-
PDF
- Split View
-
Views
-
Cite
Cite
Jeroen J Bax, Rebecca T Hahn, Nina Ajmone Marsan, Helmut Baumgartner, Great debate: symptomatic moderate aortic stenosis should undergo intervention, European Heart Journal, Volume 45, Issue 11, 14 March 2024, Pages 912–921, https://doi.org/10.1093/eurheartj/ehae050
Close - Share Icon Share
Introduction
Corresponding author. Tel: +49 251 46110, Fax: +49 251 46109, Email: [email protected]
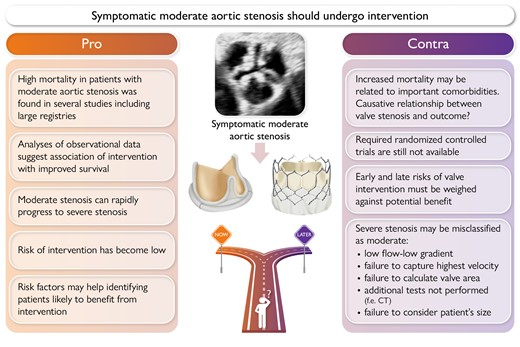
Aortic stenosis (AS) has become a major health burden with a reported prevalence of 2–6% in the population older than 65 years.1–3 Globally, 12.6 million patients with calcific AS—the most common aetiology of AS—have been estimated causing 102 700 deaths4 and the prevalence appears to increase rapidly with the aging population, particularly in Europe and North America.4,5 The dismal outcome of symptomatic severe AS was reported for the first time almost 60 years ago by Ross and Braunwald.6 The excellent outcome of this population after successful surgical aortic valve replacement (SAVR) has been demonstrated already 35 years ago.7 Although based only on observational data, the difference in survival was so striking that a randomized controlled trial (RCT) comparing surgery with conservative treatment would have been unethical and has therefore never been performed. The development of transcatheter aortic valve implantation (TAVI) however finally confirmed in an RCT including patients who were not eligible for SAVR that relief of AS by TAVI was followed by a dramatic survival improvement even in this very sick population.8 Current guidelines therefore strongly recommend intervention (SAVR or TAVI depending on patient characteristics and life expectancy) in patients with symptomatic severe AS.9,10 In contrast, moderate AS defined by an aortic valve area between 1.0 and 1.5 cm2 and a peak transvalvular velocity between 3.0 and 4.0 m/s and mean pressure gradient between 20 and 40 mmHg at normal flow has so far been considered rather benign not justifying intervention with the only exception of concomitant valve replacement when open-heart surgery is indicated for other reasons (other valve disease, aortic aneurysm, coronary artery disease).9,10 The appropriateness of such management of moderate AS has been questioned by more recent publications11–13 reporting a relatively high mortality in this population and raising the question whether intervention should be even considered in moderate AS. It has however to be taken into account that an increased mortality has been found across the spectrum of AS severities13 and even aortic valve sclerosis has been demonstrated to be associated with cardiovascular mortality and morbidity.14 Thus, other factors than the valve appear to contribute to the worse outcome of this population which may not be affected by intervention. Thus, intervention in moderate AS remains a matter of debate and it is timely to discuss Pro and Contra in detail (Graphical Abstract).
Declarations
Disclosure of Interest
H.B. received speaker fees and congress travel support from Edwards Lifesciences and Actelion.
References
Pro
The following arguments may support to intervene in symptomatic patients with moderate aortic stenosis (AS) (Figure 1):
Mortality of patients with moderate AS is high without valve replacement.
Large registries show that progression to severe AS often occurs rapidly.
Patients with moderate AS can develop left ventricular (LV) dysfunction which increases mortality.
![Moderate aortic stenosis (AS) is associated with high mortality without valve replacement and similar to severe AS (central panel). Large registries show that progression to severe AS often occurs rapidly and is associated with poorer outcome (right panel). Finally, patients with moderate AS may develop symptoms [including heart failure (HF) symptoms] and left ventricular (LV) dysfunction with an associated increased risk of adverse events (left panel)](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/45/11/10.1093_eurheartj_ehae050/1/m_ehae050f1.jpeg?Expires=1749147561&Signature=OG3TQ0VmzDqWDEFzffpD-XNL07ksOP7en0RndWkL3jwmPabvkW1FJtNVlqbA23d0IBAMBFi9dhuHccG6B2xZdrXdXO7hQGiAUBCqmZ6vlYgkfFjakKpoBi7Cs40o~2sLgjHl9JPQb7tSH2km-gjGiH3BQsA7j5bpKfCVXIAGkrTqyadNNOjDuAFuB61cogIgjnjAi3PGckS8f3ct7vNNdpubiKkFCm99OGd8H9qld8TXm6jpUrA3G2CUfP2OQH-A3gxP0KClAojNAxqe2ZD4Q7Bn4qocEPYeqRzp-GHox8lUKoWgXFPaMwcX8oWtuhHMvzudu79h034P-ZQRHmGGlQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Moderate aortic stenosis (AS) is associated with high mortality without valve replacement and similar to severe AS (central panel). Large registries show that progression to severe AS often occurs rapidly and is associated with poorer outcome (right panel). Finally, patients with moderate AS may develop symptoms [including heart failure (HF) symptoms] and left ventricular (LV) dysfunction with an associated increased risk of adverse events (left panel)
Mortality of patients with moderate aortic stenosis is significant
Various studies recently reported on the relatively high mortality in patients with moderate AS. In 2019, Delesalle and colleagues1 evaluated 508 patients (287 men, 56.5%) with preserved left ventricular ejection fraction (LVEF) and moderate AS (mean age 75 ± 11 years), which was defined as an aortic valve area (AVA) between 1 and 1.5 cm2 (mean 1.2 ± 0.15 cm2); patients had a mean pressure gradient of 24.8 ± 9 mmHg, with a peak transvalvular velocity of 3.2 ± 0.55 cm/s, and a LVEF of 64 ± 8%. During a median follow-up of 47 months, 113 patients (22%) developed severe AS and underwent aortic valve replacement (AVR). The mean time between detection of AS and surgery was 37 ± 22 months. During follow-up, 255 patients (50.2%) died. The 6-year survival of patients with moderate AS was 53 ± 2% and lower than the expected survival (65%) in a matched general population, possibly driven also by a higher comorbidity index. Interestingly, AVR was associated with better survival.
