-
PDF
- Split View
-
Views
-
Cite
Cite
Filippo Crea, Non-traditional risk factors: built environment assessed by Google Street View, syphilis, and rheumatoid arthritis, European Heart Journal, Volume 45, Issue 17, 1 May 2024, Pages 1489–1493, https://doi.org/10.1093/eurheartj/ehae261
Close - Share Icon Share
 For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
For the podcast associated with this article, please visit https://dbpia.nl.go.kr/eurheartj/pages/Podcasts.
This Focus Issue on ischaemic heart disease and epidemiology contains the State of the Art Review article ‘Patient–physician sex concordance and outcomes in cardiovascular disease: a systematic review’ by Lamia Harik from Weill Cornell Medicine in New York, USA, and colleagues.1 The authors indicate that the sex disparity in outcomes of patients with cardiovascular (CV) disease is well-described and has persisted across recent decades.2–6 While there have been several proposed mechanisms to explain this disparity, there are limited data on female patient–physician sex concordance and its association with outcomes. The authors review the existing literature on the relationship between patient–physician sex concordance and clinical outcomes in patients with CV disease, the evidence of a benefit in clinical outcomes with female patient–physician sex concordance, and the possible drivers of such a benefit, and highlight directions for future studies.
While the impact of low-grade chronic inflammation on CV risk is well established,7–10 studies on the specific impact of syphilis on the CV system in large populations are limited. In a Clinical Research article entitled ‘Syphilis and cardiovascular risk: a Taiwanese registry’, Victor Chien-Chia Wu from Chang Gung University in Taoyuan City, Taiwan, and colleagues investigated the effects of syphilis on CV outcomes.11 Medical records from 2010 to 2015 were retrieved from the Taiwan National Health Insurance Research Database, linked to the Notifiable Infectious Diseases database from the Taiwan Centers for Disease Control. Patients with syphilis were identified, excluding those with missing information, under 20 years of age, or with a history of human immunodeficiency virus (HIV) infection, acute myocardial infarction (AMI), heart failure, aortic regurgitation, replacement of the aortic valve, aneurysm and/or dissection of the aorta, atrial fibrillation, ischaemic stroke, haemorrhagic stroke, and venous thrombo-embolism. Primary outcomes included new-onset AMI, heart failure, aortic regurgitation, aneurysm and dissection of the aorta, atrial fibrillation, ischaemic stroke, haemorrhagic stroke, venous thrombo-embolism, CV death, and all-cause mortality. After exclusions and frequency matching, 20 601 syphilis patients and 20 601 non-syphilis patients were analysed. The relative rate (RR) was utilized in the analysis, as the competing risk of death was not considered. Compared with patients without syphilis, patients with syphilis had increased risks of AMI (RR 38%, P < .001), heart failure (RR 88%, P < .001), aortic regurgitation (RR 81%, P = .006), atrial fibrillation (RR 45%, P < .001), ischaemic stroke (RR 68%, P < .001), haemorrhagic stroke (RR 114%, P < .001), venous thrombo-embolism (RR 67%, P = .001), CV death (RR 155%, P < .001), and all-cause death (RR 196%, P < .001), but not for aneurysm and dissection of the aorta (Figure 1).
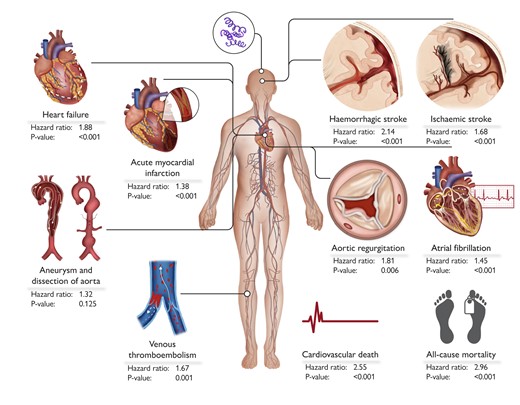
This visual summary delineates the cardiovascular risks and mortality associated with syphilis infection, presenting hazard ratios for key outcomes. These include new-onset acute myocardial infarction, heart failure, aortic regurgitation, aneurysm and/or dissection of the aorta, atrial fibrillation, ischaemic stroke, haemorrhagic stroke, venous thrombo-embolism (deep vein thrombosis or pulmonary embolism), cardiovascular death, and all-cause mortality.11
The authors point out that their study demonstrates that patients with syphilis have a higher risk of cardiovascular events and all-cause mortality compared with those without syphilis. The contribution is accompanied by an Editorial by Natalie Arnold from the University Heart & Vascular Center Hamburg and Wolfgang Koenig from the Technical University of Munich in Germany.12 The authors note that the current situation regarding syphilitic infection is alarming, not only regarding its resurgence in recent years with increasing incidence worldwide, but also regarding the severe CV complications associated with increased mortality in patients with syphilis, as impressively shown by the authors of the present work. More importantly, difficulty in pathogen detection from tissue samples, no available vaccine so far, the unpredictable course of both untreated and treated disease, as well as a huge variety of clinical presentations of syphilis, which might significantly hamper a proper diagnosis, clearly highlight that syphilis remains a public health threat. Thus, more efforts are needed to reduce the incidence of syphilis, which would allow us not only to prevent the occurrence of syphilitic cardiovascular complications, but also to lower the burden of CV mortality attributable to syphilis.
