-
PDF
- Split View
-
Views
-
Cite
Cite
Moritz Hadwiger, Nikolaos Dagres, Janina Haug, Michael Wolf, Ursula Marschall, Jan Tijssen, Alexander Katalinic, Fabian Simon Frielitz, Gerhard Hindricks, Survival of patients undergoing cardiac resynchronization therapy with or without defibrillator: the RESET-CRT project, European Heart Journal, Volume 43, Issue 27, 14 July 2022, Pages 2591–2599, https://doi.org/10.1093/eurheartj/ehac053
Close - Share Icon Share
Abstract
Cardiac resynchronization therapy (CRT) is an established treatment for heart failure. There is contradictory evidence whether defibrillator capability improves prognosis in patients receiving CRT. We compared the survival of patients undergoing de novo implantation of a CRT with defibrillator (CRT-D) option and CRT with pacemaker (CRT-P) in a large health claims database.
Using health claims data of a major German statutory health insurance, we analysed patients with de novo CRT implantation from 2014 to 2019 without indication for defibrillator implantation for secondary prevention of sudden cardiac death. We performed age-adjusted Cox proportional hazard regression and entropy balancing to calculate weights to control for baseline imbalances. The analysis comprised 847 CRT-P and 2722 CRT-D patients. Overall, 714 deaths were recorded during a median follow-up of 2.35 years. A higher cumulative incidence of all-cause death was observed in the initial unadjusted Kaplan–Meier time-to-event analysis [hazard ratio (HR): 1.63, 95% confidence interval (CI): 1.38–1.92]. After adjustment for age, HR was 1.13 (95% CI: 0.95–1.35) and after entropy balancing 0.99 (95% CI: 0.81–1.20). No survival differences were found in different age groups. The results were robust in sensitivity analyses.
In a large health claims database of CRT implantations performed in a contemporary setting, CRT-P treatment was not associated with inferior survival compared with CRT-D. Age differences accounted for the greatest part of the survival difference that was observed in the initial unadjusted analysis.
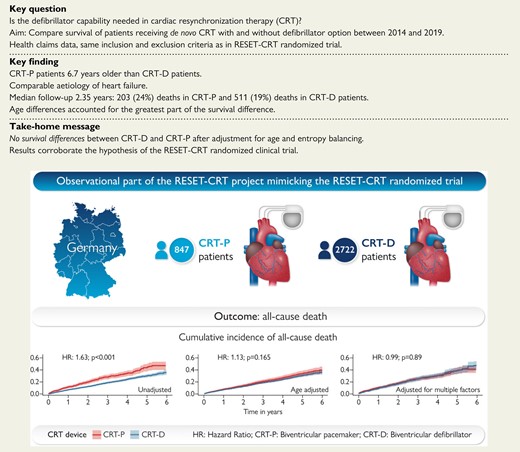
Comparison of patients undergoing de novo implantation of a cardiac resynchronisation therapy device with or without defibrillator option in a large health claims database.
See the editorial comment for this article ‘CRT-P or CRT-D in heart failure patients: the RESET-CRT project—a prelude to the randomized controlled RESET-CRT study’, by Cecilia Linde, https://doi.org/10.1093/eurheartj/ehac136.
Introduction
Cardiac resynchronization therapy (CRT) is one of the main treatment pillars for heart failure patients with reduced left ventricular ejection fraction and conduction abnormalities with broad QRS complex.1 Cardiac resynchronization therapy is delivered by biventricular pacemakers (CRT-P) or by biventricular pacemakers with additional defibrillator capability (CRT-D).
The need for the defibrillator capability in this setting is debated. Cardiac resynchronization therapy reduces per se the risk of sudden cardiac death.2 In addition, modern pharmacologic heart failure treatment further reduces that risk leading to a substantial overall decline of sudden cardiac death3–6 and a decrease of the expected benefit of the defibrillator.7
There is no randomized clinical trial (RCT) with a head-to-head comparison between CRT-P and CRT-D. The COMPANION study compared CRT-P and CRT-D devices with optimal medical therapy, but there was no direct comparison between CRT-D and CRT-P.8 In the DANISH trial in patients with non-ischaemic cardiomyopathy, no benefit for CRT-D over CRT-P devices could be shown in the large trial subgroup that received a CRT device.9 The evidence from observational studies is also ambiguous.10–14 As a result, a recent European Society of Cardiology guidelines1,15 and a recent position statement16 recommend an individual decision-making for the choice of the type of CRT device in patients undergoing CRT implantation based on parameters that are considered to be associated with the risk for sudden cardiac death and the competing risk for dying from other causes.
