-
PDF
- Split View
-
Views
-
Cite
Cite
Annette Holdgaard, Christine Eckhardt-Hansen, Christina Funch Lassen, Ingunn Eklo Kjesbu, Christian Have Dall, Kristine Lund Michaelsen, Kirstine Lærum Sibilitz, Eva Prescott, Hanne Kruuse Rasmusen, Cognitive-behavioural therapy reduces psychological distress in younger patients with cardiac disease: a randomized trial, European Heart Journal, Volume 44, Issue 11, 14 March 2023, Pages 986–996, https://doi.org/10.1093/eurheartj/ehac792
Close - Share Icon Share
Abstract
To test whether usual outpatient cardiac rehabilitation (CR) supplemented by a cognitive-behavioural therapy (CBT) intervention may reduce anxiety and depression compared with usual CR.
In this multicentre randomized controlled trial, 147 cardiac patients (67% men, mean age 54 years, 92% with coronary artery disease) with psychological distress defined as a hospital anxiety and depression scale (HADS) anxiety or depression score ≥8 were randomized to five sessions of group CBT plus usual CR (intervention, n = 74) or CR alone (control, n = 73). Patients with severe distress or a psychiatric diagnosis were excluded. The intervention was delivered by cardiac nurses with CBT training and supervised by a psychologist. A reference, non-randomized group (background, n = 41) of consecutive patients without psychological distress receiving usual CR was included to explore the effect of time on HADS score. The primary outcome, total HADS score after 3 months, improved more in the intervention than in the control group [the mean total HADS score improved by 8.0 (standard deviation 5.6) vs. 4.1 (standard deviation 7.8), P < 0.001]. Significant between-group differences were maintained after 6 months. Compared with the control group, the intervention group also had greater adherence to CR (P = 0.003), more improvement in the heart-related quality of life (HeartQoL) at 6 months (P < 0.01), and a significant reduction in cardiac readmissions at 12 months (P < 0.01). The background group had no significant change in HADS score over time.
Brief CBT provided by cardiac nurses in relation to CR reduced anxiety and depression scores, improved HeartQoL and adherence to CR, and reduced cardiovascular readmissions. The programme is simple and may be implemented by CR nurses.
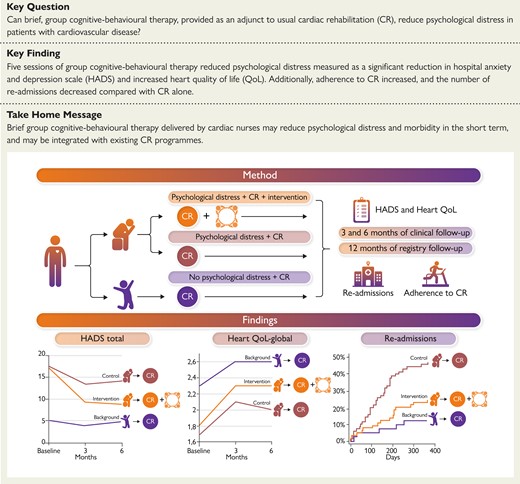
Graphical summary of the method and main findings of the present study. HADS, hospital anxiety and depression scale; HeartQoL, heart-related quality of life.
See the editorial comment for this article ‘Are we getting closer to treating heart and mind together and bridging the gap for individual patients attending cardiac rehabilitation?’, by S. S. Pedersen et al., https://doi.org/10.1093/eurheartj/ehac820.
Introduction
The psychological impact of having coronary artery disease (CAD) is substantial. Anxiety and depression affect up to one-third of patients with CAD and are linked to impaired quality of life (QoL), increased morbidity, cardiac and all-cause mortality.1–3 Among patients with psychological distress, younger patients and patients with high-risk-factor burdens and limited socioeconomic resources are overrepresented.4,5 Untreated anxiety and depression also have an impact on the patient’s work retention.6–8 Concomitantly, hospitalization time has reduced substantially which is fortunate in the general population of cardiac patients but might not leave time to address the psychological burden of having a cardiac disease making an outpatient follow-up of even more eminent importance. Cardiac rehabilitation (CR) in patients with CAD has proved to reduce mortality and morbidity and has a Class IA recommendation9 and, although less well documented, is also recommended in cardiac patients after valve surgery and other conditions.10,11 Traditionally CR interventions have focused on physical exercise and risk-factor management, whereas the management of psychological distress has received less attention. Consequently, management of psychological distress has not been systematically integrated into CR, although ESC guidelines emphasize recognition and intervention.12,13
A Cochrane review from 2017 reviewed 35 studies involving 10 703 patients with CAD randomized to multifactorial psychological interventions. The review concluded that beneficial effects of psychological interventions were found for cardiac mortality, anxiety, and depression, but due to substantial statistical heterogeneity for all psychological outcomes, future studies would benefit from testing specific psychological interventions.14 A review by Reavell et al.15 supported cognitive behavioural therapy (CBT) as an efficient treatment for anxiety and depression in patients with cardiac disease and concluded that to maximize effect, face-to-face sessions should be prioritized with a minimum of four sessions to ensure an effect. A newly published study by Wells et al.16 tested the effect of six-group metacognitive, face-to-face sessions delivered in a single-blind, parallel controlled multicentre randomized controlled trial (RCT) added to CR in 332 cardiac patients with signs of anxiety or depression defined as a hospital anxiety and depression scale (HADS) score of 8 or above. The primary outcome of change in HADS score after 4 months improved significantly and the effect was maintained at 12 months. The study did not assess effect on repeat admissions, retention in the workforce, or other cardiac outcomes.
It is imperative that psychological interventions for anxiety and depression are developed and integrated into the CR setting so that they may be offered to all cardiac patients with psychological distress. This may not only improve patients’ QoL and clinical outcomes but also has the potential to retain those with limited resources. It is well known that patients with psychological distress tend to be less adherent to CR,17,18 but it remains unknown if psychological interventions in connection with CR may increase adherence.
Psychological distress may mimic cardiac symptoms such as palpitations and chest discomfort, and it may be difficult for health providers who are not trained in cardiology to manage cardiac patients with psychological distress. After receiving CBT training, cardiac nurses can fulfil this dual role19 and probably also other CR professionals as showed in the study by Wells et al.16 Furthermore, it is important that the intervention is well described and tested in a multicentre trial to ensure that it is widely implementable.
The main aim of this study was to evaluate the effect of brief, group CBT integrated in CR and delivered by nurses to reduce psychological distress in patients with a new CAD event and/or surgically treated valvular heart disease (VHD) with symptoms of anxiety, depression, or both. Main secondary outcomes were adherence to CR, retention in the work force and repeat hospitalization.
