-
PDF
- Split View
-
Views
-
Cite
Cite
Raffaele De Caterina, Stefan Agewall, Felicita Andreotti, Dominick J Angiolillo, Deepak L Bhatt, Robert A Byrne, Jean-Philippe Collet, John Eikelboom, Alexander C Fanaroff, C Michael Gibson, Andreas Goette, Gerhard Hindricks, Gregory Y H Lip, Tatjana Potpara, Holger Thiele, Renato D Lopes, Mattia Galli, Great Debate: Triple antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting should be limited to 1 week, European Heart Journal, Volume 43, Issue 37, 1 October 2022, Pages 3512–3527, https://doi.org/10.1093/eurheartj/ehac294
Close - Share Icon Share
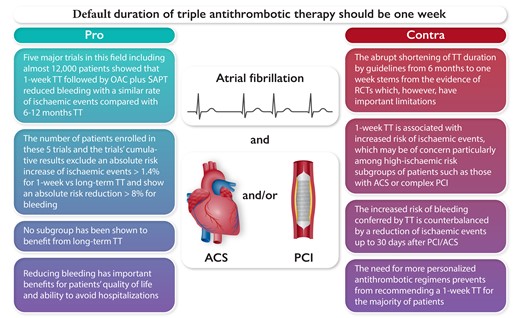
Key points in favour or against the use of one-week triple antithrombotic therapy for the majority of patients with AF and ACS/PCI. Abbreviations: TT, triple antithrombotic therapy; OAC, oral anticoagulant; DAPT, dual antiplatelet therapy; RCT, randomised controlled trials; ACS, acute coronary syndrome; PCI, percutaneous coronary intervention.
Introduction
Corresponding author. Tel.: 050-996-752; E-mail: [email protected]
How to best treat patients with atrial fibrillation (AF) and recent coronary stenting or an acute coronary syndrome (ACS) is challenging. Such condition occurs in about 10% of cases of hospital admission for an ACS and poses a continuing therapeutic dilemma. Stent thrombosis, especially subacute—1 day to 1 month after percutaneous coronary intervention (PCI), and due to stent characteristics, length, diameter, location, and inadequate deployment, as well as to patient-related factors1—is an ominous consequence of stenting. To prevent it, two seminal trials—ISAR2 and STARS3—in patients with recent stenting clearly established dual antiplatelet therapy (DAPT)—with aspirin and a P2Y12 inhibitor (at that time ticlopidine and later clopidogrel)—as a therapeutic option better than oral anticoagulation. On the other hand, stroke prevention in AF rather demands anticoagulation, in earlier times with a vitamin K antagonist (VKA) such as warfarin. In ACTIVE-W, the investigators tried to match the efficacy of warfarin in preventing stroke in AF with the more practical combination of aspirin and clopidogrel, but warfarin was there superior in efficacy and with similar rates of major bleeding.4 Therefore, stenting and AF require two different antithrombotic approaches, and the dilemma comes when the two coexist. For years, cardiologists—mostly concerned with the fear of thrombosis—embraced the dogma of ‘triple therapy’ (TT), simply stacking up aspirin, clopidogrel, and an anticoagulant in such a condition. Initial default duration was set to 1 year, after which time the risk of stent thrombosis, demanding DAPT, was considered to have largely waned out.
Many things have changed, though, in the antithrombotic scenario in more recent years. Among these:
The demonstration that more pharmacodynamically effective P2Y12 inhibitors—prasugrel and ticagrelor—ensure more profound and consistent platelet inhibition than clopidogrel, therefore becoming the default strategy after an ACS.
The introduction of non-VKA oral anticoagulants (NOACs, also called direct oral anticoagulants) associated with less major bleeding (especially intracranial) and 10% lower mortality.5
The appreciation that TT is a threat in terms of bleeding, estimated to be 20–30% per year for clinically relevant bleeding, and around 40% higher than for DAPT.6
The appreciation that bleeding can be as bad as recurrent ischaemic events and actually often causes the actual recurrence of ischaemic events for a variety of reasons, the most relevant probably being the abrupt discontinuation of antithrombotic therapy sometimes occurring even with minor bleeding.7
The introduction of more modern stent platforms for which even a very short (<3 months) DAPT duration followed by P2Y12 inhibition was reported to be the treatment of choice to reduce both major bleeding and myocardial infarction.8
As a consequence, cardiologists started to rethink the dogma, de-escalating the intensity of antithrombotic therapy in the attempt of achieving a better efficacy-to-safety balance. The WOEST trial, in a relatively small number of patients with either an ACS or a chronic coronary syndrome receiving a stent, actually showed lesser mortality by dropping one component of the antithrombotic cocktail—in this case aspirin—compared with the standard TT.7 That study also linked less bleeding with double antithrombotic therapy (DAT, clopidogrel plus a VKA) to lesser mortality, because deaths clustered particularly in patients who had bled.7
The rationale for dropping aspirin instead of clopidogrel is also a matter of current debate. A recent trial in a post-PCI setting without AF has argued for an overall superiority (on a composite primary outcome of hospital readmission due to an ACS, ischaemic and bleeding events) for dropping aspirin instead of clopidogrel,9 but here all-cause death (not a primary endpoint) was numerically higher retaining clopidogrel instead of aspirin, with a similar trend for cardiac and non-cardiac death. The combination of an anticoagulant and a P2Y12 inhibitor, however, has rapidly gained consensus in these patients. Four trials testing a NOAC instead of a VKA10–13 consistently showed a better efficacy-to-safety ratio with such a DAT strategy compared with the classical TT, but this was largely due to the obvious reduction of bleeding with two antithrombotic drugs instead of three. The use of a NOAC instead of a VKA is certainly a plus; as such, the use of DAT with a NOAC and a P2Y12 inhibitor in such patients, usually up to a year after stenting, has then become the ‘new dogma’. The alternative option of temporarily omitting the anticoagulant, using a DAPT with prasugrel or ticagrelor instead of clopidogrel, especially in patients with a low-intermediate risk of stroke,14 has not yet been formally tested.
But if we choose a DAT, renouncing to the reassuring prevention of stent thrombosis with the combination of aspirin and a P2Y12 inhibitor, how soon can we safely enact it? The European Heart Rhythm Association (EHRA) guide back in 201515 recommended an initial default TT for 6 months in ACS patients with AF after PCI, a timing modulated by the weighing of ischaemic and bleeding risks. More recently, the European Society of Cardiology (ESC) Guidelines16 and the EHRA guide17 reduced this time to 1 month and even to 1 week. This latest extreme position estimates that the risk of stent thrombosis with the current generation of drug-eluting stents in most patients wanes out so early that safety considerations prevail in allowing the early drop of aspirin. Several cardiologists, though, feel uncomfortable with such an extreme shortening of TT duration, coming at the price of a higher risk of cardiac, mainly stent-related, ischaemic occurrences.18
All guideline and consensus documents have emphasized the need of tailoring duration of TT to the individual patient, but the issue of where to put the bar for a ‘standard’ duration, the ‘default duration’, is of relevance, as most cardiologists will take this as the reference for most of their patients. Here, therefore, two groups of authors argue one way or the other, defending or opposing the latest recommendations of optimal default 1-week duration of TT after stenting for an ACS with AF. The reader should thus see the pros and cons of the two different positions, as well as the intermediate grey between them in the difficult practice of this ‘precision medicine’. Understanding the background of both opinions will allow a proper handling of the quite diverse situations encountered in such a difficult navigation between the Scylla of thrombosis and the Charybdis of bleeding.
References
Pro
Corresponding author. Tel: (919) 668-8596, Fax: 919-668-7058, E-mail: [email protected]
The default duration of antithrombotic triple therapy (TT) in patients with atrial fibrillation (AF) who undergo percutaneous coronary intervention (PCI) should be between hospital discharge and 1 week because, quite simply, this is what the evidence from randomised controlled trials demonstrates and, accordingly, this is what guidelines and expert consensus documents recommend.1–3
Five randomised controlled trials, enrolling a total of 11,542 patients, assigned patients with AF (or other reason for anticoagulation) who underwent PCI and/or had medically managed acute coronary syndrome to either TT [oral anticoagulant (OAC) + P2Y12 inhibitor + aspirin] or to double antithrombotic therapy (DAT: OAC + P2Y12 inhibitor). In each of these trials, aspirin was stopped in the DAT arm(s) at the time of randomisation. WOEST randomised patients within 4 h after PCI; RE-DUAL, 120 h (mean 1.6 days); PIONEER AF-PCI, 72 h (mean 1.6 days); ENTRUST-AF PCI, 5 days (mean 2.2 days); and AUGUSTUS, 14 days (mean 6.6 days).4–8 Therefore, each of these trials, as designed and conducted, stopped aspirin within 7 days of PCI for most patients in their DAT arms. Directly following the evidence from these trials, therefore, one should favour stopping aspirin within 7 days (if DAT is superior) or continuing aspirin for 6–12 months (if TT is superior). Because, as we argue below, DAT was superior across these five trials, aspirin should be stopped within 7 days of PCI in patients with AF.
