-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas F Lüscher, Allan Davies, Juerg H Beer, Marco Valgimigli, Christoph A Nienaber, John A Camm, Iris Baumgartner, Hans-Christoph Diener, Stavros V Konstantinides, Towards personalized antithrombotic management with drugs and devices across the cardiovascular spectrum, European Heart Journal, Volume 43, Issue 10, 7 March 2022, Pages 940–958, https://doi.org/10.1093/eurheartj/ehab642
Close - Share Icon Share
Abstract
Intravascular thrombus formation and embolization are among the most frequent events leading to a number of cardiovascular conditions with high morbidity and mortality. The underlying causes are stasis of the circulating blood, genetic and acquired coagulation disorders, and reduced antithrombotic or prothrombotic properties of the vascular wall (Virchow’s triad). In the venous system, intravascular thrombi can cause venous thrombosis and pulmonary and even peripheral embolism including ischaemic stroke [through a patent foramen ovale (PFO)]. Thrombi in the left atrium and its appendage or ventricle form in the context of atrial fibrillation and infarction, respectively. Furthermore, thrombi can form on native or prosthetic aortic valves, within the aorta (in particular at sites of ulcers, aortic dissection, and abdominal aneurysms), and in cerebral and peripheral arteries causing stroke and critical limb ischaemia, respectively. Finally, thrombotic occlusion may occur in arteries supplying vital organs such the heart, brain, kidney, and extremities. Thrombus formation and embolization can be managed with anticoagulants and devices depending on where they form and embolize and on patient characteristics. Vitamin K antagonists are preferred in patients with mechanical valves, while novel oral anticoagulants are first choice in most other cardiovascular conditions, in particular venous thromboembolism and atrial fibrillation. As anticoagulants are associated with a risk of bleeding, devices such as occluders of a PFO or the left atrial appendage are preferred in patients with an increased bleeding risk. Platelet inhibitors such as aspirin and/or P2Y12 antagonists are preferred in the secondary prevention of coronary artery disease, stroke, and peripheral artery disease either alone or in combination depending on the clinical condition. A differential and personalized use of anticoagulants, platelet inhibitors, and devices is recommended and reviewed in this article.
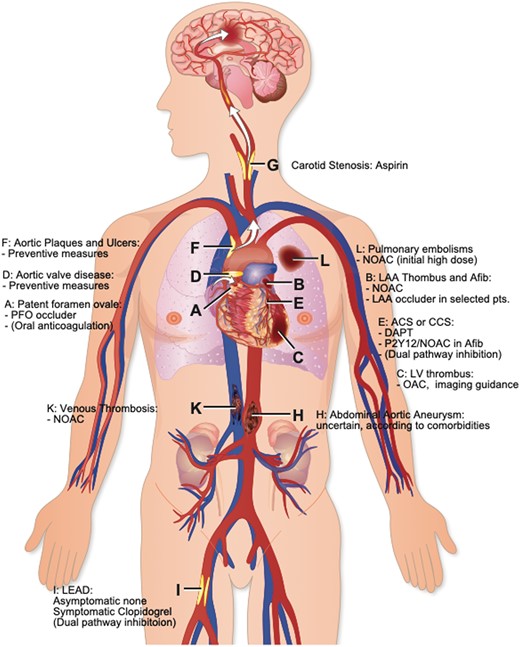
Sites of intravascular thrombus formation and/or embolization in the arterial and venous circulation. ACS, acute coronary syndrome; CCS, chronic coronary syndrome; DAPT, dual antiplatelet therapy; LAA, left atrial appendage; LEAD, lower extremity arterial disease; LV, left ventricular; NOAC, novel oral anticoagulant; PFO, patent foramen ovale.
Haemostasis and bleeding
Throughout evolution, haemostasis was essential for survival. For hunters and gatherers, the probability of dying from bleeding after injury was much higher than dying of a heart attack or stroke. Similarly, women were dependent on well-functioning haemostasis during delivery. Thus, genes promoting haemostasis provided a survival benefit and as such, for example, descendants of Scandinavian Vikings had increased frequencies of procoagulant mutations such as factor V Leiden.1 Today, with a long life expectancy and sedentary lifestyle leading to blood stasis in the lower limbs such mutations increase the risk for myocardial infarction (MI), stroke and thromboembolism. Indeed, except for vasospasm or trauma, without intravascular thrombus formation neither coronary nor cerebrovascular occlusion could occur.
On the other hand, individuals with haemophilia2 (i.e. mutations in factor VIII and IX3)—like the Zarewitsch Alexei Nikolajewitsch Romanow4—suffer from bleeding upon minimal trauma leading to haemarthrosis and fatal cerebral bleeding. Inherited or acquired von Willebrand disease is associated with nose bleedings, easy bruising, menometrorrhagias and excessive blood loss during and after interventions.5,6 Similarly, acquired or hereditary platelet disorders can cause fatal bleedings upon minimal trauma or even spontaneously.
Thus, there is a sweet spot at which both cardiovascular disorders and major bleedings are unlikely to occur. A personalized approach would minimize both thrombus formation and bleeding. The risk of thrombosis depends on lifestyle, genetics, and the presence of vascular disease. However, not all intravascular thrombi and vascular occlusions are alike and hence different dosages of different anticoagulants, platelet inhibitors, and/or their combination or implantable devices are recommended in different clinical conditions.
Major mechanisms of thrombus formation
Thrombus formation at sites of trauma or intravascular injuries involves activation of platelets and coagulation7 (Figure 1). While activated platelets change their shape and interconnect with each other in primary haemostasis together with von Willebrand factor, only fibrin will lead to an occluding clot. On the other hand, fibrinolysis inhibits fibrin formation through plasmin and dissolves an evolving thrombus. Thus, the balance between coagulation and fibrinolysis will determine whether an occluding clot will develop or not. On the other hand, any antithrombotic measure will be assessed as to its efficacy to prevent major adverse cardiovascular events (MACE) and its propensity to increase the bleeding risk.
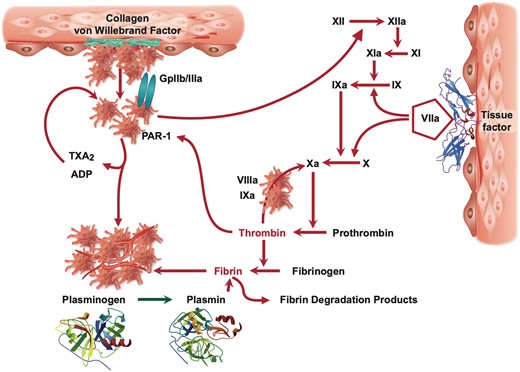
Mechanisms of thrombus formation: platelets are activated after exposure to subendothelial structures such as collagen, von Willebrand factor, and plaque components (top left), while coagulation is initiated by tissue factor (top right) leading to fibrin formation. Fibrin and activated platelets form a solid clot that can be dissolved in the presence of endogenous fibrinolysis initiated by plasminogen (bottom). ADP, adenosine diphosphate; PAR-1, protease activated receptor-1; TXA2, thromboxane A2.
Tailored antithrombotic management
Intravascular thrombi can form both in the venous and arterial circulation (Graphical Abstract). While platelets dominate on the arterial side (leading to ‘white’ thrombi), fibrin- and erythrocyte-rich thrombi stabilized by factor XII are typical on the venous side (‘red’ thrombi). Although there is overlap between the two, this has therapeutic implications as to the use of platelet inhibitors (preferentially on the arterial side) and/or oral anticoagulants (OACs; in a tailored fashion on the arterial side and commonly on the venous side), respectively. Both venous and arterial thrombi may embolize and occlude large blood vessels or the microcirculation, respectively.
On the venous side, intravascular clots preferentially form in veins of the lower limbs and abdomen, triggered by stasis (i.e. bed rest, venous obstruction), activation of coagulation by inflammation (e.g. infection including COVID-19, inflammation, wound healing, rheumatic disease including phospholipid or heparin-induced thrombocytopenia antibodies), genetic predisposition (e.g. protein C/S mutations, antithrombin III, factor V Leiden, prothrombin mutation), and cancer as well as alterations of the venous wall (Virchow’s triad). Genetically determined, clinically relevant prothrombotic mutations are summarized in Table 1.8–11
Prevalence of genetically defined thrombophilia and the relative risk for venous thromboembolism in the normal population and in pregnancy
| . | Factor V Leiden hetero-/homozygous . | Prothrombin G20210A mutation hetero-/homozygous . | Compound factor V Leiden and prothrombin G2021 mutation . | Antithrombin deficiency . | Protein C deficiency . | Protein S deficiency . | Hyperhomo cysteinemia . |
|---|---|---|---|---|---|---|---|
| Prevalence in the normal population | 2–7%/0.06–0.25% | 0.7–4%/rare | 0.1% | 0.02% | 0.2% | 0.03–0.13% | 5–10% |
| Relative risk of first VTE | 5–7/6.8–19.3 | 2–3/2.2–20.7 | 1.1–5 | 15–20 | 15–20 | 15–20 | 1.5–2.5 |
| After termination of anticoagulation | 1.4/1.8 | 1.4/not enough data | 2.7 | 1.9–2.6 | 1.4–1.8 | 1–1.4 | 2.5 |
| Relative risk of pregnancy complication | 1–2.6 | 0.9–1.3 | — | 1.3–3.6 | 1.3–3.6 | 1.3–3.6 | — |
| . | Factor V Leiden hetero-/homozygous . | Prothrombin G20210A mutation hetero-/homozygous . | Compound factor V Leiden and prothrombin G2021 mutation . | Antithrombin deficiency . | Protein C deficiency . | Protein S deficiency . | Hyperhomo cysteinemia . |
|---|---|---|---|---|---|---|---|
| Prevalence in the normal population | 2–7%/0.06–0.25% | 0.7–4%/rare | 0.1% | 0.02% | 0.2% | 0.03–0.13% | 5–10% |
| Relative risk of first VTE | 5–7/6.8–19.3 | 2–3/2.2–20.7 | 1.1–5 | 15–20 | 15–20 | 15–20 | 1.5–2.5 |
| After termination of anticoagulation | 1.4/1.8 | 1.4/not enough data | 2.7 | 1.9–2.6 | 1.4–1.8 | 1–1.4 | 2.5 |
| Relative risk of pregnancy complication | 1–2.6 | 0.9–1.3 | — | 1.3–3.6 | 1.3–3.6 | 1.3–3.6 | — |
Adapted from ref.8–11
VTE, venous thromboembolism.
Prevalence of genetically defined thrombophilia and the relative risk for venous thromboembolism in the normal population and in pregnancy
| . | Factor V Leiden hetero-/homozygous . | Prothrombin G20210A mutation hetero-/homozygous . | Compound factor V Leiden and prothrombin G2021 mutation . | Antithrombin deficiency . | Protein C deficiency . | Protein S deficiency . | Hyperhomo cysteinemia . |
|---|---|---|---|---|---|---|---|
| Prevalence in the normal population | 2–7%/0.06–0.25% | 0.7–4%/rare | 0.1% | 0.02% | 0.2% | 0.03–0.13% | 5–10% |
| Relative risk of first VTE | 5–7/6.8–19.3 | 2–3/2.2–20.7 | 1.1–5 | 15–20 | 15–20 | 15–20 | 1.5–2.5 |
| After termination of anticoagulation | 1.4/1.8 | 1.4/not enough data | 2.7 | 1.9–2.6 | 1.4–1.8 | 1–1.4 | 2.5 |
| Relative risk of pregnancy complication | 1–2.6 | 0.9–1.3 | — | 1.3–3.6 | 1.3–3.6 | 1.3–3.6 | — |
| . | Factor V Leiden hetero-/homozygous . | Prothrombin G20210A mutation hetero-/homozygous . | Compound factor V Leiden and prothrombin G2021 mutation . | Antithrombin deficiency . | Protein C deficiency . | Protein S deficiency . | Hyperhomo cysteinemia . |
|---|---|---|---|---|---|---|---|
| Prevalence in the normal population | 2–7%/0.06–0.25% | 0.7–4%/rare | 0.1% | 0.02% | 0.2% | 0.03–0.13% | 5–10% |
| Relative risk of first VTE | 5–7/6.8–19.3 | 2–3/2.2–20.7 | 1.1–5 | 15–20 | 15–20 | 15–20 | 1.5–2.5 |
| After termination of anticoagulation | 1.4/1.8 | 1.4/not enough data | 2.7 | 1.9–2.6 | 1.4–1.8 | 1–1.4 | 2.5 |
| Relative risk of pregnancy complication | 1–2.6 | 0.9–1.3 | — | 1.3–3.6 | 1.3–3.6 | 1.3–3.6 | — |
Adapted from ref.8–11
VTE, venous thromboembolism.
