-
PDF
- Split View
-
Views
-
Cite
Cite
Gerhard-Paul Diller, Stefan Orwat, Astrid Elisabeth Lammers, Robert M Radke, Fernando De-Torres-Alba, Renate Schmidt, Ursula Marschall, Ulrike M Bauer, Dominic Enders, Leo Bronstein, Gerrit Kaleschke, Helmut Baumgartner, Lack of specialist care is associated with increased morbidity and mortality in adult congenital heart disease: a population-based study, European Heart Journal, Volume 42, Issue 41, 1 November 2021, Pages 4241–4248, https://doi.org/10.1093/eurheartj/ehab422
Close - Share Icon Share
Abstract
The aim of this study was to provide population-based data on the healthcare provision for adults with congenital heart disease (ACHD) and the impact of cardiology care on morbidity and mortality in this vulnerable population.
Based on administrative data from one of the largest German Health Insurance Companies, all insured ACHD patients (<70 years of age) were included. Patients were stratified into those followed exclusively by primary care physicians (PCPs) and those with additional cardiology follow-up between 2014 and 2016. Associations between level of care and outcome were assessed by multivariable/propensity score Cox analyses. Overall, 24 139 patients (median age 43 years, 54.8% female) were included. Of these, only 49.7% had cardiology follow-up during the 3-year period, with 49.2% of patients only being cared for by PCPs and 1.1% having no contact with either. After comprehensive multivariable and propensity score adjustment, ACHD patients under cardiology follow-up had a significantly lower risk of death [hazard ratio (HR) 0.81, 95% confidence interval (CI) 0.67–0.98; P = 0.03) or major events (HR 0.85, 95% CI 0.78–0.92; P < 0.001) compared to those only followed by PCPs. At 3-year follow-up, the absolute risk difference for mortality was 0.9% higher in ACHD patients with moderate/severe complexity lesions cared by PCPs compared to those under cardiology follow-up.
Cardiology care compared with primary care is associated with superior survival and lower rates of major complications in ACHD. It is alarming that even in a high resource setting with well-established specialist ACHD care approximately 50% of contemporary ACHD patients are still not linked to regular cardiac care. Almost all patients had at least one contact with a PCP during the study period, suggesting that opportunities to refer patients to cardiac specialists were missed at PCP level. More efforts are required to alert PCPs and patients to appropriate ACHD care.
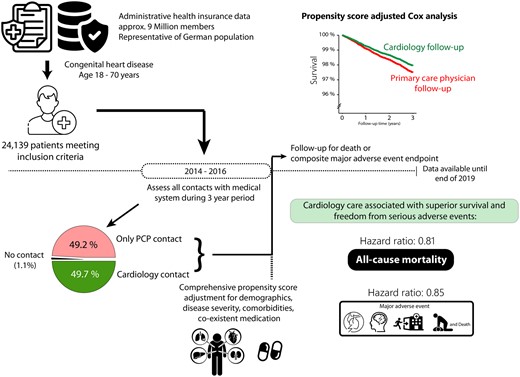
Study design and overview over the main results illustrating the unmet need of regular cardiology follow-up in 50.3% of adult congenital heart disease patients over a 3-year period. Care provided by primary care physicians was associated with worse outcome (mortality and freedom from death or major adverse events) compared to follow-up by cardiac specialists.
See page 4249 for the editorial comment for this article ‘Don’t be alarmed: the need for enhanced partnerships between medical communities to improve outcomes for adults living with congenital heart disease’, by A.D. Khan and A.M. Valente, https://doi.org/10.1093/eurheartj/ehab281.
Introduction
It is estimated that over 4 million patients with congenital heart disease are currently alive in Europe.1–3 As an increasing number of children born with congenital heart disease survive longer and enter adulthood, around two-third of the contemporary congenital patient population are adults and the number of adults with congenital heart disease (ACHD) continues to increase at a rate of 60% per decade.4–6 Unfortunately, most ACHD patients cannot be regarded as cured by the surgical or catheter interventions performed in childhood. Rather, the majority of patients continues to be afflicted by cardiac and extracardiac sequalae and complications. These chronic medical issues include heart failure, cardiac arrhythmias, ongoing risk of infections and endocarditis, neurological, respiratory as well as extracardiac end-organ disease.7–12 As a consequence, life-long specialized cardiac care is required in this vulnerable population.8 , 13 Previous studies from Australia and Canada have demonstrated the positive impact of specialized tertiary care on guideline adherence as a measure of process quality and overall mortality in ACHD patients.6 , 14 As a consequence, consolidation of ACHD care and creation of superregional tertiary centres together with a process of individual training and accreditation for adult and paediatric cardiologists is advocated and has been implemented in various countries in Europe and North America.15 , 16 Depending on the healthcare system involved, the organisation of ACHD healthcare provision may differ, however. Unlike in highly centralized health systems like the English NHS, many healthcare systems are largely decentralized with larger patient choice and ambulatory specialist medical services offered, both, on a hospital and an office-based level. Specialized follow-up provided by general, office-based cardiologists working alongside ACHD-trained cardiologists and tertiary centres is common in health care systems like the USA or Germany. Consequently, beyond the impact of tertiary care, ambulatory cardiology follow-up may affect morbidity and mortality of ACHD patients. However, robust data on this aspect of care are lacking but are required to guide health political decisions, primary care physicians (PCPs), and patients.