In meta-analyses of these trials, DAT was associated with a nearly 50% lower risk of clinically relevant bleeding [hazard ratio (HR 0.56, 95% CI 0.39–0.80)] with no significant increased risk of major adverse cardiovascular events (HR 1.07; 95% CI 0.94–1.22).9 Though absence of evidence for an increased risk of major cardiovascular events in patients treated with DAT does not imply that no increased risk exists—and indeed, it would be surprising if less potent antithrombotic therapy did not increase the risk of ischaemic events—the number of patients enrolled in these five trials and their cumulative results exclude a relative risk difference >20% or an absolute risk difference >1.4% for DAT versus TT.9 By contrast, the absolute risk reduction in clinically relevant bleeding for DAT versus TT is nearly 8% (Figure 1).
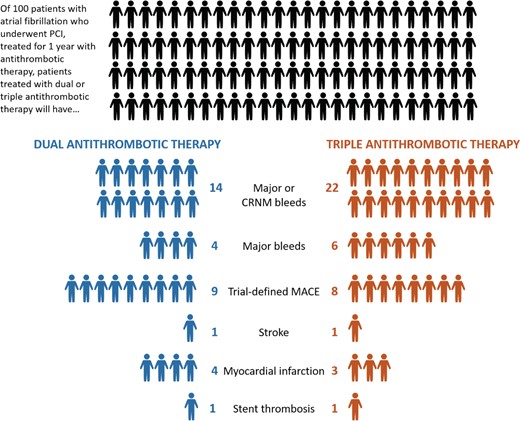
Absolute risks of bleeding and ischaemic endpoints with double and triple antithrombotic therapy in major trials of patients with atrial fibrillation undergoing percutaneous coronary intervention. Across major trials of triple versus double antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention, dual therapy is associated with a 6% absolute reduction in major or clinically relevant non-major bleeding and a 2% reduction in major bleeding compared with triple therapy, with a smaller (1%) absolute increase in trial-defined major adverse cardiovascular events and myocardial infarction. For three of the four trials included in this calculation, triple therapy comprised the combination of dual antiplatelet therapy and vitamin K antagonist, whereas dual therapy comprised a non-vitamin K antagonist oral anticoagulant plus clopidogrel. CRNM, clinically relevant non-major; MACE, major adverse cardiovascular events.
Thus, judging the relative merits of DAT versus TT requires weighing the relative importance of bleeding and ischaemic events, an ever-present dilemma in the interpretation of clinical trials of antithrombotic therapies.10 From a patient-centred standpoint, clinically relevant bleeding (Bleeding Academic Research Consortium [BARC] ≥2) is associated with approximately half the effect on quality of life of a recurrent myocardial infarction (MI); major bleeding (BARC ≥3) is associated with roughly the same decrease in quality of life.11,12 Even bleeding for which patients do not seek medical attention (BARC 1) is associated with meaningfully lower quality of life.12,13 From a prognostic standpoint, major bleeding in the 30 days following elective, urgent, or emergency PCI is associated with a four-fold higher risk of 1-year death, greater than that of recurrent MI within the same time period.14,15 Importantly, in the trials comparing DAT and TT in patients with AF undergoing PCI, bleeding occurred more than twice as often as ischaemic events: across the five major trials, there were 856 major adverse cardiovascular events (death, MI, or stroke) and 2002 major or clinically relevant non-major bleeding events, including 578 major bleeds.4–8 Even within the first 30 days after randomisation, when the risk of stent-related events is highest,16 an analysis of RE-DUAL PCI found that DAT had a net clinical benefit as compared with TT.17
The significant effect of bleeding on quality of life and prognosis, as well as its frequency relative to ischaemic events in patients with AF undergoing PCI, is captured in the effect of DAT as compared with TT on the endpoint of all-cause death or hospitalization. This endpoint levels the playing field between bleeding and ischaemic events, removing the need for weighing the relative severity of events by including all events sufficiently morbid to result in hospitalization or death. In PIONEER AF-PCI, DAT reduced the relative risk of all-cause death or hospitalization by 21% (HR 0.79; 95% CI 0.66–0.94) and the absolute risk of all-cause death or hospitalization by 7% compared with TT.18 Rather than looking broadly at all sufficiently morbid events, one could also look at the effect of DAT versus TT on only the most serious events. When data are pooled across the five trials in this area, there is no effect of antithrombotic treatment strategy on all-cause death.9,19 As small treatment effects on all-cause death can be overwhelmed by patients dying for reasons unrelated to study treatment, one can also look at the most severe treatment-related events in patients with AF and PCI, intracranial haemorrhage and stent thrombosis. Both of these events are rare, occurring in <1% of patients enrolled in the five trials testing antithrombotic therapy in patients with AF undergoing PCI: there were 108 definite or probable stent thrombosis events and 62 intracranial haemorrhages among 11,542 trial participants.4–8 Though stent thrombosis was the more common event (and hence better powered to detect a difference), DAT versus TT had no significant effect on definite, probable, or possible stent thrombosis across the five trials but did reduce intracranial haemorrhage by 66%.9,19 Other meta-analyses have found an association between DAT and a higher risk of definite or probable stent thrombosis,20,21 but, due to the rarity of stent thrombosis, the absolute risk increase is 0.4%, and one would have to treat 274 patients with AF undergoing PCI with TT to prevent one stent thrombosis.22 Among those 274 patients, there would be an additional eight major or clinically relevant non-major bleeding events, including three major bleeding events and one intracranial haemorrhage.22
The totality of the evidence from five randomised controlled trials evaluating DAT versus TT in patients with AF undergoing PCI thus indicates that DAT’s large reduction of bleeding overwhelms any small, hypothetical increased risk of ischaemic events. Moreover, subgroup analyses of these trials have failed to find a group that benefits more from TT versus DAT with respect to ischaemic events, with no modification of treatment effect by indication for PCI (acute versus chronic coronary syndrome), urgency of revascularization, location of the culprit artery, angiographic stenosis >70%, presence of bifurcation, presence of thrombus, type of stent, length of stents, number of stents, baseline DAPT score, PRECISE-DAPT score, CHA2DS2-VASc score, HAS-BLED score, renal function, diabetes, or use of proton pump inhibitor.6,7,5,8,23–31 In one trial-level meta-analysis, TT was associated with a lower risk of MI in the subgroup of patients with acute coronary syndromes (ACS),22 but this has not been shown in all meta-analyses and should be interpreted with caution. These patients made up only a fraction of the patients enrolled in WOEST (27%), PIONEER AF-PCI (29%), RE-DUAL (50%), AUGUSTUS (51%), and ENTRUST-AF PCI (52%), and individual trials were not powered to detect a treatment by subgroup interaction. A patient-level meta-analysis or dedicated clinical trial in patients witih AF and ACS is necessary to confirm any benefit of longer TT in this population, and any benefit would be counterbalanced by an increase in bleeding.