On the arterial side, platelet-rich thrombi dominate and typically form at sites of endothelial dysfunction or damage and/or exposure of subintimal structures or proteins (e.g. collagen, von Willebrand factor, fibronectin, thrombospondin, tissue factor among others) to blood components due to rupture or erosion of atherosclerotic plaques that are initiated by activation of inflammatory pathways and tissue factor—thus, inflammation begets coagulation.
While thrombi can form at any site in the cardiovascular system, their triggers, mechanisms, cellular composition, and consequences differ substantially. Furthermore, the efficacy and safety of the various antithrombotic regimens, their dosages and recommended duration of use as well as indications for devices differ as does the risk of individual patients depending on their condition, bleeding risk, comorbidities, and genetic background. Thus, the use of different anticoagulants, thrombolytics, platelet inhibitors, and devices should consider the patient profile, clinical condition, his/her ischaemic and bleeding risk, and efficacy and safety of the antithrombotic regimen to provide an individualized management.
Genetic variants can affect both the risk of thrombotic events and the effectiveness of antithombotic molecules.3 A large number of genetically determined prothrombotic dysfunctions affecting coagulation factors and platelet structure and function have been described. Table 1 lists clinically relevant genetic coagulation disorders with their frequency in the population and the incidence of thromboembolic disease.8–11 On the platelet side, gain-of-function mutations of glycoprotein (GP) IIb/IIIa (PlA1/2), GPIa/IIa,11 GPVI, and GPIb12 and combinations thereof have been described but are not applied in current clinical routine, although in the POPular Genetics trial patients undergoing primary percutaneous coronary intervention (PCI), a CYP2C19 genotype-guided strategy for selection of P2Y12 inhibitors was non-inferior to ticagrelor or prasugrel at 12 months as regards thrombotic events and resulted in a lower incidence of bleeding.13 Importantly, genetics of the cytochrome P450 complex affects the metabolism of certain antithrombotics and their interaction with other drugs (see below). As an ever increasing number of variables have to be taken into account for decision-making including data of trials with different inclusion criteria and patient profiles, machine learning and artificial intelligence may be the coming approach to integrate such big data sets.14,15
Patent foramen ovale
In RivaroxabaN vs. Asprin in secondary preVentIon of stroke and prevention of systemic embolism in pATients with rEcent Embolic Stroke of Undetermined Source (NAVIGATE ESUS)16 and DabigatRan Etexilate for Secondary PrEvention in patienTs with Embolic Stroke of Undetermined Source (RE-SPECT ESUS),17 rivaroxaban and dabigatran, respectively, were not superior to aspirin in preventing recurrent embolic strokes of unknown source (ESUS), but were associated with a higher risk of bleeding. In a meta-analysis, OAC appeared superior to aspirin in preventing stroke with a hazard ratio (HR) of 0.68, but the confidence interval crossed the line of identity and bleeding was more common with OAC (HR 1.12).18 Thus, in patients at high bleeding risk aspirin may be considered instead of OAC, while in others OAC with vitamin K antagonists (VKAs) or novel oral anticoagulants (NOACs) are more effective than platelet inhibitors. As patients with embolic stroke or thromboembolism into the coronary circulation19 or other organs20 are usually young, lifelong OAC provides a considerable bleeding risk. Thus, percutaneously implantable occluders are the treatment of choice. In patients with embolic stroke, as occluders impressively reduced the risk of a second embolic stroke in three large randomized trials compared to antiplatelet drugs and were non-inferior to anticoagulation (Figure 2).21,24,25 A sub-analysis of the CLOSE trial showed an advantage of patent foramen ovale (PFO) closure in those with large shunts and/or atrial septal aneurysms.24 A meta-analysis of all major randomized trials, however, could not confirm this but did confirm the higher rate of atrial fibrillation (AF) after PFO closure compared to antithrombotics.23
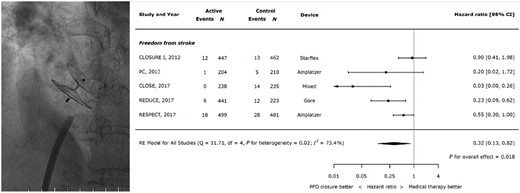
Catheter-based occlusion of a patent foramen ovale with an Amplatz occluder (left; operator TFL) and results of a meta-analysis of current trials (right21–23). CI, confidence interval; SE, systemic embolism (modified from ref. 23, by permission).
Conclusion
Patent foramen ovale closure should be considered in patients in whom other causes of stroke have been excluded, particularly those with a high Risk of Paradoxical Embolism (RoPE) score (see https://www.mdcalc.com/risk-paradoxical-embolism-rope-score), i.e. those of younger age (<60 years), with cortical stroke (particularly with simultaneous venous thrombosis), but no diabetes, hypertension, smoking or prior stroke or transient ischaemic attack (TIA) (i.e. new neurologic symptoms or deficit lasting <24 h with no new infarction on neuroimaging).26 Thrombus formation on PFO struts occurs in <3% of patients receiving an PFO occluder.27 Thrombi usually dissolve with OAC, but materials with lower thrombogenicity are an unmet medical need.
Atrial fibrillation
Anticoagulation
Atrial fibrillation (AF) is the most common indication for lifelong OAC to avoid cardioembolic strokes.28 Around 90% of thrombi are formed in the left atrial appendage (LAA),29–33 but spontaneous echo contrast reflecting low atrial blood flow is also a risk factor for embolic events.34,35 Vitamin K antagonists reduce the risk of stroke by more than 60% in AF.36–39 NOACs similarly reduce strokes and also non-gastrointestinal including intracranial bleeding (Figure 3).40 NOACs received a Class IA indication over VKAs in the 2016 European Society of Cardiology (ESC) guidelines on the management of AF for individuals with a gender-neutral CHA2DS2VASc score of 2 or greater.28 The 2020 ESC guidelines on the diagnosis and management of AF further emphasize regular assessment of bleeding risk using the HAS-BLED score [i.e. age >65 years, hypertension, renal or liver failure, coagulation disorder, unstable international normalized ratio (INR), drug interaction, alcohol].28
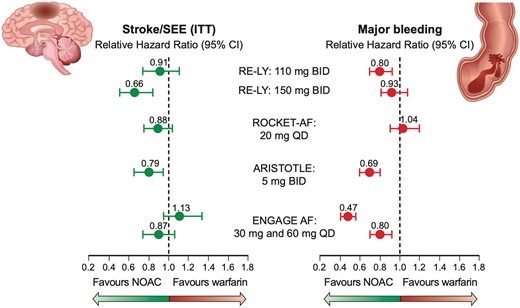
Novel oral anticoagulants compared to vitamin K antagonists in patients with atrial fibrillation. CI, confidence interval; SEE, systemic embolic event (Modified with data from ref.38, N Engl J Med 2011;365:883–891, N Engl J Med 2011;365:981–992 and N Engl J Med 2013; 369:2093–2104).
Anticoagulation or not?
Young patients with AF with no underlying cardiovascular disease (CVD) do not need OAC, although about half do receive it inappropriately. The CHA2DS2-VASc score (i.e. age, heart failure, hypertension, diabetes, aterosclerotic CVD, stroke, female sex) is used to eliminate those with a gender-neutral score of 0 who do not warrant OAC. In those with CHA2DS2-VASc score of 1 in men or 2 in women, there is little evidence, but observational studies suggest a low thromboembolic risk. Currently, it is recommended that bleeding risk (as assessed by the HAS-BLED score) should guide decisions, although a number of other scores, including GARFIELD-AF, exist.41 A score of 2 already outweighs an intermediate thromboembolic risk.42 Numerical vs. biological age, mild vs. severe hypertension, and factors not accounted for in the CHA2DS2-VASc score, i.e. high thrombosis risk, renal impairment,43 left ventricular (LV) hypertrophy,44 or left atrial dilatation might warrant OAC. If OAC is initiated, NOACs should be preferred over VKAs,42 but patients’ preferences must be taken into account. Furthermore, ablation or cardioversion should be considered.45,46 Those with a score of >2, OAC should be prescribed unless contraindicatated.28 Less used scoring systems incorporate biomarkers such as troponin or brain natriuretic peptide.47 Generally, AF patients on OAC should be monitored regularly as to their bleeding risk. In the Mobile Atrial Fibrillation Application (mAFA-II) trial, the HAS-BLED score was regularly monitored up to 1 year using a holistic app-based management which reduced bleeding events.48
Which anticoagulant?
Vitamin K antagonists are partially affected by genetics, but more so by drug–drug and food–drug interactions. Carriers of VKORC1 and CYP2C9 polymorphisms are at increased risk of adverse events when receiving VKA,49 but are not widely used in the clinic. Frequent INR assessments are essential. If not readily available, self-testing is recommended.50 Inability to afford treatment with warfarin or NOACs often leads to the use of aspirin which is cheap, but relatively ineffective51 and not recommended by ESC guidelines.28 Vitamin K antagonist is mandatory for patients with metallic valve prosthesis or moderate or severe rheumatic mitral stenosis.52 It is also useful for patients with poor compliance.
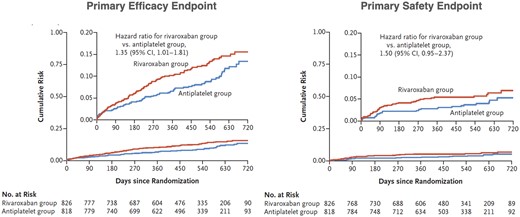
NOACs are the first choice due to lower intracranial bleeding risk compared to VKAs (Figure 4).54 NOACs can also be used in AF patients with hypertrophic cardiomyopathy and most forms of valvular heart disease (including bioprosthetic heart valves, but not mechanical or transcatheter aortic heart valves; see below). NOACs are also preferred in moderate renal impairment, because acute OAC nephropathy and progressive renal impairment due to nephrocalcinosis is less common with NOACs than VKAs.55 NOACs are better than VKAs in patients with severe renal impairment, but the value of OAC is uncertain in these patients.56,57 Like VKAs, NOACs should not be used in pregnancy [where low molecular weight heparin (LMWH) is commonly recommended].58
Outcomes in the pivotal Left Atrial Appendage System Embolic Protection in Patients with Atrial Fibrillation (PROTECT AF) trial.53 CV, cardiovascular; ITT, intention-to-treat (Reproduced from Piccini JP, Sievert H, Patel MR: Left atrial appaendage occlusion: rationale, evidence, devices and patient selection. Eur. Heart J, 2017;38: 869–876., with permission).
