-
PDF
- Split View
-
Views
-
Cite
Cite
Emanuele Barbato, Emanuele Gallinoro, Mohamed Abdel-Wahab, Daniele Andreini, Didier Carrié, Carlo Di Mario, Dariusz Dudek, Javier Escaned, Jean Fajadet, Giulio Guagliumi, Jonathan Hill, Margaret McEntegart, Kambis Mashayekhi, Nikolasos Mezilis, Yoshinobu Onuma, Krzyszstof Reczuch, Richard Shlofmitz, Giulio Stefanini, Giuseppe Tarantini, Gabor G Toth, Beatriz Vaquerizo, William Wijns, Flavio L Ribichini, Management strategies for heavily calcified coronary stenoses: an EAPCI clinical consensus statement in collaboration with the EURO4C-PCR group, European Heart Journal, Volume 44, Issue 41, 1 November 2023, Pages 4340–4356, https://doi.org/10.1093/eurheartj/ehad342
Close - Share Icon Share
Abstract
Since the publication of the 2015 EAPCI consensus on rotational atherectomy, the number of percutaneous coronary interventions (PCI) performed in patients with severely calcified coronary artery disease has grown substantially. This has been prompted on one side by the clinical demand for the continuous increase in life expectancy, the sustained expansion of the primary PCI networks worldwide, and the routine performance of revascularization procedures in elderly patients; on the other side, the availability of new and dedicated technologies such as orbital atherectomy and intravascular lithotripsy, as well as the optimization of the rotational atherectomy system, has increased operators’ confidence in attempting more challenging PCI. This current EAPCI clinical consensus statement prepared in collaboration with the EURO4C-PCR group describes the comprehensive management of patients with heavily calcified coronary stenoses, starting with how to use non-invasive and invasive imaging to assess calcium burden and inform procedural planning. Objective and practical guidance is provided on the selection of the optimal interventional tool and technique based on the specific calcium morphology and anatomic location. Finally, the specific clinical implications of treating these patients are considered, including the prevention and management of complications and the importance of adequate training and education.
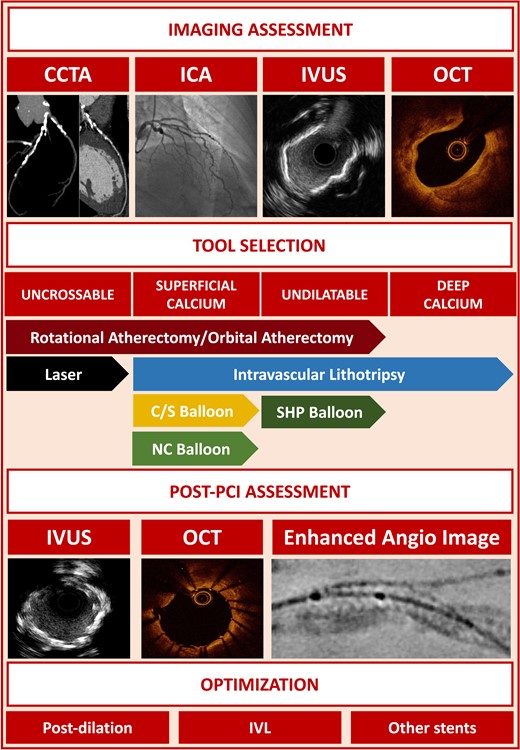
Summary of the main points of the consensus document. In the central section of the figure, the indication for each tool according to the type of calcified lesion is represented. Abbreviations: CCTA, coronary computed tomography angiography; ICA, invasive coronary angiography; IVUS, intravascular ultrasound; OCT, optical coherence tomography; NC, non-compliant; C/S, cutting/scoring; PCI, percutaneous coronary intervention; SHP, super-high-pressure; IVL, intravascular lithotripsy.
Introduction
Since the publication of the 2015 EAPCI consensus on rotational atherectomy (RA),1 the number of percutaneous coronary interventions (PCI) performed in patients with severely calcified coronary artery disease (CAD) has grown substantially.2 This has been prompted on one side by the clinical demand for the continuous increase in life expectancy and the routine performance of revascularization procedures in elderly patients. The prevalence of coronary calcification in fact is age- and sex-dependent being more common in men >70 years (>90% in men vs. 67% in women) and influenced by imaging modalities adopted. If only invasive coronary angiography (ICA) is used, the prevalence of moderate-to-severe calcification according to studies with core laboratory angiographic assessment is between 20%–30%.3–5 Therefore, advocating the need for adjunctive plaque modification techniques is likely in 2–3 out of 10 contemporary patients undergoing ICA.
The availability of new and dedicated technologies such as orbital atherectomy (OA) and intravascular lithotripsy (IVL), as well as the optimization of the RA system, has increased operators’ confidence in attempting more complex and calcified lesions, tailoring at times the device selection based on the calcification pattern. Calcification, in fact, can occur in both intimal and medial coronary layers. When occurring at the intima, calcification can lead to significant luminal obstruction and downstream ischaemia, while localization of calcification in the media and periadventitia results primarily in reduced vascular compliance.6 The mechanism of arterial calcification is complex and entails the apoptosis of macrophages within the lipid core and the dedifferentiation of vascular smooth muscle cells to an osteoblast phenotype, resulting in the development of surrounding sheets or nodules of calcium. Fractures in these calcified sheets can cause them to break through the overlying tissue and form nodules.7 Pathology and in vivo optical coherence tomography (OCT) studies have reported calcified nodules as the culprit lesion in 2%–10% of acute coronary syndrome (ACS) patients.8–10 Calcified plaques are less compliant than lipid plaques,11 and this can be associated with several technical challenges during PCI, including difficult device delivery, equipment damage or entrapment, stent underexpansion, and complications, such as coronary rupture.
This current EAPCI clinical consensus statement prepared in collaboration with the EURO4C-PCR group describes the comprehensive management of patients with heavily calcified coronary stenoses, starting with how to use non-invasive and invasive imaging to assess calcium burden and inform procedural planning to providing practical guidance on the appropriate device selection and adoption in order to optimize procedural outcomes (Graphical Abstract and Supplementary data online, Table S1).
Non-invasive imaging assessment
Coronary computed tomography angiography (CCTA) is validated as an accurate non-invasive method to detect coronary stenoses12,13 (see Supplementary data online, Figure S1). Coronary computed tomography angiography is able to assess calcium distribution and quantification, providing a roadmap of all coronary arteries.14 It provides similar information to intravascular imaging but with lower spatial resolution and a systematic overestimation of calcific plaque volume, especially when located periadventitial with limited impact on luminal obstruction.15 Calcium thickness remains challenging due to the presence of blooming artefact; preliminary studies have shown that photon-counting detector computed tomography (CT) may reduce this artefact, improving the diagnostic performance of CCTA.16 Additional limitations in CCTA are related to the presence of arrhythmias or to motion artefacts.
Heavy calcification of plaques at CCTA was associated with lower stent expansion and higher rates of adverse events after PCI.17 While visualization of a high calcium burden can predict the use of calcium modification techniques during PCI with 71% sensitivity and 97% specificity,18 further refinement of the procedural approach may require intravascular imaging. The lesion-based score of calcium severity may overcome some of the limitations encountered with the traditional calcium scoring (CS) assessment.18 In particular, a per-lesion calcium score of ≥453 is a predictor of an undilatable lesion and the need for dedicated devices such as RA.18 Despite the potential benefits for calcium assessment and procedural planning, CCTA is still underused in clinical practice.
