-
PDF
- Split View
-
Views
-
Cite
Cite
Kevin C Maki, Investigating contrasting results in REDUCE-IT and STRENGTH: partial answers but questions remain, European Heart Journal, Volume 42, Issue 47, 14 December 2021, Pages 4818–4820, https://doi.org/10.1093/eurheartj/ehab643
Close - Share Icon Share
This editorial refers to ‘A possible explanation for the contrasting results of REDUCE-IT vs. STRENGTH: cohort study mimicking trial designs’, by T. Doi et al., https://doi.org/10.1093/eurheartj/ehab555.
Schematic depicting biological effects of omega-3 fatty acids expected to favorably impact atherosclerotic cardiovascular disease outcomes.
Interest in the potential for long-chain omega-3 fatty acid intake to reduce atherosclerotic cardiovascular disease (ASCVD) risk dates back nearly 50 years to observations in Greenland Inuit, whose low rate of mortality from coronary heart disease (CHD) was found to be associated with high intakes of the fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). EPA and DHA have several biological effects that might be expected to favourably impact ASCVD outcomes, including reducing circulating triglycerides; lowering blood pressures (systolic and diastolic); dampening platelet reactivity; moderating oxidative stress; and reducing biomarkers of chronic inflammation such as C-reactive protein (CRP), tumour necrosis factor-α and proinflammatory eicosanoids and leukotrienes.1–3
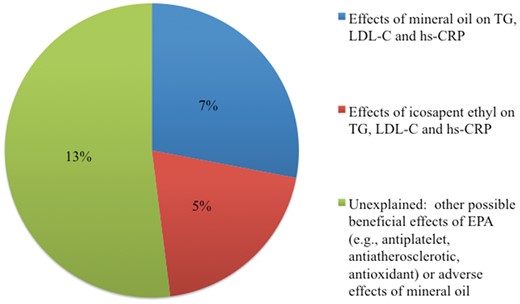
Possible explanations for the 25% difference in cardiovascular events between icosapent ethyl and mineral oil groups in REDUCE-IT. EPA, eicosapentaenoic acid; hs-CRP, high-sensitivity C-reactive protein; LDL-C, low-density lipoprotein cholesterol; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial; TG, triglycerides.
Randomized, controlled trials of omega-3 fatty acid interventions have produced mixed results. Many of the earlier trials suffered from limitations such as usual care rather than placebo controls and low dosages of EPA or EPA + DHA. A meta-analysis of data from 12 primary and secondary prevention trials with 119 298 participants employing low dosages of EPA or EPA + DHA (≤1800 mg/day, median = 858 mg/day) produced pooled estimates of effects that were consistent with modest but statistically significant (P ≤ 0.02) benefits for CHD-related outcomes (relative risks of 0.92, 0.95, and 0.92 for myocardial infarction, total CHD, and CHD death, respectively).4 However, no benefit was observed for stroke [relative risk of 1.05, 95% confidence interval (CI) 0.98–1.14).4
To date, two large-scale, randomized ASCVD outcomes trials have been completed with higher dosages of omega-3 fatty acids. These were designed to avoid the limitations of many of the previous studies by employing therapeutic dosages of EPA or EPA + DHA in well-characterized pharmaceutical formulations, compared with blinded control capsules, and in populations with elevated triglycerides at high ASCVD risk due to a history of clinical ASCVD or risk factors.
The Reduction of Cardiovascular Events with Icosapent Ethyl-Intervention Trial (REDUCE-IT) administered 4 g/day of icosapent ethyl (ethyl esters providing 3840 mg/day of EPA) or a mineral oil placebo to 8179 subjects and produced a 25% reduction in incidence of the primary composite ASCVD outcome over a median follow-up time of 4.9 years.5 Several secondary outcomes were similarly reduced, including a 28% reduction in total stroke. The Long-Term Outcomes Study to Assess Statin Residual Risk with Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia (STRENGTH) administered 4 g/day of EPA + DHA (2200 mg/day of EPA + 800 mg DHA) as carboxylic acids compared with a corn oil control in a total sample of 13 078 subjects.6 The trial was terminated early due to futility, after an interim analysis showed no evidence of benefit for the primary outcome, resulting in an estimate of a non-significant, 1% lower incidence of the primary composite ASCVD outcome after a median follow-up of 3.5 years.
Several possible explanations exist for the markedly different results from these two trials of omega-3 fatty acid interventions (Figure 1 and Graphical Abstract), including effects of the active interventions (EPA vs. EPA + DHA) and of the placebo comparator used (mineral oil in REDUCE-IT and corn oil in STRENGTH). In their study reported in this issue of the European Heart Journal, Doi et al. used data from the Copenhagen General Population Study to identify cohorts that met key trial inclusion criteria, one cohort to reflect the characteristics of REDUCE-IT participants and a second for STRENGTH participants.7 Cox proportional hazards models were used to assess relationships of biomarker levels to ASCVD risk in these cohorts, and, further, to estimate effects of the observed changes in circulating levels of three biomarkers, all believed to be causally related to ASCVD risk, in each arm of the two trials: triglyceride-rich lipoproteins (reflected by triglyceride concentration), Low-density lipoprotein cholesterol (LDL-C), and CRP, an inflammatory marker. Estimated changes in ASCVD risk were then compared with observed differences in these biomarkers in REDUCE-IT and STRENGTH.