Providing more extensive evidence on outcomes of patients with moderate AS, also larger registries/databases have been published. Also in 2019, Strange and colleagues2 performed a nationwide registry in Australia which included data from patients with different degrees of AS. The authors reported a significantly higher mortality in 3315 patients with moderate AS (defined as mean pressure gradient 20.0–39.0 mmHg and/or peak transvalvular velocity 3.0–3.9 m/s and/or AVA > 1 cm2), not much lower than in the patients with severe AS (n = 2668 collected in the same registry): the 5-year mortality was 56% vs. 67% in patients with moderate and severe AS, respectively, with a 2.6- vs. a 3.0-fold increased risk (Figure 1). However, no granular clinical data were available in this cohort.
A second registry of patients with moderate AS was published by Amanullah et al.,3 who collected information from 1245 patients with an AVA between 1.0 and 1.5 cm2, which were followed up for a median of 4.3 (range 2.4–6.9) years, with a primary endpoint of mortality and a secondary combined endpoint of mortality, stroke, heart failure, and myocardial infarction. The observed mortality in these patients was high (45%), and the combined endpoint occurred in 49.8% of the patients.
Also, Coisne and colleagues4 performed a meta-analysis of 25 studies with 12 143 patients diagnosed with moderate AS, for a period of 3.8 ± 1.7 years of follow-up. The authors showed that the pooled rates per 100 person-years were 9.0 [with 95% confidence interval (CI) of 6.9–11.7] for all-cause mortality, 4.9 (with 95% CI of 3.1–7.5) for cardiac death, 3.9 (with 95% CI of 1.9–8.2) for heart failure and 1.1 (with 95% CI of 0.8–1.5) for sudden death. Moreover, the authors showed that in patients with moderate AS, the rate of AVR was limited to 7.2 (with 95% CI of 4.3–12.2) per 100 person-years in 20 studies, including 7634 patients with a mean follow-up of 3.6 ± 1.8 years. Of interest, diabetes (P = .019), coronary artery disease (P = .017), presence of symptoms (P < .001), and LV dysfunction (P = .009) were associated with all-cause mortality. When compared with moderate AS, the incidence rate difference of all-cause mortality was −3.9 (with 95% CI of −6.7 to −1.1) for patients without AS or with mild AS, while the incidence rate was 2.2 (with 95% CI of 0.8–3.5) for patients with severe AS.
More recently from the reports of ∼600 000 patients who received an echocardiogram, Genereux et al. reported that the 4-year mortality risk associated with the diagnosis of (untreated) AS increased incrementally across the full spectrum of AS severity, with a rate of 33.5% for moderate AS vs. 45% for moderate to severe and severe AS; the difference remained significantly different even after adjusting for informative censoring caused by treatment and for comorbidities.5
These studies underscore that moderate AS is not a benign disease, although the increased mortality rate may possibly be related also to the important comorbidities which often characterize these patients.