Putting aside, for a moment, the results of these clinical trials, it is worth providing some additional insights on why aspirin can be safely stopped within 7 days after stent implantation if treatment with OAC and a P2Y12 inhibitor is maintained. First, OACs indirectly inhibit platelet activation by reducing the generation of thrombin, which acts via the protease activated receptor-1 receptor to activate platelets.32 Notably, the combination of OAC and single antiplatelet therapy, by blocking both plasma and cellular components of thrombus formation, acts synergistically.33 Indeed, the use of a P2Y12 inhibitor as the single antiplatelet agent is driven by its well-established effects on platelet activation and amplification processes which are key in arterial thrombotic processes.32 It should be noted, however, that the direct thrombin inhibitor dabigatran may not have the same antiplatelet effect as the factor Xa inhibitors.34 Regardless, early stent thrombosis is a thrombin-dependent process,35 which OAC effectively blocks even in patients on single antiplatelet therapy.36
Second, stent thrombosis, the most serious and life-threatening complication of stopping antiplatelet therapy soon after PCI, is uncommon. As noted above, <1% of patients enrolled in the five pivotal trials of antithrombotic strategies in patients with AF undergoing PCI had definite or probable stent thrombosis; this is consistent with findings from a systematic review and meta-analysis of all trials of second-generation drug-eluting stents (DES).37
Third, advances in PCI technology have since reduced the risk of stent thrombosis further. Latest-generation DES—with thinner, streamlined, cobalt-chromium struts that reduce fibrin deposition; fluoropolymers that facilitate adherence of an albumin-rich protein layer on the stent surface; and use of everolimus, biolimus, and zotarolimus, which inhibit smooth muscle proliferation and migration without a significant effect on endothelial cells—have numerous design features that increase biocompatibility and reduce the risk of thrombosis.38 Meta-analyses comparing newer DES with older technology have shown >50% lower stent thrombosis.39 An autopsy study of 204 DES-treated lesions showed that 40% of second-generation DES had <30% uncovered stent struts within 3 months of implantation, which is comparable to the proportion of first-generation DES that met these criteria more than 9 months after implantation.40 In optical coherence tomography studies enrolling patients undergoing PCI with newer DES, 70% of stent struts were covered within 2 weeks of PCI and up to 93% were covered within 1 month.41–43 These patients were not treated with OAC, and the effect of anticoagulation on endothelialization, which is a fibrin-dependent process, is unclear. Furthermore, enhanced stent deliverability, development of more supportive guide catheters and coronary wires, improvements in atherectomy devices, and the increased use of stent optimization through intravascular imaging and coronary physiology may also contribute to lower rates of stent thrombosis by increasing the proportion of PCIs with optimal results, long known to be protective against stent thrombosis.44–46
These improvements in stent technology and delivery have changed the risk-benefit calculation with respect to bleeding and stent thrombosis following PCI. In patients without AF undergoing PCI, a number of studies have evaluated increasingly shorter durations of DAPT. The STOPDAPT-2 study, which enrolled 3045 patients who underwent PCI in Japan, compared 1 month of DAPT with aspirin and clopidogrel followed by clopidogrel monotherapy to 12 months of DAPT, and found no significant difference in cardiovascular events (including stent thrombosis) with only 1 month of aspirin, but an absolute risk reduction of 1% in severe (BARC ≥3) bleeding.47 MASTER DAPT, which enrolled 4434 patients who underwent PCI at sites around the world, compared 1 month of DAPT with aspirin and clopidogrel followed by aspirin monotherapy to ≥3 months of DAPT and found no significant difference in cardiovascular events in the 1-month DAPT arm, but a lower risk of bleeding.48,49 Other studies, which stopped aspirin between 1 and 3 months following PCI, have had similar results.50 Mechanistic studies showing lower risk of stent thrombosis with new generation DESs implanted using modern techniques, and randomised clinical trials showing the safety and efficacy of single antiplatelet therapy as soon as 1 month after stent implantation, form the background against which to evaluate the results of the five major trials of double versus triple antithrombotic therapy. Against this background, the safety of an approach combining single antiplatelet therapy and OAC by 1 week after PCI is easy to understand.
Importantly, in PIONEER AF-PCI, ENTRUST-AF PCI, and RE-DUAL, the TT groups were VKA-based, and the DAT arms were NOAC-based. Therefore, only two of the five major trials of antithrombotic therapy following PCI in patients with AF can be used to discriminate the direct effect of aspirin withdrawal on bleeding complications—AUGUSTUS and WOEST. Furthermore, there are no prospective randomised controlled trials comparing 7 and 30 days of aspirin following PCI in patients with AF. In AUGUSTUS, which by virtue of its 2 × 2 factorial design allowed direct comparison between aspirin and placebo, a landmark analysis showed that there were as many excess ischaemic events in the placebo arm as excess bleeding events in the aspirin arm over the first 30 days,51 leading some to suggest that aspirin should be continued for 30 days after PCI. Though this strategy was not tested in AUGUSTUS, in the absence of a direct comparison between 7 and 30 days of TT, it is difficult to make conclusive statements for routine clinical practice.
Nevertheless, when determining how long aspirin should be continued following PCI in patients with AF, one needs only to follow the evidence from the pivotal trials in this population as they were conducted, supported by clinical and preclinical evidence of the low thrombogenicity of modern DES. To argue for longer duration of aspirin therapy requires an over-interpretation of landmark analyses, overestimation of the frequency of stent thrombosis, and under-estimation of the patient-centred and prognostic importance of bleeding events. Five clinical trials of double versus triple antithrombotic therapy in patients with AF undergoing PCI for stable coronary artery disease or ACS stopped aspirin within 7 days of PCI, and the totality of evidence from these trials shows that the net benefits of dropping aspirin outweigh any costs. The sweet spot for prevention of cardiac events and bleeding in this population is stopping aspirin within 7 days after PCI.
Conflict of interest: R.D.L. reports grants and personal fees from Bristol-Myers Squibb, Pfizer, GlaxoSmithKline, Medtronic PLC, and Sanofi; and personal fees from Amgen, Bayer, and Boehringer Ingelheim. D.J.A. has reports receiving payments as an individual for: (i) Consulting fee or honorarium from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, and Sanofi; (ii) Institutional payments for grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli-Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions and the Scott R. MacKenzie Foundation. D.L.B. discloses the following relationships - Advisory Board: Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Novartis, Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Lexicon, Lilly, Medtronic, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Philips, Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, Takeda. J.P.-L. reports research Grants to the Institution or Consulting/Lecture Fees from Abbott, AstraZeneca, Boston Scientific, Bristol-Myers Squibb, Medtronic, Pfizer. J.E. reports grants and personal fees from AZ, Bayer, BI, BMS, DSI, Janssen, Merck, Pfizer, Portola, Sanofi, Servier. A.F. receives grant funding from the American Heart Association and National Institutes of Health, and personal fees from Intercept Pharmaceuticals. M.G. reports research support from Johnson & Johnson. He receives consulting support from Astra-Zenca, Johnson & Johnson, and Janssen & Bayer. H.T. reports no conflicts of interest relevant to this manuscript.
References
Contra
Corresponding author. Tel: +39-06-3015-4275, Fax: +39-06-3015-5846, E-mail: [email protected]
The Roman poet Horace, about 2040 years ago, described ‘virtus’ (virtue) as equidistant from two extremes: ‘virtus est medium vitiorum et utrimque reductum’ (virtue lies between two defects, keeping away from both one and the other).1 In medicine, this statement is often true, as many decisions rely on the balance between treatment risks and benefits. The shortening of triple antithrombotic therapy (TT) from 6 months to 1 week as the recommended default strategy for atrial fibrillation (AF) patients with acute coronary syndrome (ACS) or undergoing percutaneous coronary intervention (PCI) represents a remarkable paradigm shift in antithrombotic therapy of the past decade (Figure 1).2–4 This recent change in recommendations was driven by the results of five randomised controlled trials (RCTs) comparing different oral anticoagulants (OACs) in combination with a P2Y12 inhibitor (mainly clopidogrel)—the so-called double antithrombotic therapy (DAT)—versus prolonged TT mainly consisting of aspirin, clopidogrel, and a vitamin K antagonist (VKA).5–9 Because non-VKA oral anticoagulants (NOACs) are currently considered the standard of care for patients with AF, the four more recent RCTs comparing NOAC-based DAT with either dabigatran, rivaroxaban, apixaban, or edoxaban after a short course TT (on average 4 days) versus prolonged TT (on average 4.7 months) are of particular clinical interest (Figure 1).5–8 We believe a careful appraisal of these four trials’ characteristics and limitations is necessary for a balanced interpretation of their results and for a reasonable application to routine clinical practice. Key issues for consideration are presented below.
Primary endpoint definition. The main concern when de-escalating or shortening antithrombotic therapy is the possible occurrence of ischaemic events (reduced efficacy), while a reduction of bleeding (increased safety) is to be expected. Major RCTs testing such de-escalation or shortening strategies among PCI patients have therefore included not only bleeding but also ischaemic outcomes in their primary endpoint, enrolling sufficiently large samples to address at a minimum their non-inferiority compared with usual care with respect to composite ischaemic endpoints or ‘net adverse clinical events’ (NACEs) (Table 1). In contrast, the four AF-PCI/ACS trials comparing NOAC-based DAT versus TT considered only bleeding as primary endpoint and were underpowered to compare ischaemic events. Therefore, while individually powered to test for differences in bleeding, all four RCTs fall short in reassuring against a possible increase of ischaemic events when TT is considerably shortened.5–8
Prolonged VKA-based TT in the comparator arm. With the exception of AUGUSTUS’ factorial design, that randomised patients scheduled to receive a P2Y12 inhibitor first to NOAC or VKA and then, each of these, to aspirin or placebo (resulting in both NOAC-based TT and VKA-based DAT), the other trials compared NOAC-based DAT versus VKA-based TT.5–8 Arguably, the use of a VKA compared to a NOAC is in itself a reason for increased bleeding risk.10 In addition, TT lasting on average 4.7 months is no longer a strategy widely used in current clinical practice (i.e. 1-month TT with a NOAC).2,7 Thus, these trials do not answer the clinical question of whether NOAC-based DAT after 1-week TT is beneficial compared to real-world 1-month NOAC-based TT, but rather overestimate the safety of early DAT by comparing it to a very long and old fashioned TT (Figure 2). It can be argued that the above trials have proven the obvious: namely, that DAT following very short TT using a safer OAC reduces bleeding events as compared to prolonged TT using a less safe OAC.