The Re-evaluation of Optimal Re-synchronization Therapy in Patients with Chronic Heart Failure (RESET-CRT) project17 addresses this clinically important evidence gap. The project consists of a large ongoing RCT18 that compares CRT-P and CRT-D in a randomized fashion with total mortality as the primary end point. The hypothesis of the RESET-CRT project is that CRT-P is non-inferior to CRT-D. In addition to the randomized trial, we compared the survival of CRT-P and CRT-D patients in a large health claims database of a statutory health insurance in Germany from 2014 to 2019 reflecting contemporary medical practice.
Methods
For the analysis of the health claims data, we applied a retrospective, non-experimental, population-based weighted cohort study design.
Setting
The survival analysis was based on health claims data of the second largest German health insurance, the BARMER, which operates nationwide and insures 10.7% of the German population, i.e. 8.9 million people.19 In Germany, health insurance is mandatory, either as private (∼10% of the population) or as statutory health insurance such as the BARMER.20 The BARMER database contains anonymized longitudinal information of all insured persons on the vital status, costs, utilization, and socio-demographics between 2005 and 2019. The database comprises generalizable information with a sex and age distribution which is comparable with the German population and has already been used for cardiovascular research.21,22 A diagnosis-related group system is used for reimbursement of inpatient treatment in Germany. Therefore, all codes of the International Classification of Diseases (ICD) and Operation and Procedure Classification (OPS) codes, an adaptation of the International Classification of Procedures in Medicine that are relevant for patient treatment are reported to the health insurance and are available in the database.
Study population
For the study population, we considered all patients in the BARMER database that underwent CRT implantation during 2014–19 (n = 7082). We operationalized the inclusion and exclusion criteria of the RESET-CRT randomized trial using ICD and OPS codes recorded in the BARMER database. The complete list of ICD and OPS codes that were used can be found in Supplementary material online, Table S1.
In accordance with the inclusion and exclusion criteria of the RESET-CRT randomized trial, we excluded patients who were younger than 18 years (n = 3), without symptomatic heart failure (n = 612), with an indication for implantation of an implantable cardioverter defibrillator for secondary prevention of sudden cardiac death (n = 1144), an implanted cardiac pacemaker, defibrillator or CRT device (n = 596), unexplained syncope (n = 477), a hospitalization with unstable heart failure with New York Heart Association (NYHA) Class IV within 1 month prior CRT implantation (n = 43), or an acute coronary syndrome or cardiac revascularization therapy by coronary angioplasty or coronary artery bypass grafting 6 weeks prior to implantation (n = 738), cardiac valve surgery or percutaneous cardiac valvular intervention such as transcatheter aortic valve replacement or transcatheter mitral valve repair within 3 month prior to CRT implantation (n = 182), severe chronic renal disease (n = 125) or on the waiting list for a heart transplant (n = 2). We further excluded patients who had not been consistently observed for 3 years prior to CRT implantation for risk adjustment (n = 104), patients without a minimum follow-up time of 3 months (or death during that period) (n = 56), as well as patients with no (n = 321) or ambiguous (n = 141) NYHA information prior to CRT implantation. After applying these inclusion and exclusion criteria, 3569 eligible CRT de novo implantations were included in the analysis. Of these, 847 were CRT-P implantations and 2722 were CRT-D implantations (Figure 1).
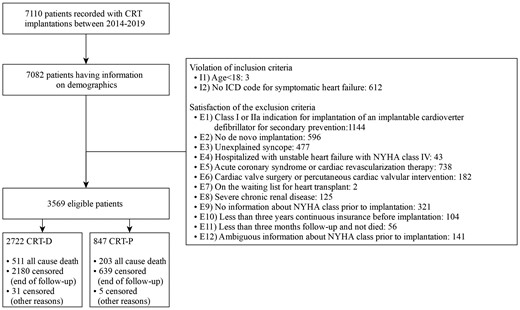
Flowchart. CRT-P, cardiac resynchronization therapy with pacemaker; CRT-D, cardiac resynchronization therapy with defibrillator.
Outcome
The outcome was all-cause death occurring between CRT implantation and 31 December 2019. For each patient in the study cohort, it was established whether the patient had died, had left the BARMER (<1.05% of the study population) by the end of the observation period, or was still alive and BARMER paid for their health expenditures.
Covariates
Comorbidities were coded if they were recorded in the BARMER database. The complete list of ICD and OPS codes used for the identification of comorbidities can be found in Supplementary material online. From the demographics, we included age and sex. From the clinical characteristics, we considered the number of hospitalizations within 1 year prior to CRT implantation (0, 1, 2, >2), NYHA Class (II, III, IV), and aetiology of heart failure (ischaemic/non-ischaemic). From the comorbidities, we included renal dysfunction (Stages III and IV), diabetes and atrial fibrillation. In addition, we added all comorbidities of the Elixhauser comorbidity groups that we had not already considered.23
Statistical analyses
The statistical analysis was performed in three phases. First, we performed an analysis of the unadjusted cumulative incidence rates for all-cause death, illustrated by Kaplan–Meier time curves and a univariate Cox proportional hazard regression. Patients who were still alive at the end of the observational period (31 December 2019) were censored. For patients who left BARMER, the leaving date was used for censoring. The follow-up time was defined as the time between CRT device implantation (index date) and death or censoring. Patients who received a CRT-D device were the control group.