Progression of moderate aortic stenosis to severe aortic stenosis
The fact that AS is a progressive condition and that moderate AS can rapidly evolve into severe AS with a poor outcome has been suggested by several studies6,7 (Figure 1). Rosenhek and colleagues,6 for example, followed up 176 asymptomatic patients with mild to moderate AS (defined by a peak transvalvular velocity of 2.5–3.9 m/s). With a mean interval between echocardiograms of 46 ± 19 months, the average increase in peak transvalvular velocity was 0.24 ± 0.30 m/s/year and a total of 46% patients developed severe AS during follow-up. Patients with moderate to severe aortic valve calcification showed a more rapid increase in peak transvalvular velocity, as compared with patients with mild calcification (0.35 ± 0.31 vs. 0.16 ± 0.19 m/s/year, P = .0004). Progression was also significantly faster in patients with coronary artery disease (0.34 ± 0.42 vs. 0.18 ± 0.19 m/s/year, P = .004) and in patients older than 50 years (0.30 ± 0.33 vs. 0.10 ± 0.14 m/s/year, P = .0005). Interestingly, in this study, diabetes, arterial hypertension, hypercholesterolaemia, gender, and aortic peak transvalvular velocity were not related to the progression of AS. During a median follow-up of 48 ± 19 months, 33 patients needed AVR and 34 patients died. The event-free survival (death or AVR) declined rapidly in these patients, from 95 ± 2% at 1 year to 75 ± 3% at 3 years and to 60 ± 5% at 5 years of follow-up, and both cardiac and non-cardiac mortality were significantly increased, with a 1.8 times higher mortality than predicted. Of interest, AS progression was faster in patients who developed an event (although combined of AVR or death): 0.45 ± 0.38 vs. 0.14 ± 0.18 m/s (P = .0001). On multivariate analysis, the following parameters were independent predictors of outcomes: moderate to severe aortic valve calcification, aortic transvalvular peak velocity, and coronary artery disease.
Also, a recent meta-analysis including more than 5000 patients8 confirmed that AS is a rapidly progressive disease with an annual pooled annualized increase in mean pressure gradient of 4.10 mmHg, a decrease in AVA of 0.08 cm2, and also a worsening in aortic valve calcification (by computed tomography) of 158.5 AU; of interest, increasing baseline severity of AS was predictive of higher rates of progression for all the abovementioned parameters. These findings suggest that patients with mild to moderate AS require close follow-up as they can rapidly progress and show excess mortality.
Associates of poor outcome in moderate aortic stenosis: findings from large registries on moderate aortic stenosis
Although a causative relationship between moderate AS and the related poorer outcome has not been demonstrated, several studies have shown important associates of adverse outcome in these patients.
Amanullah and colleagues3 in their abovementioned study in moderate AS patients also reported that extra-aortic valvular cardiac abnormalities extending from the left ventricle, to the left atrium or mitral valve, to the pulmonary vasculature or tricuspid valve, and to the right ventricle was common in these patients and independently associated with both all-cause mortality and cardiovascular events: higher mortality rates were observed with increasing extent of extra-aortic valvular cardiac abnormalities.
Additional insights into potential prognosticators came from a large database including 1961 patients with moderate AS, with 5-year clinical outcomes/survival data and with all digitized echocardiographic data available (allowing post-processing and measuring echocardiographic variables). From this large database, Stassen et al.9 stratified these patients based on the New York Heart Association (NYHA) functional class and LVEF (LVEF ≥ 60%, LVEF 50%–59%, or LVEF < 50%) and showed that when symptoms of dyspnoea developed, long-term outcomes were significantly reduced as compared with patients who remained asymptomatic: for patients in NYHA classes I, II, or III–IV, the 5-year survival rates were 69%, 51%, or 40%, respectively, while the 5-year event-free survival rates were 46%, 28%, and 16%, respectively. Interestingly, patients with low-normal LVEF (<60%) and mild symptoms (NYHA II) already showed an increased risk of adverse event (Figure 1).
The same group also evaluated the prognostic value of LV diastolic dysfunction in these patients,10 which was present in 43% of the population, and was associated with an increased 5-year mortality: 41% in patients with diastolic dysfunction vs. 29% in patients with normal diastolic function. Moreover, subtle systolic dysfunction11 was evaluated using LV global longitudinal strain (GLS, cut-off value < 16% indicating reduced GLS) in a subgroup of 760 patients of the same large database of moderate AS patients. Importantly, even in the patients with normal LVEF (≥50%) but reduced LV GLS (<16%), the 5-year survival was reduced to 58%.
These observations suggest an important component of the myocardial involvement in the poor prognosis of patients with moderate AS, which should represent a specific target of treatment but could possibly have a significant benefit from relieving the LV outflow tract obstruction due to the AS.
Prospective, randomized controlled trials to treat patients with moderate aortic stenosis
Currently, three prospective, randomized controlled trials are ongoing evaluating the outcomes of patients with moderate AS undergoing intervention vs. conservative treatment (Table 1): the TAVR UNLOAD (Edwards Lifesciences, NCT02661451), the PROGRESS Trial (Edwards Lifesciences, NCT04889872), and the EXPAND TAVR II Pivotal Trial (Medtronic, NCT05149755), including respectively 600, 750, and 650 patients with moderate AS.
