATS), 26 ± 9% (right ATS); infarct size was still reduced with plasma dialysate 4 days after ATS and 9 days after RIC. In a subgroup of six healthy volunteers, such infarct size reduction was abrogated by intravenous atropine. Infarct size reduction by RIC or ATS was also abrogated in 10 volunteers with splenectomy, but not in their 20 matched controls.
In humans, vagal innervation and the spleen as a relay organ are decisive for the cardioprotective signal transduction of RIC and ATS.
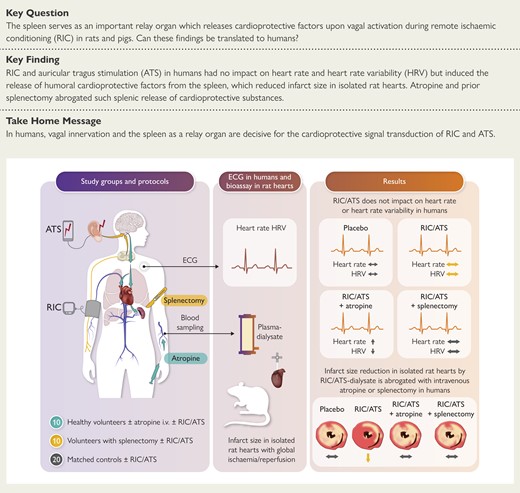
In healthy humans, remote ischaemic conditioning (RIC) by cycles of arm ischaemia/reperfusion and electrical auricular tragus stimulation (ATS) does not alter heart rate and heart rate variability (HRV) but induces the release of circulating cardioprotective factor(s) which, when transferred to an isolated perfused rat heart with global ischaemia/reperfusion, reduce infarct size. This release of cardioprotective factors requires vagal innervation and is abrogated by atropine. The cardioprotective factor(s) are released from the spleen and are absent in humans with prior splenectomy. Both RIC and ATS activate vago-splenic signal transduction for cardioprotection. ECG, electrocardiogram.





