-
PDF
- Split View
-
Views
-
Cite
Cite
Denisa Muraru, Luigi P Badano, Rebecca T Hahn, Roberto M Lang, Victoria Delgado, Nina C Wunderlich, Erwan Donal, Maurizio Taramasso, Alison Duncan, Philipp Lurz, Tom De Potter, José L Zamorano Gómez, Jeroen J Bax, Ralph Stephan von Bardeleben, Maurice Enriquez-Sarano, Francesco Maisano, Fabien Praz, Marta Sitges, Atrial secondary tricuspid regurgitation: pathophysiology, definition, diagnosis, and treatment, European Heart Journal, Volume 45, Issue 11, 14 March 2024, Pages 895–911, https://doi.org/10.1093/eurheartj/ehae088
Close - Share Icon Share
Abstract
Atrial secondary tricuspid regurgitation (A-STR) is a distinct phenotype of secondary tricuspid regurgitation with predominant dilation of the right atrium and normal right and left ventricular function. Atrial secondary tricuspid regurgitation occurs most commonly in elderly women with atrial fibrillation and in heart failure with preserved ejection fraction in sinus rhythm. In A-STR, the main mechanism of leaflet malcoaptation is related to the presence of a significant dilation of the tricuspid annulus secondary to right atrial enlargement. In addition, there is an insufficient adaptive growth of tricuspid valve leaflets that become unable to cover the enlarged annular area. As opposed to the ventricular phenotype, in A-STR, the tricuspid valve leaflet tethering is typically trivial. The A-STR phenotype accounts for 10%–15% of clinically relevant tricuspid regurgitation and has better outcomes compared with the more prevalent ventricular phenotype. Recent data suggest that patients with A-STR may benefit from more aggressive rhythm control and timely valve interventions. However, little is mentioned in current guidelines on how to identify, evaluate, and manage these patients due to the lack of consistent evidence and variable definitions of this entity in recent investigations. This interdisciplinary expert opinion document focusing on A-STR is intended to help physicians understand this complex and rapidly evolving topic by reviewing its distinct pathophysiology, diagnosis, and multi-modality imaging characteristics. It first defines A-STR by proposing specific quantitative criteria for defining the atrial phenotype and for discriminating it from the ventricular phenotype, in order to facilitate standardization and consistency in research.
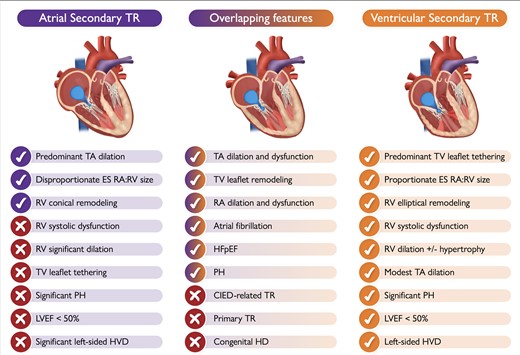
Characteristic aspects of atrial vs. ventricular secondary tricuspid regurgitation and the possible overlapping features that may occur between the two typical phenotypes (the check mark means ‘presence of’; the tick means ‘absence of’). Depending on its etiology, some of the listed features of ventricular secondary tricuspid regurgitation may be absent in some patients (LVEF < 50%, left-sided VHD etc). CIED, cardiac implantable electronic device; ES, end-systolic; HD, heart disease; HFpEF, heart failure with preserved ejection fraction; HVD, heart valve disease; LVEF, left ventricular ejection fraction; PH, pulmonary hypertension; RA, right atrium; RV, right ventricle; TA, tricuspid annulus; TR, tricuspid regurgitation; TV, tricuspid valve.
Introduction
Atrial secondary tricuspid regurgitation (A-STR) is a newly recognized type of STR predominantly involving the dilation of the right atrium (RA) and tricuspid annulus (TA), whilst the systolic function of the left ventricle (LV) and right ventricle (RV) are preserved. Clinically relevant tricuspid regurgitation (TR) (greater or equal to moderate) has a prevalence of 0.55% in the general population,1 and A-STR accounts for approximately 10%–15% of clinically relevant TRs.2,3 Atrial secondary tricuspid regurgitation occurs most commonly in elderly women with long-standing atrial fibrillation (AF) and/or heart failure with preserved ejection fraction (HFpEF).4,5 Overall, AF prevalence is increasing due to the aging of the population and improved life expectancy with chronic diseases.6,7 One-fourth of the adults free of AF at age 40 years and older will develop AF.8 Amongst patients with new-onset AF, one-third will develop clinically relevant TR during follow-up, which will place them at a higher risk of death.9 Patients with A-STR appear to derive clinical and survival benefits from rhythm control and timely valve interventions.9–11
Increased recognition of A-STR as a separate entity with distinct pathophysiology and outcomes motivated researchers to better define its phenotype, as well as its imaging characteristics and therapeutic implications for transcatheter tricuspid valve interventions (TTVIs). In recent investigations, A-STR has been considered a diagnosis of exclusion and its definition had considerable variability. Due to these inconsistencies, little is mentioned in current guidelines on how to evaluate, follow, and treat AF patients with A-STR compared with those with ventricular STR (V-STR).7,12,13
This interdisciplinary expert opinion document aims to review the available data about the pathophysiology, diagnosis, outcome, and management of A-STR and provides the rationale for its recent classification as a separate STR phenotype14 (Figure 1). A definition of A-STR is proposed to address the previous inconsistencies and facilitate research efforts aiming to identify optimal treatment strategies for this specific category of TR patients.
Delineating a new entity within the spectrum of secondary tricuspid regurgitation
Approximately 90% of all TR cases are of secondary aetiology.16 Secondary tricuspid regurgitation is independently associated with cardiovascular mortality and morbidity17; hence, understanding its anatomical and functional aspects is key for optimal clinical decision-making. For many years, STR has been primarily attributed to an abnormal geometry and/or systolic dysfunction of the RV, whilst RA remodelling has been considered a consequence of the regurgitation. Left-sided valve diseases, LV systolic dysfunction, and pulmonary hypertension (PH) are the most common aetiologies leading to V-STR.
Systematic echocardiographic assessment showed that, in some patients, a considerable enlargement of the RA and TA may be observed in the presence of relatively normal RV geometry and function without the classic tethering of the tricuspid valve (TV) leaflets commonly observed in V-STR.5 Accordingly, this entity has been called A-STR, to distinguish it from the more common V-STR phenotype. Historically, the A-STR phenotype has been variably classified as idiopathic or isolated STR. Kasai et al.18 recognized a distinct entity in 13 elderly patients with right heart failure and severe idiopathic TR, despite no underlying cardiac abnormality, except AF and TA dilation. Several authors subsequently adopted the term idiopathic to describe isolated STR occurring in elderly patients having permanent AF and dilated TA in the absence of any apparent cause.19,20 Zhou et al.21 observed that lone AF has a greater impact on TA than on mitral annulus (MA) remodelling, leading to more severe STR than secondary mitral regurgitation (SMR). Amongst patients with idiopathic STR reported by Mutlak et al.22 almost all had AF with preserved RV and LV function. This study highlighted for the first time the role of the RA in the development of STR and put forward the hypothesis that ‘AF may contribute to TA dilation by progressive RA enlargement’.22
Pathophysiology of the atrial secondary tricuspid regurgitation phenotype
In A-STR, there is a predominant TA dilation resulting in a leaflet-to-annulus imbalance, as the TV leaflets become insufficient to cover the enlarged annulus area. Tricuspid annulus enlargement is asymmetric along the RV free wall (where the annulus is more distensible and prone to dilation23), and in AF occurs mainly towards its posterior aspect.24 The main trigger for TA dilation seems to be RA dilation and dysfunction associated with atrial arrhythmias (AF or atrial flutter). Marked bi-atrial enlargement increases the total heart volume, leading to pericardial restraint that affects exertional cardiac output and filling pressures.25 Secondary tricuspid regurgitation is highly dependent on annular dilation, with clinically relevant STR occurring already with 40% dilation in the TA area, whereas 75% MA area dilation is required for SMR to develop.26 As mitral leaflets are both attached to a single papillary muscle, the coaptation defect provoked by pure annular enlargement is more limited in SMR. Conversely, the papillary muscles supporting the TV are smaller and widely separated, applying less constraint to the TV leaflets.27 In addition, the TA has less fibrotic component, and, differently from the MA, more than two-thirds of its circumference is connected with the muscular free wall. From a histological point of view, the distance separating the RA from the RV musculature widens with age and the atrial myocardium extends beyond the TA into the valve leaflets more frequently than on the left side28 (Figure 2). These differences explain why A-STR is generally more prevalent and severe than A-SMR.