Using a comprehensive inpatient and ambulatory dataset of administrative data from one of Germany’s largest health insurance companies (9 million members, representative of the German population), we aimed to provide contemporary data on the effect of ambulatory office-based cardiology follow-up on morbidity and mortality compared to isolated community-based PCP provided care across the spectrum of ACHD.
Methods
Based on the administrative data from the German BARMER GEK Health Insurance Company insuring around 1/9 of the German population, i.e. ∼9 million members, all ambulatory and inpatient procedures and diagnoses coded in the time period 2005–19 were available. The underlying dataset contains an anonymized version of data required for reimbursement purposes. The data are, therefore, complete and data quality aspects such as data integrity and representativeness for the general population of Germany have been demonstrated.17 For the scope of the analysis we focused on patients with congenital heart disease as encoded based on the German modification of ICD-10 codes (Q20-26; for detailed information see Supplementary material online, Table S1), aged ≥18 and <70 years throughout the study period. Underlying congenital cardiac defects were stratified into mild, moderate, and severe complexity based on published recommendations.8 Patients with an isolated atrial septal defect could not be included as this cardiac defect shares an ICD code with patent foramen ovale in the German ICD-10 system. Patients were included if they had a relevant congenital heart disease code provided between 2005 and 2019. Consistent with previous publications, to ensure data quality, only patients who had relevant ICD-10 codes repeatedly recorded or in whom the qualifying code was provided by a cardiologist or during an in-hospital stay were considered.2 , 18 To assess the association between level of care (i.e. community-based PCP care only vs. follow-up by a paediatric or adult cardiologist), patients were stratified based on whether they were only followed by a PCP or whether they presented at least once to an office-based cardiologist or an ACHD centre in the time period 2014–16 irrespective of PCP care. This 3-year interval was chosen to account for the fact that especially patients with simpler cardiac defects may require specialist consultations only every few years based on current guideline recommendations.8 The start point (January 2014) of the interval coincides with the availability of ambulatory codes from ACHD centres in the underlying database, while the end of the time interval (December 2016) was chosen to allow for a subsequent 3-year follow-up period after the allocation period. Patients were followed throughout 2017–19 and the occurrence of all-cause mortality or a composite of death or major complications was assessed. Details on the composite major adverse event endpoint, including death, successful resuscitation, cardiac transplantation, implantation of a cardiac assist device, myocardial infarction, acute emergency admission to a cardiology or cardiac surgery department or a composite of non-traumatic cerebral ischaemic or haemorrhagic event, are provided in the Supplementary material online. To allow for varying baseline characteristics and risk profiles, both standard multivariable adjustment and propensity score adjustment were performed adjusting for relevant co-existing diagnoses, previous interventions (such as pacemaker/implantable cardioverter defibrillators) or medication.
The study was approved by the relevant ethics committee as part of the overarching research project using anonymized administrative health insurance data (OptAHF project).
Statistical analysis
Continuous variables were summarized by medians and quartiles and tested for group differences using Mann–Whitney U tests. Group comparisons between categorical variables were performed by the Chi-square test. We fitted Cox proportional hazard models for time from beginning of the event period (01 January 2017) to first composite major adverse event endpoint or to death. For the composite major adverse event endpoint, only first events were considered. For the overall cohort and subgroups with a sufficient number of events, we fitted multivariable Cox regression models (without propensity scores), models adjusted only for the logit of the propensity score, and also combinations of both. Results were highly similar for all three variants. Since some of the subgroups considered had insufficient numbers of events to allow for a full Cox regression adjustment on all variables, we instead adjusted on the propensity score and on further important variables (age, sex, heart defect complexity, and presence of heart failure). Propensity scores were calculated using logistic regression based on all concomitant diagnoses and medications listed in Supplementary material online, Table S1. We checked the overlap of propensity score distributions in the two groups, which were found to essentially fully overlap, so that no patients were excluded from the analysis.