Second, we performed an analysis adjusted for age. The sample was divided into three groups: (i) younger than or equal to 65 years (n = 898), (ii) patients older than 65 and younger than or equal to 75 years (n = 1207), and (iii) patients older than 75 years (n = 1464). The cumulative incidence of death was illustrated by Kaplan–Meier time-to-event curves for each group and Cox proportional hazard regressions were performed. Additionally, we performed an age-adjusted Cox proportional hazard regression for the total sample. The age-adjusted cumulative incidence curves based on the Cox proportional hazard regression are illustrated, with age fixed at the sample mean.
Third, we performed an adjusted analysis using entropy balancing.24 Entropy balancing is a reweighting method, which aims to produce exact covariate balance of CRT-P and CRT-D patients. Entropy balancing is considered a generalization of propensity score weighting and uses an optimization algorithm by assigning a scalar weight to each patient in the control group to balance means and variances between CRT-P patients and the reweighted CRT-D patients. The set of weights that deviates the least from the set of uniform weights is selected. In entropy balancing, no case is discarded.25 The estimated weights can be used like survey sampling weights in the subsequent analyses. Standardized differences were used for the balancing diagnostics instead of P-values.26 A standardized difference >0.1 indicates a meaningful difference.27 The weights of entropy balancing were used to calculate a weighted Kaplan–Meier curve for the CRT-D patients and to perform a weighted univariate Cox proportional hazard regression.28
Two sensitivity analyses were conducted. First, we performed a 1 : 1 propensity score matching (caliper = 0.05) without replacement, resulting in 727 CRT-P and 727 CRT-D patients and plotted Kaplan–Meier curves. Second, we included patients with ambiguous NYHA coding prior to CRT implantation (n = 141) and performed entropy balancing and weighted Kaplan–Meier curves again. Ambiguous NYHA class coding was defined as two different consecutive NYHA class codes at baseline. For the sensitivity analysis, the higher NYHA class was chosen. A P-value of <0.05 was considered statistically significant. Statistical analyses were carried out in ‘R’ (version 4.0.3).29
Results
In total, the analysis included 3569 patients with CRT implantation from 2014 to 2019, of whom 847 were CRT-P patients and 2722 were CRT-D patients. Baseline characteristics are displayed in Table 1. Cardiac resynchronization therapy with pacemaker patients were on average 6.7 years older and more likely female (48 vs. 35%) than CRT-D patients. The aetiology of heart failure (ischaemic/non-ischaemic) was comparable between the two groups. Differences in NYHA classes and hospitalizations prior to CRT implantation were small. Cardiac resynchronization therapy with defibrillator patients were more likely to have diabetes, while Stages III and IV renal dysfunction and atrial fibrillation were more common in CRT-P patients.
Baseline characteristics of patients at cardiac resynchronization therapy implantation
| Characteristic . | CRT-P (n = 847) . | CRT-D (n = 2722) . | Standardized difference . |
|---|---|---|---|
| Age (years), mean (SD) | 76.7 (8.89) | 69.9 (9.57) | 0.75 |
| Male sex, n (%) | 440 (52) | 1768 (65) | −0.25 |
| Non-ischaemic heart failure aetiology, n (%) | 225 (27) | 678 (25) | 0.04 |
| CRT implantation year, n (%) | |||
| 2014 | 108 (13) | 496 (18) | −0.16 |
| 2015 | 106 (13) | 466 (17) | −0.14 |
| 2016 | 123 (15) | 439 (16) | −0.05 |
| 2017 | 150 (18) | 482 (18) | 0 |
| 2018 | 178 (21) | 445 (16) | 0.12 |
| 2019 | 182 (21) | 396 (15) | 0.17 |
| Number of hospitalizations one year prior to implantation, n (%) | |||
| 0 | 40 (5) | 121 (4) | 0.01 |
| 1 | 274 (32) | 846 (31) | 0.03 |
| 2 | 222 (26) | 868 (32) | −0.13 |
| >2 | 311 (37) | 887 (33) | 0.09 |
| NYHA Class, n (%) | |||
| II | 131 (15) | 420 (15) | 0 |
| III | 548 (65) | 1712 (63) | 0.04 |
| IV | 168 (20) | 590 (22) | −0.05 |
| Heart failure specific comorbidities | |||
| Diabetes, n (%) | 272 (32) | 982 (36) | −0.08 |