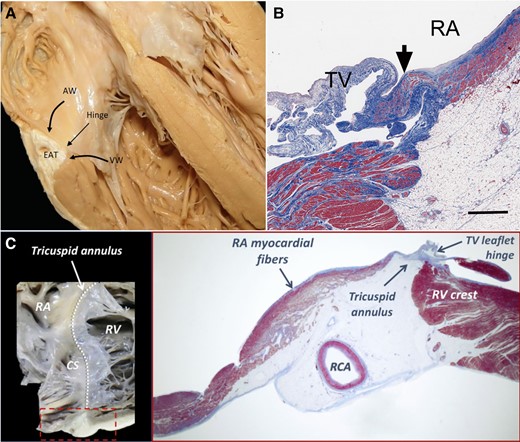
Anatomical relationship of the tricuspid annulus with right atrial and right ventricular myocardial fibres. (A) Atrioventricular junction, showing the insertion of the tricuspid valve leaflets; (B) Extension of right atrial myocardium at the base of the tricuspid valve leaflets, which is less present on the left side; (C) Close anatomical relationship of the tricuspid annulus with the atrial and ventricular myocardium (adapted with permission from Kato et al., Schlossbauer et al., and Muraru et al.28–30). AW, atrial wall; CS, coronary sinus; EAT, epicardial adipose tissue; RA, right atrial; RCA, right coronary artery; RV right ventricular; TV, tricuspid valve; VW, ventricular wall
Unfavourable TA dynamics is an additional aspect assumed to contribute to A-STR pathophysiology. In AF compared with sinus rhythm (SR), TA area shortening is significantly blunted due to the loss of atrial contraction, and the timing of TA minimal area is variable and frequently discoordinated with respect to RV systole (i.e. occurring in diastole) leading to larger TA area during systole in most cardiac cycles.31,32 Unfavourable timing of minimal TA area was more frequently associated with at least moderate TR, irrespective of the cardiac rhythm.32 Similar mechanisms have been described in A-SMR, in which the blunting of presystolic annular contraction due to the loss of atrial contraction in AF contributes to MV leaflet malcoaptation. Sinus rhythm restoration allowed gradual recovery of the presystolic annular contraction and decreased A-SMR severity by improving the annular-leaflet area imbalance, regardless of left atrial (LA) remodelling.33 Restoration of SR is associated with atrial reverse remodelling and reduction in both A-SMR and A-STR.10,34
For unclear reasons, not all patients with permanent AF and RA dilation develop significant A-STR. One hypothesis relates to the highly variable anatomy of the atrioventricular junction. Indeed, the insertion of the TV leaflets into the TA varies at the level of the mural part of the annulus (along the RV free wall). Leaflets can be inserted on the inferior margin of the atrial wall, on the superior (atrial) margin of the ventricular wall, or on both, in different annular areas of the same patient29 (Figure 2). An insertion line predominantly located in the atrial myocardium may therefore predispose to develop A-STR. Conversely, in V-STR, the thin muscular strings arising from the RV base and inserting into the right atrioventricular junction may mediate TA dilation.29 Compensatory leaflet growth through endothelial–mesenchymal transformation has been also described as an adaptive response to TA dilation or leaflet tethering, aiming to augment the area of leaflet coverage and decrease STR severity.5 Compared with patients with V-STR in SR, A-STR patients were characterized by an insufficient adaptive posterior leaflet growth, associated with predominant posterior dilation and displacement of TA.24 The different morphology and number of TV leaflets, as well as a different extent of compensatory TV leaflet growth, may contribute to the variability of A-STR development in AF patients.35 Animal studies elucidated that, although the TV leaflets grow in response to leaflet stress, there is a maladaptive increase in cellularity and fibrosis of the TV.36 This was associated with collagen up-regulation along the leaflet edges, which was in turn linked to TR severity. Animal studies have also shown that profibrotic changes of tethered MV leaflets post-myocardial infarction can be modulated by losartan,37 whilst the decrease in serotonin transporter activity may lead to increased MV stiffening and dysfunction.38 Fibrosis modulators may therefore have a role in delaying or preventing the development of the malcoaptation in the setting of A-STR.
Atrial secondary tricuspid regurgitation may occur even in the absence of AF, especially when atrial myopathy and HFpEF-related factors, such as aging, female sex, and LV diastolic dysfunction are present. The concept of RA myopathy is increasingly recognized and may explain why a significant proportion of isolated TR patients (up to 38% in the European Society of Cardiology - Heart Failure Association, Heart Failure Long-Term Registry) do not have any history of AF.1,39,40 Recent data showed that RA mechanics together with minimal RA size may be more relevant than maximal RA volume for the A-STR pathophysiology. Right atrial function, in terms of reservoir strain and haemodynamics, is more altered in A-STR patients compared with other TR aetiologies and in massive–torrential compared with severe TR.41 The minimal RA volume and TA area were reported to be the only predictors of STR severity, whilst the maximal RV volume and RA volume were not.42 Although maximal RA volume is similar in A-STR and V-STR for the same TR severity, the larger TA area coupled with insufficient TV adaptative growth leads to severe A-STR even in the absence of leaflet tethering.43 The concept of RA myopathy in relation to HFpEF and the role of rhythm control in AF to restore RA function and potentially improve A-STR need to be further clarified.44
Proposal of a standardized nomenclature and definition of atrial secondary tricuspid regurgitation
The term ‘secondary’ is used throughout this document in agreement with the current societal guidelines,12,13,45 although ‘functional’ TR has been used interchangeably with STR in the literature to distinguish patients having an abnormal leaflet coaptation secondary to RA/TA dilation or RV remodelling. The terms ‘idiopathic’ TR and ‘isolated’ TR have been variably used to describe TR occurring in isolation due to either AF, a primary disease of TV, a device, or to the TR progression after left-sided valve surgery.13,46,47 To avoid any confusion, these terms should no longer be used to describe A-STR.
The new definition of the A-STR phenotype proposed in this document includes the criteria listed in Table 1.2,3,43,48–52 Overlapping imaging features of A-STR and V-STR may present in a single patient, for instance when A-SMR (or other left-sided diseases) becomes significant during the A-STR disease course, leading to significant post-capillary PH and RV remodelling. Alternatively, a superimposed event known to cause TR may occur in A-STR patients [new LV dysfunction, pacemaker implantation with concomitant cardiac implantable electronic device (CIED)-related TR, etc.]. Finally, the chronic volume overload due to the long-standing A-STR will eventually lead to overt RV remodelling and dysfunction, and a ventricular component will be added to the initial atrial mechanism. In the presence of supporting evidence attesting to the initial A-STR mechanism, these cases should be labelled as mixed STR. Often, it may be difficult to discern the dominant STR mechanism when the patient presents in the late phase of the A-STR pathway. However, accurate phenotyping at this stage is probably less clinically relevant than in early-stage A-STR, when the reverse remodelling of the right chambers and TA (by rhythm control or valve repair) is more likely.
