-
PDF
- Split View
-
Views
-
Cite
Cite
Nils P Johnson, Jung-Min Ahn, Left main PCI: beware the circumflex!, European Heart Journal, Volume 44, Issue 41, 1 November 2023, Pages 4337–4339, https://doi.org/10.1093/eurheartj/ehad434
Close - Share Icon Share
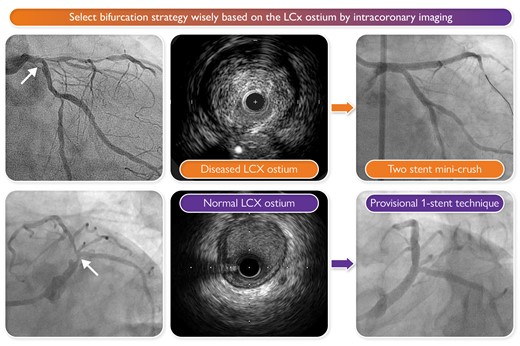
Take-home message. Select the bifurcation strategy wisely based on the left circumflex (LCx) ostium by intracoronary imaging.
This editorial refers to ‘Left main bifurcation stenting: impact of residual ischaemia on cardiovascular mortality’, by H.-Y. Wang et al., https://doi.org/10.1093/eurheartj/ehad318.
Percutaneous coronary intervention (PCI) of the left mainstem (LM) provides similar all-cause survival compared against coronary artery bypass grafting (CABG), but with higher rates of spontaneous myocardial infarction (MI) and repeat revascularization over 5 years.1 However, patients may not be CABG candidates for many reasons, including clinical circumstance, poor distal targets, or treatment preference. Because continuous plaque extends from the LM into the left anterior descending (LAD) artery in 90% of cases,2 almost all patients receive a stent along this trajectory. Consequently, treatment and prognosis of the left circumflex (LCx) side branch remain the key questions.
This issue of the European Heart Journal presents 3-year clinical outcomes from the left main registry from Fuwai Hospital in Beijing.3 Briefly, 1148 patients underwent PCI of the LM and had both complete follow-up and an analysable post-PCI angiogram. Cardiovascular death and bifurcation-related MI occurred at higher rates among patients with residual angiographic disease, but only when quantified by the simulated physiological index μQFR (quantitative flow ratio using a single angiographic projection and reference vessel diameters modelled by Murray’s law) and not by percent diameter stenosis (%DS).
QFR for the left main
As with all simulated physiology to predict fractional flow reserve (FFR), accuracy and precision provide the key metrics. In general, angiography plus physiological models can predict FFR without bias (average difference <0.01) but with an imprecision of ±0.07, as seen in a massive lesion-level pooled analysis of 1478 vessels.4 Specifically for the left main, two recent papers this year have examined the performance of QFR in modestly sized cohorts. In 73 vessels, the bias was +0.02 (FFR > QFR) with an imprecision of ±0.13,5 while 107 vessels had a bias of <0.01 with an imprecision of ±0.07.6 Importantly, in >75% of cases in both studies, the pressure wire was placed in the LAD when evaluating the LM. Additionally, neither study used μQFR that may yield smaller imprecision at ±0.05, albeit in only 330 vessels not including any LM, not compared against QFR, and predominantly studied in the main branch.7 Thus prior to the current study, post-PCI μQFR of the LM rested on a modest evidence base, especially considering the LCx side branch.
Notably, the current analysis from Fuwai Hospital employed a software package for quantitative coronary angiography that does not incorporate Murray’s law for bifurcations, potentially disadvantaging %DS as suggested by prior literature.8 Perhaps this difference accounts for a reversal of the usual observation that more lesions have abnormal anatomy than physiology, i.e. generally the percentage of vessels with high %DS will exceed the percentage of vessels with low QFR. However, in their cohort, only 72 vessels had %DS > 50% whereas 155 vessels had μQFR ≤0.8.3 It would have been intriguing to apply different methodologies for %DS and observe their associations with outcomes, as the advantage of μQFR might have become blunted.
It's the circumflex!
Numerous observations in the current study emphasize the role of the LCx in producing events after PCI of the LM.3 The vast majority of abnormal μQFR ≤0.8 occurred in the LCx alone (117 of the 155 vessels, or 75%) or in the LCx and the LAD together (another 6 vessels, total 79%), leaving only a small minority of the cohort with isolated and abnormal μQFR in the LAD (32 of the 155 vessels, or 21%). These disproportionately abnormal reductions in μQFR of the LCx developed despite starting with higher values than the LAD: the LCx went from 0.75 to 0.91 (Δ + 0.16), whereas the LAD went from 0.64 to 0.95 (Δ + 0.31) on average.