Invasive imaging assessment of calcified lesions
Angiography
On invasive coronary angiography (ICA), moderate or severe calcified lesions are detected as radiopaque densities in the coronary arterial wall, seen with or without cardiac motion, and visible prior to contrast injection (Figure 1).4,19–21 These angiographic criteria do not necessarily imply an intraluminal obstruction but are included in the SYNTAX score I assessment of lesion complexity.22
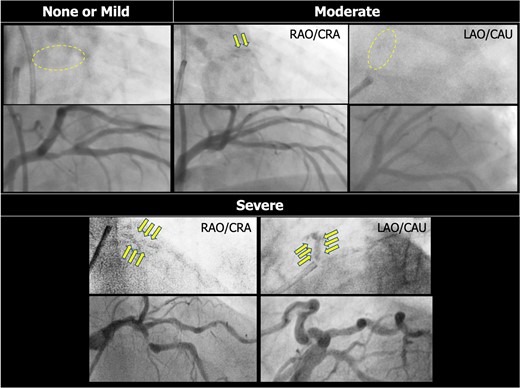
Detection of coronary calcification on invasive coronary angiography. Coronary calcification is documented as radiopaque densities in the coronary artery wall. Moderate calcification is defined as radiopaque densities noted only during cardiac motion and involving only one side of the vascular wall, which is typically visible only in a single projection. Severe calcification is defined as radiopaque densities noted without cardiac motion prior to contrast injection and involving both sides of the arterial wall. At variance, in the assessment of the SYNTAX score, severe calcification is defined as multiple persisting opacifications of the coronary wall visible in multiple projections, surrounding the complete lumen of the coronary artery at the site of the lesion.
Angiographically identified severe coronary calcification is a predictor of adverse clinical outcomes after revascularization with either PCI or bypass surgery.4,19,20 This may in part be explained by its association with a number of comorbidities [age, male sex, diabetes mellitus, and chronic kidney disease (CKD)], a larger plaque burden, increased technical complexity of PCI, post-procedural stent fracture, and stent underexpansion.4 In addition, recent pathological studies have shown that severely calcified lesions were associated with delayed healing (i.e. higher prevalence of uncovered struts) after implantation of new-generation drug-eluting stents (DES).23
Intravascular ultrasound and optical coherence tomography
The sensitivity of angiography to detect calcified plaques as well as the inter- and intraobserver reproducibility is suboptimal.24,25 Intravascular imaging has a higher calcium detection rate than angiography26,27 and thus should be used more liberally to avoid underestimation of calcification. Intravascular ultrasound (IVUS) and OCT are catheter-based imaging tools, which provide the complementary morphometric and quantitative assessment of calcified lesions (Figure 2 and Table 1) and facilitate the selection of the most appropriate techniques and devices during PCI. The inability to cross a calcified lesion with an imaging catheter often indicates the need for an upfront dedicated plaque modification technique.

Paired cross-sectional images of coronary angiography, intravascular ultrasound (A–E), and optical coherence tomography (A′–E′) in severely calcified coronary arteries. Both intravascular ultrasound and optical coherence tomography disclose significant calcification that is heavily overlooked at the baseline coronary angiogram (pre-percutaneous coronary interventions). Due to the limited penetration of ultrasound into the calcium, calcium on intravascular ultrasound is depicted as a high-echogenic edge with an acoustic shadow behind it (A–E). In contrast, on optical coherence tomography (A′–E′), light can penetrate calcium to enable measurement of the thickness, area, and volume. Panels (A) and (A′) present nodular calcification with a large lumen area. Panels (B) and (B′) demonstrate nodular eccentric calcification with a small lumen area. Panels (C) and (C′) are the images of focal superficial calcification. Panels (D) and (D′) show concentric and superficial calcification with a large lumen area. Panels (E) and (E′) represent eccentric thick calcium (>0.5 mm). The lower part of the figure shows post-percutaneous coronary intervention, angiographic, intravascular ultrasound, and optical coherence tomography findings, suggesting an adequate stent expansion and apposition despite significant calcification.
| . | Non-invasive imaging prior to the catheterization laboratory . | . | Intravascular imaging in the catheterization laboratory . | ||
|---|---|---|---|---|---|
| . | CCTA . | CS . | ICA . | OCT . | IVUS . |
| Spatial resolution | 0.2–0.5 mm | 1.25 mm | 0.5–0.6 mm | 15–20 μ | 50–200 μ |
| Contrast needed | Yes | No | Yes | Yes | No |
| Time of data acquisition | 1–5 min | 1 min | 15 mina | <5–10 s | 2–4 min |
| Availability | +++ | +++ | +++ | + | ++ |
| Additional cost | + | + | + | +++ | +++ |
| Tissue penetration (non-calcified) | +++ | +++ | +++ | + | ++ |
| Global assessment of calcification | +++ | +++ | + | - | - |
| Calcium volume quantification | + | - | - | ++ | - |
| Calcium arc | ++ | - | - | +++ | +++ |
| Calcium thickness | + | - | - | +++ | - |
| Longitudinal calcium length | + | - | - | +++ | +++ |
| . | Non-invasive imaging prior to the catheterization laboratory . | . | Intravascular imaging in the catheterization laboratory . | ||
|---|---|---|---|---|---|
| . | CCTA . | CS . | ICA . | OCT . | IVUS . |
| Spatial resolution | 0.2–0.5 mm | 1.25 mm | 0.5–0.6 mm | 15–20 μ | 50–200 μ |
| Contrast needed | Yes | No | Yes | Yes | No |
| Time of data acquisition | 1–5 min | 1 min | 15 mina | <5–10 s | 2–4 min |
| Availability | +++ | +++ | +++ | + | ++ |
| Additional cost | + | + | + | +++ | +++ |
| Tissue penetration (non-calcified) | +++ | +++ | +++ | + | ++ |
| Global assessment of calcification | +++ | +++ | + | - | - |
| Calcium volume quantification | + | - | - | ++ | - |
| Calcium arc | ++ | - | - | +++ | +++ |
| Calcium thickness | + | - | - | +++ | - |
| Longitudinal calcium length | + | - | - | +++ | +++ |
CCTA, coronary computed tomography angiography; CS, calcium scoring; ICA, invasive coronary angiography; OCT, optical coherence tomography; IVUS, intravascular ultrasound.
