-
PDF
- Split View
-
Views
-
Cite
Cite
Arjun K Pandey, Deepak L Bhatt, Francesco Cosentino, Nikolaus Marx, Ori Rotstein, Bertram Pitt, Ambarish Pandey, Javed Butler, Subodh Verma, Non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease, European Heart Journal, Volume 43, Issue 31, 14 August 2022, Pages 2931–2945, https://doi.org/10.1093/eurheartj/ehac299
Close - Share Icon Share
Abstract
Despite existing treatments, patients with heart failure and chronic kidney disease (CKD) remain at high risk for adverse outcomes and progression to end-stage disease. Steroidal mineralocorticoid receptor antagonists (MRAs) such as spironolactone and eplerenone reduce mortality but remain under-prescribed due to the perceived risk of hyperkalaemia and hormonal side effects. The discovery of non-steroidal MRAs represents a major new dimension in cardiorenal disease therapy. Non-steroidal MRAs have high affinity and specificity for the mineralocorticoid receptor (MR) and differ from both steroidal agents and each other with respect to important physiochemical, pharmacodynamic, and pharmacokinetic parameters. Similar to their steroidal counterparts, they have beneficial anti-inflammatory, anti-remodelling, and anti-fibrotic properties in the kidneys, heart, and vasculature. There are several non-steroidal MRAs under development and clinical assessment; of these, only esaxerenone and finerenone are approved for treatment globally. In Japan, esaxerenone is approved for essential hypertension and has been studied in diabetic nephropathy. Compared with steroidal MRAs, finerenone more potently inhibits MR co-regulator recruitment and fibrosis and distributes more evenly between the heart and kidneys. The landmark Phase III trials FIGARO-DKD and FIDELIO-DKD demonstrated that finerenone-reduced major kidney and cardiovascular events on top of maximally tolerated renin–angiotensin–aldosterone system inhibition in patients with CKD associated with Type 2 diabetes. Non-steroidal MRAs are currently under evaluation in heart failure and for synergistic treatment with sodium–glucose contransporter 2 inhibitors. These ground-breaking agents could become an important therapy across the spectrum of cardiorenal disease.
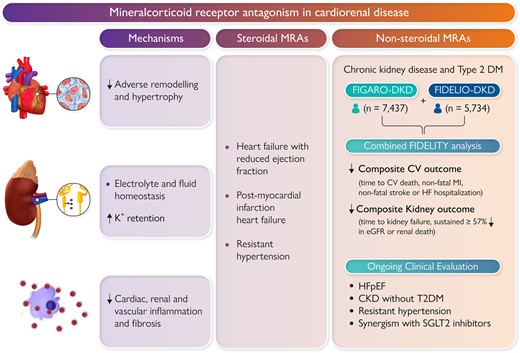
Non-steroidal mineralocorticoid antagonists (MRAs) reduce adverse remodelling, inflammation, and fibrosis in the heart, kidneys, and vasculature. Finerenone has been shown to improve cardiovascular and kidney outcomes in patients with chronic kidney disease and Type 2 diabetes. Non-steroidal MRAs are currently being investigated in other settings such as heart failure.
- Chapters
- descriptions off, selected
- captions settings, opens captions settings dialog
- captions off, selected
- en (Main), selected
This is a modal window.
Beginning of dialog window. Escape will cancel and close the window.
End of dialog window.
This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Cardiorenal syndrome characterizes the complex interplay between diseases such as chronic kidney disease (CKD) and heart failure (HF) and affects millions of people worldwide.1–3 Patients remain at persistently high risk for progression to end-stage disease even with existing treatments.1–3
Despite being launched over eight decades ago and demonstrating substantial mortality benefit in HF over two decades ago, clinical use of mineralocorticoid receptor antagonists (MRAs) has been limited by the perceived risk of hyperkalaemia and adverse hormonal side effects.4–6 The discovery of non-steroidal MRAs represents an important new dimension in the treatment of cardiorenal disease.4–6 Similar to their steroidal counterparts, these agents reduce fibrosis, inflammation, dysfunction, and adverse remodelling in the vasculature, kidney, and heart. This review will summarize the mechanisms and clinical evaluation of non-steroidal MRAs, as well as future perspectives on their role in the treatment of cardiorenal disease (Graphical Abstract).
Mineralocorticoid receptor structure, function, and physiological agonists/antagonists
Steroidal ligands interact with intracellular receptors, which normally reside in a transcriptionally inactive state in the cytoplasm.7–10 The activated receptors undergo conformational changes that enable translocation to the nucleus and interaction with a repertoire of co-regulator proteins that are receptor and cell-type-specific.7–10 The recruited co-activators or corepressors have many important functions, including regulation of transcription, epigenetics, and post-transcriptional modification.7–10 Steroidal ligands also have increasingly recognized non-genomic, rapid effects through various cellular signalling pathways as well.7–10
The mineralocorticoid receptor (MR) is a ligand-activated nuclear transcription factor expressed in many cell types around the body including in the gastrointestinal tract, heart, brain, kidneys, immune cells, and vasculature.4–6,8–10 There are at least 22 known cofactors for the MR that determine the transcriptional response in a cell-specific and ligand-specific manner.5,11 The MR has a similar affinity for many endogenous steroids including progesterone, cortisol, and aldosterone. In humans, progesterone, which served as the structural basis for preliminary steroidal MRAs, acts as a competitive MRA, although intracellular enzymatic metabolism may limit its effects.8,12–14 The relationship between aldosterone, cortisol, and the MR is more complex (Figure 1). The MR can be activated promiscuously by both aldosterone and cortisol, recruiting a largely overlapping repertoire of co-regulators, although the aldosterone-MR complex may be more stable.8,15–17 Mineralocorticoid receptor-expressing epithelial tissues such as intestinal and renal tubular cells express the enzyme 11β-HSD2 which converts cortisol into MR-inactive metabolites.8 In these cells, aldosterone therefore serves as the primary ligand for the MR. However, in other MR-expressing cell types such as macrophages and cardiomyocytes which do not express 11β-HSD2, it may be reasonable to assume that cortisol may be a critical regulator of MR activity, especially given that plasma concentration of cortisol is many-fold higher than that of aldosterone.8 Non-aldosterone-mediated MR activation in 11β-HSD2 unprotected cells such as cardiomyocytes and myeloid cells could thus be an important therapeutic target that may be unaddressed by existing renin–angiotensin–aldosterone system (RAAS)-modulating agents such as angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs).
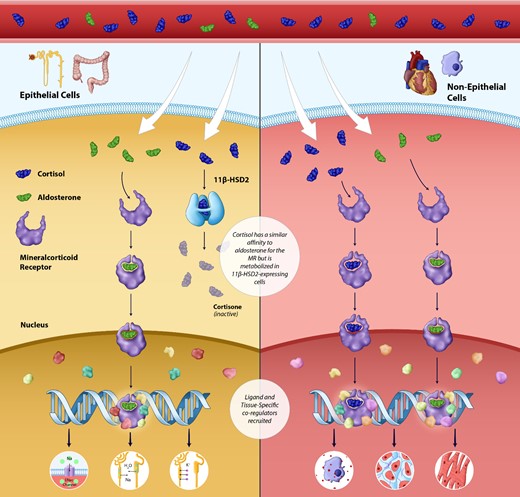
Physiology of the mineralocorticoid receptor: Activation in epithelial and non-epithelial cells. The mineralocorticoid receptor is a ligand-activated nuclear transcription factor expressed in a wide variety of tissues throughout the body. In epithelial cells, the mineralocorticoid receptor is activated solely by aldosterone (the enzyme 11 beta-hydroxysteroid dehydrogenase Type 2 metabolizes cortisol into the mineralocorticoid receptor inactive cortisone). Mineralocorticoid receptor activation in renal epithelial cells leads sodium and fluid retention and potassium excretion. In non-epithelial cells such as myeloid cells and macrophages, the mineralocorticoid receptor is likely activated by both cortisol and aldosterone; overactivation leads to pathological fibrosis, inflammation, and adverse remodelling in the heart, kidneys, and vasculature.