Methods
Study design
This was a multicentre, open-label, prospective, randomized trial with 3 and 6 months of clinical follow-up comparing group CBT plus usual CR with CR alone. The patients were recruited at three hospitals (Bispebjerg and Frederiksberg Hospital, Hvidovre and Amager Hospital, and North Zealand Hospital) in Denmark. The patients had a new CAD event and/or surgically treated VHD and psychological distress (Figure 1). Psychological distress was measured by the HADS. In the previously published protocol, the design was described in detail.20
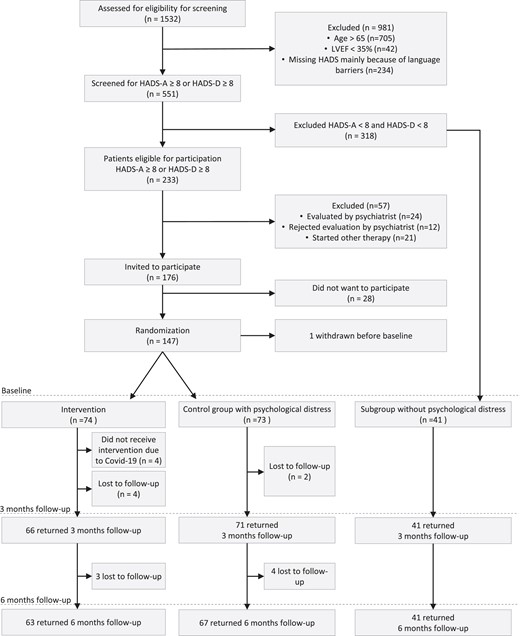
Patient flow chart. Patients in the intervention group followed cardiac rehabilitation and received five sessions of cognitive-behavioural therapy, whereas the control group followed standard cardiac rehabilitation. BDI, Beck depression inventory; CBT, cognitive-behavioural therapy; CR, cardiac rehabilitation; HADS, hospital anxiety and depression scale; HADS-A, the hospital anxiety and depression scale, anxiety subscale; HADS-D, the hospital anxiety and depression scale, depression subscale.
Patients
Consecutive patients with CAD and/or surgically treated VHD referred to CR were screened for eligibility at their first visit with the following in- and exclusion criteria.
Inclusion criteria were:
Referred to CR and accepting CR, patients with a new CAD event [ST or non-ST-elevation myocardial infarction (MI) and/or revascularized (percutaneous coronary intervention or coronary artery bypass graft)] within 3 months prior to first visit at CR and/or surgically treated VHD within 3 months prior to first visit
HADS score ≥8 for HADS-A and/or HADS-D
Age <65 years or, if >65 years to remain occupationally active
Able to speak and understand Danish
Exclusion criteria were:
Left ventricular ejection fraction <35%
Other serious comorbidity expected to have a serious impact on life expectancy
Known abuse of alcohol or euphoric drugs
Known more serious psychopathology such as schizophrenia, bipolar disorder, severe personality disorder, and treatment with psychoactive drugs including selective serotonin reuptake inhibitors
The HADS score measures symptoms of anxiety (HADS-A, seven items) and depression (HADS-D, seven items). Greater scores indicate a higher degree of distress. On each subscale, scores below eight are regarded as normal.21 A score of eight or greater is considered to be the cut-off for mild clinical symptoms and yields the optimal sensitivity and specificity for both HADS-A and HADS-D for identifying clinical caseness.22 The psychiatrist evaluated patients with score of HADS-D ≥ 11 and Beck depression inventory (BDI) > 17, made a clinical evaluation and identified patients with severe depression or other serious psychiatric diagnoses, who were then excluded. Patients who declined this evaluation, or after evaluation by the psychiatrist were recommended another treatment were excluded from the study.
Eligible patients who accepted to participate in the study after receiving written and verbal information from a study nurse signed an informed consent form before randomization. Randomization was performed by a blinded independent researcher who was not involved in the trial and was unaware of the patients’ characteristics. For randomization, we used STATA II, the Ralloc programme, with permuting random block sizes of 2, 4, and 6.
The patients were randomized to CR supplemented by five sessions of group CBT (intervention, I) or CR alone (control, C). Furthermore, a non-randomized group of consecutive patients fulfilling in- and exclusion criteria but without signs of psychological distress (i.e. HADS-A < 8 and HADS-D < 8) was recruited among the non-distressed over a shorter period of time and participated as a background group, receiving usual CR with re-evaluation after 3 and 6 months. The aim of this group was to follow the natural course, including any subsequent development of psychological distress during the follow-up period.
Usual cardiac rehabilitation
Patients in all three groups were offered the usual CR programme at their centre. CR programmes were group based and consisted of 8 weeks of exercise, twice weekly. All patients performed a cardiopulmonary exercise test (CPET) to determine the peak oxygen uptake (VO2peak, mL/kg/min)23 before beginning exercise training and most also at the end of the training programme. The exact number and time for the CPET are described in Supplementary material online, Table S1. Each session lasted 90 min and contained both moderate-intensity training (80% of VO2peak) and resistance training. The other core components of CR were patient education on heart disease including lifestyle and medical risk-factor management, psychological issues, diet counselling once a week, and up-titration of medication according to guidelines. In addition, some patients also had individual sessions with a nurse, dietician, or cardiologist. The CR programmes were slightly different (either first visit lead by a nurse or a consultant cardiologist, and CPET also after exercise training at two sites otherwise indifferent) across the three hospitals but all offered the above core components (see Supplementary material online, Table S2).
Intervention
The intervention group received group CBT, which in this trial was a psycho-educational group course provided in addition to CR. The course consisted of five sessions facilitated by an experienced cardiac nurse as one scheduled 2 h session per week. Each group comprised three to four patients. The group CBT intervention is manualized, and each session had a set structure, homework was used, and sessions always ended with the opportunity to provide feedback. The same manual was used at all three hospitals.24 Furthermore, nurses received feedback on sessions from the psychologist. The nurses had received education in CBT by a psychologist specialized in CBT and had subsequently received supervised training in delivering the intervention to a pilot group. The nurses received supervision by a psychologist as needed throughout the trial. In this trial, group CBT aimed at supporting patients thereby allowing them to cope with their heart disease.
Some of the overall components in the sessions are described in Table 1. A matrix25 was used in the first session to help the patient clarify which values were important and which difficulties their heart disease had caused. The matrix was used throughout the course.
| Session . | Theme . | Contents . |
|---|---|---|
| 1 | Introduction to CBT and mapping of own values (individual values limited/affected by cardiac disease) |
|
| 2 | Anxiety and anxiety reduction techniques |
|
| 3 | Behavioural analysis (awareness of consequences of own behaviour) |
|
| 4 | Concerns and strategies for dealing with concerns |
|
| 5 | How to cope with future psychological distress by using the CBT strategies |
|
| Session . | Theme . | Contents . |
|---|---|---|
| 1 | Introduction to CBT and mapping of own values (individual values limited/affected by cardiac disease) |
|
| 2 | Anxiety and anxiety reduction techniques |
|
| 3 | Behavioural analysis (awareness of consequences of own behaviour) |
|
| 4 | Concerns and strategies for dealing with concerns |
|
| 5 | How to cope with future psychological distress by using the CBT strategies |
|
| Session . | Theme . | Contents . |
|---|---|---|
| 1 | Introduction to CBT and mapping of own values (individual values limited/affected by cardiac disease) |
|
| 2 | Anxiety and anxiety reduction techniques |
|
| 3 | Behavioural analysis (awareness of consequences of own behaviour) |
|
| 4 | Concerns and strategies for dealing with concerns |
|
| 5 | How to cope with future psychological distress by using the CBT strategies |
|
| Session . | Theme . | Contents . |
|---|---|---|
| 1 | Introduction to CBT and mapping of own values (individual values limited/affected by cardiac disease) |
|
| 2 | Anxiety and anxiety reduction techniques |
|
| 3 | Behavioural analysis (awareness of consequences of own behaviour) |
|
| 4 | Concerns and strategies for dealing with concerns |
|
| 5 | How to cope with future psychological distress by using the CBT strategies |
|
No restrictions were placed on concomitant care during the trial period. The programme was described in detail in the previously published protocol.20
Outcomes
The primary outcome was change in psychological distress measured by HADS total score21 from baseline to the 3-month follow-up in the intervention group compared with the usual care group.