Per procedure28.
| . | Non-invasive imaging prior to the catheterization laboratory . | . | Intravascular imaging in the catheterization laboratory . | ||
|---|---|---|---|---|---|
| . | CCTA . | CS . | ICA . | OCT . | IVUS . |
| Spatial resolution | 0.2–0.5 mm | 1.25 mm | 0.5–0.6 mm | 15–20 μ | 50–200 μ |
| Contrast needed | Yes | No | Yes | Yes | No |
| Time of data acquisition | 1–5 min | 1 min | 15 mina | <5–10 s | 2–4 min |
| Availability | +++ | +++ | +++ | + | ++ |
| Additional cost | + | + | + | +++ | +++ |
| Tissue penetration (non-calcified) | +++ | +++ | +++ | + | ++ |
| Global assessment of calcification | +++ | +++ | + | - | - |
| Calcium volume quantification | + | - | - | ++ | - |
| Calcium arc | ++ | - | - | +++ | +++ |
| Calcium thickness | + | - | - | +++ | - |
| Longitudinal calcium length | + | - | - | +++ | +++ |
| . | Non-invasive imaging prior to the catheterization laboratory . | . | Intravascular imaging in the catheterization laboratory . | ||
|---|---|---|---|---|---|
| . | CCTA . | CS . | ICA . | OCT . | IVUS . |
| Spatial resolution | 0.2–0.5 mm | 1.25 mm | 0.5–0.6 mm | 15–20 μ | 50–200 μ |
| Contrast needed | Yes | No | Yes | Yes | No |
| Time of data acquisition | 1–5 min | 1 min | 15 mina | <5–10 s | 2–4 min |
| Availability | +++ | +++ | +++ | + | ++ |
| Additional cost | + | + | + | +++ | +++ |
| Tissue penetration (non-calcified) | +++ | +++ | +++ | + | ++ |
| Global assessment of calcification | +++ | +++ | + | - | - |
| Calcium volume quantification | + | - | - | ++ | - |
| Calcium arc | ++ | - | - | +++ | +++ |
| Calcium thickness | + | - | - | +++ | - |
| Longitudinal calcium length | + | - | - | +++ | +++ |
CCTA, coronary computed tomography angiography; CS, calcium scoring; ICA, invasive coronary angiography; OCT, optical coherence tomography; IVUS, intravascular ultrasound.
Per procedure28.
On IVUS, a calcified plaque is detected as an area with high echogenicity, brighter than the reference adventitia, with acoustic shadowing of deeper vessel structures. With thinner calcium, IVUS detects a smooth surface with reverberations, whereas with thick calcium, IVUS detects an irregular surface without reverberations24,29 (Figure 3). A calcified plaque attenuates the ultrasound signal and thus does not allow quantification of calcium thickness behind the leading edge.24 Intravascular ultrasound can therefore quantify calcification by the size of the circumferential arc and by the length of the calcified segment. Intravascular ultrasound can also determine whether calcium is nodular, superficial, or deep. In post-mortem validation studies, IVUS demonstrated high sensitivity (89%) and specificity (100%) for identification of dense calcified plaques or clusters of microcalcifications, with a lower accuracy for isolated microcalcifications.30,31 IVUS–detected calcification and IVUS-derived scoring system are known to predict stent underexpansion.32
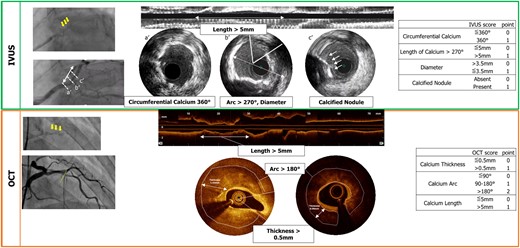
Scoring systems of calcified lesions on intravascular ultrasound and optical coherence tomography.
Optical coherence tomography, infrared light–based intravascular imaging, provides higher resolution than IVUS and detects calcified plaques as a low-intensity area with clear delineation (Figure 3). Since the tissue penetration of low-coherence light is less attenuated by calcium, the far side of the calcified plaque is detectable, and thus, the full extent of the calcium plaque can be visualized. Optical coherence tomography can therefore quantify calcification by the size of the circumferential arc, thickness, longitudinal length, depth, area, and three-dimensional volume. However, it should be noted that when calcium becomes very thick, the far side of the plaque cannot be detected. An OCT CS system has been shown to predict stent underexpansion17 (Figure 3).
Evidence of calcium fracture following lesion preparation seen either by IVUS or by OCT is associated with improved stent expansion.17 Following stent implantation, the result should be optimized using IVUS or OCT guidance.33
Interventional tools for coronary calcified lesion treatment
Vascular access and guiding catheters
Considerations regarding vascular access include (i) high bleeding risk profile of the patients (often related to advanced age), (ii) frequent calcification in larger arteries, and (iii) need to use 7 or 8F guiding catheters (GC) in some cases (Table 2). Access site complications can be reduced with the use of 6F GC via radial access, compatible with OA, small-size excimer laser, IVL, and 1.25–1.5 mm RA burrs (note that although 1.75 mm RA burr is accommodated in 6F GC, advancement can be extremely difficult, especially when there is peripheral vascular tortuosity). Slender sheaths permit the use of 7F GC through radial access if deemed necessary. The use of the ulnar artery, which has generally a larger diameter and fewer loops, could be an alternative option.34 Selection of a GC that facilitates coaxial alignment and maximizes support is important. Guiding catheter support can be enhanced further by using guide extension catheters, particularly in tortuous coronary anatomy or when treating calcified lesions in the mid- or distal vessel.
Challenges related to vascular access in patients with heavily calcific vessels undergoing PCI
| Challenge . | Potential solutions . |
|---|---|
| Frequent high bleeding risk patient profile |
|
| Calcification in femoral arteries |
|
| Need for 7F |
|
| Challenge . | Potential solutions . |
|---|---|
| Frequent high bleeding risk patient profile |
|
| Calcification in femoral arteries |
|
| Need for 7F |
|
CTA, computed tomography angiography; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; GC, guiding catheter.
Challenges related to vascular access in patients with heavily calcific vessels undergoing PCI
| Challenge . | Potential solutions . |
|---|---|
| Frequent high bleeding risk patient profile |
|
| Calcification in femoral arteries |
|
| Need for 7F |
|
| Challenge . | Potential solutions . |
|---|---|
| Frequent high bleeding risk patient profile |
|
| Calcification in femoral arteries |
|
| Need for 7F |
|
CTA, computed tomography angiography; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; GC, guiding catheter.
Peripheral IVL has been used to facilitate large bore sheath/catheter delivery through the ilio-femoral vessels for both coronary and aortic valve interventions and the insertion of haemodynamic support devices.
Balloon-based techniques
Cutting balloons and scoring balloons
Cutting balloons and scoring balloons are commonly used for the preparation of calcified lesions.35–37 The cutting balloon (Wolverine™ Cutting Balloon™, Boston Scientific) is a non-compliant (NC) balloon with three or four microblades longitudinally arranged on its surface. Scoring balloons are semi-compliant (AngioSculpt®, Philips; NSE Alpha™, Braun) or NC (ScoreFlex™ NC, OrbusNeich) balloons with scoring elements on the surface. The presence of cutting/scoring (C/S) elements on the balloon surface allows effective dilation with a lower inflation pressure.38 A recent randomized trial comparing super-high-pressure (SHP) balloons vs. scoring balloons for the preparation of calcified coronary lesions revealed comparable stent expansion on intravascular imaging.39
High- and super-high-pressure balloons
High-pressure balloons (HPB) have a more uniform and limited expansion profile than semi-compliant balloons, avoiding the dog-boning effect with both under- and overexpansion that can result in vessel perforation or dissection.35,40 A twin-layer NC SHP balloon (OPN, SIS Medical AG) allows the use of inflation pressures up to 35 atm, which may be cautiously increased up to 40–50 atm in selected cases without eccentric calcification. The main limitation of HPB and SHP balloons is their stiffness, which can make crossing and recrossing difficult.