Novel oral anticoagulants and renal function
There are more similarities than differences between NOACs (Figure 1). All NOACs can be used in mild renal failure [creatinine clearance (CrCl) >50 mL/min] at the standard dose. Dabigatran is not suitable in patients with more severe renal failure, because of its dominant renal elimination.59 Low-dose rivaroxaban (15 mg od) and edoxaban (30 mg od) can be used in patients with moderate renal impairment (i.e. CrCl 30–49 mL/min). Below moderate renal impairment (CrCl 15–30 mL/min), there are only observational data to support the use of factor Xa inhibitors and they should only be used with caution. Apixaban can be used in patients with moderate renal failure (serum creatinine ≥1.5 mg/dL [133 μmol/L]) at a reduced 2.5 mg bid dose if one other dose reduction parameter (weight ≤60 kg or age ≥80 years) is present. In the USA, both apixaban and rivaroxaban may be used for haemodialysed patients, but there is no approval for this by the European Medicines Agency. Edoxaban should be used with caution in patients with very good renal function (CrCl >95 mL/min) as strokes may not be effectively prevented.60 Many patients will prefer and adhere better to once a day regimen with rivaroxaban or edoxaban,61 but missing occasional doses is less relevant with twice daily regimens (apixaban and dabigatran).
In patients with a history of gastrointestinal bleeding, apixaban rather than other NOACs better avoids bleeding.62 It is recommended to co-prescribe a proton pump inhibitor to such patients.62 With repetitive or severe gastrointestinal bleeding an LAA occluder may be considered (see below and Figure 4).
In case of severe bleeding, dabigatran can be reversed by idarucizumab.63 This has even been used in ischaemic stroke requiring thrombolysis.64 For rivaroxaban and edoxaban, prothrombin complex concentrate was recommended (but not advised for apixaban), while now andexanet alfa is indicated for the reversal of life-threatening or uncontrolled bleeding in patients on rivaroxaban or apixaban.65 On the other hand, under-dosing out of fear of bleeding is not uncommon and increases stroke rates, especially with apixaban, without substantial decreases in major bleeding.66
Interactions and dosing
Dosing of NOACs differs for each compound depending on patient age, renal function, body weight, and concomitant drugs, e.g. permeability glycoprotein (P-Gp) inhibitors (e.g. benzothiazepines, colchicine, digoxin, diltiazem, tacrolimus, verapamil, among others) or inhibitors (see below) or inducers of the cytochrome P450 complex.28 A reduced dose of 30 mg/day for edoxaban is recommended with concomitant therapy with strong P-Gp inhibitor (e.g. cyclosporine, dronedarone, erythromycin, or ketoconazole). Rivaroxaban 15 mg/day (rather than 20 mg/day) is recommended with in combination with dronedarone.67 The lower dose of dabigatran (110 mg bid) should be used in those taking verapamil or dronedarone.
Conclusion
NOACs are first choice in AF irrespective of the bleeding risk and should be prescribed at high dose. Dose reduction may be considered in those with low body weight, markedly reduced renal function, while inhibitors and inducers of CYP3A4 and P-Gp68 should be avoided.
Follow-up
Frequency should be adapted depending on the presence of renal impairment, poor adherence, comorbidities, polypharmacy, cognitive function, and social care. Particularly younger patients, especially with paroxysmal AF and low CHA2DS2-VASc score, tend to abandon treatment.69 Older patients with comorbidities often have renal impairment. These groups need careful follow-up.
Hypertension and atrial fibrillation
Hypertension and AF commonly coexist and if occurring together carry an increased risk of stroke and systemic embolism. Of note, poorly controlled blood pressure in anticoagulated patients with AF is associated with an increased risk of bleeding. Hence, optimal blood pressure control is important in this context.70 Because of the increased risk of bleeding, NOACs should be preferred over VKA in such patients to reduce the elevated stroke and bleeding risk.
Alternatives to medical anticoagulation
Antiplatelet drugs are inadequate for stroke prophylaxis in high risk AF; indeed, apixaban reduced stroke and embolism by 55% compared to aspirin.71 When OAC is contraindicated because of bleeding, stopping co-medications which increase bleeding risk or LAA occlusion or excision can be considered (Figure 4). In support of interventional trials (see below), among the 4770 participants of the Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke (LAAOS) III trial with AF who had undergone cardiac surgery, of whom most continued to receive antithrombotic therapy, the risk of ischaemic stroke or systemic embolism was lower with surgical LAA occlusion than without it.72 Thus, evidence to support these therapies is increasing, but not strong enough yet to consider them with no valid contraindication to OACs.
Left atrial appendage occlusion
In patients with unacceptable bleeding risk with OAC, i.e. after haemorrhagic stroke, severe gastrointestinal bleeding, catheter-based, or surgical LAA occlusion can be considered.53,73,74 The procedure is associated with few, but important complications such as pericardial effusion, tamponade, LAA perforation or embolization of the occluder, device-related thrombus, and ischaemic stroke.75 Overall, in randomized trials against warfarin [Watchman Left Atrial Appendage Closure Technology for Embolic PROTECTion in Patients With Atrial Fibrillation (PROTECT-AF)]53 or NOACs (PRAGUE-17),76 LAA occluders were non-inferior in preventing embolic stroke (with, however, higher rates of ischaemic strokes), but with less bleeding complications suggesting that overall it does not provide the same protection against stroke as OAC (Figure 4).77,78 Importantly, several large-scale randomized studies of LAA occlusion devices vs. NOACs [Clinical trial of Atrial fibrillation paTients comparing Left atrial appendage occlusion therapY to non-vitamin K antagoniST oral anticoagulants (CATALYST) (NCT04226547) and WATCHMAN FLX vs. NOAC for embolic ProtectION in the management of patients with Non-Valvular Atrial Fibrillation (CHAMPION-AF) (NCT04394546)] are currently underway.
Conclusion
Currently, LAA occlusion should only be considered in patients with a strong contraindication to anticoagulation as a local solution to a generalized problem (i.e. increased coagulation and platelet reactivity, other sites of thrombus formation than LAA).
Left ventricular thrombi
Left ventricular thrombi develop after myocardial infarction (MI), especially transmural anterior MI and in patients with ischaemic or dilated cardiomyopathies, particularly LV non-compaction.79,80 In patients with hereditary and acquired thrombophilia (e.g. phospholipid antibodies), incidences may be increased.81 The incidence of LV thrombi after MI has markedly decreased with primary PCI79 with only a few causing embolic events. Their incidence is underestimated by LV angiography or echocardiography compared to magnetic resonance imaging.82 OAC to dissolve LV thrombi is not evidenced-based, but the 2017 ESC guidelines83 recommend it with IIa C for up to 6 months guided by repeated imaging. A registry analysis suggested that warfarin might be superior to NOACs in preventing stroke and systemic embolism in such patients.84 In LV non-compaction where thrombi form in persistent sinuses,80 OAC is not evidence-based, but commonly recommended.
The COMMANDER-HF trial evaluating NOACs in the prevention of LV thrombi and MACE in patients with heart failure revealed neutral results.85 Similarly, in the WARCEF trial, among 2305 patients with reduced LV ejection fraction who were in sinus rhythm, there was no significant difference in the primary outcome between warfarin and aspirin. A reduced risk of ischaemic stroke with warfarin was offset by an increased risk of major haemorrhage.86
Conclusion
NOACs are ineffective in the primary prevention of LV thrombus formation in patients with moderately to severely reduced LV ejection fraction. However, in those with LV thrombus, VKA should be considered until disappearance under imaging guidance. In the prevention of stroke of patients with heart failure and in sinus rhythm, OAC is overall not superior to aspirin as a small benefit on stroke is counterbalanced by increased bleeding. The choice between OAC and aspirin should therefore be individualized, mainly based on bleeding risk in such patients.
Valvular heart disease
Valvular heart disease is associated with a risk of stroke for several reasons: (i) thrombi on degenerated valvular leaflets; (ii) AF, particularly with mitral valve disease, and (iii) thrombi on biological and particularly on mechanical valves.
Mitral valve disease
Mitral stenosis leads to low flow and spontaneous echo contrast and left atrial thrombi even in the absence, but particularly in the presence of AF. The ESC guidelines recommend OAC with VKA, but not NOACs in these high risk patients.52 Recent registries, however, support NOACs in mitral stenosis over VKA with lower bleeding and lower mortality.87 Combined antiplatelet and anticoagulant therapy is particularly effective.88 Similarly, severe mitral regurgitation increases the risk of AF and stroke. Stroke risk is reduced after balloon dilatation of mitral stenosis or after surgical reconstruction of mitral regurgitation, while little data are available after MitraClip®. Mechanical valves in mitral position bear a high stroke risk, if OAC with VKAs is not kept within the therapeutic range.
The RIVaroxaban in Patients with Atrial Fibrillation and a BioprosthEtic MitRal Valve (RIVER) trial compared rivaroxaban (20 mg/day) in 1005 patients with AF and a bioprosthetic mitral valve with warfarin. Importantly, rivaroxaban was non-inferior to warfarin with respect to the mean time to death, MACE or major bleeding at 12 months.89 However, NOACs have not been evaluated and are not recommended in metallic valves, particularly after the results of the DabigatRan EtexilAte in patients with mechanicaL heart valves (RE-ALIGN) trial (see below).
Conclusion
In mitral valve stenosis, OAC with VKA is mandatory as it is after mechanical valve replacement for mitral stenosis or regurgitation. NOACs may be considered in patients with a bioprosthesis in mitral position.
Aortic valve disease
Calcific aortic stenosis increases the risk of stroke due embolization of thrombotic or calcific material on leaflets and/or AF.90 After aortic valve replacement (AVR) or transcatheter aortic valve implantation (TAVI), stroke risk remains increased.91 With mechanical valves, OAC with VKAs, but not NOAC, is strongly recommended as in the RE-ALIGN trial dabigatran was associated with increased thromboembolic and bleeding events compared to warfarin.92 For surgical bioprostheses and TAVI valves, OAC is not recommended. Currently, dual antiplatelet therapy (DAPT) for 3–6 months followed by aspirin is common practice. However, in a recent trial randomizing patients undergoing TAVI without indication for OAC to aspirin alone or aspirin plus clopidogrel, bleeding or thromboembolic events at 1 year were less frequent with aspirin than with DAPT for 3 months.93
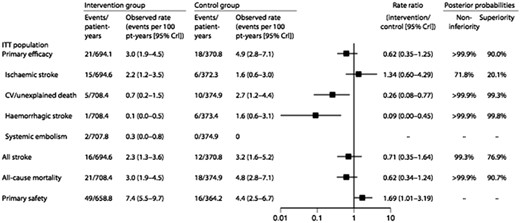
After TAVI, hypo-attenuated leaflet thickening (HALT),94,95 i.e. thrombi aligned to the leaflets that can impair valve opening leading to a transvalvular pressure gradient, may occur. HALT is reversible spontaneously or with OAC. The risk of stroke is uncertain; while some reported cerebrovascular events with HALT,96 others were unable to confirm this.97 In TAVI patients without an indication for OAC, the Global study comparing a rivAroxaban-based antithrombotic strategy an antipLatlet-based Strategy after transctheter aortIc vaLve rEplacement to Optimize clinical outcome (GALILEO) trial with rivaroxaban 10 mg plus aspirin 75–100 mg vs. DAPT was stopped prematurely due to increased mortality, thromboembolic events, and bleeding with rivaroxaban (Figure 5).99 The Anti-Thrombotic strategy after trans-aortic valve ImpLAnTatIon for aortic Stenosis (ATLANTIS) trial with apixaban 5 mg bid compared to VKA or DAPT/single antiplatelet therapy (SAPT), respectively, is still under way.100 The increased risk of bleeding in GALILEO may be due to acquired von Willebrand’s disease in patients with calcific aortic stenosis101 and after TAVI.102 In the POPular TAVI EU clinical trials, patients undergoing TAVI who did not have an indication for OAC, bleeding, and the composite of bleeding or thromboembolic events at 1 year were significantly less frequent with aspirin than with aspirin plus clopidogrel administered for 3 months,93 while in those with an indication for anticoagulation, the incidence of serious bleeding was lower with OAC alone than with OAC plus clopidogrel.103
Cumulative risk of the primary efficacy and primary safety outcomes and death from any cause in the intention-to-treat analysis in the GALILEO trial. The primary efficacy outcome was the composite of death or thromboembolic events. The primary safety outcome was major, disabling, or life-threatening bleeding. The trial was terminated prematurely by the data and safety monitoring board because of safety concerns. CI, confidence interval. Modified from ref.98
Conclusion
OAC appears contraindicated after TAVI, while DAPT or possibly SAPT for 3–6 months93 is likely to remain the standard of care, unless the ATLANTIS trial will reveal different results. However, AF is present in up to a third of patients undergoing TAVI90 and then OAC is appropriate, most likely as monotherapy. LAA occlusion might provide an attractive alternative to prevent stroke after TAVI, particularly in those with acquired von Willebrand syndrome and/or increased risk of bleeding.