Ongoing prospective, randomized controlled trials evaluating the outcomes of patients with moderate aortic stenosis undergoing transcatheter aortic valve replacement vs. conservative treatment
| Trial . | Design . | n of patients . | inclusion criteria . | Primary outcome . |
|---|---|---|---|---|
| TAVR UNLOAD | Open randomized trial; TAVR and OMT vs. OMT | 600 | NYHA class ≥ II with LVEF < 50% (>20%) | All-cause death at 1 year. Disabling stroke at 1 year. Hospitalizations related to HF, symptomatic aortic valve disease, non-disabling stroke, or clinically significant worsening of HF (HF hospitalization equivalent) at 1 year Change in KCCQ relative to baseline at 1 year |
| PROGRESS | Open randomized trial; TAVR and OMT vs. OMT | 750 | Symptoms related to AS (dyspnoea or angina) or evidence of cardiac systolic or diastolic dysfunction. Age ≥ 65 years old | Non-hierarchical composite of death, stroke, life-threatening or fatal bleeding, acute kidney injury stage 4, hospitalization due to device- or procedure-related complication, and valve dysfunction requiring reintervention at 30 days Composite of death and heart failure hospitalization or event at 2 years |
| EXPAND TAVR II Pivotal | Open randomized trial; TAVR and OMT vs. OMT | 650 | Symptoms (NYHA class ≥ II, reduced functional capacity) with either HF event in the past year or AF or LVEF < 60% (but >20%) or stroke volume index < 35 mL/m² or elevated cardiac biomarkers or reduced GLS (≤16%) or elevated LV filling pressures. Age ≥ 65 years old | Composite of all-cause mortality, all-stroke, life-threatening bleeding, acute kidney injury, hospitalization due to device or procedure-related complication, or valve dysfunction requiring reintervention at 30 days Composite rate of all-cause mortality, heart failure hospitalization or event, or medical instability leading to aortic valve replacement or reintervention at 2 years |
| Trial . | Design . | n of patients . | inclusion criteria . | Primary outcome . |
|---|---|---|---|---|
| TAVR UNLOAD | Open randomized trial; TAVR and OMT vs. OMT | 600 | NYHA class ≥ II with LVEF < 50% (>20%) | All-cause death at 1 year. Disabling stroke at 1 year. Hospitalizations related to HF, symptomatic aortic valve disease, non-disabling stroke, or clinically significant worsening of HF (HF hospitalization equivalent) at 1 year Change in KCCQ relative to baseline at 1 year |
| PROGRESS | Open randomized trial; TAVR and OMT vs. OMT | 750 | Symptoms related to AS (dyspnoea or angina) or evidence of cardiac systolic or diastolic dysfunction. Age ≥ 65 years old | Non-hierarchical composite of death, stroke, life-threatening or fatal bleeding, acute kidney injury stage 4, hospitalization due to device- or procedure-related complication, and valve dysfunction requiring reintervention at 30 days Composite of death and heart failure hospitalization or event at 2 years |
| EXPAND TAVR II Pivotal | Open randomized trial; TAVR and OMT vs. OMT | 650 | Symptoms (NYHA class ≥ II, reduced functional capacity) with either HF event in the past year or AF or LVEF < 60% (but >20%) or stroke volume index < 35 mL/m² or elevated cardiac biomarkers or reduced GLS (≤16%) or elevated LV filling pressures. Age ≥ 65 years old | Composite of all-cause mortality, all-stroke, life-threatening bleeding, acute kidney injury, hospitalization due to device or procedure-related complication, or valve dysfunction requiring reintervention at 30 days Composite rate of all-cause mortality, heart failure hospitalization or event, or medical instability leading to aortic valve replacement or reintervention at 2 years |
AF, atrial fibrillation; AS, aortic stenosis; GLS, global longitudinal strain; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association; LV, left ventricular; LVEF, left ventricular ejection fraction; OMT, optimal medical therapy; TAVR, transcatheter aortic valve replacement.
Ongoing prospective, randomized controlled trials evaluating the outcomes of patients with moderate aortic stenosis undergoing transcatheter aortic valve replacement vs. conservative treatment
| Trial . | Design . | n of patients . | inclusion criteria . | Primary outcome . |
|---|---|---|---|---|
| TAVR UNLOAD | Open randomized trial; TAVR and OMT vs. OMT | 600 | NYHA class ≥ II with LVEF < 50% (>20%) | All-cause death at 1 year. Disabling stroke at 1 year. Hospitalizations related to HF, symptomatic aortic valve disease, non-disabling stroke, or clinically significant worsening of HF (HF hospitalization equivalent) at 1 year Change in KCCQ relative to baseline at 1 year |
| PROGRESS | Open randomized trial; TAVR and OMT vs. OMT | 750 | Symptoms related to AS (dyspnoea or angina) or evidence of cardiac systolic or diastolic dysfunction. Age ≥ 65 years old | Non-hierarchical composite of death, stroke, life-threatening or fatal bleeding, acute kidney injury stage 4, hospitalization due to device- or procedure-related complication, and valve dysfunction requiring reintervention at 30 days Composite of death and heart failure hospitalization or event at 2 years |
| EXPAND TAVR II Pivotal | Open randomized trial; TAVR and OMT vs. OMT | 650 | Symptoms (NYHA class ≥ II, reduced functional capacity) with either HF event in the past year or AF or LVEF < 60% (but >20%) or stroke volume index < 35 mL/m² or elevated cardiac biomarkers or reduced GLS (≤16%) or elevated LV filling pressures. Age ≥ 65 years old | Composite of all-cause mortality, all-stroke, life-threatening bleeding, acute kidney injury, hospitalization due to device or procedure-related complication, or valve dysfunction requiring reintervention at 30 days Composite rate of all-cause mortality, heart failure hospitalization or event, or medical instability leading to aortic valve replacement or reintervention at 2 years |