Secondary outcomes included HADS-A, HADS-D, adherence to CR (participation in >80% of planned training sessions, and education sessions) and return to work as well as the following outcomes measured at the 3- and 6-month follow-ups; adherence to lifestyle goals [glycated haemoglobin (HbA1c) < 48 mmol/L, blood pressure (BP) < 135/85 mmHg, body mass index (BMI) < 30 kg/m2, and low-density lipoprotein (LDL) < 1.4 mmol/L], smoking status, physical activity (obtained in the interview with the nurse, and defined as days/week with >30 min of moderate physical activity) and heart-related quality of life (HeartQoL). The HeartQoL questionnaire measures heart-related QoL in patients with heart disease. It provides two subscales: The physical subscale consisting of 10 items and the emotional subscale consisting of 4 items, all of which are scored from 0–3. A higher score indicates a better QoL.26,27 The HeartQoL questionnaire has proved to be a reliable instrument with a Cronbach’s α between 0.80 and 0.91 for the global score and each subscale and to be responsive in patients with a wide spectrum of cardiac diagnoses.28
We assessed cardiac readmissions, all-cause mortality, fatal and non-fatal MI and coronary artery revascularization within 12 months from randomization. Cardiac readmissions covered all readmissions to emergency and cardiac departments for cardiac symptoms. Admissions to other departments of somatic disease were not included and admissions to psychiatric departments were assessed separately. Cardiac readmission was ascertained through electronic health records and adjudicated by an independent cardiologist blinded to treatment intervention.
Statistical analyses
We used descriptive statistics to compare baseline data across the groups with and without psychological distress and between the intervention and control groups. The normal distribution of all continuous variables was examined visually and by the Shapiro–Wilk test. Normally distributed variables were compared across groups using t-test or one-way analysis of variance and presented as mean ± standard deviation (SD). Data not following the normal distribution were compared using the Kruskal–Wallis test and presented as median (interquartile range). Categorical variables were compared across the groups using Pearson’s χ2 test.
To analyse the main outcome, i.e. change in total HADS from baseline to 3 months, we used linear regression mixed models, thus adjusting for the baseline HADS value. We also included centre (three sites) in the model, and analyses were tested for treatment–centre interaction. A priori, power estimates suggested that we would have 80% power to detect a 2.3 point difference in the total HADS score (delta HADS) between the intervention and control groups, due to the risk of dropout of patients included in this type of trial, the total number of patients included was increased by 15% to 148 patients as described in the previously published protocol.20 The minimal clinically important difference of change in HADS is 1.7, and serves as an indicator of treatment success for interventions intended to improve the mental health of patients with cardiovascular disease.29
Secondary outcomes, including the HeartQol and other outcomes on a continuous scale, were compared across intervention and control groups with similar analysis, whereas dichotomized secondary outcomes [adherence to CR (>80%), return to work (yes/no), HbA1c < 48, BP < 135/85 mmHg, BMI < 30 kg/m2, LDL <1.4 mmol/L, smoking status, and physical activity) were compared by logistic regression adjusting for treatment centre.
For clinical events (number of readmissions within 12 months of randomization), we compared outcomes across groups with Kaplan–Meier curves and time-to-event analyses (Cox regression) adjusting for centre.
The primary reported analyses were conducted as intention-to-treat and included all participants that attended follow-up at 3 and 6 months, respectively. In sensitivity analyses, we included information at all time points on patients’ lost to follow-up by carrying forward the most recent available measurement. We planned for a per-protocol analyses defined by participation in ≥ four of the five group sessions, but all patients fulfilled this criterion. There were no missing data on the primary outcome of HADS. For the questionnaire HeartQol, missing data in one or more of the questions were imputed as the mean value of the corresponding question across the population if there were <20% missing data in the HeartQol questionnaire for the individual in question. Analyses were repeated after excluding patients with missing data.
We compared the primary outcome in intervention and control groups with the spontaneous variation in the non-distressed subgroup of 41 participants by separate mixed-models linear regression adjusting for baseline values.
A two-tailed P-value P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata SE 17 v17.0.116 (StataCorp LP).
Ethics
The trial was performed in accordance with the Declaration of Helsinki, and all patients gave informed consent after written and oral information. The trial was initiated following approval by the Danish Data Protection Agency and the regional ethics committee (H-16042832). The trial is registered with www.ClinicalTrials.gov (NCT04254315).
Results
Patient characteristics
From February 2017 to March 2021, 1532 patients with a new CAD event and/or surgically treated VHD were screened; 176 were invited to participate; and 147 patients were enrolled in the study (Figure 1). Among the patients with a high depression score and evaluated by the psychiatrist, eight (25%) were subsequently included in the study. The remaining 57 patient with HADS-D ≥ 11 and BDI > 17 were excluded either because they did not want to be evaluated by a psychiatrist, were already started other therapy, or were recommended another treatment by the psychiatrist (Figure 1). Furthermore, among the 318 screened but non-distressed patients, a background group of 41 consecutive patients were recruited during a shorter period of time serving as a reference group (Figure 1).
After randomization, eight (12%) were excluded in the intervention group due to the COVID-19 lockdown (four patients) or were lost to follow-up as they did not wish to continue in the trial or participate in follow-ups (four patients). A total of 66 (89%) patients received the full intervention, defined as four or more of the five sessions. At 3-month follow-up, two patients in the control group were lost to follow-up. At the 6-month follow-up, three (5%), four (6%), and none were lost to follow-up in the intervention, control, and background groups, respectively. The last follow-up was conducted in September 2021.
The two groups with psychological distress, i.e. the intervention and the control groups, were similar with respect to clinical and demographic characteristics, with the exception of significantly more patients having a coronary artery bypass graft operation in the control group (Table 2). The mean age was 54 ± 8 years, 67% were males and 92% had CAD as their index event. Approximately 89% of patients scored ≥8 on HADS-A and 50% ≥8 on HADS-D with no difference between the intervention and control groups (see Supplementary material online, Table S3). The background group without psychological distress was similar to the groups with psychological distress with the exception of a male preponderance (85% vs. 67%, P = 0.03), higher age (57 ± 6 vs. 54 ± 6 years) and a higher proportion of patients working before the event (98% vs. 78%).