Inadequate characterization of procedural details. Complex PCI (such as stenting a left main stem, bifurcations or chronic total occlusions, as well as high stent number and length) is associated with an increased risk or more significant impact of adverse events that is in part mitigated by more intense antithrombotic regimens.11,12 Thus, procedural details are considered extremely relevant in studies that test antithrombotic regimens in patients undergoing PCI. The RCTs of AF-PCI/ACS patients lack such information (Table 1).5–8 A subgroup analysis of the REDUAL PCI trial showed that only 9.9% of included patients had high-risk procedural factors and only 10% had both high-risk procedural and clinical complexity factors.13 Although high-risk procedural features were not explicit exclusion criteria, the dearth of procedural details and the absence of statistically powered outcome stratification by PCI complexity prevent generalization of the four RCT results to PCI patients with high ischaemic risk PCI, which may represent up to 60% of cases according to a recent study.12
Sub-analyses and specific high-risk subgroups. Sub-analyses of the four RCTs have been published in the attempt to address some of the above unsolved issues. These analyses should be considered at best hypothesis-generating since they are burdened with the limitations of the original trials, are often not prespecified, and are affected by pronounced statistical underpower.13–15 Importantly, in the presence of low statistical power, raw data rather than P-values are more informative. Thus, a recurring trend in various studies using different NOACs should raise concern.15 Indeed, with the exception of dabigatran 150 mg bid, AF-ACS versus AF-chronic coronary syndrome (CCS) patients showed an alarming numerical trend towards increased ischaemic events with all NOAC-based DAT regimens versus prolonged TT, a trend also observed with dabigatran 110 mg bid-based DAT in the subgroup of patients with high-risk procedural and clinical complexity factors compared to those without these factors.13,16–18
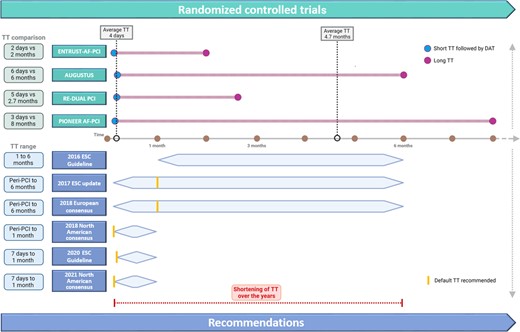
Randomised controlled trials and changes in the recommendation on the use of antithrombotic therapy among patients with atrial fibrillation and acute coronary syndrome or undergoing percutaneous coronary intervention. Upper section: The four RCTs compared very short NOAC-based TT(lasting on average 4 days) versus prolonged VKA-based TT regimen (lasting on average 4.7 months). Lower section: European consensuses and guidelines recommended TT duration up to 6 months before 2018 and up to 1 month after 2018. Specifically, the 2016 ESC Guideline recommended 1 to 6 month TT after PCI among ACS patients according to bleeding risk and 1-month TT among CCS patients; the 2017 ESC dual antiplatelet therapy update and the 2018 Joint European consensus recommended 1 month TT as default strategy peri-PCI and up to 6 months TT according to bleeding and ischaemic risks; the 2018 and 2021 North American consensuses recommended peri-PCI TT as default strategy and up to 1 month TT according to bleeding and ischaemic risks; the 2020 ESC Guideline recommended 1-week TT as default strategy and up to 1 month TT according to bleeding and ischaemic risks. Abbreviations: TT, triple therapy; DAT, double antithrombotic therapy; PCI, percutaneous coronary intervention; ESC, European Society of Cardiology; RCT, randomised controlled trial; NOAC, non-vitamin K antagonist; VKA, vitamin k antagonist; ACS, acute coronary syndrome; CCS, chronic coronary syndrome.
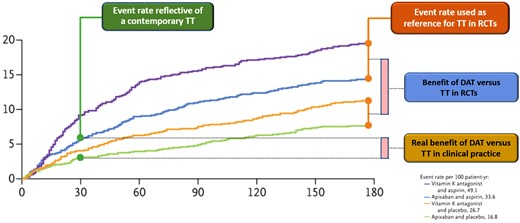
Overestimation of the safety of double versus triple antithromotic therapy in randomised controlled trials as opposed to the real-world setting. Kaplan–Meier curves from the AUGUSTUS trial for the primary outcome of major or clinically relevant non-major bleeding according to DAT or TT with either NOAC or VKA. In this study, bleeding event rates with DAT comprising either NOAC or VKA (orange and green lines) were compared to those with 6-month TT comprising either NOAC or VKA (violet and blue lines and orange box). Such a comparison is not reflective of contemporary clinical practice, in which NOAC-based DAT (green line) or 1-month NOAC-based TT (green box) are employed. As the AUGUSTUS curves diverge more sharply 30 days after randomisation and the trial included both NOAC and VKA regimens, the benefit shown by the trial (blue box) is far beyond that expected in current clinical practice (yellow box). Modified from Lopes et al.7 Abbreviations: DAT, double antithrombotic therapy; TT, triple therapy; RCT, randomised controlled trial; NOAC, non-vitamin k antagonist anticoagulant; VKA, vitamin k antagonist.
Key recent randomised controlled trials on antithrombotic strategies to reduce bleeding among patients undergoing percutaneous coronary intervention
| Study . | Publication year . | Number of patients enrolled . | Clinical presentation (%) . | Treatment arms . | Time from PCI to “de-escalation” . | Primary endpoint . | Procedural details . | Follow-up (months) . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ACS . | CCS . | . | . | . | . | . |
| Double versus triple antithrombotic therapy in atrial fibrillation patients undergoing PCI | |||||||||
| PIONEER AF-PCI | 2016 | 1389 | 40 | 60 | Dual (clopidogrel + rivaroxaban 15 mg) vs 8 months triple (aspirin + clopidogrel + VKA) therapy | 3 days | SAFETY (TIMI major or minor bleeding or bleeding requiring medical attention) | Not reported | 12 |
| RE-DUAL PCI | 2017 | 2725 | 50 | 50 | Dual (clopidogrel + dabigatran 110 mg bid or 150 mg bid) vs 2.7 months triple (aspirin + clopidogrel + VKA) therapy | 5 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 14 |
| AUGUSTUSa | 2019 | 4614 | 38 | 62 | Dual (clopidogrel + apixaban 5 mg bid/VKA) vs 6 months triple (aspirin + clopidogrel+ apixaban 5 mg bid/VKA) therapy | 6 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 6 |
| ENTRUST-AF-PCI | 2019 | 1506 | 52 | 48 | Dual (clopidogrel + edoxaban 60 mg) vs 2 months triple (aspirin + clopidogrel + VKA) therapy | 2 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 12 |
| Aspirin-free versus standard DAPT | |||||||||
| GLOBAL-LEADERS | 2018 | 15 968 | 47 | 53 | Ticagrelor monotherapy for 23 months vs DAPT with ticagrelor (ACS) or clopidogrel (CCS) for 12 months | 1 month | EFFICACY (all-cause death or MI) | Reported | 24 |
| TWILIGHT | 2019 | 7119 | 64 | 36 | Ticagrelor monotherapy vs standard DAPT in uneventful patients | 3 months | EFFICACY AND SAFETY (BARC bleeding type 2, 3, or 5 and all-cause death or MI or stroke) | Reported | 15 |
| SMART-CHOICE | 2019 | 2993 | 58 | 42 | P2Y12 inhibitor monotherapy vs standard DAPT | 3 months | EFFICACY (all-cause death, MI or stroke) | Reported | 12 |
| STOPDAPT-2 | 2019 | 3045 | 38 | 62 | Clopidogrel monotherapy vs standard DAPT | 1 month | EFFICACY AND SAFETY (CV death, MI, stroke, definite stent thrombosis, or TIMI major or minor bleeding) | Reported | 12 |
| TICO | 2020 | 3056 | 100 | 0 | Ticagrelor monotherapy vs standard DAPT | 3 months | EFFICACY AND SAFETY (TIMI major bleeding, all-cause death, MI, stent thrombosis, stroke, or target-vessel revascularization) | Reported | 12 |
| STOPDAPT-2-ACS | 2022 | 4169 | 100 | 0 | Clopidogrel monotherapy vs standard DAPT | 1–2 months | EFFICACY AND SAFETY (CV death, MI, stroke, ST and TIMI major or minor bleeding) | Reported | 12 |