Proposed definition criteria for the atrial secondary tricuspid regurgitation phenotype
| Definition criteria for A-STR . | Recommended cut-offs and caveats . |
|---|---|
| 1. Clinically relevant STR (greater or equal to moderate) | Alternative causes of STR and the typical imaging features of primary TR should be adequately ruled outa |
 |
|
| 2. Predominant TA dilation | Limits of normality for parameters of TA size depend on sex, body size, view, and timing of measurement during the cardiac cycle |
 | 2D Echo measurements at end-diastole48,50:
|
| 3. Predominant RA dilation with increased end-systolic RA:RV ratio | RA dilation is not specific for A-STR, but rather its disproportion compared with RV size |
 |
|
| 4. Absence of significant tricuspid leaflet tethering | Single-plane measurement of coaptation height might underestimate leaflet tenting in case of asymmetric tethering |
 | Absence of significant TV leaflet tethering is defined by |
| 5. RV conical remodelling with predominant enlargement of RV basal dimension | RV sphericity is a surrogate index reflecting conical (triangular) RV remodelling, and is calculated as (RV midventricular diameter × RV longitudinal diameter)/RV basal diameter |
 | |
| 6. Preserved LV and RV systolic function | Preserved bi-ventricular function criteria may not apply if the A-STR patient is evaluated during AF with relatively rapid ventricular response or in the late stages of severe to torrential A-STR (with/without significant A-SMR) |
 | (in SR or rate-controlled AF and in earlier stages of the A-STR disease) |
| Definition criteria for A-STR . | Recommended cut-offs and caveats . |
|---|---|
| 1. Clinically relevant STR (greater or equal to moderate) | Alternative causes of STR and the typical imaging features of primary TR should be adequately ruled outa |
 |
|
| 2. Predominant TA dilation | Limits of normality for parameters of TA size depend on sex, body size, view, and timing of measurement during the cardiac cycle |
 | 2D Echo measurements at end-diastole48,50:
|
| 3. Predominant RA dilation with increased end-systolic RA:RV ratio | RA dilation is not specific for A-STR, but rather its disproportion compared with RV size |
 |
|
| 4. Absence of significant tricuspid leaflet tethering | Single-plane measurement of coaptation height might underestimate leaflet tenting in case of asymmetric tethering |
 | Absence of significant TV leaflet tethering is defined by |
| 5. RV conical remodelling with predominant enlargement of RV basal dimension | RV sphericity is a surrogate index reflecting conical (triangular) RV remodelling, and is calculated as (RV midventricular diameter × RV longitudinal diameter)/RV basal diameter |
 | |
| 6. Preserved LV and RV systolic function | Preserved bi-ventricular function criteria may not apply if the A-STR patient is evaluated during AF with relatively rapid ventricular response or in the late stages of severe to torrential A-STR (with/without significant A-SMR) |
 | (in SR or rate-controlled AF and in earlier stages of the A-STR disease) |
Definite A-STR diagnosis requires the fulfilment of all six criteria. Probable A-STR is defined by at least 4 criteria. Specific thresholds for parameters describing the geometry of right chambers and TV in A-STR are based on currently limited evidence, normative data, or TVARC document49 and might be further refined by future dedicated studies on A-STR.
aSee Supplementary material.
2D, two-dimensional; 3D, three-dimensional; AF, atrial fibrillation; A-SMR, atrial secondary mitral regurgitation; A-STR, atrial secondary tricuspid regurgitation; EROA, effective regurgitant orifice area; FAC, fractional area change; LV, left ventricular; LVEF, left ventricular ejection fraction; PISA, proximal isovelocity surface area; RA, right atrial; RV, right ventricular; RVFWLS, right ventricular free wall longitudinal strain; RVEF, right ventricular ejection fraction; SR, sinus rhythm; STR; secondary tricuspid regurgitation; TA, tricuspid annulus; TAPSE, tricuspid annular plane systolic excursion; TDI S’, tissue Doppler imaging systolic velocity; TR, tricuspid regurgitation; TV, tricuspid valve; VC, vena contracta.
Proposed definition criteria for the atrial secondary tricuspid regurgitation phenotype
| Definition criteria for A-STR . | Recommended cut-offs and caveats . |
|---|---|
| 1. Clinically relevant STR (greater or equal to moderate) | Alternative causes of STR and the typical imaging features of primary TR should be adequately ruled outa |
 |
|
| 2. Predominant TA dilation | Limits of normality for parameters of TA size depend on sex, body size, view, and timing of measurement during the cardiac cycle |
 | 2D Echo measurements at end-diastole48,50:
|
| 3. Predominant RA dilation with increased end-systolic RA:RV ratio | RA dilation is not specific for A-STR, but rather its disproportion compared with RV size |
 |
|
| 4. Absence of significant tricuspid leaflet tethering | Single-plane measurement of coaptation height might underestimate leaflet tenting in case of asymmetric tethering |
 | Absence of significant TV leaflet tethering is defined by |
| 5. RV conical remodelling with predominant enlargement of RV basal dimension | RV sphericity is a surrogate index reflecting conical (triangular) RV remodelling, and is calculated as (RV midventricular diameter × RV longitudinal diameter)/RV basal diameter |
 | |
| 6. Preserved LV and RV systolic function | Preserved bi-ventricular function criteria may not apply if the A-STR patient is evaluated during AF with relatively rapid ventricular response or in the late stages of severe to torrential A-STR (with/without significant A-SMR) |
 | (in SR or rate-controlled AF and in earlier stages of the A-STR disease) |
| Definition criteria for A-STR . | Recommended cut-offs and caveats . |
|---|---|
| 1. Clinically relevant STR (greater or equal to moderate) | Alternative causes of STR and the typical imaging features of primary TR should be adequately ruled outa |
 |
|
| 2. Predominant TA dilation | Limits of normality for parameters of TA size depend on sex, body size, view, and timing of measurement during the cardiac cycle |
 | 2D Echo measurements at end-diastole48,50:
|
| 3. Predominant RA dilation with increased end-systolic RA:RV ratio | RA dilation is not specific for A-STR, but rather its disproportion compared with RV size |
 |
|
| 4. Absence of significant tricuspid leaflet tethering | Single-plane measurement of coaptation height might underestimate leaflet tenting in case of asymmetric tethering |
 | Absence of significant TV leaflet tethering is defined by |
| 5. RV conical remodelling with predominant enlargement of RV basal dimension | RV sphericity is a surrogate index reflecting conical (triangular) RV remodelling, and is calculated as (RV midventricular diameter × RV longitudinal diameter)/RV basal diameter |
 | |
| 6. Preserved LV and RV systolic function | Preserved bi-ventricular function criteria may not apply if the A-STR patient is evaluated during AF with relatively rapid ventricular response or in the late stages of severe to torrential A-STR (with/without significant A-SMR) |
 | (in SR or rate-controlled AF and in earlier stages of the A-STR disease) |
Definite A-STR diagnosis requires the fulfilment of all six criteria. Probable A-STR is defined by at least 4 criteria. Specific thresholds for parameters describing the geometry of right chambers and TV in A-STR are based on currently limited evidence, normative data, or TVARC document49 and might be further refined by future dedicated studies on A-STR.
aSee Supplementary material.