Atherectomy techniques
Atherectomy is an important technique for plaque modification not only to facilitate adequate stent expansion but also to allow crossing of tight calcific stenoses. The basic principle of these techniques is the ablation of calcific atherosclerotic plaques within the vessel lumen while also creating fractures and fissures. While intravascular imaging may help select the most appropriate technique, in some cases, it will not be possible to cross the lesion with an imaging catheter before modification,42 and in some geographic locations, the use of imaging and device choice may be limited.
Rotational atherectomy
Rotational atherectomy (RA), first described in 198743 and utilized in >1 million patients,1 has survived all subsequent developments due to its unique advantages in treating calcified coronary lesions. The main component of RA is a rapidly rotating olive-shaped metallic burr coated with small diamond crystals on its distal end (the burr diameter ranges between 1.25 and 2.5 mm). Rotation is achieved by converting highly pressurized air to rotational energy, and the system has recently been upgraded (Rotapro, Boston Scientific), resulting in improved ease of use.44 Rotational atherectomy is performed on a dedicated 0.014″ guidewire, commercially available in two different designs, floppy (ROTAWIRE™ Drive Floppy) and extra support (ROTAWIRE™ Drive Extra Support). Plaque debulking is achieved based on the physical principle of differential cutting, which enables the burr to preferentially ablate inelastic tissue. Wire bias may influence the trajectory of atherectomy, but this can potentially be adjusted by using a floppy wire or bigger burrs. The microparticles of plaques generated are <5 µm in diameter and can therefore pass through the capillary bed into the systemic venous circulation and be engulfed by the reticuloendothelial system. Key procedural steps to avoid microcirculatory disturbances leading to no or slow flow include pharmacological measures to avoid coronary spasms and hypotension and technical measures (e.g. short burr runs, appropriate rotational speed, and avoidance of decelerations), the details of which are beyond the scope of this document.1
Data from the PREPARE-CALC (Comparison of Strategies to PREPARE Severely CALCified Coronary Lesions) and ROTAXUS (ROtational Atherectomy Prior to TAXUS Stent Treatment for Complex Native Coronary Artery Disease) trials suggested that RA before stent implantation is feasible and effective nearly in all patients with heavily calcified lesions. Despite the fact that a RA strategy did not result in a better angiographic outcome compared with a balloon-based strategy, RA is recognized as an enabling tool to facilitate balloon and stent delivery and adequate stent expansion in calcified coronary lesions, particularly when a balloon-based lesion preparation strategy has failed. In randomized clinical trials, up to 20% of calcified coronary lesions required bailout RA to achieve procedural success.45,46 When optimal atherectomy techniques are applied, complication rates are low and not significantly different compared with balloon-based strategies.45,46 Importantly, RA is used to modify lesion morphology by creating a polished channel that allows adequate balloon dilatation, calcium fracture, and optimal stent expansion and is therefore utilized for limited (rather than aggressive) debulking (burr/artery ratio < 0.7). Despite a recommended rotablation speed between 135 000 and 180 000 rpm,1 greater calcium debulking has been shown with speeds < 150.000 rpm in a small OCT study.47
Data on the relative efficacy of RA compared with OA or IVL are not yet available. Identifying RA as the initial PCI strategy in a given patient, with the potential of reducing radiation, procedural time, contrast dose, and cost,48 can be facilitated by using the RotaScore, which integrates four variables (degree of calcification, lesion length, tortuosity, and involvement of a bifurcation) for the upfront prediction of the need for RA.49,50
Orbital atherectomy
Orbital atherectomy (OA) (Cardiovascular Systems, Inc., St Paul, MN, USA) uses a differential sanding mechanism to reduce the calcified plaque.51,52 A drive shaft eccentrically mounted a diamond-coated crown modifies the plaque and increases the luminal size and compliance. Orbital atherectomy is performed on a dedicated 0.014″ guidewire (VIPERWIRE Advance®). In contrast to RA, OA uses a single crown with its orbital diameter expanding radially via centrifugal force when a high rotational speed is selected; this orbital rotation also reduces the limitations of wire bias. A key aspect of OA is that it works bidirectionally, ablating plaques while being advanced and retracted. Orbital atherectomy has been shown to modify the calcified plaque, changing its morphology and compliance and ultimately facilitating stent expansion.53
The OA device has two speed settings. Low speed (80 000 rpm) should be used for the initial pass, with only some lesions requiring high speed (120 000 rpm). It is advised to avoid high speed in tortuosity, severe angulation, and vessels < 3.0 mm, as it might be associated with an increased risk of vessel perforation,54 limiting its use only to larger straight vessel segments if insufficient ablation or compliance change has been achieved after two or more runs at low speed.
Two prospective multicentre studies, ORBIT (Orbital Atherectomy System in Treating De Novo, Severely Calcified Coronary Lesions) I and II, have compared PCI with or without OA in patients with calcified stenoses.52,55 ORBIT II reported in 443 patients an in-hospital major adverse cardiac event (MACE) rate of 10% and a rate of 23% at 3-year follow-up.56 In addition, a real-world retrospective registry on the use of OA has been published.57 The ongoing ECLIPSE trial (ClinicalTrials.gov identifier NCT03108456) is the largest randomized trial to date studying coronary atherectomy for severely calcified de novo lesions, assessing OA vessel preparation compared with HPB angioplasty and/or cutting balloons.
Excimer laser coronary atherectomy
Excimer laser coronary angioplasty (ELCA) is based on the emission of monochromatic coherent light in the ultraviolet range (wavelength 308 nm) able to ablate inorganic material and break molecular bonds. While laser is thought to be more effective on thrombi and other soft or fibrotic plaques, it can be useful in calcified lesions,58 alone or in combination with or after failed RA.59 Another specific application is within underexpanded stents, where laser can be activated during slow contrast infusion (although data concerning its safety are still lacking and the indication is off-label).60
Intravascular lithotripsy
Intravascular lithotripsy has emerged as a novel therapy for the treatment of vascular calcification. The Shockwave Medical (Santa Clara, CA, USA) coronary IVL catheter consists of a 0.014″ guidewire-compatible, fluid-filled balloon angioplasty catheter with two spark gap–based lithotripsy emitters incorporated into the shaft of the 12 mm long balloon.61 The coronary IVL system is delivered on a rapid exchange catheter and is available in 2.5, 3.0, 3.5, and 4.0 mm diameters to allow 1:1 sizing to the reference vessel diameter. When the balloon is positioned at the target lesion and inflated to 4 atm, 10 IVL pulses are delivered, followed by optional brief balloon inflation to 6 atm. Intravascular lithotripsy treatment cycles are continued until full balloon expansion is achieved (up to 80 pulses per balloon or 120 with the latest generation Shockwave C2+ system), with interval deflation to allow for distal perfusion. An IVL pulse is produced when lithotripsy emitters create vapour bubbles within the integrated balloon, resulting in the formation of acoustic shockwaves with peak acoustic pressures of ∼50 atm that propagate circumferentially and transmurally through soft tissue with minimal effect while imparting compressive stress on calcified plaques as the primary mechanism of calcium fracture61 (see Supplementary data online, Figure S2).