| Guided de-escalation versus standard DAPT | |||||||||
| ANTARCTIC | 2016 | 877 | 100 | 0 | PFT guided de-escalation vs standard DAPT | 14 days | EFFICACY AND SAFETY (CV death, MI, stroke, stent thrombosis, urgent revascularization or BARC 2–5 bleeding) | Reported | 12 |
| TROPICAL-ACS | 2017 | 2610 | 100 | 0 | PFT guided de-escalation vs standard DAPT | 14 days | EFFICACY AND SAFETY (CV death, MI, stroke or BARC 2–5 bleeding) | Reported | 12 |
| POPular Genetics | 2019 | 2488 | 100 | 0 | Genotype guided de-escalation vs standard DAPT | 48 hours | EFFICACY AND SAFETY (all-cause death, MI, definite stent thrombosis, stroke, or PLATO major bleeding and PLATO major or minor bleeding) | Reported | 12 |
| Unguided de-escalation versus standard therapy | |||||||||
| TOPIC | 2017 | 646 | 100 | 0 | Clopidogrel-based DAPT vs standard DAPT | 1 month | EFFICACY AND SAFETY (CV death, urgent revascularization, stroke and BARC 2–5 bleeding) | Reported | 12 |
| HOST-REDUCE POLYTECH-ACS | 2020 | 3429 | 100 | 0 | Prasugrel 5 mg-based DAPT vs prasugrel 10 mg-based DAPT | 1 month | EFFICACY AND SAFETY (all-cause death, MI, stent thrombosis, repeat revascularization, stroke, and BARC 2–5 bleeding) | Reported | 12 |
| TALOS-MI | 2021 | 2697 | 100 | 0 | Clopidogrel-based DAPT vs ticagrelor-based DAPT | 1 month | EFFICACY AND SAFETY (CV-death, MI, stroke, BARC 2–5 bleeding) | Reported | 12 |
| Very short versus standard DAPT | |||||||||
| REDUCE | 2019 | 1496 | 100 | 0 | 3 versus 12 months DAPT | 3 months | EFFICACY AND SAFETY (all-cause death, MI, stent thrombosis, stroke, target vessel revascularization and BARC 2–5 bleeding) | Reported | 12 |
| One-month DAPT | 2021 | 3020 | 39 | 61 | 1 versus 6–12 months DAPT in non-complex PCI | 1 month | EFFICACY AND SAFETY (CV death, MI, target vessel revascularization, stroke and major bleeding) | Reported | 12 |
| MASTER-DAPT | 2021 | 4434 | 49 | 51 | 1 versus 5 months DAPT among HBR patients | 1 month | EFFICACY AND SAFETY (all-cause death, MI, stroke, or major bleeding and all-cause death, MI or stroke and major or CRNM bleeding) | Reported | 11 |
| Study . | Publication year . | Number of patients enrolled . | Clinical presentation (%) . | Treatment arms . | Time from PCI to “de-escalation” . | Primary endpoint . | Procedural details . | Follow-up (months) . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ACS . | CCS . | . | . | . | . | . |
| Double versus triple antithrombotic therapy in atrial fibrillation patients undergoing PCI | |||||||||
| PIONEER AF-PCI | 2016 | 1389 | 40 | 60 | Dual (clopidogrel + rivaroxaban 15 mg) vs 8 months triple (aspirin + clopidogrel + VKA) therapy | 3 days | SAFETY (TIMI major or minor bleeding or bleeding requiring medical attention) | Not reported | 12 |
| RE-DUAL PCI | 2017 | 2725 | 50 | 50 | Dual (clopidogrel + dabigatran 110 mg bid or 150 mg bid) vs 2.7 months triple (aspirin + clopidogrel + VKA) therapy | 5 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 14 |
| AUGUSTUSa | 2019 | 4614 | 38 | 62 | Dual (clopidogrel + apixaban 5 mg bid/VKA) vs 6 months triple (aspirin + clopidogrel+ apixaban 5 mg bid/VKA) therapy | 6 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 6 |
| ENTRUST-AF-PCI | 2019 | 1506 | 52 | 48 | Dual (clopidogrel + edoxaban 60 mg) vs 2 months triple (aspirin + clopidogrel + VKA) therapy | 2 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 12 |
| Aspirin-free versus standard DAPT | |||||||||
| GLOBAL-LEADERS | 2018 | 15 968 | 47 | 53 | Ticagrelor monotherapy for 23 months vs DAPT with ticagrelor (ACS) or clopidogrel (CCS) for 12 months | 1 month | EFFICACY (all-cause death or MI) | Reported | 24 |
| TWILIGHT | 2019 | 7119 | 64 | 36 | Ticagrelor monotherapy vs standard DAPT in uneventful patients | 3 months | EFFICACY AND SAFETY (BARC bleeding type 2, 3, or 5 and all-cause death or MI or stroke) | Reported | 15 |
| SMART-CHOICE | 2019 | 2993 | 58 | 42 | P2Y12 inhibitor monotherapy vs standard DAPT | 3 months | EFFICACY (all-cause death, MI or stroke) | Reported | 12 |
| STOPDAPT-2 | 2019 | 3045 | 38 | 62 | Clopidogrel monotherapy vs standard DAPT | 1 month | EFFICACY AND SAFETY (CV death, MI, stroke, definite stent thrombosis, or TIMI major or minor bleeding) | Reported | 12 |
| TICO | 2020 | 3056 | 100 | 0 | Ticagrelor monotherapy vs standard DAPT | 3 months | EFFICACY AND SAFETY (TIMI major bleeding, all-cause death, MI, stent thrombosis, stroke, or target-vessel revascularization) | Reported | 12 |
| STOPDAPT-2-ACS | 2022 | 4169 | 100 | 0 | Clopidogrel monotherapy vs standard DAPT | 1–2 months | EFFICACY AND SAFETY (CV death, MI, stroke, ST and TIMI major or minor bleeding) | Reported | 12 |
| Guided de-escalation versus standard DAPT | |||||||||
| ANTARCTIC | 2016 | 877 | 100 | 0 | PFT guided de-escalation vs standard DAPT | 14 days | EFFICACY AND SAFETY (CV death, MI, stroke, stent thrombosis, urgent revascularization or BARC 2–5 bleeding) | Reported | 12 |
| TROPICAL-ACS | 2017 | 2610 | 100 | 0 | PFT guided de-escalation vs standard DAPT | 14 days | EFFICACY AND SAFETY (CV death, MI, stroke or BARC 2–5 bleeding) | Reported | 12 |
| POPular Genetics | 2019 | 2488 | 100 | 0 | Genotype guided de-escalation vs standard DAPT | 48 hours | EFFICACY AND SAFETY (all-cause death, MI, definite stent thrombosis, stroke, or PLATO major bleeding and PLATO major or minor bleeding) | Reported | 12 |
| Unguided de-escalation versus standard therapy | |||||||||
| TOPIC | 2017 | 646 | 100 | 0 | Clopidogrel-based DAPT vs standard DAPT | 1 month | EFFICACY AND SAFETY (CV death, urgent revascularization, stroke and BARC 2–5 bleeding) | Reported | 12 |
| HOST-REDUCE POLYTECH-ACS | 2020 | 3429 | 100 | 0 | Prasugrel 5 mg-based DAPT vs prasugrel 10 mg-based DAPT | 1 month | EFFICACY AND SAFETY (all-cause death, MI, stent thrombosis, repeat revascularization, stroke, and BARC 2–5 bleeding) | Reported | 12 |
| TALOS-MI | 2021 | 2697 | 100 | 0 | Clopidogrel-based DAPT vs ticagrelor-based DAPT | 1 month | EFFICACY AND SAFETY (CV-death, MI, stroke, BARC 2–5 bleeding) | Reported | 12 |
| Very short versus standard DAPT | |||||||||
| REDUCE | 2019 | 1496 | 100 | 0 | 3 versus 12 months DAPT | 3 months | EFFICACY AND SAFETY (all-cause death, MI, stent thrombosis, stroke, target vessel revascularization and BARC 2–5 bleeding) | Reported | 12 |
| One-month DAPT | 2021 | 3020 | 39 | 61 | 1 versus 6–12 months DAPT in non-complex PCI | 1 month | EFFICACY AND SAFETY (CV death, MI, target vessel revascularization, stroke and major bleeding) | Reported | 12 |
| MASTER-DAPT | 2021 | 4434 | 49 | 51 | 1 versus 5 months DAPT among HBR patients | 1 month | EFFICACY AND SAFETY (all-cause death, MI, stroke, or major bleeding and all-cause death, MI or stroke and major or CRNM bleeding) | Reported | 11 |
Trials comparing double versus triple antithrombotic therapy among patients with atrial fibrillation and concomitant ACS or undergoing PCI present several distinctive features compared to trials testing de-escalated or shortened antiplatelet regimens among patients undergoing PCI. Namely, primary endpoint focused on safety, procedural details were not reported, ‘de-escalation’ of antiplatelet antiplatelet therapy started very early after PCI, and none focused on ACS patients. Bold type: features of randomised controlled trials limiting the generalization of their results to the totality of patients with ACS or undergoing PCI.