2D, two-dimensional; 3D, three-dimensional; AF, atrial fibrillation; A-SMR, atrial secondary mitral regurgitation; A-STR, atrial secondary tricuspid regurgitation; EROA, effective regurgitant orifice area; FAC, fractional area change; LV, left ventricular; LVEF, left ventricular ejection fraction; PISA, proximal isovelocity surface area; RA, right atrial; RV, right ventricular; RVFWLS, right ventricular free wall longitudinal strain; RVEF, right ventricular ejection fraction; SR, sinus rhythm; STR; secondary tricuspid regurgitation; TA, tricuspid annulus; TAPSE, tricuspid annular plane systolic excursion; TDI S’, tissue Doppler imaging systolic velocity; TR, tricuspid regurgitation; TV, tricuspid valve; VC, vena contracta.
Characteristics of the atrial secondary tricuspid regurgitation phenotype
Atrial secondary tricuspid regurgitation corresponds to the Carpentier Type 1 classification, in which leaflets structure and motion are normal and there is isolated annular dilation. Consequently, the jet direction in A-STR is commonly central, whilst in V-STR (Carpentier Type IIIb) and primary TR (Carpentier Types II and IIIa) can be either central or eccentric. An eccentric TR jet in the presence of AF and a device lead should raise the suspicion of CIED-related TR.15 Clinically relevant A-STR should be at least moderate (Table 1) since a mild degree of TR is common in healthy subjects.
The characteristics of A-STR and those overlapping with V-STR have been summarized in the Graphical Abstract and are described in detail in the Supplementary data. The proposed parameters that can be used to classify a patient with A-STR are summarized in Figure 3. In the setting of discordant measures within each anatomic or functional category proposed in Figure 3, an integrative approach should be used to define A-STR and V-STR.
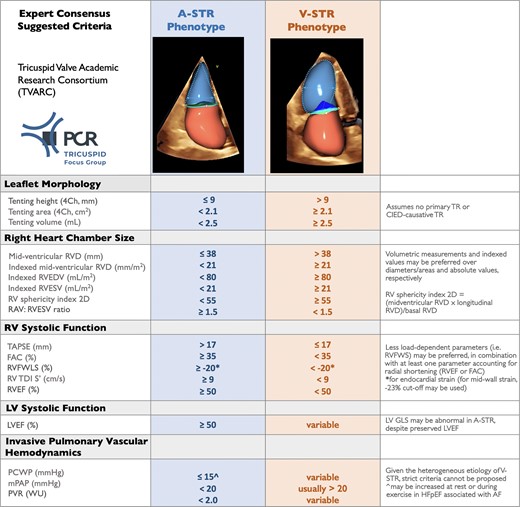
Suggested anatomic and functional parameters to discriminate atrial secondary tricuspid regurgitation from ventricular secondary tricuspid regurgitation from the PCR Tricuspid Focus Group and Tricuspid Valve Academic Research Consortium (TVARC).49 If data are discordant or incomplete (e.g. RVFWLS missing), an integrative approach based on multiple parameters is recommended. 2D, two-dimensional; 4Ch, apical four-chamber; A-STR, atrial secondary tricuspid regurgitation; CIED, cardiac implantable electronic device; EDV, end-diastolic volume; ES, end-systolic; ESV, end-systolic volume; FAC, fractional area change; LV, left ventricular; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain; mPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAV, right atrial volume; RVFWLS, right ventricular free wall longitudinal strain; RVD, right ventricular diameter; RVEF, right ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion; TDI S, tissue Doppler systolic velocity; TR, tricuspid regurgitation; V-STR, ventricular secondary tricuspid regurgitation; WU, Woods units
Importantly, A-STR is a diagnosis of exclusion and implies that alternative causes of primary TR and V-STR have been adequately ruled out (Graphical Abstract; Supplementary data).
Clinical context of atrial secondary tricuspid regurgitation
Patients who have severe A-STR may remain asymptomatic for a prolonged period. Early complaints are non-specific, such as fatigue, dyspnoea, and reduced ability to exercise due to a decrease in cardiac output.53 Compared with V-STR, patients with clinically relevant A-STR present with normal bi-ventricular function, which may contribute to their better survival.52,54 However, severe A-STR is associated with excess mortality and morbidity and its prognosis seems to improve with TTVI as compared with medical therapy, warranting heightened attention for a prompt diagnosis by quantitative methods and early referral to specialized centers.52,55
Amongst patients with new-onset AF, those who develop clinically relevant A-STR at follow-up are older, more frequently women, with chronic lung disease, a history of congestive heart failure, and permanent AF.9 For patient screening, prevention, and early diagnosis of A-STR, we propose the following list of criteria identifying patients at risk of A-STR:3,9
At risk for A-STR criteria:
Elderly patient (>70 years)
Female sex
Non-paroxysmal AF (or atrial flutter)
Rate control strategy
HFpEF
TA dilation
Mild A-STR with RA dilation and increased end-systolic RA:RV area ratio (>2.1)
A-SMR
Intermediate likelihood for PH (peak TRV 2.9–3.4 m/s)
For patients with AF who meet these criteria, close monitoring with regular clinical and echocardiographic follow-up (every year, or sooner in case of clinical worsening or HFpEF) and rhythm control management should be implemented.3,9
Imaging challenges and practical tips for evaluating patients with atrial secondary tricuspid regurgitation
Specific imaging challenges require particular attention in patients with A-STR due to the high prevalence of AF along with the complex three-dimensional (3D) geometry of the right heart structures and chronic RV volume overload.
Two-dimensional echocardiography
In patients with A-STR, the RV basal diameter is not a good surrogate of RV size and does not correlate well with RV volume. The RV basal diameter potentially leads to the overestimation of RV size due to RV conical remodelling in A-STR compared with a more elliptical (spherical) RV remodelling in V-STR.30,39 Moreover, RV basal diameter has limited inter-observer and test–retest reproducibility due to the crescentic shape of the cross-sectional cut plane of the RV. By retrospective cluster analysis, mid-RV transversal diameter has been found useful to discriminate A-STR (normal mid-RV diameter) from V-STR (increased mid-RV diameter).43,52 Right ventricular dimensions should be assessed on the RV-focused apical view,56,57 which results in the measurement of the largest RV dimensions and higher values of RV longitudinal function indices compared with the standard four-chamber view.58 Normative data for RV dimensions obtained on RV-focused view are now available.48
Both tricuspid annular plane systolic excursion (TAPSE) and the RV fractional area change are heavily affected by RV volume overload and are likely to overestimate the actual RV function in patients with severe A-STR. The ratio between RV systolic function parameters and the Doppler-estimated pulmonary arterial systolic pressure (PASP) [TAPSE/PASP or RV free wall longitudinal strain (RVFWLS)/PASP] has been proposed as a surrogate for the invasively determined RV-pulmonary artery (PA) coupling.59 Despite the limited accuracy of PASP estimation in massive/torrential STR characterized by a large effective regurgitant orifice area with low-flow velocity and rapid equalization of the RV and RA pressures,60,61 TAPSE/PASP < 0.36 mm/mmHg and RVFWLS/PASP < −0.42%/mmHg have been associated with worse outcomes in medically treated patients with clinically relevant TR,62 whilst TAPSE/PASP < 0.39–0.41 mm/mmHg was associated with increased mortality after TTVI.61,63 A RV-PA coupling using TAPSE and invasively measured PASP (proposed cut-offs of <0.29–0.30 mm/mmHg)60,61 improved the outcome prediction compared with the non-invasive TAPSE/PASP.