Consequences of activation and inhibition of the mineralocorticoid receptor
Preclinical and clinical research suggests an important dissociation between the MR-mediated effects on electrolyte and fluid homeostasis and the MR-mediated effects on organ remodelling, fibrosis, and dysfunction. The traditionally recognized function for the MR is in renal epithelial cells, where MR activation leads to sodium and fluid retention through increased epithelial sodium channel (ENaC) activity, in addition to K+ excretion.8,10,18 In the central nervous system, the MR may play a role in blood pressure regulation and in adipose tissue, overactivation may be implicated in insulin resistance and metabolic syndrome.8,10,18
In the heart, kidneys, and vasculature, overactivation of the MR is associated with tissue remodelling, dysfunction, inflammation, and fibrosis (Figure 2).6,19–21 The molecular and cellular mechanisms underscoring these processes has been reviewed in great depth previously and will be briefly summarized below.6,8,19
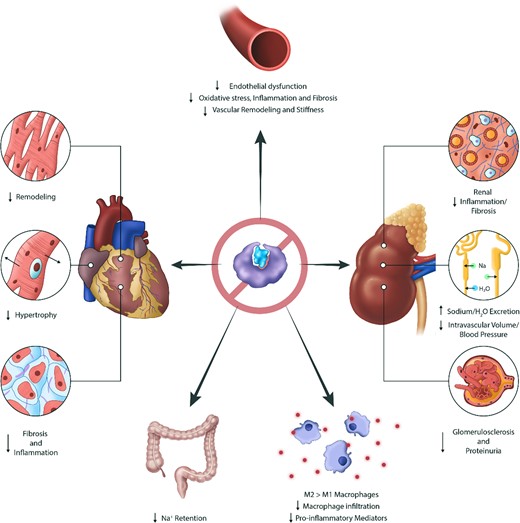
Effects of mineralocorticoid receptor inhibition across various organ systems. Mineralocorticoid receptor antagonism with steroidal or non-steroidal agents affects many organ systems. Mineralocorticoid receptor antagonists reduce sodium and fluid retention in the renal tubules and gastrointestinal tract. Mineralocorticoid receptor antagonists have beneficial anti-inflammatory, anti-remodelling, and anti-fibrotic properties in the kidneys, heart, and vasculature.
Preclinical models examining MR overexpression or tissue-selective knockout also suggest differences in MR function in epithelial compared with non-epithelial cells.22–33 In renal epithelial cells, the MR is primarily associated with electrolyte and fluid homeostasis, with knockout resulting in salt wasting due to decreased ENaC activity.22–24 Transcriptomic characterizations of MR activation in non-epithelial cells highlights its pro-inflammatory and pro-fibrotic effects: altered extracellular matrix regulation, increased M1 (pro-inflammatory) macrophage phenotype, pro-fibrotic mediators related to collagen synthesis (such as connective tissue growth factor) and oxidative stress (including NADPH oxidase) as well as pro-inflammatory cytokines and mediators including nuclear factor-κB, interleukin-1β, and tumour necrosis factor-α.8–10, 19,34–36 The confluence of these molecular pathways results in structural changes including cardiac hypertrophy, remodelling and fibrosis, vascular remodelling and endothelial dysfunction, as well as proteinuria and glomerular and tubular injury in the kidneys.8–10,34–36 Knockout of the MR in myeloid/macrophage cells is associated with reductions in renal inflammation and proteinuria (without potassium retention), as well as reductions in cardiac fibrosis, oxidative stress, and inflammation.28–33 In cardiomyocytes, MR knockout is associated with increased capillary density and reduced adverse remodelling, contractile dysfunction, and cardiac fibrosis.25–27
MR-induced inflammation, fibrosis, and remodelling represents an important treatment target in cardiorenal disease.4–6,10 Cell-type-specific MR antagonism in cardiomyocytes and myeloid cells could therefore be associated reduced inflammatory and fibrotic processes, organ dysfunction, and pathological remodelling while offering a reduced risk of hyperkalaemia that is associated with epithelial MR inhibition.5,10
Cardiorenal disease
Cardiorenal syndrome characterizes a spectrum of diseases involving metabolic, neurohormonal, haemodynamic, inflammatory, and fibrotic processes leading to heart and kidney dysfunction; one example is the complex interaction between HF and CKD.1–3 Chronic kidney disease is associated with a significantly increased risk of cardiovascular mortality and morbidity including HF. From a pathophysiological standpoint, chronic volume and pressure overload, anaemia, maladaptive neurohormonal regulation (including RAAS and sympathetic nervous system activation), oxidative stress, inflammation and fibrosis, as well as direct effects of uraemic toxins can lead to remodelling ultimately resulting in both heart and kidney failure.1–3 These metabolic, haemodynamic, and inflammatory/fibrotic processes each present important targets for therapies. Existing treatments for chronic cardiorenal disease include ACE inhibitors/ARBs, diuretics, sodium–glucose cotransporter 2 (SGLT2) inhibitors, β-blockers, as well as steroidal MRAs, which reduce morbidity and mortality.1–3
Steroidal MRAs: clinical evidence and limitations
Preliminary MRAs were analogues of progesterone, which acts as an endogenous antagonist of the MR.5,6,9 Further modification led to the discovery of potassium canrenoate, a parenterally administered MRA, as well as spironolactone which had substantially improved bioavailability.5,6,9 Spironolactone was launched as a diuretic/antihypertensive in 1960, but its anti-fibrotic and cardiac remodelling properties were not fully considered for decades.5,6,9Table 1 presents a list of published and ongoing major trials of steroidal (as well as non-steroidal) MRAs in cardiorenal disease.