Persons with rheumatoid arthritis (RA) have an increased risk of obstetric-associated complications, as well as long-term CV risk. In a Clinical Research article entitled ‘Rheumatoid arthritis and cardiovascular complications during delivery: a United States inpatient analysis’, Salman Zahid from the Oregon Health and Science University in Portland, OR, USA, and colleagues evaluate the association of RA with acute CV complications during delivery admissions.13 Data from the National Inpatient Sample (2004–2019) were queried utilizing International Classification of Diseases (ICD)-9 or ICD-10 codes to identify delivery hospitalizations and a diagnosis of RA. A total of ∼13 000 000 delivery hospitalizations were identified, of which 0.1% were among women with RA. Individuals with RA, vs. those without, were older and had a higher prevalence of chronic hypertension, chronic diabetes, gestational diabetes mellitus, obesity, and dyslipidaemia. After adjustment for age, race/ethnicity, comorbidities, insurance, and income, RA remained an independent risk factor for peripartum CV complications including pre-eclampsia [adjusted odds ratio (aOR) 1.37], peripartum cardiomyopathy (aOR 2.109), and arrhythmias (aOR 2.00) compared with no RA. Likewise, the risk of acute kidney injury and venous thrombo-embolism was higher with RA. An overall increasing trend of obesity, gestational diabetes mellitus, and acute CV complications was also observed among individuals with RA from 2004 to 2019. Finally, length of stay and cost of hospitalization were higher for deliveries among persons with RA (Figure 2).

Acute peripartum cardiovascular complications with rheumatoid arthritis. RA, rheumatoid arthritis; PCOS, polycystic ovary syndrome; GDM, gestational diabetes mellitus; NIS, National Inpatient Sample13.
Zahid et al. conclude that pregnant persons with RA have a higher risk of pre-eclampsia, peripartum cardiomyopathy, arrhythmias, acute kidney injury, and venous thrombo-embolism during delivery hospitalizations. Furthermore, there is a trend for an increase of cardiometabolic risk factors among pregnant individuals with RA. The contribution is accompanied by an Editorial by Sarah Goldstein and Kathryn Lindley from Yale University School of Medicine in New Haven, CT, USA.14 The authors note that ongoing identification of novel clinical factors associated with maternal cardiovascular adverse outcomes could lead to improved pre-conception counselling. With more accurate and patient-specific risk assessment for obstetric patients without a known history of cardiac disease, intensified and targeted monitoring and management strategies may permit avoidance or prompt treatment of maternal cardiovascular events. Expanded recognition of non-traditional maternal CV risk factors has the potential to be practice changing within the rapidly evolving field of cardio-obstetrics.
The impact of physical environment on the CV system is increasingly acknowledged.15 Tools to evaluate the built environment using machine vision and informatic approaches have been limited. In a Translational Research article entitled ‘Artificial intelligence-based assessment of built environment from Google Street View and coronary artery disease prevalence’, Zhuo Chen from the Harrington Heart and Vascular Institute in Cleveland, OH, USA, and colleagues aimed to investigate the association between machine vision-based built environment and prevalence of cardiometabolic disease in US cities.16 This cross-sectional study used features extracted from Google Street View (GSV) images to measure the built environment and link them with prevalence of coronary heart disease (CHD). Convolutional neural networks, linear mixed-effects models, and activation maps were utilized to predict health outcomes and identify feature associations with CHD at the census tract level. The study obtained 0.53 million GSV images covering 789 census tracts in seven US cities. Built environment features extracted from GSV using deep learning predicted 63% of the census tract variation in CHD prevalence. The addition of GSV features improved a model that only included census tract-level, age, sex, race, income, and education, or composite indices of social determinants of health. Activation maps from the features revealed a set of neighbourhood features represented by buildings and roads associated with CHD prevalence.
Chen and colleagues conclude that the prevalence of CHD is associated with built environment factors derived from GSV through deep learning analysis, independent of census tract demographics. Machine vision-enabled assessment of the built environment could potentially offer a more precise approach to identify at-risk neighbourhoods, thereby providing an efficient avenue to address and reduce cardiovascular health disparities in urban environments. This manuscript is accompanied by an Editorial by Rohan Khera from Yale School of Medicine in New Haven, CT, USA.17 Khera concludes that an artificial intelligence-enhanced approach to studying the physical environment and its association with CV health highlights that across our communities, measures of CV health are strongly encoded in merely the visual appearance of our neighbourhoods. It is critical to use this information wisely, both in defining strategic priorities for identifying vulnerable communities and in redoubling efforts to improve CV health in communities that need it most.