| Trial . | Design . | n of patients . | inclusion criteria . | Primary outcome . |
|---|---|---|---|---|
| TAVR UNLOAD | Open randomized trial; TAVR and OMT vs. OMT | 600 | NYHA class ≥ II with LVEF < 50% (>20%) | All-cause death at 1 year. Disabling stroke at 1 year. Hospitalizations related to HF, symptomatic aortic valve disease, non-disabling stroke, or clinically significant worsening of HF (HF hospitalization equivalent) at 1 year Change in KCCQ relative to baseline at 1 year |
| PROGRESS | Open randomized trial; TAVR and OMT vs. OMT | 750 | Symptoms related to AS (dyspnoea or angina) or evidence of cardiac systolic or diastolic dysfunction. Age ≥ 65 years old | Non-hierarchical composite of death, stroke, life-threatening or fatal bleeding, acute kidney injury stage 4, hospitalization due to device- or procedure-related complication, and valve dysfunction requiring reintervention at 30 days Composite of death and heart failure hospitalization or event at 2 years |
| EXPAND TAVR II Pivotal | Open randomized trial; TAVR and OMT vs. OMT | 650 | Symptoms (NYHA class ≥ II, reduced functional capacity) with either HF event in the past year or AF or LVEF < 60% (but >20%) or stroke volume index < 35 mL/m² or elevated cardiac biomarkers or reduced GLS (≤16%) or elevated LV filling pressures. Age ≥ 65 years old | Composite of all-cause mortality, all-stroke, life-threatening bleeding, acute kidney injury, hospitalization due to device or procedure-related complication, or valve dysfunction requiring reintervention at 30 days Composite rate of all-cause mortality, heart failure hospitalization or event, or medical instability leading to aortic valve replacement or reintervention at 2 years |
AF, atrial fibrillation; AS, aortic stenosis; GLS, global longitudinal strain; HF, heart failure; KCCQ, Kansas City Cardiomyopathy Questionnaire; NYHA, New York Heart Association; LV, left ventricular; LVEF, left ventricular ejection fraction; OMT, optimal medical therapy; TAVR, transcatheter aortic valve replacement.
All trials will prospectively evaluate a transcatheter approach (the TAVR UNLOAD and the PROGRESS trial with Sapien 3® or Sapien 3 Ultra® or Sapien 3 Ultra RESILIA® and the EXPAND TAVR II with Evolut PRO+® or Evolut FX®) vs. guideline-directed medical therapy and including a long-term follow-up. These trials required as inclusion criteria also the presence of either symptoms related to AS (mainly dyspnoea), previous heart failure hospitalization or elevated N-terminal pro B-type natriuretic peptide (NT-proBNP), or evidence of different degrees of cardiac damage/dysfunction (from a LVEF < 50% for the TAVR UNLOAD trial to myocardial systolic and diastolic dysfunction measured by advanced echocardiography in the other trials).
Conclusions
Considering the increased risk of mortality, of concomitant myocardial damage (diastolic and systolic function), and of a rapid progression, currently available evidence suggests that patients with moderate AS may benefit from early intervention, especially when symptoms related to AS are present together with signs of LV dysfunction. However, only the ongoing clinical trials in moderate AS will shed light on the actual causative and prognostic role of valve disease in these patients.
Declarations
Disclosure of Interest
J.J.B. received speaker fees from Abbott Vascular and Edwards Lifesciences and is part of the Steering Committee of the PROGRESS study (Edwards Lifesciences). N.A.M. received speaker fees from Abbott Vascular, Philips Ultrasound, GE Healthcare, and Omron.
References
Contra
Corresponding author. Email: [email protected]
Although the poor prognosis of symptomatic severe aortic stenosis (AS) is well-established,1–3 American and European guidelines are consistent in giving a IIa recommendation only for intervention on patients with moderate AS who are undergoing cardiac surgery for other indications (level of evidence C).2,3 Advocates of early intervention cite studies suggesting that patients with moderate AS have survival rates similar to patients with severe AS4; however to date, there are no prospective studies proving a survival benefit with this approach. Intervening too early exposes patients to the peri-procedural risk of the intervention such as in-hospital mortality, bleeding, stroke, and atrial fibrillation. In addition, there are long-term morbidities associated with prosthetic valves such as endocarditis, bleeding associated with chronic anticoagulation for mechanical valves, or structural valve deterioration in patients with bioprosthetic valves. On average, bioprosthetic aortic valves last between 10 and 15 years before they calcify, stenose, leak, and fail with multiple studies showing more rapid deterioration with younger age at implant as well as with prosthesis–patient mismatch.5,6 Transcatheter aortic valve implantation (TAVI) is associated with its own risks (i.e. paravalvular regurgitation and permanent pacemaker). Premature timing of bioprosthetic valve implantation therefore may increase the risk of requiring additional interventions including a redo procedure.