AUGUSTUS included a medically treated AF-ACS patient subgroup.
ACS, acute coronary syndrome; BARC, Bleeding Academic Research Consortium; CCS, chronic coronary syndrome; CV, cardiovascular; CRNM, clinically relevant non-major; DAPT, dual antiplatelet therapy; HBR, high bleeding risk; ISTH, International Society on Thrombosis and Haemostasis; MI, myocardial infarction; PFT, platelet function test; PLATO, Platelet Inhibition and Patient Outcomes; TIMI, Thrombolysis in Myocardial Infarction; VKA, vitamin K antagonists.
Key recent randomised controlled trials on antithrombotic strategies to reduce bleeding among patients undergoing percutaneous coronary intervention
| Study . | Publication year . | Number of patients enrolled . | Clinical presentation (%) . | Treatment arms . | Time from PCI to “de-escalation” . | Primary endpoint . | Procedural details . | Follow-up (months) . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ACS . | CCS . | . | . | . | . | . |
| Double versus triple antithrombotic therapy in atrial fibrillation patients undergoing PCI | |||||||||
| PIONEER AF-PCI | 2016 | 1389 | 40 | 60 | Dual (clopidogrel + rivaroxaban 15 mg) vs 8 months triple (aspirin + clopidogrel + VKA) therapy | 3 days | SAFETY (TIMI major or minor bleeding or bleeding requiring medical attention) | Not reported | 12 |
| RE-DUAL PCI | 2017 | 2725 | 50 | 50 | Dual (clopidogrel + dabigatran 110 mg bid or 150 mg bid) vs 2.7 months triple (aspirin + clopidogrel + VKA) therapy | 5 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 14 |
| AUGUSTUSa | 2019 | 4614 | 38 | 62 | Dual (clopidogrel + apixaban 5 mg bid/VKA) vs 6 months triple (aspirin + clopidogrel+ apixaban 5 mg bid/VKA) therapy | 6 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 6 |
| ENTRUST-AF-PCI | 2019 | 1506 | 52 | 48 | Dual (clopidogrel + edoxaban 60 mg) vs 2 months triple (aspirin + clopidogrel + VKA) therapy | 2 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 12 |
| Aspirin-free versus standard DAPT | |||||||||
| GLOBAL-LEADERS | 2018 | 15 968 | 47 | 53 | Ticagrelor monotherapy for 23 months vs DAPT with ticagrelor (ACS) or clopidogrel (CCS) for 12 months | 1 month | EFFICACY (all-cause death or MI) | Reported | 24 |
| TWILIGHT | 2019 | 7119 | 64 | 36 | Ticagrelor monotherapy vs standard DAPT in uneventful patients | 3 months | EFFICACY AND SAFETY (BARC bleeding type 2, 3, or 5 and all-cause death or MI or stroke) | Reported | 15 |
| SMART-CHOICE | 2019 | 2993 | 58 | 42 | P2Y12 inhibitor monotherapy vs standard DAPT | 3 months | EFFICACY (all-cause death, MI or stroke) | Reported | 12 |
| STOPDAPT-2 | 2019 | 3045 | 38 | 62 | Clopidogrel monotherapy vs standard DAPT | 1 month | EFFICACY AND SAFETY (CV death, MI, stroke, definite stent thrombosis, or TIMI major or minor bleeding) | Reported | 12 |
| TICO | 2020 | 3056 | 100 | 0 | Ticagrelor monotherapy vs standard DAPT | 3 months | EFFICACY AND SAFETY (TIMI major bleeding, all-cause death, MI, stent thrombosis, stroke, or target-vessel revascularization) | Reported | 12 |
| STOPDAPT-2-ACS | 2022 | 4169 | 100 | 0 | Clopidogrel monotherapy vs standard DAPT | 1–2 months | EFFICACY AND SAFETY (CV death, MI, stroke, ST and TIMI major or minor bleeding) | Reported | 12 |
| Guided de-escalation versus standard DAPT | |||||||||
| ANTARCTIC | 2016 | 877 | 100 | 0 | PFT guided de-escalation vs standard DAPT | 14 days | EFFICACY AND SAFETY (CV death, MI, stroke, stent thrombosis, urgent revascularization or BARC 2–5 bleeding) | Reported | 12 |
| TROPICAL-ACS | 2017 | 2610 | 100 | 0 | PFT guided de-escalation vs standard DAPT | 14 days | EFFICACY AND SAFETY (CV death, MI, stroke or BARC 2–5 bleeding) | Reported | 12 |
| POPular Genetics | 2019 | 2488 | 100 | 0 | Genotype guided de-escalation vs standard DAPT | 48 hours | EFFICACY AND SAFETY (all-cause death, MI, definite stent thrombosis, stroke, or PLATO major bleeding and PLATO major or minor bleeding) | Reported | 12 |
| Unguided de-escalation versus standard therapy | |||||||||
| TOPIC | 2017 | 646 | 100 | 0 | Clopidogrel-based DAPT vs standard DAPT | 1 month | EFFICACY AND SAFETY (CV death, urgent revascularization, stroke and BARC 2–5 bleeding) | Reported | 12 |
| HOST-REDUCE POLYTECH-ACS | 2020 | 3429 | 100 | 0 | Prasugrel 5 mg-based DAPT vs prasugrel 10 mg-based DAPT | 1 month | EFFICACY AND SAFETY (all-cause death, MI, stent thrombosis, repeat revascularization, stroke, and BARC 2–5 bleeding) | Reported | 12 |
| TALOS-MI | 2021 | 2697 | 100 | 0 | Clopidogrel-based DAPT vs ticagrelor-based DAPT | 1 month | EFFICACY AND SAFETY (CV-death, MI, stroke, BARC 2–5 bleeding) | Reported | 12 |
| Very short versus standard DAPT | |||||||||
| REDUCE | 2019 | 1496 | 100 | 0 | 3 versus 12 months DAPT | 3 months | EFFICACY AND SAFETY (all-cause death, MI, stent thrombosis, stroke, target vessel revascularization and BARC 2–5 bleeding) | Reported | 12 |
| One-month DAPT | 2021 | 3020 | 39 | 61 | 1 versus 6–12 months DAPT in non-complex PCI | 1 month | EFFICACY AND SAFETY (CV death, MI, target vessel revascularization, stroke and major bleeding) | Reported | 12 |
| MASTER-DAPT | 2021 | 4434 | 49 | 51 | 1 versus 5 months DAPT among HBR patients | 1 month | EFFICACY AND SAFETY (all-cause death, MI, stroke, or major bleeding and all-cause death, MI or stroke and major or CRNM bleeding) | Reported | 11 |
| Study . | Publication year . | Number of patients enrolled . | Clinical presentation (%) . | Treatment arms . | Time from PCI to “de-escalation” . | Primary endpoint . | Procedural details . | Follow-up (months) . | |
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | ACS . | CCS . | . | . | . | . | . |
| Double versus triple antithrombotic therapy in atrial fibrillation patients undergoing PCI | |||||||||
| PIONEER AF-PCI | 2016 | 1389 | 40 | 60 | Dual (clopidogrel + rivaroxaban 15 mg) vs 8 months triple (aspirin + clopidogrel + VKA) therapy | 3 days | SAFETY (TIMI major or minor bleeding or bleeding requiring medical attention) | Not reported | 12 |
| RE-DUAL PCI | 2017 | 2725 | 50 | 50 | Dual (clopidogrel + dabigatran 110 mg bid or 150 mg bid) vs 2.7 months triple (aspirin + clopidogrel + VKA) therapy | 5 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 14 |
| AUGUSTUSa | 2019 | 4614 | 38 | 62 | Dual (clopidogrel + apixaban 5 mg bid/VKA) vs 6 months triple (aspirin + clopidogrel+ apixaban 5 mg bid/VKA) therapy | 6 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 6 |
| ENTRUST-AF-PCI | 2019 | 1506 | 52 | 48 | Dual (clopidogrel + edoxaban 60 mg) vs 2 months triple (aspirin + clopidogrel + VKA) therapy | 2 days | SAFETY (ISTH major or CRNM bleeding) | Not reported | 12 |
| Aspirin-free versus standard DAPT | |||||||||
| GLOBAL-LEADERS | 2018 | 15 968 | 47 | 53 | Ticagrelor monotherapy for 23 months vs DAPT with ticagrelor (ACS) or clopidogrel (CCS) for 12 months | 1 month | EFFICACY (all-cause death or MI) | Reported | 24 |