| Renal dysfunction III, n (%) | 300 (35) | 749 (28) | 0.17 |
| Renal dysfunction IV, n (%) | 58 (7) | 112 (4) | 0.11 |
| Atrial fibrillation, n (%) | 497 (59) | 1105 (41) | 0.37 |
| Characteristic . | CRT-P (n = 847) . | CRT-D (n = 2722) . | Standardized difference . |
|---|---|---|---|
| Age (years), mean (SD) | 76.7 (8.89) | 69.9 (9.57) | 0.75 |
| Male sex, n (%) | 440 (52) | 1768 (65) | −0.25 |
| Non-ischaemic heart failure aetiology, n (%) | 225 (27) | 678 (25) | 0.04 |
| CRT implantation year, n (%) | |||
| 2014 | 108 (13) | 496 (18) | −0.16 |
| 2015 | 106 (13) | 466 (17) | −0.14 |
| 2016 | 123 (15) | 439 (16) | −0.05 |
| 2017 | 150 (18) | 482 (18) | 0 |
| 2018 | 178 (21) | 445 (16) | 0.12 |
| 2019 | 182 (21) | 396 (15) | 0.17 |
| Number of hospitalizations one year prior to implantation, n (%) | |||
| 0 | 40 (5) | 121 (4) | 0.01 |
| 1 | 274 (32) | 846 (31) | 0.03 |
| 2 | 222 (26) | 868 (32) | −0.13 |
| >2 | 311 (37) | 887 (33) | 0.09 |
| NYHA Class, n (%) | |||
| II | 131 (15) | 420 (15) | 0 |
| III | 548 (65) | 1712 (63) | 0.04 |
| IV | 168 (20) | 590 (22) | −0.05 |
| Heart failure specific comorbidities | |||
| Diabetes, n (%) | 272 (32) | 982 (36) | −0.08 |
| Renal dysfunction III, n (%) | 300 (35) | 749 (28) | 0.17 |
| Renal dysfunction IV, n (%) | 58 (7) | 112 (4) | 0.11 |
| Atrial fibrillation, n (%) | 497 (59) | 1105 (41) | 0.37 |
CRT-P, cardiac resynchronization therapy with pacemaker; CRT-D, cardiac resynchronization therapy with defibrillator; NYHA, New York Heart Association; SD, standard deviation.
Baseline characteristics of patients at cardiac resynchronization therapy implantation
| Characteristic . | CRT-P (n = 847) . | CRT-D (n = 2722) . | Standardized difference . |
|---|---|---|---|
| Age (years), mean (SD) | 76.7 (8.89) | 69.9 (9.57) | 0.75 |
| Male sex, n (%) | 440 (52) | 1768 (65) | −0.25 |
| Non-ischaemic heart failure aetiology, n (%) | 225 (27) | 678 (25) | 0.04 |
| CRT implantation year, n (%) | |||
| 2014 | 108 (13) | 496 (18) | −0.16 |
| 2015 | 106 (13) | 466 (17) | −0.14 |
| 2016 | 123 (15) | 439 (16) | −0.05 |
| 2017 | 150 (18) | 482 (18) | 0 |
| 2018 | 178 (21) | 445 (16) | 0.12 |
| 2019 | 182 (21) | 396 (15) | 0.17 |
| Number of hospitalizations one year prior to implantation, n (%) | |||
| 0 | 40 (5) | 121 (4) | 0.01 |
| 1 | 274 (32) | 846 (31) | 0.03 |
| 2 | 222 (26) | 868 (32) | −0.13 |
| >2 | 311 (37) | 887 (33) | 0.09 |
| NYHA Class, n (%) | |||
| II | 131 (15) | 420 (15) | 0 |
| III | 548 (65) | 1712 (63) | 0.04 |
| IV | 168 (20) | 590 (22) | −0.05 |
| Heart failure specific comorbidities | |||
| Diabetes, n (%) | 272 (32) | 982 (36) | −0.08 |
| Renal dysfunction III, n (%) | 300 (35) | 749 (28) | 0.17 |
| Renal dysfunction IV, n (%) | 58 (7) | 112 (4) | 0.11 |
| Atrial fibrillation, n (%) | 497 (59) | 1105 (41) | 0.37 |
| Characteristic . | CRT-P (n = 847) . | CRT-D (n = 2722) . | Standardized difference . |
|---|---|---|---|
| Age (years), mean (SD) | 76.7 (8.89) | 69.9 (9.57) | 0.75 |
| Male sex, n (%) | 440 (52) | 1768 (65) | −0.25 |
| Non-ischaemic heart failure aetiology, n (%) | 225 (27) | 678 (25) | 0.04 |
| CRT implantation year, n (%) | |||
| 2014 | 108 (13) | 496 (18) | −0.16 |
| 2015 | 106 (13) | 466 (17) | −0.14 |
| 2016 | 123 (15) | 439 (16) | −0.05 |
| 2017 | 150 (18) | 482 (18) | 0 |
| 2018 | 178 (21) | 445 (16) | 0.12 |
| 2019 | 182 (21) | 396 (15) | 0.17 |
| Number of hospitalizations one year prior to implantation, n (%) | |||
| 0 | 40 (5) | 121 (4) | 0.01 |
| 1 | 274 (32) | 846 (31) | 0.03 |
| 2 | 222 (26) | 868 (32) | −0.13 |
| >2 | 311 (37) | 887 (33) | 0.09 |
| NYHA Class, n (%) | |||
| II | 131 (15) | 420 (15) | 0 |
| III | 548 (65) | 1712 (63) | 0.04 |
| IV | 168 (20) | 590 (22) | −0.05 |
| Heart failure specific comorbidities | |||
| Diabetes, n (%) | 272 (32) | 982 (36) | −0.08 |
| Renal dysfunction III, n (%) | 300 (35) | 749 (28) | 0.17 |
| Renal dysfunction IV, n (%) | 58 (7) | 112 (4) | 0.11 |
| Atrial fibrillation, n (%) | 497 (59) | 1105 (41) | 0.37 |
CRT-P, cardiac resynchronization therapy with pacemaker; CRT-D, cardiac resynchronization therapy with defibrillator; NYHA, New York Heart Association; SD, standard deviation.