Major trials of steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease
| Disease state . | Major trials of steroidal MRA . | Major trials of non-steroidal MRA . |
|---|---|---|
| HFrEF | RALES37 (n = 1663): Spironolactone reduced all-cause mortality in HFrEF (<35%); RR: 0.70 (95% CI: 0.60, 0.82) EMPHASIS-HF59 (n = 2737): Eplerenone reduced the composite of CV death or HF hospitalization in HFrEF (<35%); HR: 0.63 (95% CI: 0.54, 0.74) | |
| HFpEF | TOPCAT38 (n = 3445): No significant reduction in CV death, aborted cardiac arrest, or HF hospitalization was observed with spironolactone in HFpEF (≥45%); HR: 0.89 (95% CI: 0.77, 1.04) *A significant reduction in HF hospitalization was observed, and a significant reduction in the primary outcome was observed in sub-group analysis of patients in North/South America39 Ongoing: SPIRRIT-HFpEF40: n = 3200 patients with HFpEF (≥40%) planned to be randomized to spironalactone vs. standard of care; primary outcome: CV death or first HF hospitalization Ongoing: SPIRIT-HF41: n = 1300 patients with heart failure with mid-range (40–49%) or preserved (≥50%) ejection fraction planned to be randomized to spironolactone vs. placebo; primary outcome: total CV death and HF hospitalization | Ongoing: FINEARTS-HF125: n = 5500 patients with HFpEF (≥40%) planned to be randomized to finerenone vs. placebo; primary outcome: CV death and HF hospitalization |
| Post-MI | ALBATROSS61 (n = 1603): Potassium canrenoate followed by spironolactone failed to reduce the risk of death, resuscitated cardiac arrest, significant ventricular arrhythmia, indication for implantable defibrillator, or new or worsening HF in acute MI patients; HR: 0.97 (95% CI: 0.73, 1.28) REMINDER63 (n = 1012): No significant reduction in CV death, heart failure or sustained ventricular arrhythmia with eplerenone; HR: 0.55 (95% CI: 0.23, 1.35) | |
| Post-MI HFrEF | EPHESUS58 (n = 6642): Eplerenone reduced all-cause mortality in post-MI HFrEF (<40%); RR: 0.85 (95% CI: 0.75, 0.96) | |
| Resistant hypertension | PATHWAY-264 (n = 335): Treatment with spironolactone (–8.70 mmHg, 95% CI: −9.72 to −7.69) was superior to treatment with bisoprolol, doxazosin, or placebo for BP reduction on top of three maximally tolerated BP medications in resistant hypertension (with eGFR ≥45 mL/min/1.73 m2) | Ongoing: CLARION-CKD83: n = 600 patients with treatment-resistant hypertension, with 15 ≤ eGFR ≤44 mL/min/1.73 m2 on two or more maximally tolerated BP medications will be randomized to KBP-5074 vs. placebo; primary outcome: change in SBP |
| CKD with diabetes | FIDELIO-DKD113 (n = 5734): Treatment with finerenone resulted in a lower rate (HR: 0.82, 95% CI: 0.73, 0.93) of the primary renal outcome (kidney failure, a sustained decrease of at least 40% in the eGFR from baseline over a period of at least 4 weeks, or death from renal causes) in CKD patients with either: 1. Persistent albuminuria 30 to <300 mg/g, eGFR 25–59 mL/min/1.73 m2, and known diabetic retinopathy OR 2. Persistent albuminuria 300–5000 mg/g and eGFR 25–74 mL/min/1.73 m2 FIGARO-DKD114 (n = 7437): Treatment with finerenone resulted in a lower rate (HR: 0.87, 95% CI: 0.76, 0.98) of the composite of CV death, non-fatal MI, non-fatal stroke, or HF hospitalization in patients with CKD, T2DM, and either: 1. Persistent albuminuria 30 to <300 mg/g, eGFR 25–90 mL/min/1.73 m2, and known diabetic retinopathy OR 2. Persistent albuminuria 300–5000 mg/g and eGFR of at least 60 mL/min/1.73 m2 ESAX-DN94 (n = 455): Treatment with esaxerenone increased the rate (absolute difference: 18, 95% CI: 12, 25%) of urinary albumin-to-creatinine ratio remission (<30 mg/g creatinine and ≥30% reduction from baseline on two consecutive occasions) in T2DM and a urinary albumin-to-creatinine ratio of 45 to <300 mg/g creatinine. | |
| CKD without Diabetes | Ongoing: FIND-CKD124: n = 1580 patients with non-diabetic CKD (25 ≤ eGFR <90 mL/min/1.73 m2 and proteinuria/albuminuria) will be randomized to finerenone vs. placebo; primary outcome: rate of change in eGFR | |
| ESRD | Ongoing: ALCHEMIST71: n = 825 patients on chronic haemodialysis for ESRD regardless of aetiology planned to be randomized to spironolactone vs. placebo; primary outcome: CV death, HF hospitalization, non-fatal stroke, non-fatal MI, or ACS Ongoing: ACHIEVE70: n = 2750 patients on dialysis for ≥90 days planned to be randomized to spironolactone vs. placebo; primary outcome: CV death or HF hospitalization |
| Disease state . | Major trials of steroidal MRA . | Major trials of non-steroidal MRA . |
|---|---|---|
| HFrEF | RALES37 (n = 1663): Spironolactone reduced all-cause mortality in HFrEF (<35%); RR: 0.70 (95% CI: 0.60, 0.82) EMPHASIS-HF59 (n = 2737): Eplerenone reduced the composite of CV death or HF hospitalization in HFrEF (<35%); HR: 0.63 (95% CI: 0.54, 0.74) | |
| HFpEF | TOPCAT38 (n = 3445): No significant reduction in CV death, aborted cardiac arrest, or HF hospitalization was observed with spironolactone in HFpEF (≥45%); HR: 0.89 (95% CI: 0.77, 1.04) *A significant reduction in HF hospitalization was observed, and a significant reduction in the primary outcome was observed in sub-group analysis of patients in North/South America39 Ongoing: SPIRRIT-HFpEF40: n = 3200 patients with HFpEF (≥40%) planned to be randomized to spironalactone vs. standard of care; primary outcome: CV death or first HF hospitalization Ongoing: SPIRIT-HF41: n = 1300 patients with heart failure with mid-range (40–49%) or preserved (≥50%) ejection fraction planned to be randomized to spironolactone vs. placebo; primary outcome: total CV death and HF hospitalization | Ongoing: FINEARTS-HF125: n = 5500 patients with HFpEF (≥40%) planned to be randomized to finerenone vs. placebo; primary outcome: CV death and HF hospitalization |
| Post-MI | ALBATROSS61 (n = 1603): Potassium canrenoate followed by spironolactone failed to reduce the risk of death, resuscitated cardiac arrest, significant ventricular arrhythmia, indication for implantable defibrillator, or new or worsening HF in acute MI patients; HR: 0.97 (95% CI: 0.73, 1.28) REMINDER63 (n = 1012): No significant reduction in CV death, heart failure or sustained ventricular arrhythmia with eplerenone; HR: 0.55 (95% CI: 0.23, 1.35) | |
| Post-MI HFrEF | EPHESUS58 (n = 6642): Eplerenone reduced all-cause mortality in post-MI HFrEF (<40%); RR: 0.85 (95% CI: 0.75, 0.96) | |
| Resistant hypertension | PATHWAY-264 (n = 335): Treatment with spironolactone (–8.70 mmHg, 95% CI: −9.72 to −7.69) was superior to treatment with bisoprolol, doxazosin, or placebo for BP reduction on top of three maximally tolerated BP medications in resistant hypertension (with eGFR ≥45 mL/min/1.73 m2) | Ongoing: CLARION-CKD83: n = 600 patients with treatment-resistant hypertension, with 15 ≤ eGFR ≤44 mL/min/1.73 m2 on two or more maximally tolerated BP medications will be randomized to KBP-5074 vs. placebo; primary outcome: change in SBP |
| CKD with diabetes | FIDELIO-DKD113 (n = 5734): Treatment with finerenone resulted in a lower rate (HR: 0.82, 95% CI: 0.73, 0.93) of the primary renal outcome (kidney failure, a sustained decrease of at least 40% in the eGFR from baseline over a period of at least 4 weeks, or death from renal causes) in CKD patients with either: 1. Persistent albuminuria 30 to <300 mg/g, eGFR 25–59 mL/min/1.73 m2, and known diabetic retinopathy OR 2. Persistent albuminuria 300–5000 mg/g and eGFR 25–74 mL/min/1.73 m2 FIGARO-DKD114 (n = 7437): Treatment with finerenone resulted in a lower rate (HR: 0.87, 95% CI: 0.76, 0.98) of the composite of CV death, non-fatal MI, non-fatal stroke, or HF hospitalization in patients with CKD, T2DM, and either: 1. Persistent albuminuria 30 to <300 mg/g, eGFR 25–90 mL/min/1.73 m2, and known diabetic retinopathy OR 2. Persistent albuminuria 300–5000 mg/g and eGFR of at least 60 mL/min/1.73 m2 ESAX-DN94 (n = 455): Treatment with esaxerenone increased the rate (absolute difference: 18, 95% CI: 12, 25%) of urinary albumin-to-creatinine ratio remission (<30 mg/g creatinine and ≥30% reduction from baseline on two consecutive occasions) in T2DM and a urinary albumin-to-creatinine ratio of 45 to <300 mg/g creatinine. | |
| CKD without Diabetes | Ongoing: FIND-CKD124: n = 1580 patients with non-diabetic CKD (25 ≤ eGFR <90 mL/min/1.73 m2 and proteinuria/albuminuria) will be randomized to finerenone vs. placebo; primary outcome: rate of change in eGFR | |
| ESRD | Ongoing: ALCHEMIST71: n = 825 patients on chronic haemodialysis for ESRD regardless of aetiology planned to be randomized to spironolactone vs. placebo; primary outcome: CV death, HF hospitalization, non-fatal stroke, non-fatal MI, or ACS Ongoing: ACHIEVE70: n = 2750 patients on dialysis for ≥90 days planned to be randomized to spironolactone vs. placebo; primary outcome: CV death or HF hospitalization |
ACS, acute coronary syndrome; BP, blood pressure; CKD, chronic kidney disease; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; MI, myocardial infarction; RR, relative risk; SBP, systolic blood pressure; T2DM, Type 2 diabetes mellitus.