| TWILIGHT | 2019 | 7119 | 64 | 36 | Ticagrelor monotherapy vs standard DAPT in uneventful patients | 3 months | EFFICACY AND SAFETY (BARC bleeding type 2, 3, or 5 and all-cause death or MI or stroke) | Reported | 15 |
| SMART-CHOICE | 2019 | 2993 | 58 | 42 | P2Y12 inhibitor monotherapy vs standard DAPT | 3 months | EFFICACY (all-cause death, MI or stroke) | Reported | 12 |
| STOPDAPT-2 | 2019 | 3045 | 38 | 62 | Clopidogrel monotherapy vs standard DAPT | 1 month | EFFICACY AND SAFETY (CV death, MI, stroke, definite stent thrombosis, or TIMI major or minor bleeding) | Reported | 12 |
| TICO | 2020 | 3056 | 100 | 0 | Ticagrelor monotherapy vs standard DAPT | 3 months | EFFICACY AND SAFETY (TIMI major bleeding, all-cause death, MI, stent thrombosis, stroke, or target-vessel revascularization) | Reported | 12 |
| STOPDAPT-2-ACS | 2022 | 4169 | 100 | 0 | Clopidogrel monotherapy vs standard DAPT | 1–2 months | EFFICACY AND SAFETY (CV death, MI, stroke, ST and TIMI major or minor bleeding) | Reported | 12 |
| Guided de-escalation versus standard DAPT | |||||||||
| ANTARCTIC | 2016 | 877 | 100 | 0 | PFT guided de-escalation vs standard DAPT | 14 days | EFFICACY AND SAFETY (CV death, MI, stroke, stent thrombosis, urgent revascularization or BARC 2–5 bleeding) | Reported | 12 |
| TROPICAL-ACS | 2017 | 2610 | 100 | 0 | PFT guided de-escalation vs standard DAPT | 14 days | EFFICACY AND SAFETY (CV death, MI, stroke or BARC 2–5 bleeding) | Reported | 12 |
| POPular Genetics | 2019 | 2488 | 100 | 0 | Genotype guided de-escalation vs standard DAPT | 48 hours | EFFICACY AND SAFETY (all-cause death, MI, definite stent thrombosis, stroke, or PLATO major bleeding and PLATO major or minor bleeding) | Reported | 12 |
| Unguided de-escalation versus standard therapy | |||||||||
| TOPIC | 2017 | 646 | 100 | 0 | Clopidogrel-based DAPT vs standard DAPT | 1 month | EFFICACY AND SAFETY (CV death, urgent revascularization, stroke and BARC 2–5 bleeding) | Reported | 12 |
| HOST-REDUCE POLYTECH-ACS | 2020 | 3429 | 100 | 0 | Prasugrel 5 mg-based DAPT vs prasugrel 10 mg-based DAPT | 1 month | EFFICACY AND SAFETY (all-cause death, MI, stent thrombosis, repeat revascularization, stroke, and BARC 2–5 bleeding) | Reported | 12 |
| TALOS-MI | 2021 | 2697 | 100 | 0 | Clopidogrel-based DAPT vs ticagrelor-based DAPT | 1 month | EFFICACY AND SAFETY (CV-death, MI, stroke, BARC 2–5 bleeding) | Reported | 12 |
| Very short versus standard DAPT | |||||||||
| REDUCE | 2019 | 1496 | 100 | 0 | 3 versus 12 months DAPT | 3 months | EFFICACY AND SAFETY (all-cause death, MI, stent thrombosis, stroke, target vessel revascularization and BARC 2–5 bleeding) | Reported | 12 |
| One-month DAPT | 2021 | 3020 | 39 | 61 | 1 versus 6–12 months DAPT in non-complex PCI | 1 month | EFFICACY AND SAFETY (CV death, MI, target vessel revascularization, stroke and major bleeding) | Reported | 12 |
| MASTER-DAPT | 2021 | 4434 | 49 | 51 | 1 versus 5 months DAPT among HBR patients | 1 month | EFFICACY AND SAFETY (all-cause death, MI, stroke, or major bleeding and all-cause death, MI or stroke and major or CRNM bleeding) | Reported | 11 |
Trials comparing double versus triple antithrombotic therapy among patients with atrial fibrillation and concomitant ACS or undergoing PCI present several distinctive features compared to trials testing de-escalated or shortened antiplatelet regimens among patients undergoing PCI. Namely, primary endpoint focused on safety, procedural details were not reported, ‘de-escalation’ of antiplatelet antiplatelet therapy started very early after PCI, and none focused on ACS patients. Bold type: features of randomised controlled trials limiting the generalization of their results to the totality of patients with ACS or undergoing PCI.
AUGUSTUS included a medically treated AF-ACS patient subgroup.
ACS, acute coronary syndrome; BARC, Bleeding Academic Research Consortium; CCS, chronic coronary syndrome; CV, cardiovascular; CRNM, clinically relevant non-major; DAPT, dual antiplatelet therapy; HBR, high bleeding risk; ISTH, International Society on Thrombosis and Haemostasis; MI, myocardial infarction; PFT, platelet function test; PLATO, Platelet Inhibition and Patient Outcomes; TIMI, Thrombolysis in Myocardial Infarction; VKA, vitamin K antagonists.
The four limitations considered above provide rationale for the concerns of many clinicians over the use of DAT early after PCI/ACS (e.g. after 1 week) as the default strategy for most patients. Such concerns are consolidated by the following further considerations.
Systematic reviews and meta-analyses. Although meta-analyses inherit the limitations of included studies and do not automatically lead to sufficiently large sample sizes to assess specific outcomes, they are useful to increase the statistical power of studies with similar designs comparing similar treatment arms.19 Thus, in an attempt to increase the statistical power of the four RCTs with respect to ischaemic outcomes, a number of meta-analyses have been performed.20 To this extent, pooled analyses showed early DAT versus prolonged TT to be associated with an increased risk of stent thrombosis (ST) and myocardial infarction (MI), but only in some of them this increase was statistically significant.20 Reasons behind the conflicting results of meta-analyses are mainly methodological and include the following: (i) heterogeneous outcome definitions; (ii) inclusion of heterogeneous cohorts of patients, (iii) use of different statistical methods; and (iv) lack of power to assess individual hard ischaemic outcomes, particularly in subgroups.20,21 Collectively, the evidence from the meta-analyses does not support adequate efficacy of early DAT compared to TT but suggests increased ST rates in the overall population and at least increased MI rates in the ACS subgroup with an early DAT strategy.20,22–25
14-days versus long TT regimens: A landmark analysis of the ENTRUST-AF PCI trial, comparing very early DAT to a TT strategy from 0 to 14 days and from 15 days to 1 year after randomisation, found the composite endpoint of NACE (comprising cardiovascular death, stroke, systemic embolic events, MI, ST, and major bleeding) to be reduced with TT in the first 14 days, but to be similar between treatment arms from 15 days to 1 year.26 Moreover, preliminary results from the COBRA-REDUCE trial (NCT02594501) suggest that 14 day TT is not as effective as longer TT in preventing ischaemic events among patients requiring OAC and undergoing PCI with either Cobra PzF stent and 14 days dual antiplatelet therapy (DAPT) followed by aspirin alone or standard stent and 3–6 months DAPT followed by aspirin alone.18 Although the rates of bleeding and NACE in the first 14 days in ENTRUST-AF PCI have been proposed to reflect subtherapeutic INR in the TT arm (although data on parenteral anticoagulation use during such time were not provided), and although results of COBRA-REDUCE are yet unpublished, it can be argued that these two studies support the use of TT for at least 2 weeks after PCI.