Median follow-up time was 2.35 years (interquartile range: 1.09–3.92 years). During follow-up, 203 (24%) deaths in CRT-P patients and 511 (19%) deaths in CRT-D patients were observed. In the unadjusted Kaplan–Meier time-to-event curves, CRT-P patients had a higher cumulative incidence of all-cause death than CRT-D patients (Figure 2 and Table 2) [hazard ratio (HR): 1.63, 95% confidence interval (CI): 1.38–1.92].
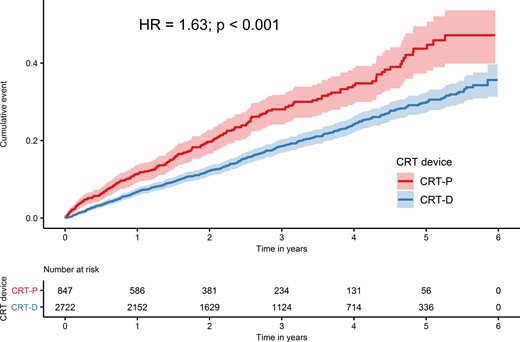
Unadjusted Kaplan–Meier time-to-event curves for the cumulative incidence of all-cause death for cardiac resynchronization therapy with defibrillator and for cardiac resynchronization therapy with pacemaker.
Cox proportional hazard regressions: hazard ratio for all-cause death in cardiac resynchronization therapy with pacemaker vs. cardiac resynchronization therapy with defibrillator
| Analysis . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|
| Unadjusted | 1.63 (1.38–1.92) | <0.001 |
| Age-adjusteda,b | 1.13 (0.95–1.35) | 0.165 |
| Age and comorbidity adjustedc | 0.99 (0.81–1.20) | 0.89 |
| Sensitivity analysis | ||
| Age and comorbidity adjusted + ambiguous NYHA patientsc | 1.04 (0.86–1.27) | 0.67 |
| PSM approach | ||
| Age and comorbidity adjustedd | 1.16 (0.93–1.44) | 0.195 |
| Analysis . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|
| Unadjusted | 1.63 (1.38–1.92) | <0.001 |
| Age-adjusteda,b | 1.13 (0.95–1.35) | 0.165 |
| Age and comorbidity adjustedc | 0.99 (0.81–1.20) | 0.89 |
| Sensitivity analysis | ||
| Age and comorbidity adjusted + ambiguous NYHA patientsc | 1.04 (0.86–1.27) | 0.67 |
| PSM approach | ||
| Age and comorbidity adjustedd | 1.16 (0.93–1.44) | 0.195 |
CI, confidence interval; CRT-P; cardiac resynchronization therapy with pacemaker; CRT-D; cardiac resynchronization therapy with defibrillator; NYHA; New York Heart Association; PSM, propensity score matching.
Bivariate Cox regression.
Hazard ratio for increasing age (per year) 1.06 (95% CI: 1.05–1.07), P < 0.001.
Univariate Cox regression using weights from entropy balancing.
Univariate Cox regression using the propensity score matched sample.