The RA volume and TA diameter50 may be grossly underestimated when using two-dimensional echocardiography measures on the conventional apical four-chamber view. Conversely, RA volumes obtained using the RV-focused apical view are closer to those obtained with 3D echocardiography and cardiac magnetic resonance (CMR).64 In patients with persistent AF, measurement of RA reservoir strain (RASr) appears to be more relevant than maximal atrial volumes to predict AF relapse after electrical cardioversion.65 An impaired RASr (<9.4%) was associated with a 3.2-fold increased risk of hospitalizations for heart failure and cardiovascular death in severe TR.41
As the TR jet velocity is about half compared with MR, the colour Doppler jet area underestimates the severity of A-STR, particularly in patients with late-stage A-STR with significant leaflet tethering.66 Marked beat-to-beat and respiratory changes in TR systolic velocities render A-STR quantification difficult (Table 2). Because TR lasts longer in systole and with higher velocity in PH-related V-STR than in A-STR, for the same effective regurgitant orifice, the regurgitant volume is larger with PH, with subsequent larger RV.39 Furthermore, due to the lower PASP and TR velocity, the A-STR jet area is much smaller. The combination of smaller RV and smaller regurgitant jet area tends to make A-STR underestimated, emphasizing the importance of A-STR quantification. Proximal isovelocity surface area method correction according to the TR velocity and leaflet tenting angle may improve its accuracy.67
Imaging challenges in atrial secondary tricuspid regurgitation patients due to persistent atrial fibrillation and potential solutions
| Challenges . | Potential Solutions . |
|---|---|
| Irregular (and short) R–R interval |
|
| Limitations of A-STR severity indices (systolic blunting of hepatic venous flow, volumetric method, intra- and inter-beat variability of PISA radius) |
|
| Risk of underestimating A-STR severity |
|
| Risk of underestimating tricuspid annulus size (inter-beat variability of the timing of maximal size) |
|
| Risk of overestimating RV size if using basal linear diameter measured by 2D echocardiography |
|
| Risk of underestimating RA size if using standard four-chamber view, particularly if severely dilated LA |
|
| Risk of under- or over-estimating PASP due to lack of TRV average or inaccurate estimation of RA pressure |
|
| Challenges . | Potential Solutions . |
|---|---|
| Irregular (and short) R–R interval |
|
| Limitations of A-STR severity indices (systolic blunting of hepatic venous flow, volumetric method, intra- and inter-beat variability of PISA radius) |
|
| Risk of underestimating A-STR severity |
|
| Risk of underestimating tricuspid annulus size (inter-beat variability of the timing of maximal size) |
|
| Risk of overestimating RV size if using basal linear diameter measured by 2D echocardiography |
|
| Risk of underestimating RA size if using standard four-chamber view, particularly if severely dilated LA |
|
| Risk of under- or over-estimating PASP due to lack of TRV average or inaccurate estimation of RA pressure |
|
2D, two-dimensional; 3D, three-dimensional; AI, artificial intelligence; AF, atrial fibrillation; A-STR, atrial secondary tricuspid regurgitation; HFpEF, heart failure with preserved ejection fraction; LA, left atrium; PASP; pulmonary arterial systolic pressure; PISA, proximal isovelocity surface area; PH, pulmonary hypertension; RA, right atrium; RV, right ventricle; TRV, tricuspid regurgitation velocity; TV, tricuspid valve.
Imaging challenges in atrial secondary tricuspid regurgitation patients due to persistent atrial fibrillation and potential solutions
| Challenges . | Potential Solutions . |
|---|---|
| Irregular (and short) R–R interval |
|
| Limitations of A-STR severity indices (systolic blunting of hepatic venous flow, volumetric method, intra- and inter-beat variability of PISA radius) |
|
| Risk of underestimating A-STR severity |
|
| Risk of underestimating tricuspid annulus size (inter-beat variability of the timing of maximal size) |
|
| Risk of overestimating RV size if using basal linear diameter measured by 2D echocardiography |
|
| Risk of underestimating RA size if using standard four-chamber view, particularly if severely dilated LA |
|
| Risk of under- or over-estimating PASP due to lack of TRV average or inaccurate estimation of RA pressure |
|
| Challenges . | Potential Solutions . |
|---|---|
| Irregular (and short) R–R interval |
|
| Limitations of A-STR severity indices (systolic blunting of hepatic venous flow, volumetric method, intra- and inter-beat variability of PISA radius) |
|
| Risk of underestimating A-STR severity |
|
| Risk of underestimating tricuspid annulus size (inter-beat variability of the timing of maximal size) |
|
| Risk of overestimating RV size if using basal linear diameter measured by 2D echocardiography |
|
| Risk of underestimating RA size if using standard four-chamber view, particularly if severely dilated LA |
|
| Risk of under- or over-estimating PASP due to lack of TRV average or inaccurate estimation of RA pressure |
|
2D, two-dimensional; 3D, three-dimensional; AI, artificial intelligence; AF, atrial fibrillation; A-STR, atrial secondary tricuspid regurgitation; HFpEF, heart failure with preserved ejection fraction; LA, left atrium; PASP; pulmonary arterial systolic pressure; PISA, proximal isovelocity surface area; PH, pulmonary hypertension; RA, right atrium; RV, right ventricle; TRV, tricuspid regurgitation velocity; TV, tricuspid valve.