When approaching a patient with symptoms of heart failure and only moderate AS by echocardiography, it is important to (i) systematically address the quantitation of AS severity and exclude true severe AS and (ii) investigate the aetiology of symptoms since patients with AS may have treatable comorbidities and concomitant cardiovascular diseases.
Quantitation of aortic stenosis severity
The mis-diagnosis of severe AS as only moderate occurs in the following situations: (i) reliance on velocity and gradient in the setting of low flow or high afterload; (ii) failure to capture the highest transaortic velocities; (iii) failure to calculate aortic valve area (AVA) or dimensionless index (DI), also known as the velocity ratio; (iv) failure to perform additional tests when discordance in velocity and gradient and AVA and DI is detected; and (v) failure to index the AVA to body size, particularly in large or tall patients.
Echocardiography is recognized as the first-line test for assessment of aortic stenosis severity.2,7 A number of echocardiographic parameters can be used to evaluate the haemodynamic severity of AS and can be divided into two general categories (Table 1): flow-dependent measurements and flow-independent measurements. Flow-dependent measurements are obtained from continuous-wave Doppler across the stenotic aortic valve and include jet velocity, peak, and mean gradients. Because gradients are related to the velocity of flow, these measurements can be derived from the use of the modified Bernoulli equation: ΔP = 4v2. Mean gradients are measured by averaging the instantaneous gradient over the systolic ejection period. Natural history studies have shown that outcomes are determined by peak transaortic velocity with progressively worse survival with increasing velocities above 4 m/s.8–10 A velocity of 4 m/s correlates well with a mean gradient of 35–40 mmHg.11 Importantly, obtaining the peak velocity by continuous-wave Doppler requires interrogation of multiple imaging windows; in one study, the maximum velocity was most frequently obtained in the right parasternal window (50%), followed by the apex (39%).12 Subjects with acute left ventricular (LV) aortic root angulation more commonly had peak velocities obtained from the right parasternal window (65% vs. 43%, P = .05) and less commonly had Vmax at the apical window (19% vs. 48%, P = .005). Failure to use non-apical windows causes 15% of patients to be mis-diagnosed as having moderate AS.
| . | Sclerosis . | Mild AS . | Moderate AS . | Severe AS . |
|---|---|---|---|---|
| Maximum velocity (m/s) | ≤2.5 | 2.6–2.9 | 3.0–4.0 | ≥4.0 |
| Mean pressure gradient (mmHg) | <20 | 20–40 | ≥40 | |
| AVA (cm2) | >1.5 | 1.0–1.5 | <1.0 | |
| AVA indexed (cm2/m2) | >0.85 | 0.60–0.85 | <0.60 | |
| Dimensionless index | >0.50 | 0.25–0.50 | <0.25 | |
| CT calcium score | Men 800–2000 AU Women 400–1200 AU | Men ≥ 2000 AU Women ≥ 1200 AU | ||
| Valvulo-arterial impedance (mmHg/mL/m2) | <3.5 | 3.5–4.5 | >4.5 |
| . | Sclerosis . | Mild AS . | Moderate AS . | Severe AS . |
|---|---|---|---|---|
| Maximum velocity (m/s) | ≤2.5 | 2.6–2.9 | 3.0–4.0 | ≥4.0 |
| Mean pressure gradient (mmHg) | <20 | 20–40 | ≥40 | |
| AVA (cm2) | >1.5 | 1.0–1.5 | <1.0 | |
| AVA indexed (cm2/m2) | >0.85 | 0.60–0.85 | <0.60 | |
| Dimensionless index | >0.50 | 0.25–0.50 | <0.25 | |
| CT calcium score | Men 800–2000 AU Women 400–1200 AU | Men ≥ 2000 AU Women ≥ 1200 AU | ||
| Valvulo-arterial impedance (mmHg/mL/m2) | <3.5 | 3.5–4.5 | >4.5 |
AS, aortic stenosis; AU, arbitrary units; AVA, aortic valve area; CT, computed tomography.
| . | Sclerosis . | Mild AS . | Moderate AS . | Severe AS . |
|---|---|---|---|---|
| Maximum velocity (m/s) | ≤2.5 | 2.6–2.9 | 3.0–4.0 | ≥4.0 |
| Mean pressure gradient (mmHg) | <20 | 20–40 | ≥40 | |
| AVA (cm2) | >1.5 | 1.0–1.5 | <1.0 | |
| AVA indexed (cm2/m2) | >0.85 | 0.60–0.85 | <0.60 | |
| Dimensionless index | >0.50 | 0.25–0.50 | <0.25 | |
| CT calcium score | Men 800–2000 AU Women 400–1200 AU | Men ≥ 2000 AU Women ≥ 1200 AU | ||
| Valvulo-arterial impedance (mmHg/mL/m2) | <3.5 | 3.5–4.5 | >4.5 |
| . | Sclerosis . | Mild AS . | Moderate AS . | Severe AS . |
|---|---|---|---|---|
| Maximum velocity (m/s) | ≤2.5 | 2.6–2.9 | 3.0–4.0 | ≥4.0 |
| Mean pressure gradient (mmHg) | <20 | 20–40 | ≥40 | |
| AVA (cm2) | >1.5 | 1.0–1.5 | <1.0 | |
| AVA indexed (cm2/m2) | >0.85 | 0.60–0.85 | <0.60 | |
| Dimensionless index | >0.50 | 0.25–0.50 | <0.25 | |
| CT calcium score | Men 800–2000 AU Women 400–1200 AU | Men ≥ 2000 AU Women ≥ 1200 AU | ||
| Valvulo-arterial impedance (mmHg/mL/m2) | <3.5 | 3.5–4.5 | >4.5 |
AS, aortic stenosis; AU, arbitrary units; AVA, aortic valve area; CT, computed tomography.