Major trials of steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal disease
| Disease state . | Major trials of steroidal MRA . | Major trials of non-steroidal MRA . |
|---|---|---|
| HFrEF | RALES37 (n = 1663): Spironolactone reduced all-cause mortality in HFrEF (<35%); RR: 0.70 (95% CI: 0.60, 0.82) EMPHASIS-HF59 (n = 2737): Eplerenone reduced the composite of CV death or HF hospitalization in HFrEF (<35%); HR: 0.63 (95% CI: 0.54, 0.74) | |
| HFpEF | TOPCAT38 (n = 3445): No significant reduction in CV death, aborted cardiac arrest, or HF hospitalization was observed with spironolactone in HFpEF (≥45%); HR: 0.89 (95% CI: 0.77, 1.04) *A significant reduction in HF hospitalization was observed, and a significant reduction in the primary outcome was observed in sub-group analysis of patients in North/South America39 Ongoing: SPIRRIT-HFpEF40: n = 3200 patients with HFpEF (≥40%) planned to be randomized to spironalactone vs. standard of care; primary outcome: CV death or first HF hospitalization Ongoing: SPIRIT-HF41: n = 1300 patients with heart failure with mid-range (40–49%) or preserved (≥50%) ejection fraction planned to be randomized to spironolactone vs. placebo; primary outcome: total CV death and HF hospitalization | Ongoing: FINEARTS-HF125: n = 5500 patients with HFpEF (≥40%) planned to be randomized to finerenone vs. placebo; primary outcome: CV death and HF hospitalization |
| Post-MI | ALBATROSS61 (n = 1603): Potassium canrenoate followed by spironolactone failed to reduce the risk of death, resuscitated cardiac arrest, significant ventricular arrhythmia, indication for implantable defibrillator, or new or worsening HF in acute MI patients; HR: 0.97 (95% CI: 0.73, 1.28) REMINDER63 (n = 1012): No significant reduction in CV death, heart failure or sustained ventricular arrhythmia with eplerenone; HR: 0.55 (95% CI: 0.23, 1.35) | |
| Post-MI HFrEF | EPHESUS58 (n = 6642): Eplerenone reduced all-cause mortality in post-MI HFrEF (<40%); RR: 0.85 (95% CI: 0.75, 0.96) | |
| Resistant hypertension | PATHWAY-264 (n = 335): Treatment with spironolactone (–8.70 mmHg, 95% CI: −9.72 to −7.69) was superior to treatment with bisoprolol, doxazosin, or placebo for BP reduction on top of three maximally tolerated BP medications in resistant hypertension (with eGFR ≥45 mL/min/1.73 m2) | Ongoing: CLARION-CKD83: n = 600 patients with treatment-resistant hypertension, with 15 ≤ eGFR ≤44 mL/min/1.73 m2 on two or more maximally tolerated BP medications will be randomized to KBP-5074 vs. placebo; primary outcome: change in SBP |
| CKD with diabetes | FIDELIO-DKD113 (n = 5734): Treatment with finerenone resulted in a lower rate (HR: 0.82, 95% CI: 0.73, 0.93) of the primary renal outcome (kidney failure, a sustained decrease of at least 40% in the eGFR from baseline over a period of at least 4 weeks, or death from renal causes) in CKD patients with either: 1. Persistent albuminuria 30 to <300 mg/g, eGFR 25–59 mL/min/1.73 m2, and known diabetic retinopathy OR 2. Persistent albuminuria 300–5000 mg/g and eGFR 25–74 mL/min/1.73 m2 FIGARO-DKD114 (n = 7437): Treatment with finerenone resulted in a lower rate (HR: 0.87, 95% CI: 0.76, 0.98) of the composite of CV death, non-fatal MI, non-fatal stroke, or HF hospitalization in patients with CKD, T2DM, and either: 1. Persistent albuminuria 30 to <300 mg/g, eGFR 25–90 mL/min/1.73 m2, and known diabetic retinopathy OR 2. Persistent albuminuria 300–5000 mg/g and eGFR of at least 60 mL/min/1.73 m2 ESAX-DN94 (n = 455): Treatment with esaxerenone increased the rate (absolute difference: 18, 95% CI: 12, 25%) of urinary albumin-to-creatinine ratio remission (<30 mg/g creatinine and ≥30% reduction from baseline on two consecutive occasions) in T2DM and a urinary albumin-to-creatinine ratio of 45 to <300 mg/g creatinine. | |
| CKD without Diabetes | Ongoing: FIND-CKD124: n = 1580 patients with non-diabetic CKD (25 ≤ eGFR <90 mL/min/1.73 m2 and proteinuria/albuminuria) will be randomized to finerenone vs. placebo; primary outcome: rate of change in eGFR | |
| ESRD | Ongoing: ALCHEMIST71: n = 825 patients on chronic haemodialysis for ESRD regardless of aetiology planned to be randomized to spironolactone vs. placebo; primary outcome: CV death, HF hospitalization, non-fatal stroke, non-fatal MI, or ACS Ongoing: ACHIEVE70: n = 2750 patients on dialysis for ≥90 days planned to be randomized to spironolactone vs. placebo; primary outcome: CV death or HF hospitalization |
| Disease state . | Major trials of steroidal MRA . | Major trials of non-steroidal MRA . |
|---|---|---|
| HFrEF | RALES37 (n = 1663): Spironolactone reduced all-cause mortality in HFrEF (<35%); RR: 0.70 (95% CI: 0.60, 0.82) EMPHASIS-HF59 (n = 2737): Eplerenone reduced the composite of CV death or HF hospitalization in HFrEF (<35%); HR: 0.63 (95% CI: 0.54, 0.74) | |
| HFpEF | TOPCAT38 (n = 3445): No significant reduction in CV death, aborted cardiac arrest, or HF hospitalization was observed with spironolactone in HFpEF (≥45%); HR: 0.89 (95% CI: 0.77, 1.04) *A significant reduction in HF hospitalization was observed, and a significant reduction in the primary outcome was observed in sub-group analysis of patients in North/South America39 Ongoing: SPIRRIT-HFpEF40: n = 3200 patients with HFpEF (≥40%) planned to be randomized to spironalactone vs. standard of care; primary outcome: CV death or first HF hospitalization Ongoing: SPIRIT-HF41: n = 1300 patients with heart failure with mid-range (40–49%) or preserved (≥50%) ejection fraction planned to be randomized to spironolactone vs. placebo; primary outcome: total CV death and HF hospitalization | Ongoing: FINEARTS-HF125: n = 5500 patients with HFpEF (≥40%) planned to be randomized to finerenone vs. placebo; primary outcome: CV death and HF hospitalization |
| Post-MI | ALBATROSS61 (n = 1603): Potassium canrenoate followed by spironolactone failed to reduce the risk of death, resuscitated cardiac arrest, significant ventricular arrhythmia, indication for implantable defibrillator, or new or worsening HF in acute MI patients; HR: 0.97 (95% CI: 0.73, 1.28) REMINDER63 (n = 1012): No significant reduction in CV death, heart failure or sustained ventricular arrhythmia with eplerenone; HR: 0.55 (95% CI: 0.23, 1.35) | |
| Post-MI HFrEF | EPHESUS58 (n = 6642): Eplerenone reduced all-cause mortality in post-MI HFrEF (<40%); RR: 0.85 (95% CI: 0.75, 0.96) | |
| Resistant hypertension | PATHWAY-264 (n = 335): Treatment with spironolactone (–8.70 mmHg, 95% CI: −9.72 to −7.69) was superior to treatment with bisoprolol, doxazosin, or placebo for BP reduction on top of three maximally tolerated BP medications in resistant hypertension (with eGFR ≥45 mL/min/1.73 m2) | Ongoing: CLARION-CKD83: n = 600 patients with treatment-resistant hypertension, with 15 ≤ eGFR ≤44 mL/min/1.73 m2 on two or more maximally tolerated BP medications will be randomized to KBP-5074 vs. placebo; primary outcome: change in SBP |
| CKD with diabetes | FIDELIO-DKD113 (n = 5734): Treatment with finerenone resulted in a lower rate (HR: 0.82, 95% CI: 0.73, 0.93) of the primary renal outcome (kidney failure, a sustained decrease of at least 40% in the eGFR from baseline over a period of at least 4 weeks, or death from renal causes) in CKD patients with either: 1. Persistent albuminuria 30 to <300 mg/g, eGFR 25–59 mL/min/1.73 m2, and known diabetic retinopathy OR 2. Persistent albuminuria 300–5000 mg/g and eGFR 25–74 mL/min/1.73 m2 FIGARO-DKD114 (n = 7437): Treatment with finerenone resulted in a lower rate (HR: 0.87, 95% CI: 0.76, 0.98) of the composite of CV death, non-fatal MI, non-fatal stroke, or HF hospitalization in patients with CKD, T2DM, and either: 1. Persistent albuminuria 30 to <300 mg/g, eGFR 25–90 mL/min/1.73 m2, and known diabetic retinopathy OR 2. Persistent albuminuria 300–5000 mg/g and eGFR of at least 60 mL/min/1.73 m2 ESAX-DN94 (n = 455): Treatment with esaxerenone increased the rate (absolute difference: 18, 95% CI: 12, 25%) of urinary albumin-to-creatinine ratio remission (<30 mg/g creatinine and ≥30% reduction from baseline on two consecutive occasions) in T2DM and a urinary albumin-to-creatinine ratio of 45 to <300 mg/g creatinine. | |