Clinical outcomes of IVL for the treatment of severely calcified de novo coronary stenoses have been reported across three prospective studies.21,62,63 Pooled results from the Disrupt CAD studies were evaluated in a patient-level pooled analysis of 628 patients enrolled across 72 sites from 12 countries.64 The primary safety endpoint of the pooled analysis (30-day MACE) was 7.3% and was driven primarily by the rate of in-hospital non-Q-wave myocardial infarction (5.7%). The primary effectiveness endpoint of procedural success, defined as stent delivery with angiographic core lab–assessed residual in-stent stenoses ≤ 30% without in-hospital MACE, was achieved in 92.4% of the cases, with low rates of flow-limiting dissection (0.2%) and perforation (0.2%) and no slow-flow or no-reflow events at the end of the procedure.64
Acute outcomes following IVL treatment have been promising, and with 1-year outcomes confirming safety and effectiveness, the simplicity of use is prompting widespread adoption.64 No randomized clinical trials comparing IVL with other calcium-modifying therapies have currently been reported.65
Optimal management of calcified lesions
Management of calcified lesions based on pre-procedural coronary computed tomography angiography
When available, CCTA may facilitate pre-procedural planning of PCI by assessing the global calcium burden, coronary anatomical complexity,66–69 distribution of calcium at each coronary segment, remaining lumen, morphology and dimension of the aortic root, and extension of calcification at the left main or right coronary ostia. Fractional flow reserve CT (FFRCT), in addition, can provide a haemodynamic assessment of the severity of the lesions (see Supplementary data online, Figure S3). Specifically, a detailed CT assessment of the aortic root and coronary anatomy can guide the interventional cardiologist on the most suitable guiding catheter and best angiographic view for lesion visualization and stent placement.70 Extensive calcification on CCTA (defined as a cross-section with calcium > 270°) could anticipate the need for adjunctive devices and thus facilitate logistical and case planning in busy interventional cardiology programmes. Finally, CCTA can be useful for PCI of coronary chronic total occlusions (CTOs), providing information on the vessel course, tortuosity, length of the occluded segment, and quality of the distal landing zone, in addition to the extent and distribution of calcification.71
Optimal management based on angiography
In centres with limited or no access to intravascular imaging, angiography is used to guide PCI procedures (Figure 4). Angiography can be used for detection of calcium, a qualitative assessment of severity, and balloon and stent expansion; the latter is further facilitated by acquisition of computer-assisted X-ray images enhancing metallic structures (e.g. stent boost).72 The adequacy of lesion preparation is evaluated by the visual expansion of a balloon appropriately sized to the vessel. Eccentric calcification may not be detected in a single angiographic view and can result in subsequent asymmetric stent expansion.73 Therefore, angiographic evaluation of balloon expansion in multiple views during lesion preparation is essential.
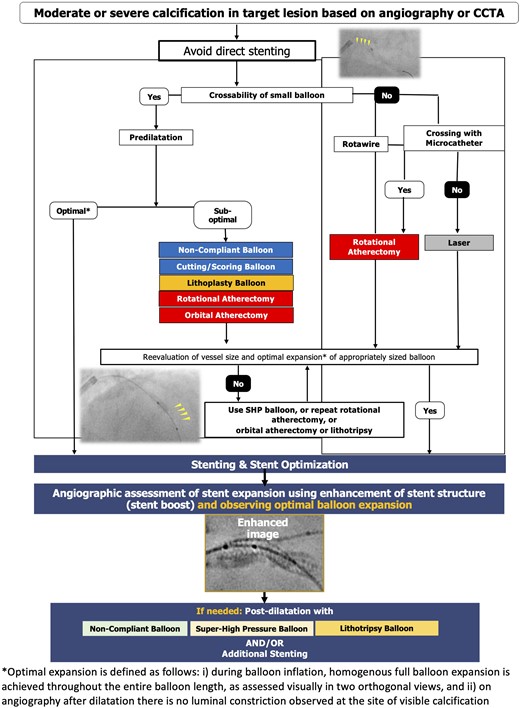
Optimal interventional management of calcified lesions based on coronary angiography.
Optimal management based on intravascular imaging
Intravascular imaging can be used to guide each step of complex PCI (at baseline, after lesion preparation, and post-stent implantation), informing decisions regarding the most appropriate technique for calcium modification and confirming optimal stent expansion (Figure 5). IVUS and OCT allow the assessment of calcium in both cross-sectional and longitudinal views. When a stenosis fulfils the criteria of high calcification burden with significant luminal narrowing, the use of an adjunctive device (e.g. RA, OA, and IVL) should be considered.17,70 When calcium is deeper in the plaque or vessel wall, IVL may be preferred, whereas with superficial calcification, atherectomy may be more effective (Figure 5). After plaque modification, intravascular imaging can be repeated, with the detection of calcium fracture and/or sufficient lumen gain, indicating that a stent can be implanted (see Supplementary data online, Figure S3B–D). Post-stent intravascular imaging can confirm that optimal stent expansion has been achieved according to the EAPCI consensus guidelines74 (Figure 5). It should be noted that in OCT studies of severely calcified lesions, the mean final stent expansions of 68%–84% were reported, highlighting the challenge of achieving these optimal stent expansion goals in resistant lesions.39,42,63,75 If stent underexpansion occurs, additional post-dilatation with HPB or off-label use of IVL can be performed.
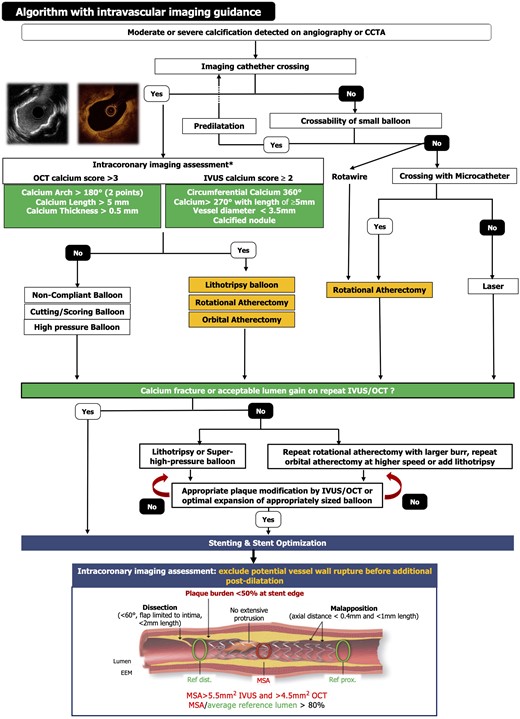
Optimal interventional management of calcified lesions based on intravascular imaging.
When dealing with a calcified lesion, stent choice is a crucial step to achieve successful revascularization. The ideal stent, in this scenario, should have the following characteristics: (i) high deliverability to be advanced through tortuous and calcified segments, (ii) high radial force to achieve an adequate expansion even in the presence of calcium, and (iii) flexibility to conform to the vessel.
Consensus statements
With a high coronary stenosis calcium score (i.e. ≥453 or a cross-section with calcium > 270°) on CCTA (when available), PCI with advanced plaque modification techniques (e.g. IVL and/or atherectomy devices) should be considered before stent implantation.