Acute and chronic coronary syndromes
Acute coronary syndromes
Acute ischaemia with total or near total occlusion of a major coronary artery is the underlying cause of Acute Coronary Syndromes (ACS),83,104 which is due to thrombus formation at sites of rupturing plaques and/or de-endothelialization with activation of platelets and coagulation (Figure 1).105 In non-ST-elevation MI (NSTEMI), aggregating platelets embolize into the microcirculation leading to increased troponins. In ST-elevation MI (STEMI), an interaction of aggregating platelets with fibrin forms an occluding clot (Figure 1). Thus, in the ISIS-2 trial, aspirin and streptokinase led to an additive 10% plus 10%, respectively, absolute reduction of vascular death.106
DAPT reduces ischaemic events after ACS by preventing the risk of MI in the culprit and non-culprit arteries and stent thrombosis.107–110 The 2020 ESC guidelines for the management of ACS in patients presenting without persistent ST-segment elevation do not recommend pre-treatment with DAPT, but after PCI gave preference to prasugrel 10 mg rather than ticagrelor 90 mg bid (based on ISAR-REACT 5111) or clopidogrel [based on a comparison of Prasugrel (CS-747) and clopidogrel in acute coronary syndrome subjecTs who aRe to undergo percutaneous coronary IntervenTiON (TRITON-TIMI 38)112] with aspirin 75–100 mg up to 1 year (Table 2).113 – 119 Clopidogrel in addition to aspirin also provides benefit in those undergoing thrombolysis rather than PCI [CLopidogrel as Adjunctive ReperfusiIon TherapY Thrombolysis in Myocardial Infarction 28 study (CLARITY-TIMI 28)113]. In patients at high bleeding risk, DAPT can be de-escalated from prasugrel or ticagrelor to clopidogrel with aspirin. Indeed, in the Assessment of Loading with the P 2Y12 inHibitor TicagrElor or Clodipogrel to Halt Events in Patients Undergoing Elective Coronary Stenting (ALPHEUS) trial,120 ticagrelor was not superior to clopidogrel after PCI. In the Testing Responsiveness tO Platelet Inhibition on Chronic AntiplateLet treatment for Acute Coronary Syndromes (TROPICAL-ACS) trial, platelet function-guided de-escalation from prasugrel to clopidogrel after 1 week was non-inferior to standard prasugrel at 1 year.116 In the GLOBAL LEADERS trial, SAPT with ticagrelor and aspirin for 1 month followed by ticagrelor alone for 23 months overall was not superior to 12 months of DAPT in the prevention of MACE after PCI in ACS or stable coronary patients (Table 2),117 with, however, a possible benefit in those undergoing complex PCI.121 In those at high risk of bleeding complications and an indication for prolonged DAPT beyond 1 year, a reduced P2Y12 inhibitor dose (i.e. ticagrelor 60 mg bid) may be considered.122
Most important randomized clinical trials on antiplatelet therapy in patients with chronic or acute coronary syndromes
| Study . | Publication year . | Size . | Follow-up . | Treatment and comparator . | Primary endpoint . | Outcome . |
|---|---|---|---|---|---|---|
| Major studies comparing antiplatelet agents in acute coronary syndromes | ||||||
| ISIS-2106 | 1988 | 17 187 | 5 weeks | Aspirin vs. placebo | Vascular mortality at 5 weeks | 23% odds reduction |
| CURE110 | 2001 | 12 562 | 12 months | Aspirin + clopidogrel vs. aspirin + placebo | CV death, non-fatal MI, or stroke | 9.3% vs. 11.4%; RR 0.80 (0.72–0.90) |
| COMMIT109 | 2005 | 45 852 | 4 weeks | Aspirin + clopidogrel vs. aspirin + placebo | Death, re-infarction, or stroke | 9.2% vs. 10.1%: RR 0.91 (0.86–0.97) |
| CLARITY-TIMI 28113 | 2005 | 3491 | 30 days | Aspirin + clopidogrel vs. aspirin + placebo | Failed reperfusion or reocclusion, death from any cause | 15% vs. 21.7%; OR 0.64 (0.53–0.76) |
| TRITON-TIMI 38112 | 2007 | 13 608 | 1 year | Aspirin + prasugrel vs. aspirin + clopidogrel | CV death, non-fatal MI, or stroke | 9.9% vs. 12.1%; HR 0.81 (0.73–0.90) |
| PLATO114 | 2009 | 18 624 | 1 year | Aspirin + ticagrelor vs. aspirin + clopidogrel | CV death, MI stroke | 9.8% vs. 11.7%; HR 0.84 (0.77–0.92) |
| ISAR-REACT 5111 | 2019 | 4018 | 1 year | Aspirin + ticagrelor vs. aspirin + prasugrel | Death, MI, stroke | 9.3% vs. 6.9%; HR 1.36 (1.09–1.70) |
| Major studies comparing antiplatelet strategies in either chronic or acute coronary syndromes | ||||||
| PEGASUS-TIMI 54115 | 2017 | 21 162 | 33 months | Aspirin + ticagrelor 90 mg, aspirin + ticagrelor 60 mg, aspirin + placebo |
|
|
| DAPT108 | 2014 | 9961 | 30 months | After 12 months DAPT, aspirin + P2Y12 vs. aspirin + placebo for further 18 months | Death, MI, stroke, ST |
|
| TROPICAL-ACS116 | 2017 | 2610 | 1 year | Aspirin + prasugrel vs. aspirin + prasugrel 1 week then PFT guided clopidogrel | CV death, MI, stroke, BARC 2, or greater bleeding | 9% vs. 7%; HR 0.81 (0.62–1.06) |
| GLOBAL LEADERS117 | 2018 | 15 968 | 2 years | Aspirin + ticagrelor 1 month, then ticagrelor 23 months vs. aspirin + P2Y12 for 12 months, then aspirin monotherapy | All-cause death, non-fatal MI | 3.81% vs. 4.37%; rate ratio 0.87 (0.75–1.01) |
| Study . | Publication year . | Size . | Follow-up . | Treatment and comparator . | Primary endpoint . | Outcome . |
|---|---|---|---|---|---|---|
| Major studies comparing antiplatelet agents in acute coronary syndromes | ||||||
| ISIS-2106 | 1988 | 17 187 | 5 weeks | Aspirin vs. placebo | Vascular mortality at 5 weeks | 23% odds reduction |
| CURE110 | 2001 | 12 562 | 12 months | Aspirin + clopidogrel vs. aspirin + placebo | CV death, non-fatal MI, or stroke | 9.3% vs. 11.4%; RR 0.80 (0.72–0.90) |
| COMMIT109 | 2005 | 45 852 | 4 weeks | Aspirin + clopidogrel vs. aspirin + placebo | Death, re-infarction, or stroke | 9.2% vs. 10.1%: RR 0.91 (0.86–0.97) |
| CLARITY-TIMI 28113 | 2005 | 3491 | 30 days | Aspirin + clopidogrel vs. aspirin + placebo | Failed reperfusion or reocclusion, death from any cause | 15% vs. 21.7%; OR 0.64 (0.53–0.76) |
| TRITON-TIMI 38112 | 2007 | 13 608 | 1 year | Aspirin + prasugrel vs. aspirin + clopidogrel | CV death, non-fatal MI, or stroke | 9.9% vs. 12.1%; HR 0.81 (0.73–0.90) |
| PLATO114 | 2009 | 18 624 | 1 year | Aspirin + ticagrelor vs. aspirin + clopidogrel | CV death, MI stroke | 9.8% vs. 11.7%; HR 0.84 (0.77–0.92) |
| ISAR-REACT 5111 | 2019 | 4018 | 1 year | Aspirin + ticagrelor vs. aspirin + prasugrel | Death, MI, stroke | 9.3% vs. 6.9%; HR 1.36 (1.09–1.70) |
| Major studies comparing antiplatelet strategies in either chronic or acute coronary syndromes | ||||||
| PEGASUS-TIMI 54115 | 2017 | 21 162 | 33 months | Aspirin + ticagrelor 90 mg, aspirin + ticagrelor 60 mg, aspirin + placebo |
|
|
| DAPT108 | 2014 | 9961 | 30 months | After 12 months DAPT, aspirin + P2Y12 vs. aspirin + placebo for further 18 months | Death, MI, stroke, ST |
|
| TROPICAL-ACS116 | 2017 | 2610 | 1 year | Aspirin + prasugrel vs. aspirin + prasugrel 1 week then PFT guided clopidogrel | CV death, MI, stroke, BARC 2, or greater bleeding | 9% vs. 7%; HR 0.81 (0.62–1.06) |
| GLOBAL LEADERS117 | 2018 | 15 968 | 2 years | Aspirin + ticagrelor 1 month, then ticagrelor 23 months vs. aspirin + P2Y12 for 12 months, then aspirin monotherapy | All-cause death, non-fatal MI | 3.81% vs. 4.37%; rate ratio 0.87 (0.75–1.01) |
BARC, Bleeding Academic Research Consortium; CV, cardiovascular; DAPT, dual antiplatelet therapy; HR, hazard ratio; MACE, major adverse cardiovascular events; MI, myocardial infarction; OR, odds ratio; PFT, platelet function testing; RR, relative risk; ST, stent thrombosis; TIMI, Thrombolysis in Myocardial Infarction.