Cox proportional hazard regressions: hazard ratio for all-cause death in cardiac resynchronization therapy with pacemaker vs. cardiac resynchronization therapy with defibrillator
| Analysis . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|
| Unadjusted | 1.63 (1.38–1.92) | <0.001 |
| Age-adjusteda,b | 1.13 (0.95–1.35) | 0.165 |
| Age and comorbidity adjustedc | 0.99 (0.81–1.20) | 0.89 |
| Sensitivity analysis | ||
| Age and comorbidity adjusted + ambiguous NYHA patientsc | 1.04 (0.86–1.27) | 0.67 |
| PSM approach | ||
| Age and comorbidity adjustedd | 1.16 (0.93–1.44) | 0.195 |
| Analysis . | Hazard ratio (95% CI) . | P-value . |
|---|---|---|
| Unadjusted | 1.63 (1.38–1.92) | <0.001 |
| Age-adjusteda,b | 1.13 (0.95–1.35) | 0.165 |
| Age and comorbidity adjustedc | 0.99 (0.81–1.20) | 0.89 |
| Sensitivity analysis | ||
| Age and comorbidity adjusted + ambiguous NYHA patientsc | 1.04 (0.86–1.27) | 0.67 |
| PSM approach | ||
| Age and comorbidity adjustedd | 1.16 (0.93–1.44) | 0.195 |
CI, confidence interval; CRT-P; cardiac resynchronization therapy with pacemaker; CRT-D; cardiac resynchronization therapy with defibrillator; NYHA; New York Heart Association; PSM, propensity score matching.
Bivariate Cox regression.
Hazard ratio for increasing age (per year) 1.06 (95% CI: 1.05–1.07), P < 0.001.
Univariate Cox regression using weights from entropy balancing.
Univariate Cox regression using the propensity score matched sample.
After adjustment for age, the HR for all-cause death in Cox regression was 1.13 (95% CI: 0.95–1.35), and the difference in survival was no longer significant (Figure 3 and Table 2). The HR was independent of age (P for interaction = 0.371). The cumulative incidence of death in the three age groups is depicted in Figure 4. No significant difference between CRT-D and CRT-P in the cumulative incidence of death was observed in any of the three age groups (for patients ≤ 65 years: HR: 1.45; 95% CI: 0.75–2.82; for patients >65 and ≤75 years: HR: 1.29; 95% CI: 0.92–1.81; for patients >75 years: HR: 1.19; 95% CI: 0.98–1.47). The HRs were similar in the three age groups (P for interaction = 0.598).
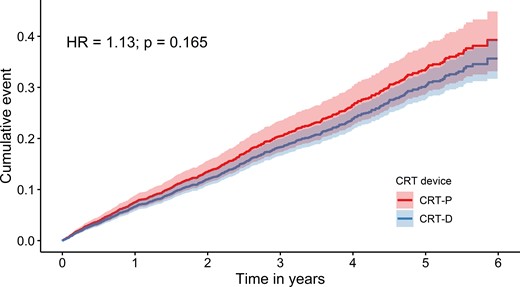
Age-adjusted cumulative incidence of all-cause death based on the Cox proportional hazard model (fixed at a mean age) for cardiac resynchronization therapy with defibrillator and for cardiac resynchronization therapy with pacemaker.
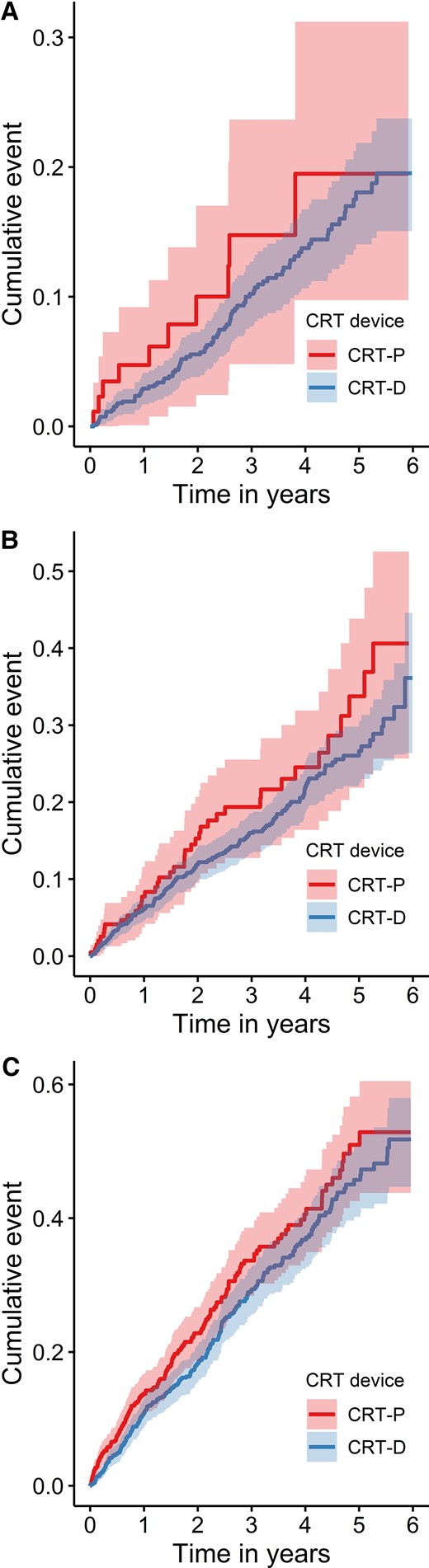
Unadjusted Kaplan–Meier time-to-event curves for the cumulative incidence of all-cause death for cardiac resynchronization therapy with defibrillator and for cardiac resynchronization therapy with pacemaker: (A) patients ≤65 years; (B) patients between >65 years and ≤75 years; (C) patients >75 years. A χ 2 test comparing a regression model with only the device and age groups as an ordinal variable and a regression with an additional interaction between age groups and device was not statistically significant (P = 0.598). A χ 2 test comparing a regression model with only the device and age groups as categorial variables for the middle and oldest age group and a regression model with additional interaction between the device and the age groups was not statistically significant (P = 0.843).