Three-dimensional echocardiography
Multi-beat 3D acquisition commonly achieves optimal spatial and temporal resolution for automated quantitation of chamber volumes and ventricular ejection fraction. However, since most A-STR patients are in AF with irregular R-R interval, the reproducibility and accuracy of volume quantitation may be affected by stitching artefacts (Table 2). Recent advances allowing high temporal resolution single-beat 3D imaging made possible the (automated) measurement of RA, RV volumes, and RV ejection fraction (RVEF)64,68,69 with excellent accuracy and reproducibility compared with CMR.70 When the transthoracic apical acoustic window is satisfactory, 3D echocardiography is the preferred modality for an accurate and reproducible quantification of RA and RV volumes and for reliably assessing the right chamber reverse remodelling in response to treatment.71 Vena contracta area by 3D colour Doppler provides significant advantages with respect to single plane vena contracta width in case of asymmetric or complex regurgitant orifices.15 The ratio between forward RV stroke volume (i.e. total RV stroke volume—tricuspid regurgitant volume) and RV end-systolic volume by 3D echocardiography (RV forward stroke volume/end-systolic volume proposed cut-off of <0.40) was more strongly associated with outcome than non-invasive TAPSE/PASP and RVFWLS/PASP in patients with clinically relevant STR.62 Three-dimensional transoesophageal echocardiography can reveal anatomic details of TV leaflets not readily apparent by conventional transthoracic approach (small leaflet prolapse, flail, endocarditis, CIED interference, supernumerary scallops, gap localization, and size), which may refine the classification of TR phenotype and help the decision-making regarding TV repair.14
Cardiac computed tomography
Using a dedicated imaging protocol for enhancing right chambers with a triphasic injection of contrast/saline mixture, cardiac computed tomography (CCT) provides accurate measurements of the dimensions of TA and right heart chambers that are essential for pre-procedural planning of transcatheter and minimally invasive surgical TV interventions (Figure 4).72 The TV leaflets are thin structures that may be challenging to visualize by CCT.73,74 Cardiac computed tomography may add relevant anatomic information (such as accurate visualization of pulmonary veins and LA appendage morphology and measurement of ostial diameters) for AF ablation planning in A-STR patients. In A-STR patients undergoing CCT, adequate rate control of AF is mandatory and retrospective, low-pitch approach is preferred over prospective scanning to minimize the artefacts.73
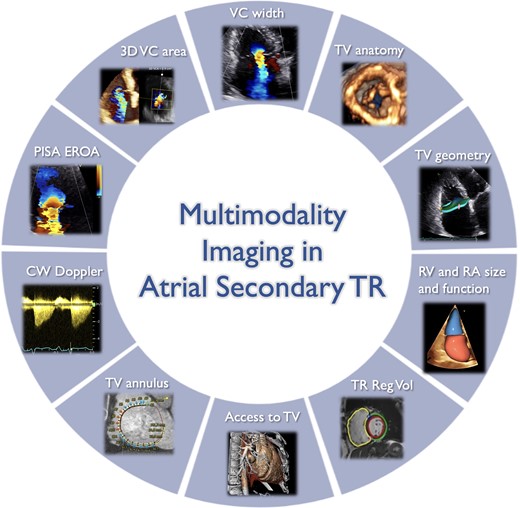
Role of multi-modality imaging for the comprehensive characterization of atrial secondary tricuspid regurgitation patients. 3D, three-dimensional; A-STR, atrial secondary tricuspid regurgitation; CW, continuous wave; EROA, effective regurgitant orifice area; PISA, proximal isovelocity surface area; RA, right atrium; Reg Vol, regurgitant volume; RV, right ventricle; TV, tricuspid valve; VC, vena contracta. Image prepared with BioRender software
Cardiac magnetic resonance
The role of CMR in the assessment of patients with A-STR includes the volumetric and functional assessment of the RA and ventricle, quantification of TR, and assessment of PH.75 By an accurate quantification of RV volumes and RVEF (especially when echocardiographic findings are suboptimal) and evaluation of the presence, pattern, and extent of myocardial fibrosis, CMR may aid the differentiation of A-STR vs. V-STR phenotype and the risk stratification before TV intervention and may guide the timing for intervention.15 Although RVEF is the standard parameter to report RV performance with CMR, it can overestimate RV function in severe TR. Effective RVEF corrected for tricuspid regurgitant volume could potentially detect earlier RV dysfunction and had a stronger association with outcomes (heart failure and cardiovascular mortality) than conventional RVEF.76 As CMR depends heavily on ECG gating, the irregularity of the cardiac rhythm during AF can make its application problematic in A-STR patients, as the quality of cine CMR images may be significantly affected by blurring and motion artefacts. Real-time CMR during free breathing emerged as a promising alternative to ECG-synchronized balanced steady-state free precession acquisitions in patients with an irregular rhythm, although it tends to provide lower volumes compared with the standard cine CMR method due to undersampling.77 To assess the tricuspid regurgitant volume and regurgitant fraction, phase-contrast CMR or four-dimensional flow CMR are the preferred techniques. In patients with A-STR undergoing surgical TV intervention, CMR-derived tricuspid regurgitant fraction and RV longitudinal strain were independently associated with worse survival.78 Furthermore, CMR-derived regurgitant fraction of ≥30% and regurgitant volume of ≥35 mL were the optimal thresholds associated with mortality during follow-up.78 Cardiac magnetic resonance can provide information on the extent of LA remodelling and fibrosis, as a powerful predictor of the success of AF ablation up to 5 years of follow-up. In patients with advanced atrial fibrosis by late gadolinium enhancement CMR, AF ablation is associated with a high procedural failure rate.79 Therefore, CMR may inform the decision to attempt rhythm control vs. to proceed to TTVI in patients with severe A-STR.
Treatment of atrial secondary tricuspid regurgitation
The management of severe symptomatic A-STR should be evaluated on a case-by-case basis within an extended multi-disciplinary heart team with expertise in transcatheter and surgical treatment of the TV at dedicated Heart Valve Centers, comprising clinical and interventional cardiologists, cardiac surgeons, imaging specialists with expertise in interventional imaging, heart failure specialists, and electrophysiologists.
Whilst valvular interventions are currently applied irrespective of the A-STR or V-STR phenotype, medical treatment and the relative importance of rhythm control strategy may vary according to the predominant aetiology of the TV disease. Acknowledging the paucity of data on this topic, a proposed treatment algorithm in A-STR is presented in Figure 5 and the clinical pathway and management of patients with A-STR are illustrated in Figure 6.
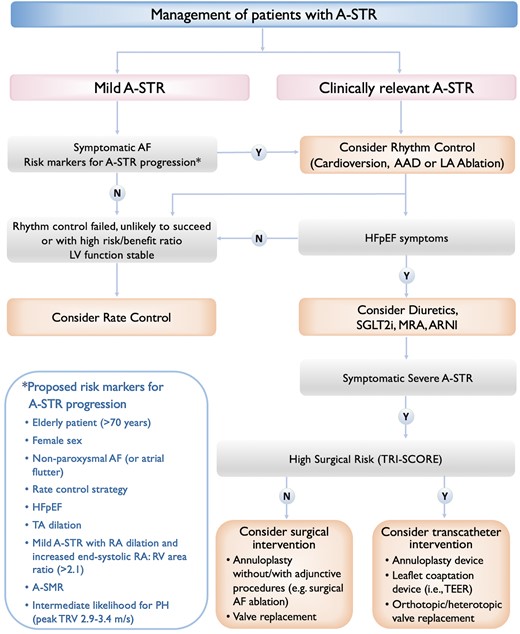
Proposed algorithm for managing patients with atrial secondary tricuspid regurgitation. AAD, antiarrhythmic drugs; ARNI, angiotensin receptor–neprilysin inhibitor; A-SMR, atrial secondary mitral regurgitation; A-STR, atrial secondary tricuspid regurgitation; HFpEF, heart failure with preserved ejection fraction; LA, left atrial; LV, left ventricle; MRA, mineralocorticoid receptor antagonist; PH, pulmonary hypertension; RA, right atrium; RV, right ventricle; SGLT2i, sodium–glucose co-transporter-2 inhibitor; TEER, transcatheter edge-to-edge repair; TRV, tricuspid regurgitation velocity
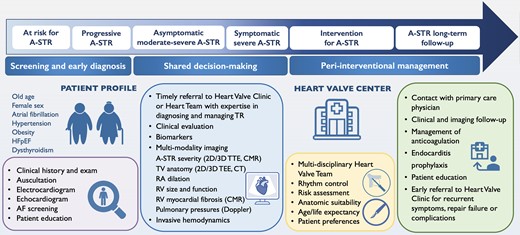
Clinical pathway and management of patients with atrial secondary tricuspid regurgitation. 2D, two-dimensional; 3D, three-dimensional; AF, atrial fibrillation; A-STR, atrial secondary tricuspid regurgitation; CMR, cardiac magnetic resonance; CT, computed tomography; HFpEF, heart failure with preserved ejection fraction; RA, right atrium; RV, right ventricle; TEE, transoesophageal echocardiography; TV, tricuspid valve; TTE, transthoracic echocardiography
Medical therapy
The main approach to treating A-STR consists of therapies targeting the underlying disease process (i.e. AF and HFpEF) and of diuretics to address the volume overload. Thiazide diuretic agents may be useful in combination with loop diuretics for more effective diuresis. As part of the sequential tubular blockade, the mineralocorticoid receptor antagonists (MRAs) may further improve symptoms and diastolic dysfunction, reduce hospitalizations in HFpEF patients with A-STR, and counterbalance the side effects of loop and thiazide diuretics. As hyperaldosteronism contributes to the pathogenesis of ascites and resistance to loop diuretics, MRA efficacy is augmented in case of hyperaldosteronism related to HFpEF and/or hepatic dysfunction. Limited data suggest that MRAs may be also beneficial to prevent recurrent AF episodes.80 Sodium–glucose co-transporter-2 inhibitors can be beneficial in selected patients with A-STR, HFpEF, and right-sided heart failure due to their diuretic, nephroprotective, and symptomatic effects. Women with HFpEF may respond more favourably to MRAs and angiotensin receptor–neprilysin inhibitor.81
If a rate control strategy is chosen, care should be taken to avoid aggressive heart rate reduction in severe A-STR (particularly in HFpEF81) due to low LV stroke volume and stroke volume reserve. Beta-blockers may be used as first-line treatment, but chronotropic incompetence may worsen exercise intolerance. Whilst digoxin in combination with beta-blockers can be useful for the management of poorly controlled ventricular rate patients with AF, digoxin does not reduce symptoms, hospitalizations, or mortality in HFpEF and may promote AF recurrence in patients with paroxysmal AF. Rate control might improve cardiac output due to improved RV filling; however, it will have no significant impact on TR severity. Atrial fibrillation in patients with A-STR/A-SMR is considered a non-valvular type of AF and the use of direct oral anticoagulants is recommended before valvular intervention based on CHA2D2-VASc score unless contraindicated.