Most important randomized clinical trials on antiplatelet therapy in patients with chronic or acute coronary syndromes
| Study . | Publication year . | Size . | Follow-up . | Treatment and comparator . | Primary endpoint . | Outcome . |
|---|---|---|---|---|---|---|
| Major studies comparing antiplatelet agents in acute coronary syndromes | ||||||
| ISIS-2106 | 1988 | 17 187 | 5 weeks | Aspirin vs. placebo | Vascular mortality at 5 weeks | 23% odds reduction |
| CURE110 | 2001 | 12 562 | 12 months | Aspirin + clopidogrel vs. aspirin + placebo | CV death, non-fatal MI, or stroke | 9.3% vs. 11.4%; RR 0.80 (0.72–0.90) |
| COMMIT109 | 2005 | 45 852 | 4 weeks | Aspirin + clopidogrel vs. aspirin + placebo | Death, re-infarction, or stroke | 9.2% vs. 10.1%: RR 0.91 (0.86–0.97) |
| CLARITY-TIMI 28113 | 2005 | 3491 | 30 days | Aspirin + clopidogrel vs. aspirin + placebo | Failed reperfusion or reocclusion, death from any cause | 15% vs. 21.7%; OR 0.64 (0.53–0.76) |
| TRITON-TIMI 38112 | 2007 | 13 608 | 1 year | Aspirin + prasugrel vs. aspirin + clopidogrel | CV death, non-fatal MI, or stroke | 9.9% vs. 12.1%; HR 0.81 (0.73–0.90) |
| PLATO114 | 2009 | 18 624 | 1 year | Aspirin + ticagrelor vs. aspirin + clopidogrel | CV death, MI stroke | 9.8% vs. 11.7%; HR 0.84 (0.77–0.92) |
| ISAR-REACT 5111 | 2019 | 4018 | 1 year | Aspirin + ticagrelor vs. aspirin + prasugrel | Death, MI, stroke | 9.3% vs. 6.9%; HR 1.36 (1.09–1.70) |
| Major studies comparing antiplatelet strategies in either chronic or acute coronary syndromes | ||||||
| PEGASUS-TIMI 54115 | 2017 | 21 162 | 33 months | Aspirin + ticagrelor 90 mg, aspirin + ticagrelor 60 mg, aspirin + placebo |
|
|
| DAPT108 | 2014 | 9961 | 30 months | After 12 months DAPT, aspirin + P2Y12 vs. aspirin + placebo for further 18 months | Death, MI, stroke, ST |
|
| TROPICAL-ACS116 | 2017 | 2610 | 1 year | Aspirin + prasugrel vs. aspirin + prasugrel 1 week then PFT guided clopidogrel | CV death, MI, stroke, BARC 2, or greater bleeding | 9% vs. 7%; HR 0.81 (0.62–1.06) |
| GLOBAL LEADERS117 | 2018 | 15 968 | 2 years | Aspirin + ticagrelor 1 month, then ticagrelor 23 months vs. aspirin + P2Y12 for 12 months, then aspirin monotherapy | All-cause death, non-fatal MI | 3.81% vs. 4.37%; rate ratio 0.87 (0.75–1.01) |
| Study . | Publication year . | Size . | Follow-up . | Treatment and comparator . | Primary endpoint . | Outcome . |
|---|---|---|---|---|---|---|
| Major studies comparing antiplatelet agents in acute coronary syndromes | ||||||
| ISIS-2106 | 1988 | 17 187 | 5 weeks | Aspirin vs. placebo | Vascular mortality at 5 weeks | 23% odds reduction |
| CURE110 | 2001 | 12 562 | 12 months | Aspirin + clopidogrel vs. aspirin + placebo | CV death, non-fatal MI, or stroke | 9.3% vs. 11.4%; RR 0.80 (0.72–0.90) |
| COMMIT109 | 2005 | 45 852 | 4 weeks | Aspirin + clopidogrel vs. aspirin + placebo | Death, re-infarction, or stroke | 9.2% vs. 10.1%: RR 0.91 (0.86–0.97) |
| CLARITY-TIMI 28113 | 2005 | 3491 | 30 days | Aspirin + clopidogrel vs. aspirin + placebo | Failed reperfusion or reocclusion, death from any cause | 15% vs. 21.7%; OR 0.64 (0.53–0.76) |
| TRITON-TIMI 38112 | 2007 | 13 608 | 1 year | Aspirin + prasugrel vs. aspirin + clopidogrel | CV death, non-fatal MI, or stroke | 9.9% vs. 12.1%; HR 0.81 (0.73–0.90) |
| PLATO114 | 2009 | 18 624 | 1 year | Aspirin + ticagrelor vs. aspirin + clopidogrel | CV death, MI stroke | 9.8% vs. 11.7%; HR 0.84 (0.77–0.92) |
| ISAR-REACT 5111 | 2019 | 4018 | 1 year | Aspirin + ticagrelor vs. aspirin + prasugrel | Death, MI, stroke | 9.3% vs. 6.9%; HR 1.36 (1.09–1.70) |
| Major studies comparing antiplatelet strategies in either chronic or acute coronary syndromes | ||||||
| PEGASUS-TIMI 54115 | 2017 | 21 162 | 33 months | Aspirin + ticagrelor 90 mg, aspirin + ticagrelor 60 mg, aspirin + placebo |
|
|
| DAPT108 | 2014 | 9961 | 30 months | After 12 months DAPT, aspirin + P2Y12 vs. aspirin + placebo for further 18 months | Death, MI, stroke, ST |
|
| TROPICAL-ACS116 | 2017 | 2610 | 1 year | Aspirin + prasugrel vs. aspirin + prasugrel 1 week then PFT guided clopidogrel | CV death, MI, stroke, BARC 2, or greater bleeding | 9% vs. 7%; HR 0.81 (0.62–1.06) |
| GLOBAL LEADERS117 | 2018 | 15 968 | 2 years | Aspirin + ticagrelor 1 month, then ticagrelor 23 months vs. aspirin + P2Y12 for 12 months, then aspirin monotherapy | All-cause death, non-fatal MI | 3.81% vs. 4.37%; rate ratio 0.87 (0.75–1.01) |
BARC, Bleeding Academic Research Consortium; CV, cardiovascular; DAPT, dual antiplatelet therapy; HR, hazard ratio; MACE, major adverse cardiovascular events; MI, myocardial infarction; OR, odds ratio; PFT, platelet function testing; RR, relative risk; ST, stent thrombosis; TIMI, Thrombolysis in Myocardial Infarction.
Pre-treatment with DAPT before PCI is no longer recommended based on data from the randomized PrehospitAl TicagreLor in ST-Segment ElevATIon Myocardial InfarCtion (ATLANTIC) trial in STEMI (where it reduced stent thrombosis, but not MACE123) and from the Timing of Oral P2Y12 Inhibitor Administration in Patients With Non-ST-Segment Elevation Acute Coronary Syndrome (DUBIUS) trial in NSTEMI124 as well as in real-world registries such as Swedish Coronary Angiography and Angioplasty Registry (SCAAR).125 Today, stent thrombosis occurs in only 1% or less. However, in the DAPT trial, stent thrombosis was responsible for 15% of recurrent ischaemic events in the placebo arm.108 Long-term DAPT mainly prevents ischaemic events in untreated coronary segments, thereby reducing non-stent-related MIs with marginal effects on stroke and venous thromboembolism (VTE).126 Yet, in the Prevention of cardiovascular Events (eG, death from heart and vascular disease, heart Attack, or stroke) in patientS with prior heart attack USing ticagrelor compared to placebo on a background of Aspirin (PEGASUS-TIMI) 54 trial, prolonged ticagrelor 60 mg bid, but not 90 mg bid, led to a significant stroke reduction on top of aspirin compared to aspirin alone.122
Conclusion
The benefit of continued DAPT after ACS is counterbalanced by increased major bleedings, which accrues over time and affects MACE, quality of life, and costs. Thus, an individualized DAPT duration using scores (i.e. PRECISE-DAPT score, among others) is recommended (Figure 6). Risk stratification for selection of intensity and duration of treatment should start with bleeding risk. Those at high bleeding risk should receive low intensity and shorter duration of DAPT, irrespective of ischaemic risk, while those at lower bleeding risk should receive intensive and/or prolonged DAPT.127 In the future, artificial intelligence may provide an even better risk prediction and hence a more personalized decision of SAPT or DAPT and the duration of each of these strategies.128
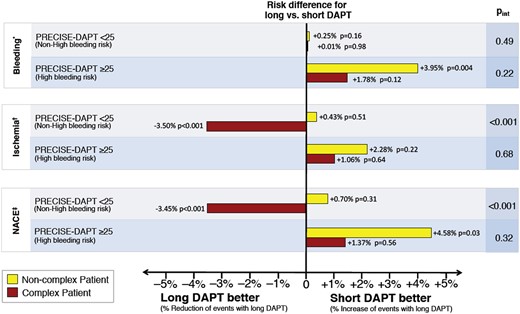
In patients at high bleeding risk, such as those with high PRECISE-DAPT score, dual antiplatelet therapy duration should be shortened to 6 months, whereas in those with low bleeding risk, prolonged dual antiplatelet therapy, possibly with a reduced dose of the P2Y12 inhibitor (i.e. ticagrelor 60 mg bid122), may be considered, especially with high-risk features such as multiple prior infarctions, multivessel disease, or the need for complex percutaneous coronary intervention. In patients in whom prolonged dual antiplatelet therapy poses concern, monotherapy with a P2Y12 inhibitor is an emerging treatment paradigm, especially in those with complex percutaneous coronary intervention.121 The primary endpoint of this analysis was out-of-hospital bleeding according to the Thrombosis in Myocardial Infarction definition, and occurring 7 days or later after the initial invasive procedure, while bleeding occurring earlier was censored. *Thrombosis in Myocardial Infarction major and/or minor bleeding definition. †Composite of myocardial infarction, stent thrombosis, stroke, or target vessel revascularization. ‡NACE, net adverse clinical events (composite of myocardial infarction, definite stent thrombosis, stroke, target vessel revascularization, or TIMI major and/or minor bleeding). DAPT, dual antiplatelet therapy; MACE, major adverse cardiovascular events (from reference 127, with permission).
Novel oral anticoagulants in acute coronary syndromes
Besides platelets and fibrin, neutrophils and macrophages contribute to an occluding clot129,130 due to neutrophil extracellular traps (NETs).131 Thus, inflammation begets coagulation via tissue factor expression and NET formation. Clot firmness is a crucial prognostic factor in ACS. Indeed, coagulation tests of clot turbidity and lysis time predict mortality and MACE.132,133 Thus, clot firmness is a novel risk factor for MACE in STEMI and a potential target of personalized anticoagulation in those with high thrombotic and low bleeding risk.
The role of NOACs in ACS without AF has been investigated in the ATLAS ACS 2-TIMI 51 trial. Rivaroxaban 2.5 mg bid, and less so 5 mg bid, reduced MACE and overall led to a mortality benefit but led—due to the triple antithrombotic regimen—to increased bleeding.134 Thus, NOACs are not recommended in ACS without AF. However, personalized OAC with SAPT rather than DAPT in patients with a propensity to form solid clots and a low bleeding risk might provide novel opportunities.132,133
Chronic coronary syndromes
In patients with chronic coronary syndromes, DAPT up to 6 months is recommended after PCI except in those at high bleeding risk in whom treatment for 1 month can be considered.135 In the Rivaroxaban for the prevention of major CardiOvascular Major events in coronary and Peripheral Artery disease (COMPASS) trial in patients with stable coronary artery disease (CAD), rivaroxaban 2.5 mg bid plus aspirin 100 mg reduced MACE by 10% but was associated with increased bleeding compared to aspirin alone. Rivaroxaban 5 mg bid alone did not result in better CV outcomes than aspirin alone and resulted in more major bleeding.134,136 Rivaroxaban 2.5 mg bid plus aspirin 100 mg reduced the risk of stroke, whereas that of new or recurrent MI was not significant. A sub-analysis of patients with prior ischaemic stroke showed that such patients treated with rivaroxaban plus aspirin had an absolute risk reduction of 2.8%.137
Genotyping of P2Y12 antagonists
Genetic variation in CYP2C19 leads in carriers of these loss-of-function alleles to reduced clopidogrel metabolite levels and high on-treatment platelet reactivity associated with an increased risk of MACE after PCI.138 Thus, prasugrel or ticagrelor should be considered in CYP2C19 poor metabolizers. Although such patients can be identified by genotyping, this is not recommended by ESC guidelines nor is it current practice. Proton pump inhibitors that inhibit CYP2C19, particularly omeprazole and esomeprazole, may reduce the pharmacodynamic response to clopidogrel and should be avoided. To help decision-making on the use clopidogrel using point-of-care genotyping, the TAILORed antiplatelet therapy following PCI (TAILOR-PCI) study found that in CYP2C19 loss-of-function carriers with ACS or CAD undergoing PCI, genotype-guided selection of a P2Y12 inhibitor, compared with conventional clopidogrel use, resulted in similar rates of CV death, MI, stroke, stent thrombosis, and severe recurrent ischaemia at 12 months.139
Conclusion
In chronic coronary syndromes, DAPT is recommended up to 6 months after PCI, except in those with high bleeding risk in whom it may be reduced to 1 month. Genetic testing for slow metabolizers does help in selecting P2Y12 inhibitors but is not common practice. Low-dose NOACs plus aspirin may be considered in those at high MACE and low bleeding risk.