After the application of entropy balancing, the weighted average of the baseline characteristics of CRT-D patients was the same as that of CRT-P patients (see Supplementary material online, Table S2). Detailed information on the distribution of baseline characteristics of CRT-D patients according to the weight assigned to them is included in Supplementary material online, Table S3. Figure 5 shows the Kaplan–Meier curve for all-cause death for CRT-P patients and the weighted Kaplan–Meier curve for CRT-D patients. There was no difference in the cumulative incidence of all-cause death. The hazard ratio for all-cause death, calculated using weighted univariate Cox proportional hazard regression, was 0.99 (95% CI: 0.81–1.20) (Table 2 and Figure 5).
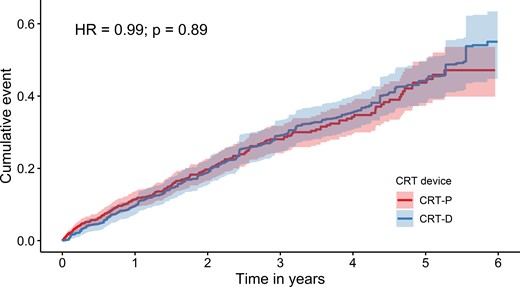
Weighted Kaplan–Meier time-to-event curves for the cumulative incidence of all-cause death for cardiac resynchronization therapy with defibrillator and for cardiac resynchronization therapy with pacemaker.
In the sensitivity analyses, the results were virtually identical to the results of entropy balancing. First, propensity score matching as a different method to adjust for the baseline imbalances was applied. In the propensity score-matched population, no significant difference in mortality could be found either (HR: 1.16; 95% CI: 0.93–1.44) (see Supplementary material online, Figure S1B and Table 2). The distributions of the propensity scores can be found in the statistical Supplementary material online, Figure S2. Second, when repeating the entropy balancing analysis with the additional patients with ambiguous NYHA class prior to CRT implantation, the HR was 1.04 (95% CI: 0.86–1.27) (see Supplementary material online, Figure S1A and Table 2).
Discussion
This analysis of a large German health claims database of 3569 de novo CRT implantations provides real-world data on the survival of CRT patients. In the unadjusted analysis, treatment with CRT-P was associated with a higher incidence of all-cause death. Our inference is based on the results of the final balancing analysis, which showed no difference in survival between CRT-P and CRT-D treated patients. The difference in survival in the unadjusted analysis could primarily be explained by the difference in age between CRT-P and CRT-D recipients. After full adjustment for age and comorbidities, (cumulative) mortality was virtually identical for CRT-P and CRT-D treated patients. The results were robust in sensitivity analyses (Structured Graphical Abstract).
The choice between CRT-P and CRT-D is a frequent clinical dilemma. Despite the large number of CRT implantations, there is no RCT with a head-to-head comparison of CRT-P and CRT-D survival. In a post hoc analysis of the randomized COMPANION study, no differences in survival were found in the overall population.30 The analysis of the CRT subgroup of the DANISH trial indicated no survival difference either. Observational studies have provided contradictory results. The unadjusted analysis of an individual patient data network meta-analysis31 and the evaluation of large samples of administrative data of the National Health Service Digital and National Health Service Hospital Episode Statistics reported a survival benefit of CRT-D devices compared with CRT-P devices.10,11 In contrast and very similar to our results, a large multinational analysis of patients who survived the first 5 years after implantation reported identical late survival of CRT-P and CRT-D patients.12 In line with our results, the survival benefit of CRT-D could not be confirmed in another study in older patients13 and in a Medicare analysis of non-ischaemic patients.14
Cardiac resynchronization therapy may significantly affect the risk for sudden cardiac death. In the CARE-HF trial, CRT was associated with a significantly decreased risk for sudden death.2 The decrease of life-threatening arrhythmias is more pronounced in patients with lower left ventricular ejection fraction and non-responders experience more ventricular arrhythmias than CRT responders.32,33 Interestingly, in a recent systematic review on sudden cardiac death risk in CRT patients, the absolute decrease in sudden cardiac death risk was more pronounced in CRT-P than CRT-D patients34 and the CeRtiTuDe study reported that 95% of the excess mortality in CRT-P recipients was not associated with sudden cardiac death.35 Thus, this evidence indicates that CRT exerts per se an antiarrhythmic effect and may render the addition of defibrillation capability unnecessary.