The two omega-3 formulations produced similar changes from baseline for triglyceride, LDL-C, and CRP levels: –20, –1, and –14%, respectively, for the EPA arm in REDUCE-IT and –19, +1, and –20%, respectively, for the EPA + DHA arm in STRENGTH. Accordingly, the predicted changes in ASCVD event risk were similar: –4% (95% CI –1% to –7%) in REDUCE-IT and –6% (95% CI –2% to –9%) in STRENGTH.
In contrast, the comparator oils produced differing effects on some biomarkers, with changes of 0, +10, and +32% for triglycerides, LDL-C, and CRP, respectively, for the mineral oil arm in REDUCE-IT, compared with changes of –1, –1, and –6%, respectively, for the corn oil arm in STRENGTH. Predicted changes in ASCVD event risk from these biomarker changes were an increase of 7% (95% CI 4–10%) in the mineral oil arm of REDUCE-IT and a small reduction in risk in the corn oil arm of STRENGTH (–1%, 95% CI –2% to –1%).
The between-arm differences in biomarker responses in REDUCE-IT produced a net predicted effect on ASCVD risk of –12% (95% CI –7% to –16%), which is roughly half of the reported effect of –25% (95% CI –17% to –32%). For STRENGTH, the predicted between-arm difference in ASCVD event risk was –4% (95% CI –1% to –7%), compared with the reported difference of –1% (95% CI –10% to +9%). Findings were similar in several sensitivity analyses that substituted non-high-density lipoprotein cholesterol (non-HDL-C) or apolipoprotein B for triglyceride and LDL-C changes, limited the study cohorts to those with baseline triglyceride levels that met trial inclusion criteria, and used changes rather than percentage changes in biomarker values.
The approach taken by Doi et al. addressed observed effects of the two interventions on lipid traits and CRP but did not exclude the possibility of additional effects of both the active and comparator oils.7 The corn oil comparator in STRENGTH is high in linoleic acid (omega-6 polyunsaturated fatty acid), which accounts for ∼600 mg/g of oil and would thus contribute ∼1% of daily energy for the study participants. In observational studies, each 1% increment in energy from polyunsaturated fatty acids is associated with an ∼3–7% lower incidence of CHD.8–11 Thus, corn oil may have been a weakly active beneficial intervention. As the authors point out, the mineral oil comparator in REDUCE-IT may interfere with statin absorption, which could account for its observed effects to raise LDL-C and CRP. To the degree that statins have other, pleiotropic effects, the adverse effect of the mineral oil comparator may not be fully accounted for in this analysis.
A second possibility is that there are additional beneficial effects of EPA beyond those on the biomarkers investigated. Icosapent ethyl in REDUCE-IT produced higher plasma EPA levels than did EPA + DHA carboxylic acids in STRENGTH. Geometric mean or median on-treatment plasma EPA concentrations in REDUCE-IT and STRENGTH active arms were 144 and 89 μg/mL, respectively. A post-hoc analysis of the REDUCE-IT data showed a strong relationship between plasma EPA concentration and ASCVD risk reduction, particularly in the range of 140–200 μg/mL.12 This association retained statistical significance after adjustment for changes in other biomarkers of ASCVD risk.12 These results are consistent with those from a post-hoc analysis of data from the Japan EPA Lipid Intervention Study, which compared 1800 mg/day of EPA with usual care, in which a plasma EPA level ≥150 μg/dL was associated with a significant reduction in CHD event risk.13 However, tertiles of changes in plasma EPA and DHA in STRENGTH showed no significant associations with ASCVD event risk.14
One notable difference between the REDUCE-IT and STRENGTH results was a trend toward greater incidence of serious bleeding events in REDUCE-IT that was not observed in STRENGTH or in the Omega-3 Fatty Acids in Elderly with Myocardial Infarction trial, a smaller ASCVD outcomes trial that used 4 g/day of EPA + DHA as ethyl esters.5 , 6 , 15 Thus, one possible explanation for at least a portion of the unexplained effect on ASCVD risk in REDUCE-IT might be greater antiplatelet effects of the higher dosage of EPA in REDUCE-IT compared with EPA + DHA in STRENGTH.
As noted earlier, EPA and DHA have several physiological effects that are potentially antiatherothrombotic. The Effect of Vascepa on Improving Coronary Atherosclerosis in People with High Triglycerides Taking Statin Therapy (EVAPORATE) trial demonstrated significantly less progression of coronary plaques with EPA treatment compared with a mineral oil placebo.16 No comparable studies have been completed with EPA + DHA. The use of a mineral oil placebo and the small sample size in EVAPORATE are reasons for caution in interpretation, but the findings nevertheless add some evidence to support the biological plausibility of the observed benefits in REDUCE-IT.
Current European guidelines recommend consideration of icosapent ethyl in combination with statin therapy for high- and very-high-risk patients with triglyceride concentrations in the range of 135–499 mg/dL (1.5–5.6 mmol/L) despite statin treatment for reduction of ASCVD risk.17 The analysis by Doi et al. provides support for this recommendation, although important questions remain.7 Additional randomized, controlled trials of ASCVD outcomes and surrogate indicators, such as coronary plaque progression, will be needed to further clarify the magnitude of the effect of icosapent ethyl on ASCVD incidence, and the mechanisms responsible for such benefits.
Conflicts of interest: K.C.M. has been an advisor to and received research funding from Matinas BioPharma, Acasti Pharma, and Pharmavite.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References