Coronary angiography underestimates the severity of coronary calcification. Response to PCI in angiographically ambiguous or calcified stenoses might be unpredictable; therefore, direct stenting (especially in ACS patients) should be discouraged.
Target calcified lesions are best evaluated in multiple angiographic projections.
Failure to dilate the lesion with a semi-compliant or NC balloon inflated at high pressure (i.e. dog-boning or asymmetric balloon expansion in at least two projections) should prompt the use of intracoronary (IC) imaging and/or the adoption of advanced plaque modification techniques (e.g. IVL and/or atherectomy devices) rather than the use of more aggressive balloon dilatation.
Liberal use of angiographic enhancement systems (e.g. stent boost, etc.) is advised pre- and post-stenting to facilitate visualization of stent expansion.
Inability of intravascular imaging tools to cross a coronary stenosis suggests the need for dedicated plaque modification tools and techniques.
The adjunctive use of advanced plaque modification techniques (e.g. IVL and/or atherectomy devices) before stent implantation is advised when the calcified lesion fulfils the IVUS or OCT criteria of high calcification burden (Figure 5) with lumen narrowing.
Transradial arterial access is advised in PCI of calcified coronary lesions to reduce bleeding risk.
Cutting balloons are suitable for (i) proximal lesions, (ii) aorto-ostial lesions, and (iii) straight coronary segments and (iv) after RA, OA, or IVL.76
Scoring balloons are suitable for (i) proximal and distal lesions, (ii) aorto-ostial lesions, and (iii) tortuous coronary segments and (iv) after RA, OA, or IVL.
High-pressure balloons and SHP balloons are suitable for (i) crossable undilatable calcified stenoses (excluding eccentric calcification patterns) and (ii) underexpanded stents.
Rotational atherectomy is suitable for (i) undilatable and/or balloon uncrossable lesions, (ii) superficial or nodular calcifications (by intravascular imaging), (iii) very tight calcified stenoses, (iv) long calcified lesions, and (v) selected calcified bifurcation lesions, if side-branch wire protection is not mandatory.
Orbital atherectomy is suitable for (i) undilatable lesions and (ii) superficial or nodular calcification (by intravascular imaging).
Intravascular lithotripsy is suitable for (i) deep calcification and calcified nodules; (ii) large vessels; (iii) stent underexpansion (currently off-label indication); (iv) bifurcation lesions, if side-branch wire protection is mandatory; and (v) aorto-ostial calcified stenoses.
Excimer laser coronary angioplasty is suitable for (i) microcatheter uncrossable fibrocalcific lesions and (ii) stent underexpansion.
Specific clinical and anatomical settings
Acute coronary syndromes/thrombotic lesions
A recent OCT analysis found that 13% of patients with ACS have a calcified plaque as the culprit lesion, with three subtypes described: superficial calcific sheets (67%), eruptive calcified nodules (26%), and calcified protrusions (7%).77 In addition, when the culprit lesion was a calcified plaque, non-culprit lesions had a higher calcium burden and less vulnerability than in patients presenting with a plaque rupture or erosion as the culprit lesion.78 A pooled analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) and HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trials found that 32% of patients had angiographic moderate or severe calcium, which was an independent predictor of stent thrombosis and ischaemic target lesion revascularization at 1 year.4
Therefore, in patients with ACS, there should be no exception to proper lesion preparation before stent implantation. More specifically, in ACS patients, direct stenting should be discouraged to prevent stent undersizing (a consequence of vessel hypoperfusion downstream to the coronary occlusion or stenosis, along with vasoconstriction) and/or underexpansion (due to unanticipated underlying calcification). In the case of an uncrossable or undilatable lesion, we advise that the threshold for ad hoc plaque modification techniques (including RA or OA) is lowered even during primary PCI procedures (https://www.pcronline.com/Cases-resources-images/Cases/Euro4C/2021/How-manage-uncrossable-lesions-ACS?auth=true). Concerns regarding the possible risk of increased distal thrombotic embolization with ablative techniques are largely offset by the achievement of an optimal acute stenting result and by the available antithrombotic treatment strategies. This also avoids the need for a staged procedure and possible subsequent rehospitalization. If an operator has limited experience with plaque modification techniques, deferral of stenting is advised after achieving coronary TIMI (Thrombolysis In Myocardial Infarction) 3 flow with balloon angioplasty. Nevertheless, full training on plaque modification techniques is advised for the interventional cardiologist participating in an emergency PCI programme to enable optimal and timely treatment of heavily calcified lesions also during the acute procedure.
Chronic total occlusions
Severe calcification is one of the main characteristics of increased complexity during CTO PCI. This in turn contributes significantly to the lower success rates in calcified CTO lesions, reflected by its inclusion in all the major CTO complexity scores.79,80 Based on histopathological findings, calcium is predominantly located at the proximal cap.81 To overcome proximal cap calcification, high-penetration force (>9 g) tapered-tip CTO wires have been developed to be used with a single- or double-lumen microcatheter to further increase the penetration force. In cases of an impenetrable cap, alternative dissection algorithms, such as the ‘move-the-cap’ technique, are often effective but may not be applicable for occlusions involving bifurcations or with an ostial location.82,83 The occlusive segment can be crossed intra- or extraplaque, as defined in the CTO-ARC consensus.84 In heavily calcified CTOs, intentional ‘extraplaque tracking’ with knuckle wire dissection is often necessary to overcome long calcified segments. Special caution should be taken during plaque modification after ‘extraplaque tracking’ to avoid severe and longitudinal perforation in large dissection planes. Balloon inflation pressures over 14–16 atm and RA or OA are generally not advised after ‘extraplaque tracking’. There are limited data on the use of IVL during CTO PCI85 and for ELCA on impenetrable proximal caps or microcatheter or balloon uncrossable lesions.86
Intravascular ultrasound is an indispensable tool during CTO PCI to understand the position of the wire and the composition and morphology of the occlusive plaque and to identify calcified nodules that are at high risk for perforation during intensive plaque modification.
Bifurcation lesions
The presence of calcification in coronary bifurcation lesions increases the complexity of these already challenging PCI procedures.87 Data from the COBIS II (COronary BIfurcation Stent) registry showed that severe calcification in bifurcation lesions was associated with a higher rate of target lesion failure (TLF) after PCI as compared with mild or no calcification.88,89 An OCT study revealed that the presence of calcium at a bifurcation segment of the main branch is an independent predictor of side-branch stenosis after main branch stenting.90 The subanalysis of the PREPARE-CALC trial reported that when comparing RA to scoring/cutting balloons (SCB) in calcified bifurcation lesions, side-branch compromise (i.e. any significant stenosis, dissection, or TIMI flow < 3) was more frequently observed after lesion preparation with SCB.91 In addition, a multicentre retrospective registry of 1156 patients treated with OA for calcified lesions reported that patients treated for bifurcation lesions had comparable outcomes to those treated for non-bifurcation lesions, demonstrating that atherectomy devices appear to be both safe and effective for calcium modification in bifurcation lesions.92
However, further data concerning the optimal management of calcium in bifurcation lesions are lacking. Plaque modification is critically important in this lesion subset before stent implantation.92 Once adequate lesion preparation is achieved in the main vessel, plaque modification with atherectomy is only advised in the side branch if a two-stent strategy is decided upfront and the vessel has moderate-to-severe calcification.93 Of note, while the side-branch wires should be removed for RA or OA to avoid wire fracture or burr entrapment, protective wires can be maintained throughout the procedure when using IVL. For atherectomy, sequential rota- or viper-wiring of the branches can be performed if needed.