Sinus rhythm restoration
In AF patients with A-STR, SR restoration should be attempted as the first step in treatment, if indicated and likely to succeed. Rhythm control strategy by cardioversion or catheter ablation may reduce the risk of incident significant A-STR and, once present, reduce its severity and slow its progression by preventing chamber remodelling.82 Restoration of SR was associated with a significant decrease in TR at 12 months, especially in patients with active restoration by cardioversion or catheter ablation, who showed reverse remodelling of the LA, RA, and RV.10 Sustained AF tripled the risk of clinically relevant TR compared with recurrent paroxysmal AF,9 suggesting that AF burden and duration, as well as RA size, should be considered for decision-making. Patients with long-standing persistent AF with a predominant RA enlargement relative to LA size [RA volume index/LA volume index (RAVI/LAVI) > 1] are less likely to achieve successful AF ablation.83 Right atrial volume and reservoir strain appear to have an incremental role compared with respective LA parameters to predict AF recurrence after cardioversion (suggested cut-offs: RAVI ≥ 42 mL/m2 and LAVI ≥ 48 mL/m2; RASr ≤ 15% and LA reservoir strain ≤ 10%).65,84 A first episode and a shorter duration of AF (<6–12 months) are associated with a higher probability of successful maintenance of SR after cardioversion. Atrial reverse remodelling was best predicted by an intervention to restore SR within the first year after the index hospitalization.10 Early rhythm control significantly contributed to risk reductions of both incident significant TR and all-cause mortality by approximately 30% each.9 Although additional data are needed, these preliminary studies open the path towards early interventional rhythm control as a specific treatment strategy for patients with A-STR.
Surgery
The role of surgery in patients with A-STR still needs to be elucidated. Whilst isolated TR surgery has been associated with increased in-hospital mortality (10%),85,86 it is now well established that patients who are referred early to an experienced centre can be safely treated.87 In an international registry of 2413 patients with severe isolated STR (TRIGISTRY), an early and successful intervention—either surgical or transcatheter—improved 2-year survival only in patients with low, and to a lower extent, intermediate TRI-SCORE, whilst no benefit was observed in the patients with high TRI-SCORE. The main drivers of postoperative TR outcome, as captured by the TRI-SCORE, are the severity of clinical presentation and the TR consequences on the RV, kidneys, and liver.88
Surgical TV annuloplasty is appealing both as preventive (at the time of mitral valve surgery) and curative treatment in A-STR, since it counteracts the exact dilative annular mechanism leading to TV dysfunction. Annuloplasty alone may be enough to treat most patients and should be preferred over suture techniques only, as it provides longer durability.89 In a recent randomized controlled trial, surgical TV annuloplasty has been associated with an increased risk of new pacemaker implantation90 with potential CIED-related TR worsening.
As AF often leads to dual valve disease (A-STR and A-SMR), surgery can address both regurgitations in a single intervention. On the left side, recurrent regurgitation after surgical mitral annuloplasty occurred less frequently in A-SMR compared with V-SMR (residual MR ≥ moderate in 2.5% vs. 7% at 2 years).91 Additional techniques, such as surgical AF ablation, LA appendage ligation, or LA plication around the MA, play an important role in this specific population of patients.92 Surgical ablation procedures for paroxysmal and persistent AF during concomitant MV and TV surgery include full bi-atrial Cox–Maze procedure, pulmonary vein isolation, alone or combined with LA lesion sets, and hybrid procedure.93 Patients with A-SMR undergoing surgical MV repair and concomitant Maze procedure had a lower incidence of heart failure and AF and lower recurrence rates of moderate–severe MR and TR at follow-up than those who underwent catheter ablation procedure.94 Surgical AF ablation can be performed also in patients undergoing surgery for isolated A-STR, although its effectiveness in maintaining SR might be lower in late-stage disease with severely dilated atria.95 Right-sided AF ablation techniques are important when AF is due to triggers or substrate present in the RA.
In advanced cases with late-stage A-STR, the decision for TV annuloplasty combined with additional techniques (such as leaflet augmentation) or TV replacement instead of repair is largely driven by anatomic factors, including the presence of severe leaflet tethering and extreme annular dilation. In these patients, TV annuloplasty alone can be ineffective or even worsen the TR. If TV replacement is indicated, a bioprosthetic valve is generally used, due to its lower rate of structural deterioration in elderly patients with A-STR. If structural degeneration occurs, the large size of bioprosthetic valves implanted to treat A-STR allows also a second tricuspid valve-in-valve intervention procedure to be safely performed without the risk of significant valve stenosis.
In most A-STR patients undergoing surgical or transcatheter bioprosthetic valve implantation, oral anticoagulation (OAC) is recommended lifelong due to concomitant AF. In the absence of other indications, OAC should be considered in all patients in the first 3–6 months after bioprosthetic TV implantation.12,13 However, due to the relatively low-flow state of the right chambers and the severe RA dilation and dysfunction in A-STR patients, the risk of thromboembolic events may be higher for tricuspid compared with mitral bioprostheses. Since A-STR patients are often old and fragile, with multiple co-morbidities (e.g. hypertension and renal and hepatic dysfunction), the actual risk should be evaluated on an individual basis, considering the type of prosthetic valve and the patient’s bleeding risk. Prospective studies are needed to evaluate if more prolonged OAC duration may be advised after tricuspid bioprosthetic valve implantation, particularly in the absence of high bleeding risk features.
As TV surgery is associated with an increased risk of advanced atrioventricular block, implantation of an epicardial pacemaker lead should be considered in A-STR patients undergoing TV replacement and in selected patients undergoing TV annuloplasty.