| CKD without Diabetes | Ongoing: FIND-CKD124: n = 1580 patients with non-diabetic CKD (25 ≤ eGFR <90 mL/min/1.73 m2 and proteinuria/albuminuria) will be randomized to finerenone vs. placebo; primary outcome: rate of change in eGFR | |
| ESRD | Ongoing: ALCHEMIST71: n = 825 patients on chronic haemodialysis for ESRD regardless of aetiology planned to be randomized to spironolactone vs. placebo; primary outcome: CV death, HF hospitalization, non-fatal stroke, non-fatal MI, or ACS Ongoing: ACHIEVE70: n = 2750 patients on dialysis for ≥90 days planned to be randomized to spironolactone vs. placebo; primary outcome: CV death or HF hospitalization |
ACS, acute coronary syndrome; BP, blood pressure; CKD, chronic kidney disease; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; MI, myocardial infarction; RR, relative risk; SBP, systolic blood pressure; T2DM, Type 2 diabetes mellitus.
The landmark RALES trial in 1999 demonstrated that spironolactone was associated with a 30% all-cause mortality reduction in HF with reduced ejection fraction (HFrEF, <35%) and New York Heart Association (NYHA) Class III–IV symptoms.37 The ground-breaking results of RALES has led to a substantial amount of research of steroidal MRAs in HF. In the TOPCAT trial, treatment with spironolactone in HF with preserved ejection fraction (HFpEF, ≥45%) did not significantly reduce the primary outcome, although a post hoc sub-group regional analysis did suggest a significant improvement in the primary outcome of cardiovascular mortality, HF hospitalization, or aborted cardiac arrest in a North and South American sub-groups.38,39 The ongoing SPIRRIT-HFpEF and SPIRIT-HF trials in HFpEF/HF with mid-range ejection fraction should further inform the efficacy of spironolactone in this setting.40,41 In the HOMAGE trial, spironolactone treatment was associated with reduced B-type natriuretic peptide (BNP) levels, inflammatory, thrombotic, and collagen metabolism biomarkers in patients at a high risk of HF.42 In several trials across various settings including post-myocardial infarction (MI), early-stage CKD, haemodialysis, and resistant hypertension, spironolactone has been shown to induce reverse remodelling, resulting in regression of elevated left ventricular mass index and reduced arterial stiffness. 43–48 In the observational COFFEE-IT study and the small randomized trial AREA-IN-CHF, canrenone (a metabolite of spironolactone) also suggested benefit in patients with HFpEF and HFrEF, respectively, with a lower associated rate of mortality and less remodelling.49,50 Two major limitations of first-generation MRAs such as spironolactone are the perceived risk of hyperkalaemia, especially in patients with reduced kidney function and using additional RAAS-blockade therapy, as well as hormonal side effects.4–6,10,51,52 Spironolactone has relatively poor specificity for the MR, and cross-reactivity with antagonism at the androgen receptor and agonism at the progesterone receptor result in side effects including erectile dysfunction, menstrual irregularities, and painful gynaecomastia.10,37,38,52,53
The second generation of MRAs were also steroid-based but were designed to be more specific to the MR to reduce these off-target side effects.5,6 The most prominent example is eplerenone, which contains an epoxide modification to the steroid backbone, resulting in a conformational alteration leading to much higher specificity for the MR at the cost of decreased affinity (as much as 20- to 40-fold lower affinity for the MR compared with spironolactone).54–57 Eplerenone has a much shorter half-life which has been suggested to be beneficial in reducing hyperkalaemia risk.5,56 In the 2003 EPHESUS trial, eplerenone, in the setting of post-MI HFrEF (<40%), reduced mortality and morbidity on top of standard of care therapy.58 In the setting of chronic HFrEF (≤30% or 30–35% with QRS >130 ms) in the EMPHASIS-HF trial, eplerenone reduced the risk of death and hospitalization on top of optimal therapy with ACE inhibitors/ARBs and β-blockers.59 Compared with spironolactone, treatment with eplerenone is associated with a lower rate of hormonal side effects such as erectile dysfunction and dysmenorrhoea due to increased MR specificity.56–60
Steroidal MRAs have been evaluated in other settings beyond HF as well (Table 1). In post-MI patients, intravenous potassium canrenoate and spironolactone failed to show any additional benefit on top of standard of care in the ALBATROSS and MINIMIZE STEMI trials.61,62 In the REMINDER trial, eplerenone did not demonstrate any clear clinical benefit in post-MI patients without HF, with reductions in the primary endpoint largely driven by lower BNP/N-terminal proBNP (NT-proBNP) levels.63 In resistant hypertension with estimated glomerular filtration rate (eGFR) ≥45 mL/min (on top of three maximally tolerated blood pressure medications), spironolactone was superior to bisoprolol, doxazosin, and placebo for blood pressure control in the PATHWAY-2 trial.64 Further mechanistic analysis suggested that the reason for this treatment benefit is because resistant hypertension may frequently be a salt-retaining, hyper-aldosterone state.65 Steroidal MRAs have also been evaluated to reduce CKD progression. In pooled analyses, spironolactone treatment is associated with reductions in blood pressure, left ventricular mass index, and proteinuria; the effect of steroidal MRAs on eGFR, major cardiovascular events and overall mortality when added to ACE inhibitors/ARBs in CKD has been less clear.66,67 While the risk of hyperkalaemia in this population is significant, the Spin-D and MiREnDa trials demonstrated this risk may be manageable even in end-stage renal disease when used in a protocolized manner.68,69 Evaluation of spironolactone on cardiovascular outcomes in dialysis patients is still an area of ongoing research with the ACHIEVE and ALCHEMIST trials.70,71 Other ongoing trials evaluating steroidal MRAs include post-percutaneous coronary intervention MI patients in the CLEAR-SYNERGY trial and post-operative patients (evaluating atrial fibrillation) in ALDOCURE.72,73
In summary, current-generation steroidal MRAs have beneficial anti-inflammatory, anti-remodelling and anti-fibrotic effects and have demonstrated life-saving properties in HF. Despite this, steroidal MRAs remain under-prescribed for treatment of HFrEF.74,75 After publication of the RALES trial, an increase in hyperkalaemia-associated morbidity and mortality was reported in Ontario, Canada.51 Further investigation demonstrated that a significant proportion of patients prescribed spironolactone had either relative or absolute contraindications to MRA therapy (such as elevated serum creatinine or elevated potassium in hospital).76 A similar analysis from Scotland showed no increase in hospital admissions for hyperkalaemia and decreasing rates of outpatient hyperkalaemia following the publication of RALES.77 Although hyperkalaemia is an important complication of MRA therapy, these results suggest that that the risk of hyperkalaemia could be minimized by careful monitoring and prescription of steroidal MRAs only in appropriate candidates. Limited educational initiatives as well as apprehension over the perceived risk of hyperkalaemia may have impeded adoption of steroidal MRAs, which remains significantly under-prescribed in patients with HF despite their demonstrated mortality benefit.74,75