Calcified coronary lesions in patients with aortic stenoses
Degenerative aortic stenoses (AS) and CAD are the most prevalent cardiovascular diseases in developed countries, and they coexist in around 40%–75% of patients.94 The pathophysiological basis behind both degenerative AS and calcified CAD is atherosclerosis. The association is so strong that calcification of the aortic valve has been proposed as a surrogate marker of CAD.94 There are limited data regarding the indication for and timing of PCI in stable patients planned for transcatheter aortic valve implantation (TAVI),95 particularly in patients with complex calcified CAD. The feasibility, efficacy, and safety of RA after TAVI have been reported.96 When revascularization is indicated in patients with AS, anticipating the presence of calcified lesions, optimal plaque modification techniques using dedicated devices should be employed as in patients without AS.
Role of mechanical circulatory support
Percutaneous coronary interventions in heavily calcified coronary arteries are technically challenging due to the need for meticulous vessel preparation and stent optimization that may lead to haemodynamic compromise and/or life-threatening complications (e.g. sustained hypotension, malignant arrhythmias, coronary rupture, and no reflow), especially in patients with multiple comorbidities and severely reduced left ventricular ejection fraction.97
The potential benefit of mechanical circulatory support (used upfront or as bailout) is related to the maintenance of an adequate blood pressure, the unloading of the left ventricle, and the increased coronary perfusion pressure in the presence of severe CAD.98
However, definitive demonstration of benefits remains weak and hampered by an increased rate of vascular complications; their use therefore should be carefully weighed within the heart team discussion.
Clinical implications
Pharmacologic treatment
Since calcified coronary lesions may be involved in both acute and chronic coronary syndromes,50 optimal pharmacological treatment should be given to patients according to their clinical presentation and respective standard protocols.
The use of glycoprotein IIb/IIIa inhibitors, previously demonstrated in small studies to reduce periprocedural cardiac enzyme elevation and slow/no reflow, should be limited to bailout situations of thrombotic complications.99 In PCI for chronic coronary syndromes, potent P2Y12 inhibitors failed to demonstrate in recent studies a benefit over clopidogrel in terms of myocardial necrosis or clinical outcomes.28,41 In the specific setting of RA for calcified lesions, ticagrelor failed to demonstrate a reduction in periprocedural necrosis over clopidogrel100 (NCT02505399). Thus, data do not support systematic use of potent P2Y12 inhibitors over thienopyridines for PCI of calcified lesions in chronic coronary syndromes.
Prevention and management of procedural complications
The presence of calcified lesions increases the complexity of PCI and, therefore, is associated with an increased risk of procedural complications (Table 3).4,19,41 These include vascular, coronary, and renal complications and are often related to the inherent complexity of the patient and lesion setting. Implementing all possible measures to prevent these complications and to promptly recognize and manage them is of paramount importance. An in-depth description of the prevention and treatment of periprocedural complications is beyond the scope of this document and can be found elsewhere; the main recommendations are summarized in Table 3.
Complications potentially occurring during PCI of calcified lesions with related management
| . | Prevention . | Management . |
|---|---|---|
| Coronary | ||
| Rupture |
|
|
| Perforation |
|
|
| Late pericardial tamponade |
|
|
| Periprocedural myocardial infarction |
|
|
| No/slow flow |
|
|
| AV block |
|
|
| Vascular and haemodynamic | ||
| Major bleeding |
|
|
| Renal | ||
| Contrast-induced nephropathy |
|
|
| . | Prevention . | Management . |
|---|---|---|
| Coronary | ||
| Rupture |
|
|
| Perforation |
|
|
| Late pericardial tamponade |
|
|
| Periprocedural myocardial infarction |
|
|
| No/slow flow |
|
|
| AV block |
|
|
| Vascular and haemodynamic | ||
| Major bleeding |
|
|
| Renal | ||
| Contrast-induced nephropathy |
|
|
RA, rotational atherectomy; IV, intravenous; IC, intracoronary; DUS, Doppler ultrasound; CKD, chronic kidney disease; GFR, glomerular filtration rate; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; OA, orbital atherectomy; RCA, right coronary artery; LCX, left circumflex artery; ACT, activated clotting time; AV, atrioventricular; IVL, intravascular lithotripsy; IVUS, intravascular ultrasound.
Complications potentially occurring during PCI of calcified lesions with related management
| . | Prevention . | Management . |
|---|---|---|
| Coronary | ||
| Rupture |
|
|
| Perforation |
|
|
| Late pericardial tamponade |
|
|
| Periprocedural myocardial infarction |
|
|
| No/slow flow |
|
|
| AV block |
|
|
| Vascular and haemodynamic | ||
| Major bleeding |
|
|
| Renal | ||
| Contrast-induced nephropathy |
|
|
| . | Prevention . | Management . |
|---|---|---|
| Coronary | ||
| Rupture |
|
|
| Perforation |
|
|
| Late pericardial tamponade |
|
|
| Periprocedural myocardial infarction |
|
|
| No/slow flow |
|
|
| AV block |
|
|
| Vascular and haemodynamic | ||
| Major bleeding |
|
|
| Renal | ||
| Contrast-induced nephropathy |
|
|
RA, rotational atherectomy; IV, intravenous; IC, intracoronary; DUS, Doppler ultrasound; CKD, chronic kidney disease; GFR, glomerular filtration rate; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; OA, orbital atherectomy; RCA, right coronary artery; LCX, left circumflex artery; ACT, activated clotting time; AV, atrioventricular; IVL, intravascular lithotripsy; IVUS, intravascular ultrasound.
Coronary rupture or perforation may occur after atherectomy, high-atmosphere balloon inflation, and occasionally IVL. Coronary rupture may require urgent pericardiocentesis if associated with cardiac tamponade. Treatment can be surgical or percutaneous with covered stent implantation. Small perforations may be treated with prolonged balloon inflation with or without covered stent implantation. In case of distal perforation, occlusion of the distal vessel with coils, fats, or microspheres is an effective treatment. In all cases, serial echocardiograms should be performed to assess for haemopericardium or tamponade. The risk of perforation and coronary rupture can be minimized by performing careful RA and OA techniques and by avoiding excessive HPB inflations.
Late pericardial tamponade can occur following temporary pacemaker implantation, during RA/OA of a right coronary artery (RCA) or dominant left circumflex artery (LCX). Following the use of a temporary pacemaker, an echocardiogram is recommended 2 h after the procedure. Some operators have abandoned the systematic use of temporary pacemakers by administrating intraprocedural atropine or using wire pacing.