Transcatheter tricuspid valve interventions
Transcatheter procedures are emerging as an appealing alternative to treat patients without other therapeutic options due to either advanced disease or increased surgical risk. The 2021 Valvular Heart Disease guidelines of the European Society of Cardiology included a IIb level C recommendation for transcatheter treatment of severe symptomatic TR in inoperable patients (IIb level C).12 Whilst they reinforced the importance of early referral of TR patients, no specific mention regarding A-STR patients was made. Several treatment modalities will certainly be needed to appropriately address the high anatomical variability of the TV complex, as well as the different disease aetiologies (Figure 7). Currently, transcatheter tricuspid edge-to-edge repair (TEER) and direct transcatheter annuloplasty (Cardioband) are approved treatments in Europe, yet their availability varies widely across European countries and is currently limited to specialized centres in TTVI. Evidence that different clinical scenarios may need different treatment approaches and may have diverging outcomes is emerging.96 Patients with A-STR have better survival when treated conservatively compared with V-STR patients, whilst the presence of A-STR remained independently associated with a lower rate of the combined endpoint of mortality and heart failure hospitalization following TTVR compared with non-A-STR patients.52,54,97
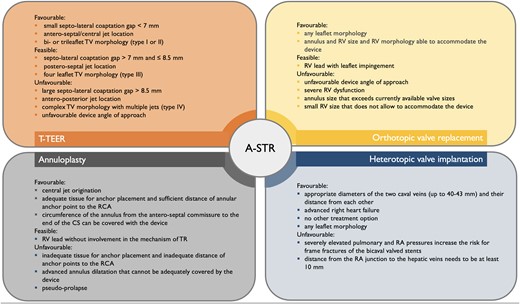
Anatomical criteria for device selection for TTVI in patients with atrial secondary tricuspid regurgitation. A-STR, atrial secondary tricuspid regurgitation; CS, coronary sinus; RA, right atrium; RCA, right coronary artery; RV, right ventricle/ventricular; T-TEER, tricuspid transcatheter edge-to-edge repair; TTVI, transcatheter tricuspid valve intervention; TV, tricuspid valve
Even if all treatment modalities including replacement have been used in patients with A-STR, only limited dedicated data have been published thus far. Atrial secondary tricuspid regurgitation patients likely represent a relevant proportion of the patients included in the TRILUMINATE trial,98 and further analyses of this trial will provide additional insights regarding the treatment response of this specific subgroup compared with medical treatment. Although patients with A-STR are older and less symptomatic as compared with patients with other TR phenotypes, it has been shown that they potentially benefit from TTVI and have lower mortality and heart failure hospitalization rates, probably due to more preserved bi-ventricular function and more efficient RV-PA coupling.52,54,99 In contrast, functional results including residual TR grade after the intervention, New York Heart Association class, and 6-min walk distance do not differ between A-STR and non-A-STR.52,99
In principle, any kind of TTVI procedure can be used for the treatment of A-STR, but specific anatomical characteristics must be taken into account (Figure 7). Of particular relevance are leaflet morphology with the definition of the number of leaflets,35,100 the origin100,101 and the effective regurgitant orifice area of the TR jet(s),72 and the size of the coaptation defect.100–102 Since coaptation gap and tethering are usually not pronounced during the initial disease stages, A-STR patients are overall good candidates for TEER or direct annuloplasty; TEER may also induce favourable changes in annular geometry, improving the leaflet-to-annulus mismatch.103 If TEER and direct annuloplasty are equally feasible, a direct annuloplasty device may be more appropriate since it does not preclude an orthotopic valve replacement, if needed later. The use of transcatheter replacement may currently be limited in some A-STR patients, due to too large TA dimensions compared to device-specific annular size criteria. Future developments of valves for transcatheter replacement will certainly target patients with A-STR with very large coaptation gaps not amenable to TEER.
Evidence gaps and future directions
The excess mortality and cardiac events associated with untreated severe isolated TR and the prognostic importance of quantitative echocardiographic parameters of TR severity55 emphasized the need to conduct clinical trials in the A-STR patients using a unifying definition for A-STR and dedicated imaging protocols accounting for the AF-related challenges (see Table 2). Disparities in the development of A-STR in patients with AF and comparable RA size need to be investigated prospectively using dedicated imaging techniques. The role of genetics and proteomics in identifying the molecular triggers for TA dilation and TV leaflet growth may further allow the identification of patients at risk.
The role of SR restoration on A-STR progression and the clinical/imaging characteristics of patients who will benefit from early aggressive rhythm control to treat and prevent A-STR need to be better characterized and possibly investigated in randomized controlled trials. Longitudinal prospective studies investigating the effect of permanent AF and SR maintenance on atrial remodelling and TR severity may help untangle the ‘chicken-and-egg’ presentation of AF and A-STR. Finally, the timing, most appropriate approach (either surgical or transcatheter), treatment modality, and immediate and preventive effect (besides quality-of-life improvement) need to be compared in A-STR and V-STR.
Conclusions
Due to its distinct pathophysiology, clinical presentation, prognosis, and potentially therapeutic approach, A-STR emerges as a separate STR phenotype first defined in the present document as an attempt to unify future prospective and retrospective research. New data suggest the need for specific interdisciplinary management to control AF, prevent disease progression, and mitigate patient symptoms due to chronic volume overload and frequently associated HFpEF.
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
D.M. declares research institutional support from GE Healthcare and Philips Medical System and speakers’ fees from GE Healthcare, Philips Medical Systems, Janssen-Cilag, and Bristol Myers Squibb. L.P.B. declares research institutional support from GE Healthcare and Philips Medical System and speakers’ fees from GE Healthcare, Philips Medical Systems, and ESAOTE. R.H. declares speaker fees from Abbott Structural, Baylis Medical, Edwards Lifesciences, Medtronic, Philips Healthcare, and Siemens Healthineers; she has institutional consulting contracts for which she receives no direct compensation with Abbott Structural, Edwards Lifesciences, Medtronic, and Novartis; she is Chief Scientific Officer for the Echocardiography Core Laboratory at the Cardiovascular Research Foundation for multiple industry-sponsored tricuspid valve trials, for which she receives no direct industry compensation. R.M.L. declares grant support from Philips Medical Systems and Ultrasight. V.D. declares speaker fees from Edwards Lifesciences, GE Healthcare, Medtronic, Novartis, and Philips and consulting fees from Edwards Lifesciences and Novo Nordisk. N.C.W. declares speaker fees from GE Healthcare, Philips Medical Systems, Abbott Vascular, Edwards Lifesciences, Boston Scientific, and Siemens Healthcare. A.D. has received consultancy fees from Abbott, Medtronic, Edwards Lifesciences, and NeoChord. M.T. declares consultancy fees from Abbott, Medtronic, Edwards Lifesciences, Boston Scientific, Shenqi Medical, MEDIRA, CoreMedic, VentriMend, CorQuest, Simulands, PiCardia, and CardioValve. P.L. has received institutional grants from Abbott Structural, ReCor, and Edwards Lifesciences, has received honoraria from ReCor and Innoventric, and hold share options of Innoventric. J.J.B. has received speaker fees from Abbott and Edwards Lifesciences. R.S.v.B. is PI or steering committee to trials with IIT (University of Göttingen, Germany) Abbott Structural, Edwards Lifesciences, Jenscare, Medtronic, and NeoChord; he declares consultancy fees from Abbott, Edwards Lifesciences, Jenscare, Medtronic, NeoChord, Philips Medical Systems, and Siemens Healthineers. F.M. declares grant and/or research institutional support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific Corporation, NVT, Terumo, and Venus; consulting fees and honoraria personal and institutional from Abbott, Medtronic, Edwards Lifesciences, Xeltis, CardioValve, Occlufit, Simulands, Mtex, Venus, and Squadra; royalty income/IP rights Edwards Lifesciences; shareholder (including share options) of Cardiogard, CardioValve, Magenta, SwissVortex, Transseptalsolutions, 4Tech, and Perifect. F.P. has received travel expenses from Edwards Lifesciences, Abbott Vascular, Medira, Polares Medical, and Siemens Healthineers. M.S. has received speaker and consultancy fees from Edwards Lifesciences, GE Healthcare, Medtronic, and Abbott. The other authors have nothing to declare.
Data Availability
No data were generated or analysed for this manuscript.
Funding
All authors declare no funding for this contribution.