Because velocity and gradient are flow dependent, the AS severity criteria outlined above are appropriate in patients with normal flow rates and blood pressure. Low gradients may occur when valve areas are severely reduced if flow rate across the valve is reduced (i.e. significant mitral regurgitation or reduced LV function). In these instances, flow-independent measurements are necessary to determine the severity of stenosis. The flow-independent measurements include the AVA calculated from the continuity equation13–16 and the DI. The continuity equation normalizes for flow by calculating the stroke volume in the numerator [measured as the product of the LV outflow tract (LVOT) area and the LVOT velocity time integral (VTI)] and dividing by the stroke distance (measured as the peak transaortic VTI). Numerous outcomes studies have shown that a continuity equation valve area of ≤1.0 cm2 predicts outcomes.17,18 When discordant haemodynamics are found, the DI may be an additional useful parameter to express the size of the effective valvular area. Calculated as the LVOT VTI divided by the aortic valve VTI, this index has a greater sensitivity (97%) than gradient alone (81%) for detecting severe AS.15 A DI ≤ 0.25 is consistent with severe AS irrespective of mean aortic valve or LVOT gradients19,20 and is associated with outcomes. The risk of events including cardiovascular death or need for aortic valve replacement increased linearly with DI < 0.25 [adjusted hazard ratio (HR): 1.14; 95% confidence interval (CI): 1.05–1.29) per 0.05 DI decrement; P = .015].21
In the setting of discordant haemodynamics, AVA < 1.0 cm2 with a mean pressure gradient < 40 mmHg, additional testing should be performed. Low-flow, low-gradient severe AS with a left ventricular ejection fraction (LVEF) < 50%, low-dose dobutamine stress echocardiography (DSE) is indicated to exclude pseudo-severe AS.22 With an appropriate increase in flow, pseudo-severe (i.e. moderate) AS is defined as an AVA > 1.0 cm2 with a mean gradient < 40 mmHg.3,23 Low-flow, low-gradient severe AS in the setting of normal LVEF may warrant further evaluation with DSE; however, it should be performed with caution, avoiding patients with very small LV cavities. For discordant grading with reduced or normal LVEF, computed tomography calcium score can be used to differentiate moderate from severe AS. A calcium score between 800 and 2000 AU in men and 400–1200 in women is consistent with moderate AS,24,25 but scores above these cut-offs are more consistent with severe AS.
Because the cardiac output required in an individual is dependent on body size, indexing the AVA to body surface area (BSA) is another important measure of severity. Indexing the valve area is particularly important in smaller patients with height < 135 cm (65 inches), BSA < 1.5 m2, or body mass index < 22 kg/m². To account for variations of body size from the population average, an AVA-indexed BSA < 0.6 cm/m2 has been proposed to identify severe AS.7 More recently, however, Vulesevic et al. explored the use of height (H) to adjust AVA to patient size. This multinational study used 1298 patients to explore the AVA/BSA cut-offs for severe AS (AVA ≤ 1.0 cm2) in obese and non-obese patients and define the severe AVA/H cut-off. The AVA/BSA values that corresponded to an AVA of 1.0 cm2 were markedly different in obese and non-obese patients (0.48 and 0.59 cm2/m2) but not with AVA/H (0.61 cm2/m for both). Agreement for the diagnosis of severe AS (AVA ≤ 1 cm2) was significantly higher with AVA/H than with AVA/BSA (P < .05). An AVA/H cut-off value of 0.6 cm2/m [HR 8.2 (5.6–12.1)] provided the best predictive value for the occurrence of AS-related events [absolute AVA of 1 cm2: HR 7.3 (5.0–10.7); AVA/BSA of 0.6 cm2/m2 HR 6.7 (4.4–10.0)]. Thus, in large and/or tall patients, AVA > 1.0 cm2 may still be consistent with severe AS indexed to body size.