The treatment of periprocedural myocardial infarction is described in dedicated guidelines,101 this complication can usually be mitigated by using appropriate periprocedural antithrombotic treatment, careful RA techniques, and low-speed OA to reduce distal embolization of debris and plaque microparticles. Periprocedural myocardial infarction has also been described with IVL in up to 6.8% of the cases, possibly related to the treatment of longer calcified lesions.56
Slow-flow/no-reflow phenomena can be related either to embolization of plaque material to the distal coronary bed or to microvascular dysfunction and/or arteriolar spasm. After excluding other causes of no reflow (i.e. thrombosis, air embolization, dissection, and intramural haematoma), various pharmacological treatment options can be used such as adenosine, nitroprusside, nicardipine, and verapamil, injected through the guiding catheter or distally through a microcatheter. Yet, the comparative effectiveness of these agents has never been tested. In order to avoid this complication, it is recommended to (i) optimize systemic blood pressure allowing a sufficient coronary perfusion pressure during PCI, (ii) keep the activated clotting time (ACT) value > 250 s, (iii) perform short runs of RA or OA, and (iv) use vasodilator in the flushing saline solution.
Intravascular lithotripsy can be associated with mechanical ventricular captures often with a transient drop in systemic blood pressure. The IVL-induced ventricular capture has been associated with episodes of non-sustained tachyarrhythmias.61
Education and training
To safely and effectively perform PCI in the setting of calcified coronary stenoses, it is paramount to do the following: first, identify and quantify the calcium, and second, understand the application of plaque modification tools and techniques. Accordingly, education should start with training in intravascular imaging that can be done both intraprocedurally and offline to boost the caseload. Training should not only start with the theory on the state-of-the-art use of tools and techniques but also focus on algorithms for potential pitfalls and troubleshooting and bailout situations. This can be best acquired through structured educational platforms, such as dedicated seminars or webinars. Practical training with specific plaque modification devices can be acquired through simulation-based learning and should include significant initial experience as second operators (at least 20–30 cases), followed by senior-assisted cases as first operators (at least 20–30 cases). Extensive experience with one device (e.g. RA) can facilitate training with another (e.g. OA). Considering that operator volume is an independent predictor of outcomes in complex procedures,102 sharing experience by working with two operators might be a favourable approach, whenever logistically possible.
Consensus statements
In calcified CTOs crossed with ‘extraplaque tracking’, balloon inflation pressures over 14–16 atm and rotational, orbital, or laser atherectomy are not advised.
In bifurcation lesions, protective side-branch wiring should be avoided during RA or OA of the main branch.
Percutaneous coronary interventions of calcified left main coronary lesions are best guided by intravascular imaging.
Advanced plaque modification techniques, if required, can be safely used in TAVI patients undergoing PCI of calcified lesions.
Patients undergoing complex high-risk PCI of calcified coronary stenoses with severely reduced left ventricular function or where an adequate blood pressure cannot be maintained might benefit from mechanical circulatory support.
Procedural pharmacologic treatment of patients undergoing complex PCI of calcified coronary stenoses should be guided by the clinical presentation.
Given the risk of severe complications in PCI of complex and calcified lesions, each centre should establish dedicated protocols for the management of complications that must include availability of dedicated materials and resources. In some cases, especially elective complex procedures, these protocols should also include the possibility of standby emergency cardiac surgery.
Interventional teams performing 24/7 emergency PCI in ACS should be proficient in dealing with complex and calcified lesions using dedicated technologies, enabling ad hoc optimal revascularization also during off-hours. In case of limited experience with plaque modification techniques, deferral of stenting after achieving coronary TIMI-3 flow with plain balloon angioplasty is advisable.
Limitations
Although the treatment of heavily calcified lesions remains challenging, the broad armamentarium of dedicated tools has improved the therapeutic approach to these patients over the last years. Some limitations of the present document should be acknowledged. First, the studies and registries available in the literature have clearly shown the indispensable adjunctive value of these dedicated tools to achieve a successful PCI. Nevertheless, none of these studies was designed to detect differences in terms of hard endpoints on long-term follow-up (see Supplementary data online, Table S2). Second, especially in the context of an elective PCI, before embarking on a potentially complex and demanding procedure, a careful evaluation aimed at balancing the risks, benefits, and costs should always be performed. Third, the consensus points provided are based on expert operator opinions, best practices, and the available evidence but do not represent clinical recommendations, which can be provided by scientific societal guidelines.
Conclusions
The availability of imaging tools to anticipate and refine the accurate assessment of calcified lesions, along with a broader armamentarium of devices, currently enables the optimal percutaneous treatment of even the most challenging calcified coronary anatomy. Yet, this remains one of the most difficult PCI procedures, and therefore, rigorous training is warranted in order to master all possible techniques, develop a superior knowledge of the technologies, and acquire advanced skills in anticipating and treating potential complications.
Of particular prognostic impact is the adequate management of severely calcified lesions in patients presenting with ACS and needing emergency PCI around the clock, a situation that imposes an urgent need for adequate training of the entire interventional team, including younger operators, nurses, and technicians.
Acknowledgements
The authors wish to thank Dr Kai Ninomiya for his assistance in the preparation of the algorithm and flow charts.
Supplementary data
Supplementary data is available at European Heart Journal online.
Declarations
Disclosure of Interest
E.B. reports consulting fees from Insight Lifetech and honoraria for lectures from Insight Lifetech, Abbott, and Boston Scientific. M.A.W. reports consulting fees from Medtronic, Boston Scientific, and Abbott; honoraria from Medtronic, Boston Scientific, Abbott, Edwards Lifesciences, and Shockwave; and participation to DSMB from Medtronic and Boston Scientific. C.D.M. reports research grants to the Careggi University Hospital from Abbott Vascular, Amgen, Behring, Chiesi, Daiichi Sankyo, Edwards, Medtronic, Shockwave, Volcano Philips, and Vectorious. J.E. reports honoraria for lectures from Shockwave Medical and Boston Scientific. G.G. reports research grants from Abbott and Amgen; consulting fees from Abbott, Infraredx, Gentuity, and Panovision; and honoraria or support for attending meetings from Abbott and Infraredx. J.H. reports research grants from Shockwave Medical, Abbott, and Boston Scientific; consulting fees from Shockwave Medical, Abbott, Boston Scientific, and Abiomed; honoraria from Shockwave Medical, Abbott, and Boston Scientific; and stock options from Shockwave Medical. M.M. reports honoraria for lectures from Boston Scientific, Biosensors, Shockwave Medical, Teleflex, Abbott Vascular, and Medtronic. G.S. reports honoraria from Abbott, Boston Scientific, and Pfizer7BMS. G.T. reports consulting fees from Medtronic, Edwards Lifesciences, and GADA and honoraria from Medtronic, Edwards Lifesciences, and GADA. G.T. reports consulting fees from Abbott, Medtronic, and Terumo and honoraria from Abbott, Medtronic, and Terumo. B.V. reports honoraria from Boston Scientific. W.W. reports honoraria from Microport, and he is a medical advisor of Corrib Core Lab and Rede Optimus Research and a co-founder of Argonauts (an innovation facilitator). F.L.R. reports consulting fees from Medtronic and Edwards and honoraria from Medtronic, Edwards, and Boston Scientific. E.G., D.A., D.C., D.D., J.F., K.M., N.M., Y.O., and R.S. report no potential conflict of interest.
Data Availability
The data supporting the findings of this manuscript were derived from previously published manuscripts, which have been listed in the references.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethical Approval
Ethical approval was not required.
Pre-registered Clinical Trial Number
None supplied.