| . | With psychological distress . | Without psychological distress . | |
|---|---|---|---|
| Intervention . | Control . | Background . | |
| (n = 74) . | (n = 73) . | (n = 41) . | |
| Male sex | 45 (61%) | 54 (74%) | 35 (85%) |
| Age (years), mean (SD) | 54 (8) | 55 (7) | 57 (6)* |
| BMI (kg/m2), mean (SD) | 28,2 (5,2) | 27.1 (5.0) | 27.2 (4.8) |
| Living alone | 24 (34%) | 31 (43%) | 9 (23%) |
| Education | |||
| ȃNo education | 6 (9%) | 13 (18%) | 7 (17%) |
| ȃShort-term education | 25 (37%) | 22 (31%) | 13 (32%) |
| ȃMedium-term education | 20 (29%) | 19 (27%) | 11 (27%) |
| ȃHigher education | 17 (25%) | 17 (24%) | 10 (24%) |
| Working status at event | |||
| ȃWorking | 60 (86%) | 51 (71%) | 40 (98%)* |
| ȃUnemployed | 8 (11%) | 14 (19%) | 1 (2%) |
| ȃSick leave | 2 (3%) | 7 (10%) | 0 (0%) |
| LVEF (%), median (IQR) | 55 (50–60) | 50 (50–60) | 60 (50–60) |
| LVEF ≤ 40% | 8 (11%) | 7 (9%) | 4 (10%) |
| Index event | |||
| ȃSTEMI | 25 (34%) | 31 (42%) | 13 (32%) |
| ȃNSTEMI | 29 (39%) | 16 (22%) | 9 (22%) |
| ȃUAP | 9 (12%) | 8 (11%) | 6 (15%) |
| ȃStable AP | 4 (5%) | 13 (18%) | 12 (29%) |
| ȃHeart valve disease | 6 (8%) | 6 (8%) | 1 (2%) |
| ȃAorta aneurysm | 1 (1%) | 0 (0%) | 0 (0%) |
| PCI | 52 (74%) | 46 (63%) | 30 (73%) |
| CABG | 5 (7%) | 14 (19%) | 7 (17%) |
| Heart valve replacement | |||
| ȃAorta | 3 (4%) | 3 (4%) | 3 (7%) |
| ȃMitral | 4 (5%) | 3 (4%) | 0 (0%) |
| NYHA class | |||
| ȃ≥ NYHA Class II | 16 (23%) | 8 (11%) | 6 (15%) |
| CCS class | |||
| ȃCCS class <1 | 64 (93%) | 66 (92%) | 41 (100%) |
| Cardiac history prior to index event | 6 (9%) | 7 (10%) | 3 (7%) |
| COPD | 3 (4%) | 0 (0%) | 3 (7%) |
| Kidney disease | 2 (3%) | 4 (6%) | 4 (10%) |
| Hypertension | 31 (44%) | 33 (46%) | 22 (54%) |
| Dyslipidaemia | 42 (59%) | 43 (60%) | 30 (73%) |
| Diabetes mellitus | 10 (14%) | 9 (13%) | 6 (15%) |
| Family history | 34 (50%) | 32 (47%) | 18 (45%) |
| Current smoker | 12 (17%) | 13 (18%) | 5 (13%) |
| Medication | |||
| ȃOther antiplatelet medication | 52 (75%) | 51 (71%) | 31 (76%) |
| ȃStatin | 61 (87%) | 65 (90%) | 38 (93%) |
| ȃBeta-blocker | 35 (50%) | 34 (49%) | 15 (37%) |
| ȃACE inhibitor | 28 (40%) | 31 (44%) | 12 (30%) |
| ȃDiabetes medication | 9 (12%) | 8 (11%) | 5 (12%) |
| VO2peak (mL/kg/min), mean (SD) | 23.0 (6.4) | 23.9 (6.4) | 25.9 (6.3)* |
| Physical activity (>30 min/day) | 2 (0–5) | 2 (0–5) | 3 (0–5) |
| HADS total, median (IQR) | 17 (14–21) | 17 (13–21) | 5 (3–7)* |
| HADS anxiety, median (IQR) | 10 (8–12) | 10 (8–13) | 3 (2–5)* |
| HADS depression, median (IQR) | 7 (5–10) | 8 (5–10) | 1 (1–3)* |
| . | With psychological distress . | Without psychological distress . | |
|---|---|---|---|
| Intervention . | Control . | Background . | |
| (n = 74) . | (n = 73) . | (n = 41) . | |
| Male sex | 45 (61%) | 54 (74%) | 35 (85%) |
| Age (years), mean (SD) | 54 (8) | 55 (7) | 57 (6)* |
| BMI (kg/m2), mean (SD) | 28,2 (5,2) | 27.1 (5.0) | 27.2 (4.8) |
| Living alone | 24 (34%) | 31 (43%) | 9 (23%) |
| Education | |||
| ȃNo education | 6 (9%) | 13 (18%) | 7 (17%) |
| ȃShort-term education | 25 (37%) | 22 (31%) | 13 (32%) |
| ȃMedium-term education | 20 (29%) | 19 (27%) | 11 (27%) |
| ȃHigher education | 17 (25%) | 17 (24%) | 10 (24%) |
| Working status at event | |||
| ȃWorking | 60 (86%) | 51 (71%) | 40 (98%)* |
| ȃUnemployed | 8 (11%) | 14 (19%) | 1 (2%) |
| ȃSick leave | 2 (3%) | 7 (10%) | 0 (0%) |
| LVEF (%), median (IQR) | 55 (50–60) | 50 (50–60) | 60 (50–60) |
| LVEF ≤ 40% | 8 (11%) | 7 (9%) | 4 (10%) |
| Index event | |||
| ȃSTEMI | 25 (34%) | 31 (42%) | 13 (32%) |
| ȃNSTEMI | 29 (39%) | 16 (22%) | 9 (22%) |
| ȃUAP | 9 (12%) | 8 (11%) | 6 (15%) |
| ȃStable AP | 4 (5%) | 13 (18%) | 12 (29%) |
| ȃHeart valve disease | 6 (8%) | 6 (8%) | 1 (2%) |
| ȃAorta aneurysm | 1 (1%) | 0 (0%) | 0 (0%) |
| PCI | 52 (74%) | 46 (63%) | 30 (73%) |
| CABG | 5 (7%) | 14 (19%) | 7 (17%) |
| Heart valve replacement | |||
| ȃAorta | 3 (4%) | 3 (4%) | 3 (7%) |
| ȃMitral | 4 (5%) | 3 (4%) | 0 (0%) |
| NYHA class | |||
| ȃ≥ NYHA Class II | 16 (23%) | 8 (11%) | 6 (15%) |
| CCS class | |||
| ȃCCS class <1 | 64 (93%) | 66 (92%) | 41 (100%) |
| Cardiac history prior to index event | 6 (9%) | 7 (10%) | 3 (7%) |
| COPD | 3 (4%) | 0 (0%) | 3 (7%) |
| Kidney disease | 2 (3%) | 4 (6%) | 4 (10%) |
| Hypertension | 31 (44%) | 33 (46%) | 22 (54%) |
| Dyslipidaemia | 42 (59%) | 43 (60%) | 30 (73%) |
| Diabetes mellitus | 10 (14%) | 9 (13%) | 6 (15%) |
| Family history | 34 (50%) | 32 (47%) | 18 (45%) |
| Current smoker | 12 (17%) | 13 (18%) | 5 (13%) |
| Medication | |||
| ȃOther antiplatelet medication | 52 (75%) | 51 (71%) | 31 (76%) |
| ȃStatin | 61 (87%) | 65 (90%) | 38 (93%) |
| ȃBeta-blocker | 35 (50%) | 34 (49%) | 15 (37%) |
| ȃACE inhibitor | 28 (40%) | 31 (44%) | 12 (30%) |
| ȃDiabetes medication | 9 (12%) | 8 (11%) | 5 (12%) |
| VO2peak (mL/kg/min), mean (SD) | 23.0 (6.4) | 23.9 (6.4) | 25.9 (6.3)* |
| Physical activity (>30 min/day) | 2 (0–5) | 2 (0–5) | 3 (0–5) |
| HADS total, median (IQR) | 17 (14–21) | 17 (13–21) | 5 (3–7)* |
| HADS anxiety, median (IQR) | 10 (8–12) | 10 (8–13) | 3 (2–5)* |
| HADS depression, median (IQR) | 7 (5–10) | 8 (5–10) | 1 (1–3)* |
The group of distressed patients compared with the group of patients with no distress.
ACE, angiotensin-converting enzyme; BMI, body mass index; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society Angina Classification; COPD, chronic obstructive pulmonary disease; HADS, hospital anxiety and depression scale; IQR, interquartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SD, standard deviation; UAP, unstable angina pectoris; VO2peak, peak oxygen uptake.