Cardioprotection in patients with AMI remains an unmet need.18–22 The ecto-nucleoside triphosphate diphosphohydrolases of the CD39 family degrade ATP and ADP into AMP, which is converted into adenosine by the extracellular CD73/ecto-5-nucleotidase. This pathway has been explored in antithrombotic treatments but little in myocardial protection. In a second Translational Research article entitled ‘Recombinant human soluble domain of CD39L3 and ticagrelor: cardioprotective effects in experimental myocardial infarction’, Gemma Vilahur from the Hospital de la Santa Creu i Sant Pau in Barcelona, Spain, and colleagues investigated whether the administration of solCD39L3 (AZD3366) confers additional cardioprotection to that of ticagrelor alone in a pre-clinical model of AMI.23 Ticagrelor-treated pigs underwent balloon-induced AMI (90 min) and, before reperfusion, received intravenously either vehicle, 1 mg/kg AZD3366, or 3 mg/kg AZD3366. All animals received ticagrelor twice daily for 42 days. A non-treated AMI group was run as a control. Serial cardiac magnetic resonance (baseline, Day 3, and Day 42 post-MI), light transmittance aggregometry, bleeding time, and histological and molecular analyses were performed. Ticagrelor reduced oedema formation and infarct size at Day 3 post-AMI compared with controls. A 3 mg/kg dose of AZD3366 provided an additional 45% reduction in oedema and infarct size compared with ticagrelor and a 70% reduction compared with controls (P < .05). At Day 42, infarct size declined in all ticagrelor-administered pigs, particularly in 3 mg/kg AZD3366-treated pigs (P < .05). Left ventricular ejection fraction was diminished at Day 3 in placebo pigs and worsened at Day 42, whereas it remained unaltered in ticagrelor ± AZD3366-administered animals. Pigs administered with 3 mg/kg AZD3366 displayed higher left ventricular ejection fraction upon dobutamine stress at Day 3 and minimal dysfunctional segmental contraction at Day 42 (χ2P < .05 vs. all). Cardiac and systemic molecular readouts supported these benefits. Interestingly, AZD3366 abolished ADP-induced light transmittance aggregometry without affecting bleeding time.
The authors conclude that infusion of AZD3366 on top of ticagrelor leads to enhanced cardioprotection compared with ticagrelor alone. The contribution is accompanied by an Editorial by Gerd Heusch and Petra Kleinbongard from the University of Essen Medical School in Germany.24 The authors remind us that in a consensus paper of the COST ACTION on CARDIOPROTECTION (CA16225), guidelines to improve the translation of cardioprotection from pre-clinical studies to patient benefit have been elaborated. Use of pigs, which have haemodynamics and spatial and temporal development of AMI comparable with humans, was proposed as the most suitable species, with infarct size and coronary microvascular obstruction as the most robust endpoints. Use of cardiac magnetic resonance imaging to assess infarct size and coronary microvascular injury in pre-clinical studies was also recommended, as it would be the same measurement method and technology as in clinical studies. Finally, longer observation periods would also permit the study of adverse remodelling and for an estimate of clinically relevant events, such as mortality and heart failure, to be obtained. The authors note that the study by Vilahur et al. complies with many of the above proposals for better translation of cardioprotection. The authors conclude by indicating that they are confident that clinical trials in AMI in those who really need cardioprotection beyond that offered by reperfusion will close the translational gap in cardioprotection research.
The last contribution is a Rapid Communications contribution entitled ‘Acid-sensing ion channel 1a blockade reduces myocardial injury in rodent models of myocardial infarction’ by Meredith A. Redd from the University of Queensland, and colleagues.25 The authors note that while blockade of acid-sensing ion channel 1a (ASIC1a) is known to protect the brain and heart against ischaemic injury, the cardioprotective effect of ASIC1a inhibition during AMI remains unknown. The authors tested this using a mouse model involving 40 min ischaemia induced by ligation of the left anterior descending coronary artery followed by reperfusion. Hi1a (1 mg/kg), an ASIC1a inhibitor, was administered (intravenous bolus) 5 min prior to ligation, 5 min after, onset of ischaemia, or 5 min prior to reperfusion. Twenty-four hours post-MI, myocardial viability was assessed as a fraction of the area at risk (AAR). While all groups had comparable AAR, Hi1a treatment reduced infarct size with equal efficacy at all time points.
The authors conclude that ASIC1a inhibitor, Hi1a, might have a role in cardioprotection. Consistent with EU-CARDIOPROTECTION guidelines, future studies should evaluate Hi1a in comorbid and large animal MI models to de-risk the path to clinical translation.
The editors hope that this issue of the European Heart Journal will be of interest to its readers.
Dr. Crea reports speaker fees from Abbott, Amgen, Astra Zeneca, BMS, Chiesi, Daiichi Sankyo, Menarini outside the submitted work.
With thanks to Amelia Meier-Batschelet, Johanna Huggler, and Martin Meyer for help with compilation of this article.