Non-steroidal MRAs: mechanisms and clinical evaluation
Recent research has focused on non-steroidal MRA, which are compounds with MRA activity that have important physiochemical, pharmacodynamic, and pharmacokinetic differences compared with steroidal MRAs.4–6,10,11, Many of these non-steroidal MRAs were identified through high-throughput screening and their properties (including tissue distribution, half-life, affinity, specificity, and effect on co-factor regulation) differ both compared with steroidal MRAs and between each other as well.4–6,10,11 These compounds were designed to reduce hyperkalaemia and unwanted off-target effects; as a result, general commonalities of non-steroidal MRAs include high affinity, improved MR specificity and an improved therapeutic index [generally defined as the ratio of (drug) needed to cause a significant increase in potassium to the ratio of the (drug) needed to improve parameters such as albuminuria] compared with steroidal MRAs.4–6,10,11 There are several non-steroidal MRAs in various stages of development and clinical assessment; of these, esaxerenone and finerenone have the most comprehensive clinical data and are the only two that are currently approved for treatment in various regions.4–6,10,11
The clinical and preclinical assessment of several unapproved non-steroidal MRAs has been previously reviewed and will be briefly discussed. PF03882845 and LY2623091 were selective and orally bioavailable non-steroidal MRAs assessed at various preclinical and preliminary clinical stages before apparent discontinuation.4–6,78–80 In an animal model, the non-steroidal MRA KBP5074 demonstrated an improved therapeutic index compared with eplerenone, with less potassium retention associated with reductions in albuminuria.81 In the Phase IIB BLOCK-CKD trial, KBP5074 successfully reduced systolic blood pressure in Stage 3b/4 CKD patients with treatment-resistant/uncontrolled hypertension by 10.2 mmHg (placebo-corrected) and was associated with a relatively low hyperkalaemia risk.82 The ongoing Phase 3 Clarion-CKD is evaluating KBP5074 in the same setting.83 Apararenone is a non-steroidal MRA that was investigated in non-alcoholic steatohepatitis and diabetic nephropathy, where it was safe, tolerable, and significantly reduced the urinary albumin-to-creatinine ratio (UACR).5,84 AZD9977, proposed to be a selective-MR modulator, is a non-steroidal partial antagonist of the MR, resulting in an intermediate degree of co-factor recruitment (with less inhibition of co-regulator recruitment than the steroidal MRA eplerenone).85–88 This molecule has a notable effect on the AF2 region of the MR which is a crucial region for co-regulator interaction.78,85 AZD9977 reduced albuminuria and improved renal histopathological markers to a similar degree as eplerenone, and was associated with a smaller increase in urinary Na+/K+ ratio than eplerenone (indicating that it may result in reduced potassium retention).85–88 A new Phase 2 trial called MIRACLE has been launched in the setting of HF and CKD investigating the combination treatment with both dapagliflozin and AZD9977.89
Esaxerenone is a non-steroidal MRA which was approved in Japan for the treatment of essential hypertension in 2019.90 In preclinical investigations, esaxerenone was shown to inhibit MR-induced transcription more potently than spironolactone and eplerenone, with substantially higher selectivity for the MR as well.91 Esaxerenone has a long half-life (18–22 h), and in a preclinical mouse model demonstrated significant reductions in blood pressure, adverse cardiac remodelling and fibrosis.92 Compared head-to-head with spironolactone in a mouse model of Type 2 diabetes mellitus (T2DM), esaxerenone was associated with similar reductions in blood pressure but greater attenuation of renal fibrosis and inflammation as well as albuminuria.93 In the 52-week Phase III ESAX-DN trial, esaxerenone reduced albuminuria in patients with diabetic nephropathy and an elevated UACR, but in 4% of patients was associated with discontinuation due to hyperkalaemia (compared with 0.4% in the placebo arm).94 The Phase III ESAX-HTN trial in patients with essential hypertension showed that esaxerenone was well tolerated and at least as effective for blood pressure reduction as eplerenone; incidence of hyperkalaemia requiring discontinuation was low (<1%) with either drug.95 A post hoc analysis of this trial suggested that esaxerenone may reduce nocturnal blood pressure more significantly than eplerenone, especially in those with a non-dipping 24 h pattern.96
Finally, finerenone is a non-steroidal MRA approved by the Food and Drug Administration (FDA) to treat patients with CKD associated with T2DM.97 Finerenone was derived from the chemical optimization of a group of dihydropyridines identified to be potent MRAs through high-throughput screening.4,5,6,98 Preclinical investigations of finerenone have been previously reviewed in detail. Finerenone is more specific for the MR than eplerenone and spironolactone, with very little cross-reactive activity at additional steroid receptors and various other receptors and ion channels including the L-type Ca2+ channel.98,99 Finerenone has a comparable potency to spironolactone (both of which are significantly more potent than eplerenone) regardless of whether the MR steroidal agonist tested against was cortisol or aldosterone.6 Finerenone exhibits so-called ‘bulky,’ passive MR antagonism resulting in greater reductions in nuclear translocation as well as co-factor and RNA polymerase recruitment compared with spironolactone and eplerenone (Figure 3).100–102 Finerenone appears to act as inverse agonist, inhibiting basal co-factor recruitment even in absence of aldosterone; steroidal agonists such as eplerenone, by contrast, appear to act as partial agonists, inducing coactivator binding at high concentrations.100–103 This appears to have important downstream effects; transcriptomic characterization in several preclinical models demonstrated greater inhibition of pro-fibrotic gene expression with finerenone compared with spironolactone and eplerenone.100–102
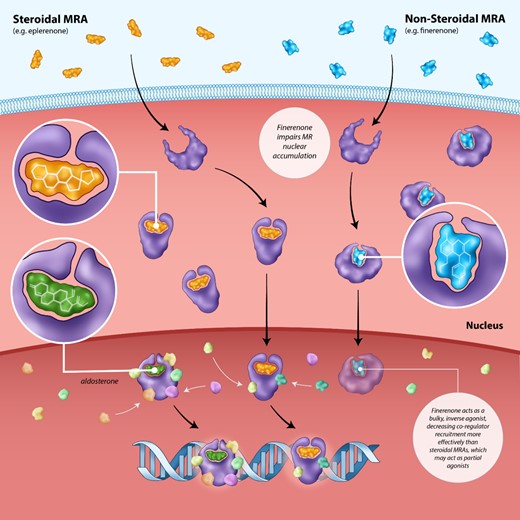
Inhibition of co-regulator recruitment by the non-steroidal mineralocorticoid receptor antagonist finerenone vs. steroidal mineralocorticoid receptor antagonists. The binding of aldosterone or cortisol to the mineralocorticoid receptor results in nuclear translocation and the recruitment of tissue-specific co-regulator which are critical to its activity. The non-steroidal mineralocorticoid receptor antagonist finerenone impairs nuclear translocation and co-regulator recruitment more efficiently than steroidal mineralocorticoid receptor antagonists such as spironolactone. Finerenone acts as a bulky, inverse agonist, inhibiting co-regulator recruitment even in the absence of aldosterone. On the other hand, steroidal mineralocorticoid receptor antagonists may serve as partial agonists, resulting in some level of co-regulator recruitment at high concentrations.