P < 0.05.
| . | With psychological distress . | Without psychological distress . | |
|---|---|---|---|
| Intervention . | Control . | Background . | |
| (n = 74) . | (n = 73) . | (n = 41) . | |
| Male sex | 45 (61%) | 54 (74%) | 35 (85%) |
| Age (years), mean (SD) | 54 (8) | 55 (7) | 57 (6)* |
| BMI (kg/m2), mean (SD) | 28,2 (5,2) | 27.1 (5.0) | 27.2 (4.8) |
| Living alone | 24 (34%) | 31 (43%) | 9 (23%) |
| Education | |||
| ȃNo education | 6 (9%) | 13 (18%) | 7 (17%) |
| ȃShort-term education | 25 (37%) | 22 (31%) | 13 (32%) |
| ȃMedium-term education | 20 (29%) | 19 (27%) | 11 (27%) |
| ȃHigher education | 17 (25%) | 17 (24%) | 10 (24%) |
| Working status at event | |||
| ȃWorking | 60 (86%) | 51 (71%) | 40 (98%)* |
| ȃUnemployed | 8 (11%) | 14 (19%) | 1 (2%) |
| ȃSick leave | 2 (3%) | 7 (10%) | 0 (0%) |
| LVEF (%), median (IQR) | 55 (50–60) | 50 (50–60) | 60 (50–60) |
| LVEF ≤ 40% | 8 (11%) | 7 (9%) | 4 (10%) |
| Index event | |||
| ȃSTEMI | 25 (34%) | 31 (42%) | 13 (32%) |
| ȃNSTEMI | 29 (39%) | 16 (22%) | 9 (22%) |
| ȃUAP | 9 (12%) | 8 (11%) | 6 (15%) |
| ȃStable AP | 4 (5%) | 13 (18%) | 12 (29%) |
| ȃHeart valve disease | 6 (8%) | 6 (8%) | 1 (2%) |
| ȃAorta aneurysm | 1 (1%) | 0 (0%) | 0 (0%) |
| PCI | 52 (74%) | 46 (63%) | 30 (73%) |
| CABG | 5 (7%) | 14 (19%) | 7 (17%) |
| Heart valve replacement | |||
| ȃAorta | 3 (4%) | 3 (4%) | 3 (7%) |
| ȃMitral | 4 (5%) | 3 (4%) | 0 (0%) |
| NYHA class | |||
| ȃ≥ NYHA Class II | 16 (23%) | 8 (11%) | 6 (15%) |
| CCS class | |||
| ȃCCS class <1 | 64 (93%) | 66 (92%) | 41 (100%) |
| Cardiac history prior to index event | 6 (9%) | 7 (10%) | 3 (7%) |
| COPD | 3 (4%) | 0 (0%) | 3 (7%) |
| Kidney disease | 2 (3%) | 4 (6%) | 4 (10%) |
| Hypertension | 31 (44%) | 33 (46%) | 22 (54%) |
| Dyslipidaemia | 42 (59%) | 43 (60%) | 30 (73%) |
| Diabetes mellitus | 10 (14%) | 9 (13%) | 6 (15%) |
| Family history | 34 (50%) | 32 (47%) | 18 (45%) |
| Current smoker | 12 (17%) | 13 (18%) | 5 (13%) |
| Medication | |||
| ȃOther antiplatelet medication | 52 (75%) | 51 (71%) | 31 (76%) |
| ȃStatin | 61 (87%) | 65 (90%) | 38 (93%) |
| ȃBeta-blocker | 35 (50%) | 34 (49%) | 15 (37%) |
| ȃACE inhibitor | 28 (40%) | 31 (44%) | 12 (30%) |
| ȃDiabetes medication | 9 (12%) | 8 (11%) | 5 (12%) |
| VO2peak (mL/kg/min), mean (SD) | 23.0 (6.4) | 23.9 (6.4) | 25.9 (6.3)* |
| Physical activity (>30 min/day) | 2 (0–5) | 2 (0–5) | 3 (0–5) |
| HADS total, median (IQR) | 17 (14–21) | 17 (13–21) | 5 (3–7)* |
| HADS anxiety, median (IQR) | 10 (8–12) | 10 (8–13) | 3 (2–5)* |
| HADS depression, median (IQR) | 7 (5–10) | 8 (5–10) | 1 (1–3)* |
| . | With psychological distress . | Without psychological distress . | |
|---|---|---|---|
| Intervention . | Control . | Background . | |
| (n = 74) . | (n = 73) . | (n = 41) . | |
| Male sex | 45 (61%) | 54 (74%) | 35 (85%) |
| Age (years), mean (SD) | 54 (8) | 55 (7) | 57 (6)* |
| BMI (kg/m2), mean (SD) | 28,2 (5,2) | 27.1 (5.0) | 27.2 (4.8) |
| Living alone | 24 (34%) | 31 (43%) | 9 (23%) |
| Education | |||
| ȃNo education | 6 (9%) | 13 (18%) | 7 (17%) |
| ȃShort-term education | 25 (37%) | 22 (31%) | 13 (32%) |
| ȃMedium-term education | 20 (29%) | 19 (27%) | 11 (27%) |
| ȃHigher education | 17 (25%) | 17 (24%) | 10 (24%) |
| Working status at event | |||
| ȃWorking | 60 (86%) | 51 (71%) | 40 (98%)* |
| ȃUnemployed | 8 (11%) | 14 (19%) | 1 (2%) |
| ȃSick leave | 2 (3%) | 7 (10%) | 0 (0%) |
| LVEF (%), median (IQR) | 55 (50–60) | 50 (50–60) | 60 (50–60) |
| LVEF ≤ 40% | 8 (11%) | 7 (9%) | 4 (10%) |
| Index event | |||
| ȃSTEMI | 25 (34%) | 31 (42%) | 13 (32%) |
| ȃNSTEMI | 29 (39%) | 16 (22%) | 9 (22%) |
| ȃUAP | 9 (12%) | 8 (11%) | 6 (15%) |
| ȃStable AP | 4 (5%) | 13 (18%) | 12 (29%) |
| ȃHeart valve disease | 6 (8%) | 6 (8%) | 1 (2%) |
| ȃAorta aneurysm | 1 (1%) | 0 (0%) | 0 (0%) |
| PCI | 52 (74%) | 46 (63%) | 30 (73%) |
| CABG | 5 (7%) | 14 (19%) | 7 (17%) |
| Heart valve replacement | |||
| ȃAorta | 3 (4%) | 3 (4%) | 3 (7%) |
| ȃMitral | 4 (5%) | 3 (4%) | 0 (0%) |
| NYHA class | |||
| ȃ≥ NYHA Class II | 16 (23%) | 8 (11%) | 6 (15%) |
| CCS class | |||
| ȃCCS class <1 | 64 (93%) | 66 (92%) | 41 (100%) |
| Cardiac history prior to index event | 6 (9%) | 7 (10%) | 3 (7%) |
| COPD | 3 (4%) | 0 (0%) | 3 (7%) |
| Kidney disease | 2 (3%) | 4 (6%) | 4 (10%) |
| Hypertension | 31 (44%) | 33 (46%) | 22 (54%) |
| Dyslipidaemia | 42 (59%) | 43 (60%) | 30 (73%) |
| Diabetes mellitus | 10 (14%) | 9 (13%) | 6 (15%) |
| Family history | 34 (50%) | 32 (47%) | 18 (45%) |
| Current smoker | 12 (17%) | 13 (18%) | 5 (13%) |
| Medication | |||
| ȃOther antiplatelet medication | 52 (75%) | 51 (71%) | 31 (76%) |
| ȃStatin | 61 (87%) | 65 (90%) | 38 (93%) |
| ȃBeta-blocker | 35 (50%) | 34 (49%) | 15 (37%) |
| ȃACE inhibitor | 28 (40%) | 31 (44%) | 12 (30%) |
| ȃDiabetes medication | 9 (12%) | 8 (11%) | 5 (12%) |
| VO2peak (mL/kg/min), mean (SD) | 23.0 (6.4) | 23.9 (6.4) | 25.9 (6.3)* |
| Physical activity (>30 min/day) | 2 (0–5) | 2 (0–5) | 3 (0–5) |
| HADS total, median (IQR) | 17 (14–21) | 17 (13–21) | 5 (3–7)* |
| HADS anxiety, median (IQR) | 10 (8–12) | 10 (8–13) | 3 (2–5)* |
| HADS depression, median (IQR) | 7 (5–10) | 8 (5–10) | 1 (1–3)* |
The group of distressed patients compared with the group of patients with no distress.
