-
PDF
- Split View
-
Views
-
Cite
Cite
Giuseppe Danilo Norata, David Sancho, Jan Van den Bossche, Daniel F J Ketelhuth, on behalf of the European Immunometabolism Network (EIMN), Understanding immunometabolism in cardiovascular disease: translating research into practice, European Heart Journal, Volume 45, Issue 26, 7 July 2024, Pages 2276–2278, https://doi.org/10.1093/eurheartj/ehae131
Close - Share Icon Share
Chronic low-grade inflammation is a particularly relevant risk factor in cardiovascular disease (CVD), contributing to unresolved inflammation and autoimmune responses in the arterial wall that can drive the progression of atherosclerosis as well as heart failure.1 Strategies to harness the innate and adaptive response, such as anti-inflammatory therapies, have been tested to reduce the cardiovascular risk burden. For instance, anti-IL-1β therapy with canakinumab and colchicine was shown to be effective in reducing the incidence of cardiovascular events in high-risk patients. Nevertheless, broad targeting of inflammation in the context of CVD has shown clear limitations, for example, canakinumab significantly increased the risk of death from sepsis in the CANTOS trial,2 whereas the use of colchicine is not an option for individuals with impaired liver and kidney function. Thus, while targeting unresolved arterial inflammation in atherosclerosis seems like a key strategy for patients with residual risk, beyond the control of classical risk factors, other more targeted-specific therapies to control the immunity driving CVD need to be developed.3
Emerging research reveals that metabolism and immunity are completely interrelated processes that play a crucial role in health and disease. This is particularly relevant in cardiometabolic disorders, in which changes in systemic metabolism not only promote inflammation but also contribute to the reprogramming of the cellular metabolism and function of immune cells. In this context, a new field of research, ‘immunometabolism’, has emerged to study how metabolic pathways influence immune cells to carry out their functions, as well as how alterations in immune cell metabolism can influence immunity and inflammation, and their impact on disease (Figure 1).4 It is important to note that the metabolic rewiring induced by immune cell activation fulfils a key role beyond the production of energy and building blocks for biosynthesis; it can regulate the redox balance and control the activity of enzymes and metabolite production that orchestrate signalling, for example, by serving as cofactors and mediating post-translational or epigenetic modifications.
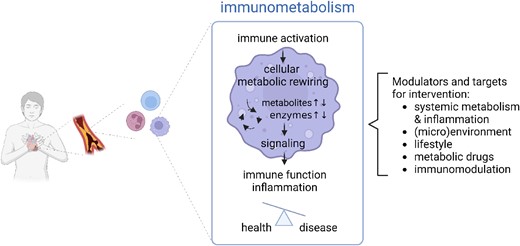
Implication of immunometabolism for cardiometabolic disease. Immunometabolism refers to the interplay between metabolism and immunity. It comprises the studies of metabolic alterations that are induced upon immune activation and regulate inflammatory signalling and immune function, together controlling the balance between health and disease. Cellular immunometabolism is affected by systemic immunometabolism and other external factors and regulate immune cell function, providing multiple opportunities for targeting and disease management
Recent results in the immunometabolism field indicate that the pro-inflammatory signalling associated with CVD triggers a metabolic switch in both myeloid and lymphoid cells, including bone marrow precursors, influencing their differentiation, signalling, and immune effector functions.5 It is well known now that inflammatory macrophages, monocytes, activated B cells, and effector T cells prioritize glycolysis and the conversion of pyruvate to lactate for rapid ATP generation, even in the presence of oxygen (the Warburg effect), which can be accompanied by the reprogramming of sterol, lipid, and amino acid metabolism to support quick cell activation and/or proliferation. In contrast, oxidative phosphorylation and fatty acid oxidation are prevalent in resting and naïve cells and are associated with the maintenance of the anti-inflammatory activity of, for instance, pro-resolution macrophages and regulatory T cells.5 Considering that intracellular metabolic switching is well known and considered a target to combat cancer, the manipulation of immune cell metabolism emerges as a promising strategy to fight uncontrolled infections and autoimmune and autoinflammatory disorders, including CVDs, in the near future.