Because a provisional one-stent technique was dominant (74% of the cohort), this finding indicates that the LCx—in addition to its baseline atherosclerosis—often becomes affected by plaque shift, carina repositioning, and jailing by stent struts. As seen in Supplementary Table S3 of the study by Wang et al., a provisional one-stent crossover strategy dominated the low μQFR columns, including >90% of techniques leading to intact μQFR of the LAD but reduced μQFR in the LCx. Relatedly, a provisional one-stent crossover strategy predicted μQFR ≤0.8 in the multivariable analysis. In addition, even μQFR did not appear to capture all the relevant parameters affecting the LCx—the reference vessel diameter of the LCx before PCI remained a significant predictor of μQFR ≤0.8 in the multivariable analysis. For the small cohort of 32 patients with intact μQFR of the LCx but reduced μQFR in the LAD after PCI, 84% received provisional one-stent crossover—probably from the LM to the LCx—further highlighting the challenges for the jailed side branch.
The frequency of μQFR ≤0.8 in the LCx at 123 of 1170 vessels (11%) after LM PCI in this study matches two modestly sized series of post-LM PCI FFR in the LCx: 3 of 43 vessels (7%) in one series9 and 14 of 83 vessels (17%) in another series.10 While the current μQFR study used a variety of bifurcation techniques but the FFR cohorts both used a one-stent crossover strategy, a consistent message emerges: only a small minority of LCx have reduced μQFR or FFR after PCI of the LM.
Can we change outcomes?
Careful inspection of Table 4 in Wang et al.3 reveals a surprising result, namely that low μQFR ws associated only with a reduction in bifurcation-related MI (TBMI) but not target bifurcation revascularization (TBR) after 3 years.3 In contrast, 5-year outcome data after assessment of the jailed LCx following a one-stent crossover strategy—albeit in a much smaller cohort of 83 patients—suggested an increased risk of both TBMI and TBR associated with low residual FFR.10 Potential explanations include a much larger cohort size in Wang et al., and the development of high-sensitivity troponin assays that generally recategorize TBR to TBMI.
Long-term events arise from the LCx, since continuous μQFR only predicted outcomes for the LCx and not for the LAD.3 Additionally, it is difficult to understand the absence of acute events when analysing the cohort by μQFR as opposed to %DS. The complete lack of peri-procedural events in these 72 vessels contrasts with an approximate 3% event rate in the rest of the cohort.
The authors propose that a randomized controlled trial of n = 3000 subjects could determine the superiority of a μQFR-guided strategy for LM PCI.3 Based on the preceding discussion, only 10%–15% of patients would be expected to have μQFR ≤0.8 after PCI of the LM—in other words, 300–450 total subjects. The remaining 2550–2700 subjects with final μQFR > 0.8 would be followed in a registry. The bifurcation-oriented composite endpoint of TBMI (excluding peri-procedural events that generally have no clinical relevance) and TBR might reach 10%–15% after 3 years as suggested, and consistent with the provisional stenting arm of DKCRUSH-V.11 Given the uncommon occurrence of μQFR ≤0.8 and modest event rates, the effect size of incremental optimization would need to be substantial (such as a hazard ratio of ≤0.5) to demonstrate a statistically significant benefit.
Conclusion
While awaiting further evidence, we propose the following take-home message summarized in the Graphical Abstract. The most important decision for PCI of the LM revolves around the strategy: provisional single stent vs. upfront two stents. A potential resolution to the apparent conflict between DKCRUSH-V11—an upfront two-stent strategy is superior—and EBC MAIN12—a provisional single stent strategy is superior—is that both are correct. For more complex, true LM bifurcation disease, make a two-stent plan; for less complex scenarios, begin with a single crossover stent. Wise selection of the strategy involves a thoughtful evaluation of the side branch (usually the LCx), and intravascular imaging brings the most clarity,2 although intracoronary physiology and anatomic features can help too.
If starting with a provisional approach, evaluate all branches after initial PCI and be prepared to react—for example, 26% of patients in the recent EBC MAIN trial needed further treatment of the side vessel after provisional one-stent crossover.12 Generally the LCx remains the Achilles heel, with higher rates of suboptimal acute results and long-term events. Advanced techniques such as physiology, intravascular imaging, and perhaps μQFR—as we now learn from our colleagues in Fuwai3—can help beyond basic angiography when judging the result as adequate.
Declarations
Disclosure of Interest
N.P.J. reports no direct relationships, but outside of the present work receives internal funding from the Weatherhead PET Center for Preventing and Reversing Atherosclerosis; has patents pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology, and on methods to correct pressure tracings from fluid-filled catheters; receives significant institutional research support from CoreAalst (PPG Global, NCT04789317) and Neovasc/Shockwave (PET core lab for COSIRA-II, NCT05102019); and had an institutional licensing agreement with Boston Scientific for the smart minimum FFR algorithm commercialized under 510(k) K191008. J.M.A. reports no relevant disclosures.
References
Author notes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.