ACE, angiotensin-converting enzyme; BMI, body mass index; CABG, coronary artery bypass grafting; CCS, Canadian Cardiovascular Society Angina Classification; COPD, chronic obstructive pulmonary disease; HADS, hospital anxiety and depression scale; IQR, interquartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SD, standard deviation; UAP, unstable angina pectoris; VO2peak, peak oxygen uptake.
P < 0.05.
Outcomes
At 3 months, the mean total HADS score improved by 8.0 (SD 5.6) in the intervention group vs. 4.1 (SD 7.8) in the control group (P for difference <0.001). Both subscales, i.e. anxiety and depression, also improved significantly more in the intervention group (Figure 2 and Supplementary material online, Tables S3 and S4). These differences were maintained at the 6-month follow-up. No difference was observed in treatment effect across the three sites (no treatment–centre interaction), between genders or between CAD and VHD; however, the statistical power to detect interaction was limited. The background group without psychological distress had no significant change in total HADS score at the 3- and 6-month follow-ups. Results were similar when carrying forward the most recent available measurement for patients’ lost to follow-up.
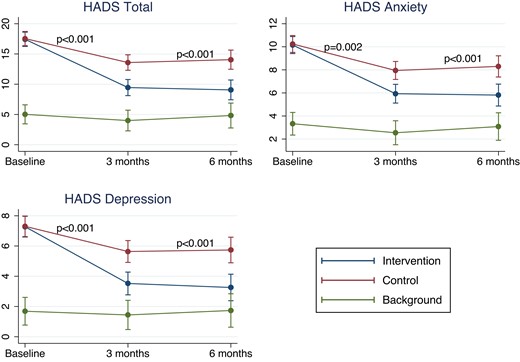
Change in hospital anxiety and depression scale score in the intervention, control, and background groups. Estimated total hospital anxiety and depression scale score, subscore on depression, and subscore on anxiety at baseline, and after 3 and 6 months for the three groups from mixed models adjusting for centre. P-values denote comparison of Δ hospital anxiety and depression scale between the intervention and control groups.
HeartQol improved significantly in all three groups with no significant difference at the 3-month follow-up between the intervention and control groups in global score or physical/emotional subscores (Figure 3 and Supplementary material online, Table S5). At the 6-month follow-up, significant differences were detected in favour of the intervention group for the total and emotional score but not for the physical score (P = 0.05). The background group had a significantly (P < 0.001) higher HeartQoL at baseline and remained higher than the groups with psychological distress at the 3- and 6-month follow-up. There was no difference in effect between genders (no treatment–sex interaction) and results were similar after excluding participants with missing data (not imputed) and carrying forward the most recent available measurement for patients’ lost to follow-up.
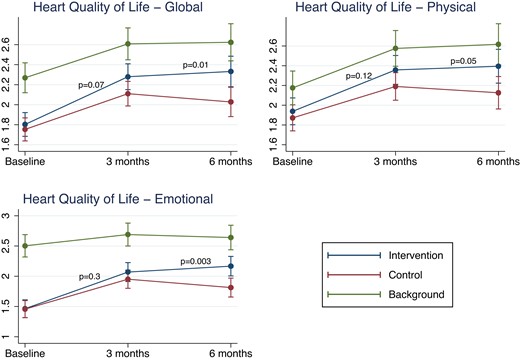
Change in HeartQoL score in the intervention, control and background group. HeartQoL-global score, subscore on HeartQol-physical and subscore on HeartQol-emotional at baseline, and after 3 and 6 months for the three groups. P-values denote comparison of ΔHeartQoL between the intervention and control groups from mixed models adjusted for centre effect.
No significant differences were observed between the intervention and control groups with respect to risk-factor control (HbA1c, BP, LDL, and physical activity) at the 3- and 6-month follow-up (Table 3). We did, however, detect a significant difference in adherence to the physical training sessions and education sessions in favour of the intervention group (P = 0.02). Among the distressed patients, 78% had returned to work at 6 months in the intervention group vs. 67% in the control group (P = 0.11).
| . | Baseline . | 3-Month follow-up . | 6-Month follow-up . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention n = 74 . | Control n = 73 . | P-value . | Intervention n = 66 . | Control n = 71 . | P-value . | Intervention n = 63 . | Control n = 67 . | P-value . | |
| HbA1c < 48 mmol/mol | 52 (76%) | 65 (93%) | 0.007 | 54 (86%) | 65 (92%) | 0.28 | 56 (88%) | 57 (90%) | 0.59 |
| BP < 135/85 mmHg | 42 (61%) | 44 (61%) | 0.98 | 42 (65%) | 47 (67%) | 0.76 | 33 (52%) | 40 (62%) | 0.30 |
| LDL < 1.4 mmol/L | 21 (30%) | 25 (35%) | 0.59 | 27 (42%) | 30 (43%) | 0.82 | 26 (41%) | 30 (46%) | 0.53 |
| BMI <30 kg/m2 | 56 (76%) | 50 (68%) | 0.27 | 48 (72%) | 49 (69%) | 0.75 | 43 (68%) | 47 (70%) | 0.92 |
| Current smoker | 12 (17%) | 13 (18%) | 0.92 | 10 (15%) | 8 (11%) | 0.52 | 7 (11%) | 8 (12%) | 0.83 |
| Physical activity 30 min > 4 days/week | 23 (31%) | 25 (34%) | 0.68 | 34 (46%) | 42 (58%) | 0.16 | 42 (57%) | 43 (59%) | 0.79 |
| Rehabilitation completed (training sessions > 80%) | 51 (77%) | 40 (56%) | 0.010 | ||||||
| . | Baseline . | 3-Month follow-up . | 6-Month follow-up . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention n = 74 . | Control n = 73 . | P-value . | Intervention n = 66 . | Control n = 71 . | P-value . | Intervention n = 63 . | Control n = 67 . | P-value . | |
| HbA1c < 48 mmol/mol | 52 (76%) | 65 (93%) | 0.007 | 54 (86%) | 65 (92%) | 0.28 | 56 (88%) | 57 (90%) | 0.59 |
| BP < 135/85 mmHg | 42 (61%) | 44 (61%) | 0.98 | 42 (65%) | 47 (67%) | 0.76 | 33 (52%) | 40 (62%) | 0.30 |
| LDL < 1.4 mmol/L | 21 (30%) | 25 (35%) | 0.59 | 27 (42%) | 30 (43%) | 0.82 | 26 (41%) | 30 (46%) | 0.53 |
| BMI <30 kg/m2 | 56 (76%) | 50 (68%) | 0.27 | 48 (72%) | 49 (69%) | 0.75 | 43 (68%) | 47 (70%) | 0.92 |
| Current smoker | 12 (17%) | 13 (18%) | 0.92 | 10 (15%) | 8 (11%) | 0.52 | 7 (11%) | 8 (12%) | 0.83 |
| Physical activity 30 min > 4 days/week | 23 (31%) | 25 (34%) | 0.68 | 34 (46%) | 42 (58%) | 0.16 | 42 (57%) | 43 (59%) | 0.79 |
| Rehabilitation completed (training sessions > 80%) | 51 (77%) | 40 (56%) | 0.010 | ||||||
WHO risk > 29.9. P-value denotes Δ in risk factors between the intervention and control groups.