Acute or chronic coronary syndromes with atrial fibrillation
At particularly high risk are patients with AF undergoing PCI. Although not perfect, a reduced NOAC dose with a P2Y12 inhibitor without aspirin markedly reduces bleeding risk.140,141 In the PreventION of blEEding in patients with atRial fibrillation undergoing PCI (PIONEER AF-PCI) trial, 2124 patients with AF and prior PCI were assigned to rivaroxaban 15 mg plus a P2Y12 inhibitor for 12 months, rivaroxaban 2.5 mg bid plus DAPT for 1, 6, or 12 months, or a VKA plus DAPT for 1, 6, or 12 months. There was less bleeding with rivaroxaban than VKA, while MACE were similar in all groups140 (Table 2). In EdoxabaN TReatment versUs vitamin K antagonist in patients with aTrial fibrillation undergoing Percutaneous Coronary Intervention (ENTRUST-AF PCI), patients with AF were randomly assigned after PCI to either edoxaban (60 or 30 mg/day with impaired CrCl) or VKA. Edoxaban-based regimen was non-inferior for bleeding to the VKA-based regimen, with a similar rate of ischaemic events.142 The A stUdy of apixaban in patients with atrial fibrillation, not caused by a heart valve problem, who are at risk for thrombosis (blood clots) due to havinG had a recent coronary event, sUch aS a heart aTtack or a procedUre to open the veSsels of the heart (AUGUSTUS) trial assigned in a two-by-two factorial design 4614 patients with AF and a recent ACS and/or PCI to apixaban 5 mg (or 2.5 mg bid in elderly, with low body weight or renal failure) or a VKA and to receive aspirin 81 mg or matching placebo for 6 months. Bleeding occurred in 10.5% receiving apixaban, but 14.7% in those receiving a VKA and in 16.1% of those receiving aspirin, but only 9.0% with placebo. Patients on apixaban had a 17% lower incidence of death or hospitalization than those on VKA, but a similar incidence of ischaemic events (Table 2). In contrast, patients on aspirin had a similar incidence of death or hospitalization and ischaemic events as those on placebo.141 Thus, patients with AF, a recent ACS, and/or PCI treated with a P2Y12 inhibitor, apixaban without aspirin, result in less bleeding and fewer hospitalizations without significant differences in MACE compared to VKA, aspirin, or both and seem currently the optimal management in these patients.
Finally, the RE-DUAL trial randomized 2725 CAD patients of whom half had an ACS to dabigatran 110 or 150 mg bid with a P2Y12 inhibitor, or warfarin with P2Y12 inhibitor and aspirin for 14 months. Major or clinically relevant non-major bleeding events were reduced with dual therapy with both dosages of dabigatran compared to warfarin triple therapy with HRs between 0.47 and 0.67 in ACS and 0.57 and 0.76 with elective PCI, respectively. For MACE, systemic embolism, or unplanned revascularization, dabigatran dual therapy was comparable to warfarin triple therapy (Table 2).143 Although these trials were not powered to show efficacy in preventing ischaemic events, there is clearly less bleeding with dual regimens.
Finally, a systematic review and meta-analysis encompassing 10 234 patients concluded that DAPT, in particular with an NOAC instead of a VKA and a P2Y12 inhibitor, is associated with reduced major and intracranial bleeding, but counterbalanced by a higher risk of cardiac, mainly stent related, but not cerebrovascular ischaemic events.144
Conclusion
The 2020 ESC guidelines for the management of ACS in patients presenting without persistent ST-segment elevation do recommend triple antithrombotic therapy for 1 week followed by an NOAC and preferably clopidogrel (to reduce bleeding).119 Beyond 12 months only oral anticoagulation may be considered.
Prevention of coronary artery disease
Until recently, aspirin has been recommended in the primary prevention of CAD or stroke. In the HOT trial enrolling patients with hypertension, aspirin 75 mg on top of antihypertensives reduced MACE by 15% and MI by 36%, with no effect on stroke. There were not more fatal, but more non-fatal major bleeds with aspirin.145 The more recent A Study of Cardiovascular Events In Diabetes (ASCEND) and AspiRin for pRImary preVEntion in a moderate cardiovascular risk cohort (ARRIVE) trials in diabetics and non-diabetics, respectively, showed no net benefit when MACE and bleeding were considered together.146,147 In the ASpirin in Reducing Events in the Elderly (ASPREE) trial enrolling elderly patients aged >70 years, aspirin was associated with increased bleeding without CV benefit (Figure 7).148 On the other hand, an analysis of patients with ACS showed that those with prior aspirin (particularly those with prior aspirin and statins) were less likely to experience STEMI, but rather NSTEMI.149 Of note, in the open-label, pragmatic Aspirin Dosing: A Patient-Centric Trial Assessing Benefits and Long-Term Effectiveness (ADAPTABLE) trial randomizing 15 076 patients with atherosclerotic CVD to 81 or 325 mg/day of aspirin, there were no differences in MACE or major bleeding between groups.150
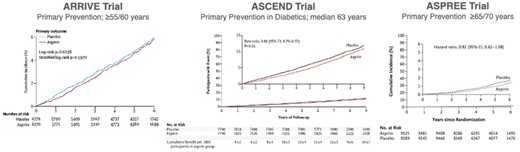
Effect of aspirin in primary prevention in apparently healthy individuals 55 (males) or 60 (females) years or older (ARRIVE trial),182 diabetics with a median age of 63 years (ASCEND trial)147 and healthy elderly of 70 years or older or 65 years and older in those with Afroamerican or Hispanic ethnicity (ASPREE trial).148 In ARRIVE, the primary efficacy endpoint was a composite outcome of time to first occurrence of cardiovascular death, myocardial infarction, unstable angina, stroke, or transient ischaemic attack. Safety endpoints were haemorrhagic events and incidence of other adverse events with no significant difference.146 The primary efficacy endpoint of ASCEND was the first myocardial infarction, stroke or transient ischaemic attack, or death from any vascular cause which reached significance (P < 0.01). However, the primary safety endpoints (i.e. first major or intracranial haemorrhage, sight-threatening eye or gastrointestinal, or other serious bleeding) counterbalanced the protective effects of aspirin.121 Secondary outcomes included gastrointestinal tract cancer. In ASPREE, the primary efficacy endpoint was a composite of death, dementia, or persistent physical disability. Secondary endpoints included major haemorrhage and fatal coronary artery disease, non-fatal myocardial infarction, fatal or non-fatal stroke, or hospitalization for heart failure (Modified from references 147, 148 and 182, with permission).148
Conclusion
Although aspirin may not prevent infarctions, it may transform STEMIs into NSTEMIs with less myocardial damage. However, in a recent meta-analysis,151 aspirin was not protective in primary prevention—possibly except for hypertensives and those at high ACS risk—and was associated with increased bleeding. In secondary prevention, 81 mg/day should be used.
Aortic plaques and ulcers
Aortic plaques and ulcers are prone to thrombus formation and embolization. Based on imaging,152 aortic plaques are staged in grade I (no or minimal intimal thickening), grade II (intimal thickening 1–3.9 mm without atheroma), grade III (Atheroma <4 mm), grade IV (intimal thickening or atheroma >4 mm), and grade V (mobile or ulcerated atheroma).153,154
The prospective APRIS study155 found a prevalence of 62% of aortic plaques in stroke-free patients, and the Framlingham Offspring Heart Study in 46% in those free of CVD.156 Aortic arch disease has a stronger association with cerebrovascular disease,157 but the evolution of aortic atheroma over time is dynamic depending on comorbidities such as diabetes, hypertension or smoking. The risk of embolization of plaques, cholesterol crystals, or thrombi158 increases with increasing grade and size of atheromas, particularly with grade V plaques, but not with low grade plaques.159,160 Recent guidelines161,162 make no recommendation on how to prevent aortic embolism, but recommend aspirin in patients with asymptomatic peripheral artery disease [PAD; ankle-brachial index (ABI) <0.9]. While the mere presence of aortic plaques should not mandate antiplatelet therapy, it should lower the threshold to start SAPT, while DAPT is not recommended and associated with an increased bleeding without reducing embolic events.163
While some trials suggested that anticoagulation might be superior to antiplatelet therapy,164 others could not confirm this.165 Regardless of the strategy, the risk of recurrent stroke remains high in those with high grade plaques. Similarly, large vessel vasculitis is associated with CV events due to inflammation-driven atherothrombosis, but warfarin had limited benefit in retrospective studies.166–169 More evidence is required to guide medical management of aortic plaques, particularly on the clinical effectiveness of statins in stabilizing high grade plaques as they reduce aortic plaque burden.170
Conclusion
Aortic plaques are a source of emboli, particularly with increasing size and mobility. There is no solid evidence for the use of antithrombotics; thus, the ESC guidelines on the diagnosis and treatment of aortic diseases recommend preventive measures like lifestyle modification and drugs to control hypertension and hypercholesterolaemia (Class I, Level C).171 Aspirin can be considered in those with comorbidities, particularly PAD.
Carotid artery disease
In carotid artery disease, antiplatelet therapy is indicated irrespective of clinical symptoms or revascularization. The benefits of SAPT in preventing stroke in asymptomatic patients with luminal narrowing >50% are not supported by randomized trials (Class IIa, level of evidence C).172 However, as patients are also at double risk of MI,173 aspirin or clopidogrel (particularly in those not supporting aspirin) seems justified. The combination of low-dose rivaroxaban (2.5 mg bid) with aspirin 100 mg was superior to monotherapy with aspirin in patients with asymptomatic carotid stenosis or after revascularization.174
DAPT is recommended for 1 month after carotid stenting (but not after endarterectomy) to prevent stent thrombosis.175,176 The usefulness of DAPT in patients with carotid stenosis is less clear. In the Clopidogrel for High Atherothrombotic Risk and Ischaemic Stabilization Management and Avoidance (CHARISMA) trial, 7% had asymptomatic carotid stenosis with no difference between DAPT and SAPT.177 The Clopidogrel and Aspirin for the Reduction of Emboli in Symptomatic carotid Stenosis (CARESS) study enrolled only 107 patients and found that DAPT reduced silent cerebral micro-emboli compared to aspirin.178
Finally, it appears that mechanical thrombectomy of acute intracranial internal carotid artery occlusion using retriever devices, alone or in combination with adjunctive endovascular therapy, could be considered as in smaller series it improved outcomes after stroke.179
Conclusion
SAPT is recommended in carotid artery disease, while DAPT has not consistently been shown to be superior but is used after stenting. Low-dose NOAC with aspirin is superior to aspirin alone in those with carotid disease and PAD. In selected patients with acute thrombotic internal carotid artery occlusion, mechanical thrombectomy might be considered.