Determination of symptom aetiology
Patients with moderate aortic valve calcification or fibrosis are likely to have multiple comorbidities such as hypertension, atrial fibrillation, diabetes, chronic lung disease, and coronary artery disease. In addition, significant concomitant valvular regurgitation (aortic, mitral, or tricuspid) or stenosis (in particular degenerative mitral stenosis) may also accompany moderate AS and may not only be the cause of symptoms but would warrant appropriate therapy. The coexistence of wild-type transthyretin cardiac amyloidosis is common in patients with severe AS undergoing TAVI,27 and some investigators believe these patients’ phenotype likely reflects an early stage of amyloid infiltration and transthyretin amyloid-specific therapy is therefore likely to be beneficial.28
Outcomes of patients with moderate AS are known to be driven by patients with reduced LVEF.29–32 A multi-centre collaborative study including 305 patients with moderate AS and reduced LVEF reported a 61% composite event rate that included all-cause death, aortic valve replacement, and heart failure hospitalization at 4-year follow-up.29 In addition, in 262 patients with heart failure with reduced ejection fraction (HFrEF), moderate AS was associated with a marked incremental risk of mortality when matched to a population with HFrEF having no AS.30
Given the effect of any increase in load on ventricular remodelling, structural myocardial changes, even in the setting of normal LVEF, may be the aetiology of symptoms. Studies using cardiac magnetic resonance have demonstrated that LV fibrosis may be present in patients with AS despite preserved LVEF.33–35 Among patients with moderate AS and preserved LVEF, indices of LV diastolic dysfunction, reduced LV myocardial strain, elevated natriuretic peptides, and atrial fibrillation predict outcomes.36–38 In addition, the staging scheme of AS applied to asymptomatic patients with LVEF ≥ 50% with at least moderate AS suggests that concomitant diseases are important predictors of outcomes in these patients.39
Summary
The clinician presented with a patient with symptomatic, moderate AS (Figure 1) should thus (i) confirm that the stenosis is moderate by using both flow-dependent and flow-independent measures as recommended by the guidelines, indexing AVA by body size when appropriate; (ii) ensure that peak transaortic velocities, gradients, and VTI have been obtained by the compulsive acquisition of both apical non-apical Doppler imaging windows; and (iii) use adjunctive imaging tools to confirm the severity of AS. If moderate AS is confirmed, then other aetiologies of symptoms should be aggressively sought and treated when possible, including clinical or subclinical LV dysfunction, diastolic dysfunction, infiltrative cardiomyopathy, hypertension, and atrial fibrillation. Current guidelines give a IIa class of recommendation for surgical aortic valve replacement (SAVR) in patients with moderate AS undergoing coronary artery bypass grafting or surgical intervention on the ascending aorta or another valve after heart team discussion. How to manage moderate AS when transcatheter therapies are used to treat significant concomitant valvular disease or coronary artery disease requires further study
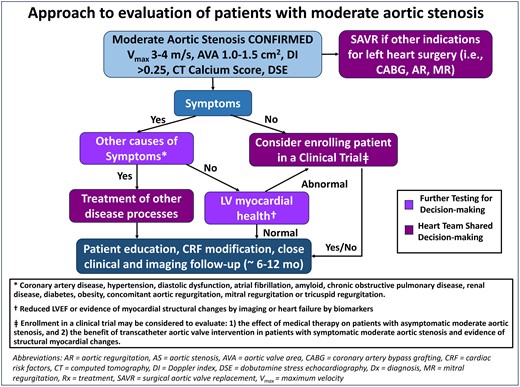
Approach to evaluation of patients with moderate aortic stenosis (AS). Patients with confirmed moderate AS with another indication for cardiac surgery (i.e. other significant left valve disease or coronary artery disease) may be considered for surgical aortic valve replacement (SAVR). If there is no other indication for open-heart surgery and the patient is symptomatic, then other causes of symptoms should be sought and treated. For asymptomatic patients, there are ongoing trials of medical therapy for AS for which the patient may be eligible. Patients with moderate AS, without clear cause of symptoms and with evidence for myocardial structural changes, may be eligible for clinical trials investigating different management options such as transcatheter aortic valve implantation. All patients with moderate AS should be followed closely for changes in valve haemodynamics or symptoms
Clinically, moderate AS is a heterogeneous population and patients with these risk factors likely have more advanced structural heart disease and, accordingly, they may have a more rapid progression to major adverse cardiac events. However, given the lack of robust data to support either surgical or transcatheter intervention for this disease, whether intervention vs. watchful waiting with treatment of comorbidities is the preferred management strategy requires validation. Early intervention is not without risk; younger patients may have a mechanical valve placed or suffer from early structural valve deterioration of a bioprosthetic valve with the need for redo surgery or valve-in-valve procedure. Until the completion of ongoing randomized controlled trials of interventions for moderate AS vs. medical therapy, these patients should be followed closely. Although guidelines suggest moderate AS be followed every 1–2 years, it may be more appropriate to shorten that follow-up in the setting of symptoms and/or concomitant disease processes that predict adverse outcomes.
Declarations
Disclosure of Interest
R.H. reports speaker fees from Abbott Structural, Baylis Medical, Edwards Lifesciences, and Philips Healthcare; she has institutional consulting contracts for which she receives no direct compensation with Abbott Structural, Boston Scientific, Edwards Lifesciences, Medtronic, and Novartis; she has stock options with Navigate; and she is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored trials, for which she receives no direct industry compensation.
References
Author notes
Jeroen J. Bax and Rebecca T. Hahn contributed equally to the study.