BMI, body mass index; BP, blood pressure; HbA1c, glycated haemoglobin; LDL, low-density lipoprotein.
| . | Baseline . | 3-Month follow-up . | 6-Month follow-up . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention n = 74 . | Control n = 73 . | P-value . | Intervention n = 66 . | Control n = 71 . | P-value . | Intervention n = 63 . | Control n = 67 . | P-value . | |
| HbA1c < 48 mmol/mol | 52 (76%) | 65 (93%) | 0.007 | 54 (86%) | 65 (92%) | 0.28 | 56 (88%) | 57 (90%) | 0.59 |
| BP < 135/85 mmHg | 42 (61%) | 44 (61%) | 0.98 | 42 (65%) | 47 (67%) | 0.76 | 33 (52%) | 40 (62%) | 0.30 |
| LDL < 1.4 mmol/L | 21 (30%) | 25 (35%) | 0.59 | 27 (42%) | 30 (43%) | 0.82 | 26 (41%) | 30 (46%) | 0.53 |
| BMI <30 kg/m2 | 56 (76%) | 50 (68%) | 0.27 | 48 (72%) | 49 (69%) | 0.75 | 43 (68%) | 47 (70%) | 0.92 |
| Current smoker | 12 (17%) | 13 (18%) | 0.92 | 10 (15%) | 8 (11%) | 0.52 | 7 (11%) | 8 (12%) | 0.83 |
| Physical activity 30 min > 4 days/week | 23 (31%) | 25 (34%) | 0.68 | 34 (46%) | 42 (58%) | 0.16 | 42 (57%) | 43 (59%) | 0.79 |
| Rehabilitation completed (training sessions > 80%) | 51 (77%) | 40 (56%) | 0.010 | ||||||
| . | Baseline . | 3-Month follow-up . | 6-Month follow-up . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention n = 74 . | Control n = 73 . | P-value . | Intervention n = 66 . | Control n = 71 . | P-value . | Intervention n = 63 . | Control n = 67 . | P-value . | |
| HbA1c < 48 mmol/mol | 52 (76%) | 65 (93%) | 0.007 | 54 (86%) | 65 (92%) | 0.28 | 56 (88%) | 57 (90%) | 0.59 |
| BP < 135/85 mmHg | 42 (61%) | 44 (61%) | 0.98 | 42 (65%) | 47 (67%) | 0.76 | 33 (52%) | 40 (62%) | 0.30 |
| LDL < 1.4 mmol/L | 21 (30%) | 25 (35%) | 0.59 | 27 (42%) | 30 (43%) | 0.82 | 26 (41%) | 30 (46%) | 0.53 |
| BMI <30 kg/m2 | 56 (76%) | 50 (68%) | 0.27 | 48 (72%) | 49 (69%) | 0.75 | 43 (68%) | 47 (70%) | 0.92 |
| Current smoker | 12 (17%) | 13 (18%) | 0.92 | 10 (15%) | 8 (11%) | 0.52 | 7 (11%) | 8 (12%) | 0.83 |
| Physical activity 30 min > 4 days/week | 23 (31%) | 25 (34%) | 0.68 | 34 (46%) | 42 (58%) | 0.16 | 42 (57%) | 43 (59%) | 0.79 |
| Rehabilitation completed (training sessions > 80%) | 51 (77%) | 40 (56%) | 0.010 | ||||||
WHO risk > 29.9. P-value denotes Δ in risk factors between the intervention and control groups.
BMI, body mass index; BP, blood pressure; HbA1c, glycated haemoglobin; LDL, low-density lipoprotein.
After 12 months, one patient in the intervention group died due to cancer. Sixteen (25%) patients in the intervention group had one or more cardiovascular readmissions vs. 33 (49%) in the control group (Figure 4 and Supplementary material online, Table S6). The hazard ratio for hospital admission for the intervention group vs. control group adjusted for centre was 0.43 (95% confidence interval 0.24–0.80) and the between-group difference was mainly due to higher rate of emergency room contact, hospital admission due to angina and angiography in the control group, whereas no differences were seen for MI or revascularization. There was no treatment– gender interaction. Only five patients in the background group had one or more cardiovascular readmissions. No fatal and two non-fatal MIs were recorded (one in the control and one in the background group). Twenty-two subacute coronary angiographies were performed among all participants after their primary event. The number of revascularisations was equally distributed among the participants: two in the intervention group, two in the control group, and one in the background group.
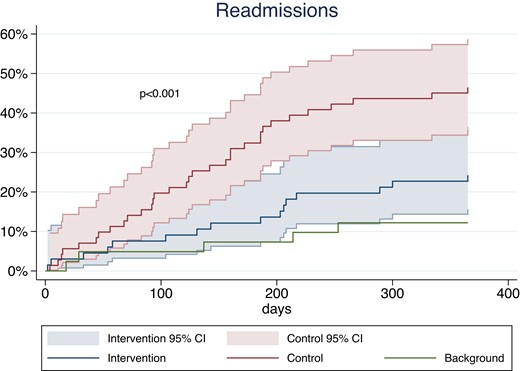
Time-to-event curves for cardiac readmission in the intervention group compared with the control group at the 12-month follow-up change in hospital anxiety and depression scale score points. CI, confidence interval.
Discussion
In this trial, five sessions of group CBT added to CR significantly reduced anxiety and depression compared with CR alone among patients with psychological distress. Improvements in the primary outcome of total HADS score at 3 months as well as subscales on anxiety and depression were maintained at the 6-month follow-up. Corresponding improvements were seen for HeartQoL at 6 months and adherence to the physical training sessions during CR, whereas no differences were recorded in other risk-factor-related secondary outcomes. Furthermore, we found a significant reduction in cardiac readmissions after 12 months in the intervention group compared with the control group (Structured Graphical Abstract).