Transient ischaemic attacks and stroke
Transient ischaemic attack (i.e. a neurological deficit of <1 h) or stroke has many causes, i.e. cardiac, aortic, or carotid emboli, small vessel disease, or ESUS.180 These conditions should be managed differently, both in primary and secondary prevention. In patients with suspected embolic stroke, the work-up may reveal a PFO, aortic or carotid plaques, while AF is difficult to exclude in those in sinus rhythm at presentation. In such cases, artificial intelligence has provided an algorithm that detects prior AF in an electrocardiogram (ECG) on sinus rhythm and may help to decide on OAC.181
Primary prevention
In the primary prevention of stroke, aspirin is not effective and has an increased bleeding risk. In the ARRIVE trial randomizing 12 546 individuals to aspirin or placebo for 60 months, stroke rates were identical as was bleeding.182 The ASPREE trial that recruited 19 114 healthy elderly to aspirin 100 mg or placebo found a non-significant reduction of stroke with aspirin from 3.9% to 3.5%, but a trend to more intracranial bleedings (2.5% vs. 1.7%).148 Finally, the ASCEND trial randomized 15 480 diabetics without CVD to aspirin 100 mg or placebo for 7 years. While overall, serious vascular events were lower with aspirin, strokes were not reduced, although numbers tended to be lower. There was more bleeding on aspirin, but similar rates of haemorrhagic stroke.147 Thus, aspirin cannot be recommended for the primary prevention of stroke and may increase the risk of bleeding.
Atherosclerotic intracranial stenosis is another cause of stroke, in particular in Asians. In the SAMMPRIS trial, 451 patients with a recent TIA or stroke and a 70–99% stenosis of a major intracranial artery were randomized to aspirin 325 mg, clopidogrel 75 mg for 90 days and CV risk factor control with or without balloon angioplasty and stenting. Enrolment was stopped prematurely because the 30-day rate of stroke or death was 14.7% in the stented and 5.8% in the medical group. Thus, in patients with intracranial arterial stenosis, aggressive medical management is superior to angioplasty and stenting.183
Secondary prevention
The 5-year risk of stroke and MACE after TIA or minor stroke (NIHHS scale 1–4) is currently 6.4% in the first year and 12.9% up to 5 years.184 The ProFESS trial investigated in a 2-by-2 factorial design recurrent stroke with either aspirin 25 mg plus extended release dipyridamole 200 mg bid or clopidogrel 75 mg in 20 332 patients followed for 2.5 years. Recurrent stroke occurred in 9.0% receiving aspirin plus extended release dipyridamole and in 8.8% on clopidogrel suggesting that both antiplatelet regimens are equal.185 In the Acute Stroke Or transient isChaemic Attack tReated with Aspirin or Ticagrelor and patient outcomES (SOCRATES) trial in patients with acute ischaemic stroke or TIA, ticagrelor was not found to be superior to aspirin in reducing stroke, MI, or death at 90 days.186
Whether DAPT is superior to SAPT was assessed in the MATCH trial in 7599 patients with recent ischaemic stroke or TIA and at least one additional CV risk factor. Ischaemic stroke, MI, vascular death, or rehospitalization for acute ischaemia (including TIA, angina pectoris, or worsening PAD) occurred in 15.7% of patients on aspirin plus clopidogrel compared to 16.7% with clopidogrel alone, while life-threatening and major bleedings were higher on DAPT compared to SAPT. Thus, adding aspirin to clopidogrel in high-risk patients with recent ischaemic stroke or TIA is associated with a non-significant reduction in vascular events, but with increased life-threatening or major bleeding.163
Short-term DAPT with clopidogrel and aspirin after a minor ischaemic stroke or TIA has been evaluated in the Platelet-Oriented INhibition in new TIA and minor ischaemic stroke (POINT) trial randomizing 4881 patients to clopidogrel at a loading dose of 600 mg on Day 1, followed by 75 mg plus aspirin at a dose of 50–325 mg/day or aspirin alone for 90 days. The trial was halted prematurely because clopidogrel and aspirin therapy was associated with a slightly lower risk of ischaemic events of 5.0% vs. 6.5%, but increased bleeding. Major haemorrhage occurred in 0.9% receiving clopidogrel plus aspirin and in 0.4% with aspirin alone.187
The Clopidogrel in High-risk patients with Acute Non-disabling Cerebrovascular Events (CHANCE) trial that randomized 5170 Chinese patients within 24 h after a minor stroke or high-risk TIA to either clopidogrel and aspirin for 90 and 21 days, respectively, or to placebo plus aspirin for 90 days reported a reduction of stroke from 11.7% to 8.2% with DAPT. Moderate or severe haemorrhage was rare and identical with both approaches both for ischaemic and haemorrhagic stroke (0.3%).188 The differences between the POINT and CHANCE trials as regards the risk of haemorrhage with DAPT could be due to ethnic and/or genetic differences. In patients with mild-to-moderate acute non-cardioembolic stroke or TIA not undergoing i.v. or endovascular thrombolysis, a most recent trial showed that the risk of stroke or death within 30 days was lower with DAPT including clopidogrel than with aspirin alone, but disability did not differ. DAPT with ticagrelor was associated with more severe bleeding.189
However, in the secondary prevention of minor ischaemic strokes or TIA, short-term DAPT for 3 months reduces the risk of recurrent ischaemic stroke by 41% and major vascular events by 30%, without increasing the risk of intracranial haemorrhage. On the other hand, DAPT beyond 1 year reduces the risk of recurrent ischaemic stroke only by 12% and major vascular events by just 10%, while the risk of major bleeding and intracranial haemorrhage is increased.190
In conclusion, DAPT after TIA or minor stroke is recommended for 10–21 days. For long-term prevention of recurrent stroke, SAPT with aspirin or clopidogrel is recommended. In acute ischaemic stroke or TIA, ticagrelor is not superior to aspirin in reducing stroke, MI, or death at 90 days.186 The benefit of prolonged DAPT in stroke is questionable as benefit and harm are almost equal.
Abdominal aortic aneurysms
Thoracic or abdominal aortic aneurysm (AAA) commonly develop intramural thrombi.191 The role of antiplatelets is controversial as they do not change growth and rupture of AAA.192,193 AAA are common, particularly in male smokers of advanced age and associated with a considerable mortality, if they grow larger.171 Commonly, AAA are covered with thrombi that can embolize and occlude distal arteries. Cholesterol emboli originating from aortic plaques or AAA may lead to renal failure and intestinal ischaemia, affect skeletal muscles and skin (i.e. livedo reticularis and blue toe syndrome), and may lead to an acute inflammatory response with fever, malaise, hypereosinophilia, and elevated erythrocyte sedimentation rate.194
AAA is lacking effective medical treatment. Although both anticoagulants and antiplatelets were promising in animal models, clinical studies showed no benefit.195 Even after endograft implantation, thromboembolic events or intramural thrombus accumulation is unaffected by OAC or SAPT.196 Therefore, intensification of antiplatelet therapy is not justified in patients with endografts and mural thrombus, but rather general CV prevention should be followed. Similarly, the role of OAC in AAA is controversial. An efficacy and safety study of RivarOxaban with warfarin for the prevention of stroKe and non-central nervous system systemic embolism in patients with non-valvular Atrial Fibrillation (ROCKET AF) and COMPASS with rivaroxaban showed benefit in PAD, but the number of patients with AAA has not been reported.174,197 Importantly, OAC in endovascular aneurysm repair-treated patients is of no advantage.198,199
Further reservation to the use of OAC is due to the difficulty to reverse in case of haemorrhage or contained rupture; these concerns might be mitigated with idarucizumab for dabigatran (see above) and in the future with reversible OAC such as RNA aptamers.200 After open endovascular aneurysm repair the situation is even more challenging, as OAC may lead to endoleaks and sac instability.199,201 Thus, neither antiplatelet nor OAC is recommended in the pre- or post-surgical or interventional setting of AAA aside from low-dose aspirin as secondary prevention of associated cerebrovascular disease.202
Conclusion
In AAA, antithrombotics should be used with caution and the indication rather based on comorbidities, e.g. aspirin in concomitant PAD or lower extremity arterial disease (LEAD) and OAC with concomitant AF.
Lower extremity arterial disease (LEAD)
LEAD is commonly due to atherosclerosis and rarely embolism.203 Regardless of symptoms, patients with LEAD have an increased CV risk.203,204 In symptomatic LEAD, the annual rate of MACE in the trial was 4–5%, and rates of major adverse limb events (MALE) only are 1–2%.205 Antithrombotic therapy in LEAD differs from CAD and has to be individualized depending on presentation and concomitant CAD or cerebrovascular disease.206,207
Asymptomatic lower extremity arterial disease
Two randomized trials, one in a general population including 3350 subjects with ABI <0.95208 and another one in 1276 diabetics with an ABI ≤0.99 found no benefit of aspirin as compared to placebo.209 However, in asymptomatic LEAD with symptomatic CAD or cerebrovascular disease, antithrombotics are recommended by current guidelines.172
Symptomatic lower extremity arterial disease
Single antiplatelet therapy is indicated in patients with symptomatic LEAD or after revascularization. In the A randomized, blinded, trial of Clopidogrel vs. Aspirin in Patients at Risk of Ischaemic Events (CAPRIE) recruiting patients with symptomatic LEAD clopidogrel was more effective than aspirin in reducing MACE—in contrast to those with coronary or cerebrovascular disease.210 The A study comparing cardiovascular Effects of ticagrelor and CLopIDogrel in patients with peripheral artery disease (EUCLID) trial compared ticagrelor with clopidogrel in 13 885 patients with symptomatic LEAD and found—in contrast to the A comParison of ticagreLor (AZD6140) and clopidogrel in pATients with acute cOronary syndrome (PLATO) trial in coronary patients114—no difference between the P2Y12 inhibitors.205 Based on the CAPRIE and EUCLID trial, clopidogrel is the preferred antiplatelet drug in symptomatic LEAD as it is more effective than aspirin with similar benefit as ticagrelor, comparable bleeding rate and lower costs. In a sub-analysis of the COMPASS trial, involving 7470 patients with symptomatic LEAD, rivaroxaban 2.5 mg bid plus aspirin compared with aspirin alone significantly reduced MACE (5.1% vs. 6.9%), MALE (0.9% vs. 2.4%) and major amputation (0.2% vs. 0.7%).174 However, the combined treatment minimally increased bleeding compared with aspirin. In the Rivaroxaban in Peripheral Arterial Disease after revascularization (VOYAGER PAD) trial, 6564 patients with LEAD who had undergone lower extremity revascularization were randomly assigned to rivaroxaban (2.5 mg bid) plus aspirin or placebo plus aspirin. Rivaroxaban plus aspirin was associated with a significantly lower incidence of the composite outcome of acute limb ischaemia, major amputation for vascular causes, MI, ischaemic stroke, or death from CV causes than aspirin alone (17.3% vs. 19.9% at 3 years), while TIMI major bleeding did not differ between groups.211 Of note, half of the patients were on clopidogrel at randomization.
Vorapaxar, a protease-activated receptor-1 antagonist that inhibits thrombin-induced platelet aggregation, was studied in combination with aspirin, clopidogrel, or both in 26 449 patients with a history of LEAD, MI, or ischaemic stroke. In those with symptomatic LEAD, vorapaxar did not reduce MACE but reduced acute limb ischaemia (2.3% vs. 3.9%) and peripheral revascularization (18.4% vs. 22.2.%), but the drug is not in clinical use.212
Currently, there are no data supporting the superiority of DAPT over aspirin or clopidogrel alone in reducing MACE in patients with LEAD before or after bypass surgery. In the CHARISMA trial recruiting 3096 patients with LEAD, DAPT reduced MI with no effect on other vascular events, while bleeding was increased.213 Similarly, after below-the-knee bypass grafting aspirin plus clopidogrel was not superior vs. aspirin in the Clopidogrel and Acetyl Salicylic acid in byPass surgery for peripheral ARterial disease (CASPAR) trial regarding index graft occlusion or revascularization, amputation or death.214
Current guideline recommendations after revascularization of LEAD vary and are not always evidence based.172,215 In the PEGASUS-TIMI 54 trial of 21 162 patients with prior MI, adding ticagrelor to aspirin reduced MACE.115 Among 1143 patients with MI and LEAD, ticagrelor plus aspirin also reduced acute limb ischaemia and peripheral revascularization (0.7% vs. 0.5% at 3 years). Whereas the relative risk reduction in MACE with ticagrelor was consistent, regardless of LEAD, patients with LEAD had a greater absolute risk reduction of 4.1% (number needed to treat: 25) due to their higher absolute risk. The absolute excess of TIMI major bleeding was 0.12%.216 Thus, DAPT in patients with LEAD should be restricted to those at risk of MI and after LEAD stenting or below-the-knee angioplasty.