It should be noted that cardiometabolic disorders represent a more complicated setting for manipulating immunometabolism, as changes in systemic metabolism (as a consequence of diabetes, dyslipidaemia, metabolic syndrome, and obesity) influence the nutrient and metabolite landscape in the circulation and tissues, potentially affecting protective cellular metabolic feedback loops that could help prevent these diseases.6
The big question is how can emerging immunometabolism knowledge be translated into clinical practice? It is known that several drugs targeting metabolism can improve CVD, for example statins, which not only reduce plasma LDL-C levels, but also dampen inflammation.7 However, given the mechanism of action of statins and their primary target (hepatocytes), the likelihood that they can exert robust immunometabolic regulation in immune cells in atherosclerotic plaques, independently of the reduction of lipoprotein levels, is limited. Indeed, other drugs that lower LDL-C by other mechanisms of action, such as ezetimibe, bempedoic acid, or PCSK9 inhibitors, have been suggested to improve the immuno-inflammatory profile independently of their systemic lipid-lowering effects.8 Interestingly, immunomodulatory effects have also been proposed for SGLT2i9 and GLP1RA,10 but it is not clear whether these result from the improvement of the metabolic landscape or may depend on direct effects on cellular immunometabolism.
While repurposing of drugs to modulate the metabolism of immune cells is a possibility, the lack of specificity for many of them may limit their use without wanted or non-wanted systemic effects. In this context, the growing field of immunometabolism research aims at advancing our understanding of the role of specific cellular immunometabolic checkpoints and to develop novel therapeutics that selectively target immune cell subsets. The exciting basic research findings from the field are paving the way for a new era of cardio-immunometabolic pharmacology and better ways to improving CVD outcomes associated with immune activation and inflammation, which are clearly not sufficiently targeted by current guideline therapies.
Declarations
Disclosure of Interest
G.D.N. reports Research Grants from Novartis; consultant to MSD, Amarin, and Viatris. D.S. serves as a scientific adviser for Pulmobiotics and has research collaboration agreements with Inmunotek and Adendra Therapeutics.
Funding
G.D.N. is supported by Progetti di Rilevante Interesse Nazionale (PRIN 2022 7KTSAT), Ricerca Finalizzata, Ministry of Health (RF-2019-12370896), PNRR Missione 4, (Progetto CN3-National Center for Gene Therapy and Drugs based on RNA Technology), PNRR Missione 4 (Progetto MUSA-Multilayered Urban Sustainability Action), PNRR Missione 6 (PNRR-MAD-2022-12375913), European Commission (EUROPEAID/173691/DD/ACT/XK Nanokos), and European Research Area for Health (ERA4Health; GA No. 101095426 of the EU Horizon Europe Research and Innovation Programme). D.F.J.K. is supported by grants from the Novo Nordisk Foundation (0064142; 0075258); Independent Research Fund Denmark (2034-00136B), Simon Fougner Hartmanns Familiefond (2023-0066), and the University of Southern Denmark. J.V.d.B. was funded by a consortia grant from European Research Area Network on Cardiovascular Diseases (ERA-CVD 2019T108), The Netherlands Heart Foundation senior fellowship (2017T048), and an ENW-Klein-1 grant from NWO (OCENW.KLEIN.268). Work in the D.S. laboratory is funded by the CNIC; by MCIN PID2022-137712OB-I00, CPP2021-008310, and CPP2022-009762 MCIN/AEI/10.13039/501100011033 Unión Europea NextGenerationEU/PRTR; by Comunidad de Madrid (P2022/BMD-7333 INMUNOVAR-CM); and by ‘la Caixa’ Foundation (LCF/PR/HR23/52430012, LCF/PR/HR22/52420019, and LCF/PR/HR20/52400015).