Compared with steroid-based MRAs, finerenone also has vastly different physiochemical properties, such as markedly lower lipophilicity and higher polarity, that confer important differences in distribution and tissue penetration.5,6 Unlike lipophilic steroidal MRAs, finerenone does not cross the blood brain barrier. Compared with [H3] labelled spironolactone and [C14] labelled eplerenone which have much greater (at least three-fold) accumulation in the kidney, [C14] labelled finerenone has an equal distribution in the heart and kidneys.5,6,103 Finerenone has no active metabolites and has a short half-life, which may enable more rapid reversal of hyperkalaemia; spironolactone, in contrast, along with its metabolites, may remain present in the body for up to 3 weeks after treatment.103,104 Some have suggested that the shorter half-life and more balanced tissue distribution could result in a reduced risk of hyperkalaemia compared with steroidal MRAs, although this has yet to be clinically validated in an appropriately powered study.
Preclinical studies suggest that these differences in co-factor recruitment and tissue distribution may have important end-organ cardiorenal consequences.100–103,105–109 At equinatriuretic doses, finerenone-reduced myocardial and renal hypertrophy, BNP, and proteinuria, as well as pro-inflammatory/pro-fibrotic gene expression in both cardiac and renal tissue more than eplerenone in animal models.101,105,106
The clinical evaluation of finerenone has been conducted in the setting of CKD, diabetes, and HF. In the Phase II ARTS-HF trial, among patients with HFrEF and coexisting T2DM/CKD, treatment with finerenone and eplerenone were associated with a similar reduction in NT-proBNP.110 Notably, treatment with finerenone at a dosage of 10 to 20 mg was associated with a significant reduction in the composite of all-cause mortality, cardiovascular hospitalization, or emergency HF presentation compared with eplerenone (although the trial was not designed/powered to assess for this outcome).110 Both drugs were associated with a similar rate of hyperkalaemia.110 A proposed trial entitled FINESSE-HF on this question, comparing eplerenone with finerenone in HFrEF, was discontinued before enrolling any patients, presumably for sponsor strategic reasons.111 In the ARTS-DN trial, which examined CKD patients with T2DM, finerenone was associated with reduced albuminuria and a low rate of hyperkalaemia (<2%).112 No significant reduction in blood pressure was observed with finerenone, and improvements in UACR were independent of changes in blood pressure/eGFR suggesting a non-haemodynamic (i.e. anti-fibrotic/remodelling) mechanisms.112 The Phase II ARTS in patients with CKD and HFrEF demonstrated similar reductions in NT-proBNP and albuminuria compared with spironolactone, with a smaller increase in serum potassium.104 The dosage of finerenone most patients received in the ARTS trial was lower than is being investigated in Phase III trials such as FIDELIO-DKD and FIGARO-DKD, however, and the small sample size of ARTS precludes any definitive conclusions on hyperkalaemia.104,113,114 Overall, these preliminary trials showed promising results for finerenone in several settings, but were not powered to draw informed conclusions about effects on clinical outcomes and hyperkalaemia compared with steroidal agents.
In the landmark Phase III FIDELIO-DKD and FIGARO-DKD trials of CKD patients with diabetes, finerenone, when used on top of maximally tolerated RAAS inhibitor treatment, was shown to be both renally protective (reducing CKD progression as assessed by the composite of kidney failure/death from renal causes) and to reduce cardiovascular outcomes (including the composite of cardiovascular death, HF hospitalization, non-fatal MI and non-fatal stroke).113,114 In FIDELIO-DKD, patients either had a UACR of 30–300 mg/g with an eGFR of 25–60 mL/min/1.73 m2 of body surface area, as well as diabetic retinopathy or a UACR of 300–5000 mg/g with an eGFR of at least 25–75 mL/min/1.73 m2 of body surface area.113 The inclusion criteria of FIGARO-DKD were similar, with patients either having a UACR of 30–300 mg/g with an eGFR of 25–90 mL/min/1.73 m2 of body surface area or a UACR of 300–5000 mg/g with an eGFR of at least 60 mL/min/1.73 m2 of body surface area.114 Importantly, patients with symptomatic (NYHA Class II–IV) HFrEF were not eligible for inclusion in either trial.113,114 The results of these two trials were combined in the pre-specified FIDELITY analysis (n = 13,026), which confirmed the clinical benefits of this agent, reduction in composite cardiovascular [hazard ratio (HR): 0.86, 95% confidence interval (CI): 0.78, 0.95] and composite kidney outcomes (HR: 0.77, 95% CI: 0.67, 0.88), across the spectrum of CKD in diabetes patients (already on maximally tolerated dose of RAAS inhibitor).115 Reductions in the composite cardiovascular outcome were driven by reductions in HF hospitalization (HR: 0.78, 95% CI: 0.66, 0.92).115 Change in blood pressure with finerenone were only modest.113–115
In the pooled FIDELITY analysis, investigator-reported hyperkalaemia occurred in 14.0% of patients receiving finerenone compared with 6.9% of patients in the placebo arm, although only led to discontinuation in 1.7 and 0.6%, respectively.115 Hyperkalaemia resulting in hospitalization occurred in 0.9% of patients receiving finerenone and 0.2% of patients on placebo and was not fatal in any patients in the FIDELITY analysis. 115 In multivariate analysis of the FIDELIO-DKD trial, higher baseline potassium and lower baseline eGFR were associated with higher risks of hyperkalaemia whereas baseline treatment with SGLT2 inhibitor or diuretic was associated with a lower risk of hyperkalaemia.116 Finerenone was otherwise well tolerated across the FIDELITY analysis, with no increased rate of acute kidney injury or gynaecomastia reported compared with placebo.115
Importantly, although 45.6% of patients in FIDELITY had a history of cardiovascular disease and 7.7% had a history of HF, patients with symptomatic (NYHA Class II–IV) HFrEF were not eligible for inclusion in either trial.113,114 A pre-specified sub-group analysis of the FIDELIO-DKD demonstrated consistent benefits in the composite cardiac and kidney endpoint irrespective of baseline HF history.117 A sub-analysis of the FIGARO-DKD trial demonstrated a significant reduction in the risk of new-onset HF (HR: 0.68, 95% CI: 0.50, 0.93); interestingly, an exploratory analysis suggested that this reduction may have been even greater in patients receiving SGLT2 inhibitors at baseline.118 Finerenone also reduced the incidence of new-onset atrial fibrillation or flutter (HR: 0.71, 95% CI: 0.53, 0.94) in a pre-specified analysis of the FIDELIO-DKD trial as well.119
In FIDELITY, patients had T2DM for a mean 15.4 years [standard deviation (SD): 8.7] with a mean baseline glycated haemoglobin (HbA1c) of 7.7% (SD: 1.4).115 Reductions in the composite cardiac and kidney endpoint were irrespective of baseline HbA1c in a sub-analysis of FIDELIO-DKD.120 While 97.7% of patients were on glucose-lowering therapies, only 6.7 and 7.2% were receiving SGLT2 inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists, respectively.115,121,122 In two sub-analyses of FIDELIO-DKD, there was no significant interaction between the effects of finerenone on the composite cardiac and kidney endpoints and treatment with either SGLT2 inhibitors or GLP-1 receptor agonists.121,122
Based on the results of these trials, finerenone was approved by the FDA to reduce the risk of sustained eGFR decline, end-stage kidney disease, cardiovascular death, non-fatal MI and hospitalization for HF in CKD patients with TD2M (an indication not granted for any previous MRA).97 These results seem applicable to a broad range of patients in actual clinical practice. A recent estimate using data sets from the National Health and Nutrition Examination Survey suggested that over 2 million patients in the United States would qualify for treatment based on either FIDELIO-DKD or FIGARO-DKD trial criteria.123
The recently announced Phase III trial FIND-CKD is designed to investigate the effectiveness of finerenone to prevent progression of kidney disease in patients with non-diabetic CKD.124 The ongoing Phase III trial FINEARTS-HF is evaluating the effects of finerenone on HF hospitalization/cardiovascular death in the setting of HFpEF.125
In summary, the preclinical and clinical data for finerenone suggest it may be an important therapeutic agent in cardiorenal disease, exerting its effects through a unique, blood pressure-independent mechanism via potent inhibition of MR-induced cardiac and kidney fibrosis, inflammation, and remodelling. The FIGARO-DKD and FIDELIO-DKD trials indicate that this drug may substantially improve clinical outcomes in a broad spectrum of patients with T2DM and CKD already treated with ACE inhibitors or ARBs. Direct head-to-head comparisons of non-steroidal MRAs and steroidal MRAs in other settings such as HFrEF are warranted before finerenone can be definitively established as superior or safer than steroidal MRAs for these indications.