OAC should only be considered if there is a concomitant indication and may be combined with aspirin or clopidogrel for a limited time after revascularization. Indeed, in the Dutch Bypass Oral Anticoagulants or Aspirin Study, no difference in peripheral graft patency, amputation, or mortality was found between aspirin or aspirin plus dipyridamole compared to a VKA, while major bleedings doubled with the latter.217 There were fewer venous bypass occlusions with a VKA compared to aspirin alone, a finding that could not be confirmed in another study.218 The WAVE trial randomized 2161 patients with LEAD to OAC plus antiplatelet therapy or antiplatelet therapy alone to prevent MACE.219 After 35 months, there were no differences in MACE or limb related acute ischaemic events. Life-threatening bleedings were, however, three-fold higher with warfarin.
Conclusion
In asymptomatic LEAD, no antithrombotics are recommended, while in symptomatic LEAD, clopidogrel is the antiplatelet drug of choice. Low-dose NOAC plus aspirin reduces MACE and MALE and major amputations, without significantly increasing bleeding risk. In LEAD patients after bypass surgery, OAC should only be considered if there is a comorbidity requiring it, but patient preferences should also be considered.
Venous thromboembolism
Venous thrombosis
Venous thrombi, typically in the lower limbs, cause swelling, pain, and trophic skin changes. They may embolize into the pulmonary circulation causing acute pulmonary hypertension, right ventricular failure, dyspnoea, syncope, haemodynamic shock, and sudden death. The major triggers of VTE are surgery or traumatic vascular injury, immobilization, inflammation and infection, cancer, and/or genetic predisposition (Table 1). Antiphospholipid syndrome is an acquired autoimmune disease that is characterized by thromboembolic events and the presence of antiphospholipid antibodies.220 While NOACs are commonly used in venous thrombosis, the use of rivaroxaban in high-risk patients with antiphospholipid syndrome provided no benefit and excess bleeding compared to warfarin.221 Further details on the management of deep vein thrombosis (DVT) are described in the joint consensus document of the ESC working groups of aorta and peripheral vascular diseases, and pulmonary circulation and right ventricular function.222
Pulmonary embolism
The algorithm proposed by the 2019 ESC guidelines for a risk-adjusted therapeutic approach to acute pulmonary embolism (PE) recommend parenteral or oral anticoagulation in the absence of haemodynamic compromise (Figure 8).223 While aspirin does reduce MACE in PE,224 NOACs such as rivaroxaban are much more effective.225 Subcutaneous, weight-adjusted LMWH or fondaparinux or NOACs are preferred over i.v. unfractionated heparin. Phase 3 trials on acute and long-term treatment of PE and DVT demonstrated non-inferior efficacy and superior safety of all NOACs compared to LMWH and VKA. Overall, recurrence of symptomatic or lethal VTE was 2.0% for NOACs and 2.2% for VKA, while major bleeding occurred in 1.1% and 1.7, respectively.226 Phase 3 trials with rivaroxaban and apixaban established the efficacy and safety of a single-oral-drug anticoagulation strategy with higher initial dosages (3 weeks rivaroxaban 30 mg, 7 days apixaban 10 mg).67,227,228 Normotensives with at least one risk factor for PE, or aggravating conditions or comorbidity, should be hospitalized (Figure 8). Rescue thrombolytic therapy or surgical embolectomy or percutaneous catheter-directed treatment should be reserved for those with haemodynamic instability.229 In high-risk PE, reperfusion, in most cases with thrombolysis, is the treatment of choice. Surgical pulmonary embolectomy or percutaneous catheter-directed treatment are alternatives in those with contraindications to systemic thrombolysis, if expertise and resources are available. Following haemodynamic stabilization, patients recovering from high-risk PE can be switched, based on clinical parameters, from parenteral to oral anticoagulation.
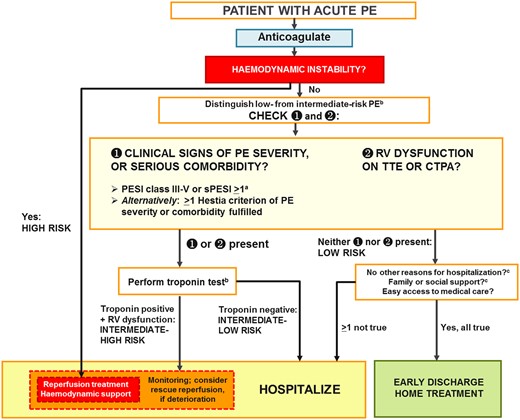
Risk-adjusted management strategy for acute pulmonary embolism. CTPA, computed tomography pulmonary angiography; PE, pulmonary embolism; PESI, Pulmonary Embolism Severity Index; RV, right ventricular; sPESI, simplified Pulmonary Embolism Severity Index; TTE, transthoracic echocardiogram. aCancer, heart failure, and chronic lung disease are the comorbidities included in the PESI and sPESI. bA cardiac troponin test may already have been performed during initial diagnostic work-up (e.g. in the chest pain unit). Troponin is proposed as the preferred biomarker, because it is the only one to have been used in an interventional trial.228 cIncluded in the Hestia criteria. Adapted from the 2019 ESC Guidelines on the diagnosis and management of acute pulmonary embolism (with permission).223
Home-based vs. hospital management
Early discharge of acute PE and home-based OAC should be considered if the risk of early death or serious complications is low, there are no serious comorbidities or aggravating conditions. The Hestia criteria230 or the Pulmonary Embolism Severity Index (PESI), in its original231 or simplified form (sPESI),232 may be used for clinical triage. Prognostic sensitivity may be further increased when clinical criteria are combined with imaging or laboratory biomarkers indicating right ventricular dysfunction.233 A multicentre trial investigated efficacy and safety of early discharge and outpatient rivaroxaban in patients selected by clinical criteria and no right ventricular dysfunction. The 3-month rate of symptomatic or fatal recurrent VTE was 0.6 with no PE-related deaths permitting early termination of the trial.234
Duration of oral anticoagulant
All PE patients should be anticoagulated for at least 3 months.235 Beyond this, the risk of VTE recurrence and that of bleeding has to be taken into consideration. Rivaroxaban (20 or 10 mg/day) was compared with aspirin (100 mg/day) in 3365 patients.225 Both dosages of rivaroxaban reduced symptomatic recurrent fatal or non-fatal VTE by ∼70% compared to aspirin with no differences in major or clinically relevant non-major bleeding.225 Similarly, apixaban (2.5 or 5 mg bid) reduced symptomatic recurrent VTE or death compared with placebo, with no safety concerns.236 Today, extended treatment, usually with an NOAC, is recommended after recurrent VTE and should at least be considered after a first event of DVT or PE in the absence of strong transient or reversible risk factors (i.e. major surgery, trauma, immobilization). Exceptions are patients with the antiphospholipid antibody syndrome, in whom indefinite anticoagulation with VKAs is recommended.237 Edoxaban or rivaroxaban are an effective and safe alternative to LMWH in cancer-associated DVT or PE, except patients with gastrointestinal tumours.238,239 Suggestions for anticoagulation and overall management of acute PE in specific clinical situations, for which no solid evidence exists, are summarized by the 2019 ESC guidelines.223
Conclusion
In uncomplicated PE, a single-oral-drug NOAC strategy with higher initial dosages (3 weeks rivaroxaban 30 mg, 7 days apixaban 10 mg) is recommended. If the risk of death or complications is low and without comorbidities or aggravating conditions, outpatient care is recommended. All PE patients should be anticoagulated for at least 3 months and prolonged OAC should be considered in those with a second PE or not obvious trigger.
Overall Conclusion
Personalized anticoagulation has to consider the clinical context, the patient characteristics (including genetics where appropriate), the expected efficacy vs. risk of bleeding related to different antithrombotic medications, and the patient’s preference. The fact that in many patients other clinical conditions such as ACS, CAD, AF, valvular heart disease, and/or PAD may also be present complicates matters substantially. Unfortunately, many trials have excluded such multimorbid patients and hence the evidence is somewhat limited in this setting. Particularly, challenging situations are patients with cerebral bleeding and ACS undergoing PCI. Similarly, some patients may receive several devices simultaneously or in staged procedures such as TAVI valves, LAA, and PFO occluders or MitraClips. Undoubtedly, the risk of complications and bleeding might be considerably higher in such patients requiring good clinical judgement and personalized decision-making within a Heart Team. Last but not least, patient-centred care has to consider the patient’s interests and preferences, particularly as regards the risks of lifelong antithrombotic medication vs. efficacy and safety of implantable devices.
Conflict of interest: T.F.L. has received honoraria and unrestricted educational and/or grants from Abbott, Amgen, Astrazeneca, Boehringer Ingelheim, Daichi-Sankyo, Medtronic, Novartis, Pfiter, Sanofi, and Servier unrelated to this article. J.A.C. discloses that he has received institutional grants personal fees from Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer/BMS and personal fees from Boston Scientific and Abbott. M.V. reports personal fees from Astra Zeneca, grants and personal fees from Terumo, personal fees from Alvimedica/CID, personal fees from Abbott Vascular, personal fees from Daiichi-Sankyo, personal fees from Opsens, personal fees from Bayer, personal fees from CoreFLOW, personal fees from Idorsia Pharmaceuticals Ltd, personal fees from University Basel, Department of Clinical Research, personal fees from Vifor, personal fees from Bristol Myers Squib SA, personal fees from iVascular, personal fees from Medscape, and personal fees from Biotronik, outside the submitted work. H.-C.D. reports grants and personal fees from Multiple, outside the submitted work; and in the last 3 years, H.-C.D. received honoraria for participation in clinical trials, contribution to advisory boards or oral presentations from: Abbott, BMS, Boehringer Ingelheim, Daiichi-Sankyo, Medtronic, Pfizer, Portola, Sanofi-Aventis, and WebMD Global. Financial support for research projects was provided by Boehringer Ingelheim. H.-C.D. received research grants from the German Research Council (DFG), German Ministry of Education and Research (BMBF), European Union, NIH, Bertelsmann Foundation, and Heinz-Nixdorf Foundation. S.V.K. reports grants and personal fees from Bayer AG, grants and personal fees from Daiichi-Sankyo, personal fees from Boston Scientific, personal fees from Bristol-Myers Squibb—Pfizer, personal fees from Novartis, and grants from Janssen (formerly Actelion), outside the submitted work. All other authors declared no conflict of interest.
References
- anticoagulants
- anticoagulation
- aorta
- aspirin
- atrial fibrillation
- anticoagulants, oral
- peripheral vascular diseases
- pulmonary embolism
- clopidogrel
- fibrinolytic agents
- coronary arteriosclerosis
- cardiovascular diseases
- left atrium
- left auricular appendage
- venous thrombosis
- hemorrhage
- blood platelets
- cerebrovascular accident
- ischemic stroke
- patent foramen ovale
- ulcer
- platelet inhibitors
- embolization
- cardiovascular system
- limb
- infarction
- brain
- genetics
- heart
- leg
- mortality
- secondary prevention
- thrombus
- embolism
- antagonists
- vitamin k antagonists
- rivaroxaban
- venous thromboembolism
- risk of excessive or recurrent bleeding
- dual anti-platelet therapy
- direct oral anticoagulants