Early (<30 days) severe ischaemic versus bleeding event rates. AUGUSTUS is the largest trial comparing DAT versus TT among AF-PCI/ACS patients.7 In this study, 80% of ST occurred within the first 30 days after PCI and Kaplan–Meier curves of severe ischaemic events started to diverge immediately after PCI.7,27 On the other hand, Kaplan-Meier curves of severe bleeding events were similar with DAT or TT during the first weeks after PCI, starting to diverge only thereafter in favour of DAT.7 Therefore, as underscored by the absolute risk difference of severe ischaemic and bleeding events, which were balanced within the first 30 days after PCI but not afterwards, the trade-off between bleeding and ischaemic events favoured TT up to 30 days after PCI, and DAT 30 days after PCI (Figure 3).7,28 Moreover, pooled trial analyses have shown that when the risk of ST is compared with the risk of the most feared bleeding event (intracranial haemorrhage) occurring over the entire duration of the trials, the numbers needed to treat or to harm are similar (274 and 314, respectively).22 Because ST occurs mostly within 30 days post PCI, the trade-off between ischaemic events and intracranial haemorrhages again favours a 30-day TT strategy.22,27 Finally, the recently reported MASTER DAPT trial showed that prolonging TT to 3 months does not reduce cardiovascular events, but may increase bleeding, compared to 1 month TT among AF patients undergoing PCI at high bleeding risk29,30.
Platelet response to clopidogrel. Clopidogrel was the most used P2Y12 inhibitor in the four RCTs and thus its use is recommended over other P2Y12 inhibitors in this setting.2,5–8 Up to 40% of patients treated with clopidogrel, however, show suboptimal platelet P2Y12 inhibition leading to high platelet reactivity (clopidogrel poor-responders), which is associated with an increased risk of ischaemic events.31 Aspirin withdrawal may further enhance the risk of ischaemic events in patients who respond poorly to clopidogrel, especially in the early phase after PCI.27,31–33 Of note, OACs (with or without aspirin) are not as effective as DAPT in preventing ischaemic events such as ST after PCI.34,35 This is the rationale for using DAPT after PCI in the first place. The use of genetic or platelet function tests to rule out poor responders to clopidogrel may be a reasonable step towards precision medicine in the use of antithrombotic therapy.33,36 Nevertheless, routine guided selection of P2Y12 inhibitor therapy is not currently recommended by guidelines and studies testing this strategy are still underway (SWAP-AC-2, NCT04483583).2,37 Whether a strategy of potent P2Y12 inhibitor for 1 month followed by unguided de-escalation to clopidogrel may reduce ischaemic events without any relevant increase in bleeding—among AF patients with ACS undergoing PCI and NOAC-based DAT after a short course of TT—will be tested in EPIDAURUS (NCT04981041).
Antithrombotic strategies to reduce bleeding among patients undergoing PCI. The low thrombogenicity of new stent platforms, together with the increasing awareness of the prognostic impact of bleeding events have prompted the design of many RCTs testing a de-escalation or shortening of antithrombotic therapy, aiming at reducing bleeding without any trade-off in ischaemic events (Table 1).38 Among them, the RCTs including patients with AF-PCI/ACS were the only ones in which: (i) the primary endpoints did not include ischaemic outcomes; (ii) procedural details were not reported; (iii) ACS patients were not well represented, as none focused entirely on the acute setting; (iv) ‘de-escalation’ was started very early after PCI (Table 1). The evidence showing heightened risk of ischaemic events in the first month after PCI has led all, but the RCTs on AF-PCI/ACS, to postpone antithrombotic therapy de-escalation/shortening to at least 30 days (1–3 months) after PCI, unless this was guided by genetic or platelet function tests.27,32 Of note, since OACs are not as effective as DAPT in preventing coronary ischaemic events, such as ST, their use does not represent a sufficient reason for an early unguided de-escalation of antithrombotic therapy as adopted in AF-PCI/ACS trials.34,35 It is worth noting that the recently published STOPDAPT-2 ACS trial, enrolling 4136 patients with ACS and sinus rhythm, failed to meet non-inferiority for the primary endpoint of NACE with clopidogrel monotherapy after 1 to 2 months of DAPT versus standard 12-month DAPT.39 These results are in contrast with those, more reassuring, found in the STOPDAPT-2 trial testing the same strategy among both ACS and CCS patients (38% and 62%, respectively), underlining the key role of clinical status in determining ischaemic events and the need for high ischaemic-risk patients (such as ACS) to be well represented among bleeding avoidance RCTs.40
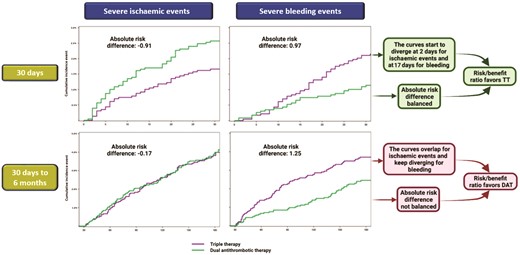
Trade-off between severe bleeding and ischaemic events from randomisation to 30 days and from 30 days to 6 months. Kaplan–Meier curves from the AUGUSTUS trial comparing DAT versus TT for the composite outcomes of severe bleeding (fatal, intracranial, or major) or severe ischaemic events (cardiovascular death, stent thrombosis, myocardial infarction, or stroke) from randomisation to 30 days (upper section) and from 30 days to 6 months (lower section). Both Kaplan-Meier curves and absolute risk differences support a risk/benefit ratio favouring a 30-day TT followed by DAT. Modified from Alexander et al.28 Abbreviations: DAT, double antithrombotic therapy; TT, triple therapy.
In conclusion, the evidence shows that a default very early DAT strategy does not represent a safe option for many patients, particularly those with ACS or undergoing high-risk PCI, placing these patients at undue risk of adverse ischaemic events. These findings are consistent with the well-known lower efficacy of OACs (with or without aspirin) compared to DAPT in preventing coronary ischaemic events and with the highest incidence of such events in the early weeks following PCI.34,35
In an era of personalized therapy representing a priority goal in medicine, a ‘one-size-fits-all’ approach for most patients is sub-optimal. Bleeding and ischaemic risks vary widely among patients and significantly impact their response to antithrombotic agents. We propose a pragmatic algorithm to select optimal TT duration for AF-PCI/ACS that considers both ischaemic (particularly among stable patients) and bleeding (particularly in the acute setting) risk, based on individual patient characteristics (Figure 4). We believe such a personalized approach is balanced, in line with Horace’s ‘virtus’, spanning two different TT durations.
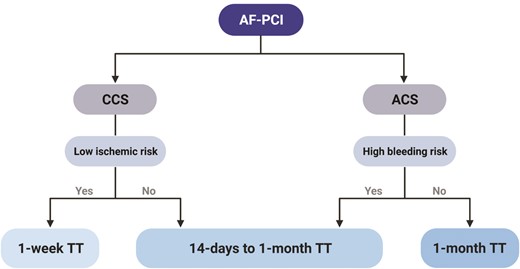
Proposed algorithm for tailoring the duration of triple antithrombotic therapy among atrial fibrillation patients undergoing percutaneous coronary intervention according to clinical setting. Abbreviations: AF, atrial fibrillation; PCI, percutaneous coronary intervention; CCS, chronic coronary syndrome; ACS, acute coronary syndrome; TT, triple therapy.
Conflict of interest: M.G. reports receiving personal speaker honoraria from Terumo, outside the present work. S.A. reports receiving personal speaker or consultancy fees from Boehringer-Ingelheim, Bayer, Thermofisher and Daiichi Sankyo; F.A. reports receiving consultant or speaker fees from Amgen, Bayer, BMS-Pfizer, Daiichi-Sankyo, Medscape and Radcliffe Cardiology; R.A.B. has not received personal payments from medical device or pharmaceutical companies and reports research grants to the institution of employment from Abbott Vascular, Biosensors, Biotronik, Boston Scientific; A.G. reports receiving EU Grant Horizon 2020 MAESTRIA Consortium; grant number 965286. Speaker fees from Abbott, Astra Zeneca, Bayer Health Care, Berlin Chemie, Biotronik, Boehringer Ingelheim, BMS/Pfizer, Boston Scientific, Daiichi-Sankyo, Medtronic, Omeicos, and Sanofi-Aventis. G.H. has nothing to disclose; G.Y.H.L. reports receiving Consultant and speaker fees for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally; T.P. reports consultancy for Bayer and BMS/Pfizer (no fees).
References
Author notes
Conflict of interest: R.D.C. co-authored ESC Guidelines on Atrial Fibrillation 2010–2012, acted as a Steering Committee member and National Coordinator for Italy, and co-authored manuscripts published on APPRAISE-2, ARISTOTLE, AVERROES, ENGAGE AF-TIMI 48 and Re-DUAL PCI. R.D.C. has received fees, honoraria and research funding from Sanofi-Aventis, Boehringer Ingelheim, Bayer, Bristol-Myers Squibb/Pfizer, Daiichi-Sankyo, Novartis, Portola, Roche, and Merck. He is co-principal investigator of ETNA-AF Europe.