Anxiety, depression and quality of life
The latest Cochrane review on the effectiveness of psychological interventions14 included 35 RCT trials with a broad range of psychological interventions and concluded that these resulted in small to moderate improvements in depressive symptoms, anxiety and stress; however, considerable uncertainty surrounded these effects due to methodological and statistical heterogeneity. For interventions by CBT a recent meta-analysis of 22 RCTs of nearly 5000 patients with CAD found that CBT significantly improved anxiety, depression and health-related QoL.30 The included trials were highly variable in their mode of delivery; however, most were individual, had a treatment course > 12 weeks, comprised more than ten sessions; and in the majority of the trials, a therapist or psychologist delivered the intervention. Consequently, most of these interventions are not accessible or standardized for implementation to a CR setting. The current trial addresses these barriers to implementation by testing the effect of brief, group CBT delivered by CR nurses and integrated into usual CR. The results support the evidence that CBT is an effective treatment for depression and anxiety in cardiac patients. The most comparable study is a recent multicentre RCT with the participation of 332 cardiac patients in UK.16 This study found similar effects of brief, group-based intervention delivered by CR staff including cardiac nurses and the intervention consisted of six sessions. The intervention was metacognitive therapy, which focuses on the purpose of one’s thinking rather than the content of a given thought. In the present study the CBT method comprised both content and the purpose of a given thought to give patients more options for dealing with distressing thoughts and emotions.20 The two studies have very similar results on the primary outcome of HADS after 3–4 months, with a difference in HADS score of >3 between groups. This is a considerably greater effect than reported in previous studies using individual or group CBT, as reported in the meta-analyses.30
The aim of this trial was to test a simplified delivery of CBT. A widely accessible and more inexpensive form of CBT is internet-based CBT (iCBT). This approach was tested in the U-CARE study (n = 239) with 14 weeks of iCBT vs. usual care, but no significant reduction in anxiety and depression was found.31 Also the above-mentioned meta-analysis included four trials using iCBT, showing mixed results and a higher dropout rate.30 In this trial we had only five sessions, in the study by Wells et al. only six, yet had significant effect of the intervention. Interestingly, in a recent Danish RCT of patients with a newly implanted cardioverter defibrillator, CBT delivered by cardiac nurses with number of sessions individualized to a target HADS score found that the therapy was effective with a median of six sessions (range 2–15).32 In the meta-analyses, duration of treatment course was not predictive of effect.30 Thus, there is consistent data to support that CBT is effective also when delivered as in relatively few group sessions.
We saw a reduction in HADS also in the control group. As also shown by others,33 this indicates that the natural course of psychological distress after a cardiac event is stress alleviation, perhaps reinforced by CR, but that the distressed patients do not spontaneously normalize their level of distress. In contrast, the background group without distress at baseline did not change their level of distress within the first 6 months indicating also that screening for psychological distress at the initiation of CR is necessary and the right time to find those with distress.
Adherence to CR and risk-factor management
Anxiety and depression have been shown to adversely affect risk-factor management and adherence to CR.34,35 We found that the patients in the intervention group were significantly more adherent to the training sessions, but we found no difference between the groups in terms of improvement of cardiovascular risk factors. However, risk-factor control was very good in both groups and left little room for improvement. At 6-month follow-up, most patients had returned to work with no significant difference between the intervention and control groups (78% vs. 67%, P = 0.11). In the non-distressed background group 98% had returned to work. The proportion that returns to work is similar to the EUROASPIRE IV study,36 in which 76% of patients had returned to work within 6 months to 3 years. We have only followed the patient’s working status for 6 months.
Readmission
Previous studies have found a strong association between psychological distress and readmissions due to cardiac events.37 Our study was in size and follow-up period not designed for morbidity and mortality as primary outcomes. However, we did find significantly higher rates of readmission among patients with psychological distress compared with the reference group; and we also found that the intervention group experienced a 57% lower risk of readmissions within the first 12 months after randomization. Interestingly, no differences were observed in rates of MI or revascularization. This may indicate that by targeting and alleviating psychological distress early after an event, many unnecessary readmissions may be avoided. Our findings are consistent with findings from another study reporting that patients with psychological distress are more likely to experience and report chest pain and might have a lower threshold for emergency visits and readmissions.38 A high number of patients with panic attacks were found in another study through routine psychological screening with HADS in the emergency room. And also an association between a certain type of personality (Type D personality) and readmissions which we did not test for.39
In perspective, CBT seems an effective treatment for anxiety and depression in patients with CAD and VHD. The brief, CBT was provided by experienced cardiac nurses at three different centres and with an equal effect at all three centres. The programme is simple, feasible, and may be integrated within existing CR programmes. Whether a similar effect can be achieved in other cardiac patients with psychological distress and whether the effects are maintained are yet to be discovered.
Strengths and limitations
Although the results of the present trial are encouraging, some limitations should be acknowledged. Our study targeted patients with signs of psychological distress at entry but without psychiatric diagnosis or treatment. With this approach the intervention group were more homogenous and expected to benefit from the group session and at the same time ensuring that the cardiac experienced nurses but unexperienced in relation to psychiatric disease could deliver a manageable CBT-based intervention. However, this also means that the results may not apply to patients with more severe psychological distress. Patients were further selected as only patients who were referred to and accepted to participate in CR were included. Patients who do not participate in CR are known to have greater comorbidity and more psychological distress.40 According to a mandatory national clinical registry (Danish Heart Rehabilitation Database41), 32% of eligible CAD patients in 2017 and 46% in 2021 were referred to CR in our capital region covering the participating hospitals of this study. Thus, it is likely that many cardiac patients with psychological distress were not identified, and our results do not apply to these. We did, however, systematically screen all patients referred to CR and among those found eligible, only 16% declined. Thus, we believe that the results would apply to cardiac patients with psychological distress referred to CR. However, patients with VHD constitute a minor part of the study population and although we found no signs of effect modification, results may mainly apply to patients with CAD. The interpretation should also take into account that this study has a limited diversity of race due to a single country trial.
Due to the nature of the intervention, it was not possible to blind patients and CR staff to group allocation. Randomization and statistical outcome analyses were, however, all blinded to reduce the risk of selection, detection, and interpretation bias. We did not measure the negative impact of the focus on psychological distress in the intervention nor did we determine the effectiveness of CBT treatment in relation to a sham intervention. The patients in the intervention group received both CBT and CR, which gives greater number of patient contact hours and this may have contributed to the effect and believer bias from both patients and from the healthcare system. It is well known from psychotherapy that the character of the therapeutic relationship has influence on the treatment effect. Common factors such as empathy, patient expectations, and agreement about goals among other factors influence the outcome.42
The intervention was conducted in accordance with a structured manual for each session, but we did not measure adherence to the manual. To ensure the quality of the intervention, supervision was conducted by a psychologist specializing in the CBT methodology. Finally, the methods of measuring anxiety, depression, and QoL are validated and widely used.26,27,43
In the control group, revascularization by coronary artery bypass grafting was more frequent than in the intervention group. This could have caused more frequent hospitalizations but the causes we found were not related to postoperative complications and therefore we do not expect it to impact results.
The background group of non-distressed patients were consecutively recruited over a shorter period of time compared with the distressed group, and consisted of less females, were older and to a higher degree at work before the event and therefore not completely matched with the distressed group. As women experience CAD almost 10 years later than men44 and psychological distress is less frequent in men45,46 our gender distribution in a consecutive group of younger CAD patients is as expected. Furthermore, lower socioeconomic status including non-employment is associated with a higher degree of distress.5 The background group should therefore not be viewed as a control group but mirrors non-distressed younger CAD patients in every-day clinic.
Conclusion
Brief CBT delivered by cardiac nurses in relation to CR to cardiac patients with psychological distress had a clinically relevant effect on anxiety and depression, improved HeartQoL at 6 months, and adherence to CR and reduced cardiovascular readmissions. The CBT programme is simple, brief and feasible with existing CR programmes.
Supplementary data
Supplementary data are available at European Heart Journal online.
Acknowledgements
The authors are very grateful to the nurses who conducted the inclusion, group sessions, and follow-up visits for the included patients at Hvidovre and Amager Hospital and at the North Zealand Hospital: Suzette Carmohn Späth and Bettina Büllow. They are indebted to psychiatrist Lisbeth Nüchel Petersen for doing the clinical evaluations of patients with a high BDI score.
Funding
This work was supported by ‘TrygFonden’ [grant ID: 124341] and ‘Helsefonden’ [grant ID: 2013-A-0290].
Data availability
Danish legislation does not allow us to share data even if anonymized. Therefore, the data will be made available in aggregated form by the authors upon request.
References
Author notes
Conflict of interest: All authors declare no conflict of interest for this contribution.