Future perspectives
The FIDELIO-DKD and FIGARO-DKD trials demonstrated that a non-steroidal MRA can safely improve cardiac and kidney outcomes in patients already treated with a maximally tolerated ACE inhibitor or ARB in diabetic CKD patients113,114; evaluation of this combination is ongoing in non-diabetic CKD and may be warranted in other disease states such as HFrEF as well, especially in comparison to steroidal MRAs which have already been shown to save lives in this population.37,59
Various other proposed methods of inhibiting the MR signal transduction pathway have been proposed or are currently under evaluation.10 These include aldosterone synthesis inhibitors, which have shown promising anti-remodelling properties in preclinical work but are currently limited by poor selectivity for the aldosterone synthase enzyme, as well as alterations to the MR chaperone state, epigenetic regulation, miRNAs, and targeted inhibition of effector molecules downstream from the MR.10,126 In addition, antibody-complexed MRAs or other mechanisms such as liposomes to deliver inhibitors to specific cell types (e.g. cardiomyocytes) could help to limit the risk of hyperkalaemia or hormonal side effects.10 Finally, evaluation of the potential synergistic properties of non-steroidal MRAs with other agents such as SGLT2 inhibitors and GLP-1 agonists is also warranted, especially in light of sub-group analyses from the FIDELIO-DKD trial which showed lower rates of hyperkalaemia in patients receiving SGLT2 inhibitors and potential synergism for reductions in new-onset HF.116,121,122 In a mouse model of hypertension-induced end-organ damage, finerenone and empagliflozin provided synergistic and in some cases, over-additive effects in terms of reductions in proteinuria, as well as in cardiac and renal fibrosis.107 The MIRACLE trial is presently investigating the non-steroidal MRA AZD9977 and dapagliflozin in the setting of HF and CKD.89 The recently announced CONFIDENCE trial will investigate finerenone in combination with empagliflozin compared with each medication individually in the setting of diabetes and CKD.127 The combination of non-steroidal MRAs with potassium-binding agents could potentially enable high-dose MRA therapy with a reduced risk of hyperkalaemia; such trials are already underway with steroidal MRAs and the potassium-binding agent patiromer.128–130 The recently presented results from the DIAMOND trial showed that, in patients with HFrEF and either a previous history of hyperkalaemia or hyperkalaemia at baseline, co-administration of patiromer was associated with a smaller change in serum potassium, a lower rate of hyperkalaemia and less frequent reduction in MRA dose below target compared with placebo.129,130 As further evidence accumulates, potassium-binding agents such as patiromer may become more broadly applied for managing the risk of hyperkalaemia in patients on MRA therapy.
Thus, although non-steroidal MRAs remain under active investigation, the accumulating preclinical and clinical evidence points to a bright future for these agents in the treatment of cardiorenal disease. Important pharmacokinetic and pharmacodynamic differences between individual non-steroidal MRAs may lead specific agents to be more effective in certain settings. Those with long half-lives such as KBP5074 or esaxerenone may be more effective in blood pressure reduction and could prove to be beneficial in reducing ischaemic events, although further clinical evaluation and evidence are needed.11 On the other hand, the non-steroidal agent finerenone has a short half-life and a modest effect on blood pressure, but very potent anti-fibrotic and anti-remodelling effects.11 In two ground-breaking trials, finerenone has demonstrated efficacy in reducing major cardiac and kidney outcomes in patients with CKD and T2DM, a treatment indication unique to this agent which should soon be reflected in updated clinical guidelines. Based on trial inclusion criteria, estimates suggest that these results may be applicable to millions of patients in the United States alone.123 Evaluation of finerenone in HFpEF and non-diabetic CKD, two settings where clinical outcomes and progression to end-stage disease remain problematic, is ongoing.124,125 For the time being, steroidal MRAs, which are generic and significantly less expensive, will likely maintain an important role in the treatment of cardiorenal disease. Spironolactone and eplerenone remain the only two MRAs proven to reduce mortality in HFrEF. Further head-to-head evaluation is needed to establish whether non-steroidal MRAs are superior to steroidal agents in settings such as HFrEF, as well as whether they are associated with reduced rates of hyperkalaemia. Physician education and judicious, evidence-based usage of MRAs will be crucial to maximize the benefit of these agents. As the clinical evidence continues to evolve and amass, non-steroidal MRAs could become an important therapy across the spectrum of cardiorenal disease.
Acknowledgements
The authors would like to thank Cassie Hillock-Watling for assistance with preparing the figures.
Funding
N/A
References
Author notes
Conflict of interest: A.K.P. has no relevant disclosures. D.L.B. discloses the following relationships—Advisory Board: Bayer, Boehringer Ingelheim, Cardax, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, Janssen, Level Ex, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, Stasys; Board of Directors: Boston VA Research Institute, DRS.LINQ (stock options), Society of Cardiovascular Patient Care, TobeSoft; Chair: Inaugural Chair, American Heart Association Quality Oversight Committee; Data Monitoring Committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical), Novartis, Population Health Research Institute; Rutgers University (for the NIH-funded MINT Trial); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees), Wiley (steering committee); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Aker Biomarine, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, 89Bio; Royalties: Elsevier (Editor, Braunwald’s Heart Disease); Site Co-Investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Takeda. Dr. Francesco Cosentino has received research grants from the Swedish Research Council, Swedish Heart & Lung Foundation, and King Gustav V and Queen Victoria Foundation; and has received consulting fees from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Merck Sharp & Dohme, Eli Lilly, Novo Nordisk, and Pfizer Inc. N.M. has received support for clinical trial leadership from Boehringer Ingelheim, Novo Nordisk, served as a consultant to Bayer, Boehringer Ingelheim, Merck, Novo Nordisk, AstraZeneca, BMS, received grant support from Boehringer Ingelheim, Merck, Novo Nordisk, and served as a speaker for Bayer, Boehringer Ingelheim, Merck, Novo Nordisk, Lilly, BMS, and AstraZeneca. N.M. is supported by the Deutsche Forschungsgemeinschaft [German Research Foundation; TRR 219; Project-ID 322900939 (M03, M05)]. O.R. has no relevant disclosures. B.P. reports receiving consulting fees from AstraZenca, Bayer, Boehringer Ingelheim/Lilly, Brainstorm Medical, Cereno Scientific, G-3 Pharmaceuticals, Lexicon, KBP Biosciences, Merck, Phasebio, ProtonIntel, SCPharmaceuticals, SQinnovations, and Tricida. A.P. reports receiving research grants and/or consulting fees from Applied Therapeutics, Gilead Sciences, Lilly, Pfizer, Rivus, Roche Diagnostics, and Tricog Health. J.B reports receiving speaking honoraria and/or consulting fees from Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Roche, Sequana Medical, and Vifor. S.V. holds a Tier 1 Canada Research Chair in Cardiovascular Surgery; and reports receiving research grants and/or speaking honoraria from Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, EOCI Pharmacomm Ltd, HLS Therapeutics, Janssen, Novartis, Novo Nordisk, Pfizer, PhaseBio, Sanofi, Sun Pharmaceuticals, and the Toronto Knowledge Translation Working Group. He is the President of the Canadian Medical and Surgical Knowledge Translation Research Group, a federally incorporated not-for-profit physician organization.