Our analysis differs from previous studies in that it was restricted to the time period from 2014 to 2019 to reflect contemporary clinical practice, thus taking into account current technological and medical treatment standards. Indeed, the risk of sudden cardiac death in heart failure patients has decreased over time,3 most probably due to advances in pharmacological and non-pharmacological treatment.36 The distribution of baseline characteristics between CRT-P and CRT-D patients in our study was similar with the distribution in previous CRT studies as the ESC CRT Survey II.37
In Germany, the vast majority of patients undergoing CRT implantation receive a CRT-D device. In 2019, approximately 61% of all CRT implantations were CRT-D implantations.38 The underlying rationale is the desired protection from sudden cardiac death. However, this comes with additional costs and risks as CRT-D devices have a higher risk of device-related problems such as infections,39 a shorter device longevity and cause significantly higher costs for the healthcare system. Additionally, the quality of life of CRT-D patients could be impaired due to inappropriate shocks.40 Our results further indicate a considerable bias in the device selection in clinical practice in favour of CRT-D in younger and of CRT-P in older patients.
Our study has several limitations. First, we used a retrospective cohort design for our analysis, and the limitations associated with this design and health claims data should be considered when interpreting the results. The conclusions of the entropy balancing weighted analysis are based on the assumption that all relevant baseline characteristics were included and that there are no other, unobserved confounders. Second, we could not consider further potentially relevant parameters such as QRS duration, or left ventricular function because these are not included in claims data. Third, a possible incorrect coding, for example of ICD codes, cannot be excluded. Fourth, a sensitivity analysis restricting the end point to arrhythmic or cardiovascular death would have been informative for our study and would have provided additional insight into the mechanisms by which the choice of the device type may affect the outcome. Unfortunately, the BARMER database as a claims database does not contain information on the cause and mode of death. The absence of this sensitivity analysis does not invalidate our conclusions with regard to all-cause death, which is the conventional primary end point in almost all randomized arrhythmia trials. Fifth, the results of the analysis can only be applied to patients with similar characteristics as those in the group analysed in our study. In particular, our study specifically excluded patients who received a device for secondary prevention of sudden cardiac death because in this patient population, the implantation of a device with defibrillation capability appears to be mandatory. Sixth, the size of the study groups was determined by the availability in the BARMER health claims database and not by a formal sample size calculation. Nevertheless, the power of the study can be retrospectively established on the basis of the numbers of fatalities in the final entropy balancing adjusted analysis, with adjustments for age and comorbidities. This weighed analysis is statistically the equivalent of an observational cohort study with 406 (=2 × 203) fatalities. The power calculation for the RESET-CRT trial required 361 fatalities to achieve 80% for testing non-inferiority of CRT-P (vs. CRT-D) with a non-inferiority limit of 1.34 for the HR. Therefore, this observational study, with the equivalent of 406 fatalities in the final analysis, matches 80% power of the randomized RESET-CRT trial. Furthermore, we note that the upper boundary of the 95% CI for the HR in the final (entropy balancing) adjusted analysis (1.20) easily meets the non-inferiority criterion of 1.34 of the RESET-CRT trial.
Our analysis also has major strengths. It comprises a relatively long time frame with a large number of patients in a real-world setting and reflecting contemporary therapy. As an innovative element, in our analysis, we attempted to mimic an RCT by applying entropy balancing rendering the type of CRT independent of the measured covariates.
Conclusions
Using health claims data of 3569 patients in a period reflecting contemporary clinical practice (2014–19), the HR for all-cause death for CRT-P and CRT-D recipients was close to 1 after adjusting for age and further potential confounders. The survival difference in favour of the CRT-D patients that was observed in the unadjusted analysis was primarily due to the younger age of the CRT-D patients. Thus, the results of this observational study corroborate the hypothesis of the RESET-CRT randomized clinical trial that CRT-P is non-inferior to CRT-D with regard to survival.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
The authors thank Laura Schumann, Johann Mattutat, and Christiane Rudolph for providing support for our statistical analysis.
Funding
The project on which this publication is based was funded by the Innovation Committee of the Federal Joint Committee under the funding code 01VSF17050. The Federal Joint Committee (G-BA) is the highest decision-making body of the joint self-government of physicians, dentists, hospitals and health insurance funds in Germany.
Conflict of interest
none declared.
Data availability statement
The data that support the findings of this study are owned by the BARMER (Wuppertal, Germany) and are not publicly available.
References
Author notes
Moritz Hadwiger and Nikolaos Dagres contributed equally and are considered shared first authors.
Fabian-Simon Frielitz and Gerhard Hindricks contributed equally and are considered shared senior authors.