Analysis was exploratory and partly data driven. No adjustment for multiple comparisons was performed and P-values <0.05 were considered statistically noticeable/significant. Analyses were performed using the software R version 3.6.2 (R Foundation, Vienna, Austria).
Results
Overall, 24 139 patients meeting the inclusion criteria (age 18–70 years throughout the study period and with relevant congenital heart disease diagnoses) were identified and formed the basis of the analysis. The median age of the population was 43 years [IQR 29–57 years] and 54.8% of the patients were female. The majority of patients had simple underlying heart defects (n = 16 748 patients [69.4%]), while 5489 (22.7%) and 1902 (7.9%) of patients had moderate and severe complexity cardiac defects. Only 260 patients (1.1%) had no contact to the health care system, whereas 11 872 (49.2%) patients were only in contact with at least one PCP and the remaining 12 007 patients (49.7%) consulted a cardiologist (adult, paediatric or an ambulatory cardiology service at an ACHD centre; Supplementary material online, Appendix S3) at least once during the allocation period (2014–2016; Graphical abstract). Patients who had cardiology contact were significantly older (median 46 [IQR 31–59] years) compared to PCP only follow-up patients (median 40 [IQR 28–55] years, P < 0.001). In addition, exclusively PCP-treated ACHD patients had a significantly lower proportion of moderate/severe complexity underlying cardiac lesions (73.7% mild, 20.5% moderate, and 5.8% severe complexity lesions) compared to ACHD patients under cardiology follow-up (65.0% simple, 25.0% medium complexity, and 10% high complexity lesions, P < 0.001). As illustrated in Table 1, patients linked to cardiology follow-up had a significantly higher proportion of cardiac and extracardiac co-morbidities compared to those only followed by PCPs. While, by study design, no patients under exclusive PCP follow-up presented to a cardiologist accredited for ACHD or a tertiary ACHD centre, 51.8% of patients presenting to a tertiary ACHD centre also consulted a (general) office-based cardiologist during the study period. Similarly, 30.5% of patients presenting to an ACHD accredited paediatric or general adult cardiologist also consulted a general cardiologist during the study period.
Overview over the study population stratified by primary care physician and cardiology follow-up groups
| Variable . | Primary care physician follow-up . | Cardiology follow-up . | P-value . |
|---|---|---|---|
| Patients, n | 11 872 | 12 007 | |
| Age (Q1–Q3) | 40 (28–55) years | 46 (31–59) years | <0.001 |
| Female sex | 6784 (57.1) | 6381 (53.1) | <0.001 |
| Complexity (simple/moderate/high %) | 73.7%/20.5%/5.8% | 65.0%/25.0%/10.0% | <0.001 |
| Comorbidities, n (%): | |||
| Myocardial infarction | 148 (1.3) | 447 (3.7) | <0.001 |
| Heart failure | 557 (4.7) | 2264 (18.9) | <0.001 |
| Cardiac arrhythmias | 1934 (16.3) | 5110 (42.6) | <0.001 |
| Arterial hypertension | 4012 (33.8) | 6418 (53.5) | <0.001 |
| Renal failure | 544 (4.6) | 1133 (9.4) | <0.001 |
| Hepatic failure | 1354 (11.4) | 1745 (14.5) | <0.001 |
| Stroke or intracranial bleeding | 830 (6.7) | 1409 (11.7) | <0.001 |
| Diabetes | 945 (8.0) | 1276 (10.6) | <0.001 |
| Obesity | 2111 (17.8) | 2562 (21.3) | <0.001 |
| Nicotine abuse | 1585 (13.4) | 1938 (16.1) | <0.001 |
| Alcohol abuse | 510 (4.3) | 514 (4.5) | 0.95 |
| Pre-existing cardiac pacemaker | 40 (0.3) | 383 (3.2) | <0.001 |
| Pre-existing cardioverter defibrillator (ICD) | 14 (0.1) | 181 (1.5) | <0.001 |
| Co-existing medication, n (%): | |||
| ACE-Inhibitors or ARBs | 2137 (18.0) | 4254 (35.4) | <0.001 |
| Diuretic medication | 749 (6.3) | 2099 (17.5) | <0.001 |
| Beta-blockers | 1781 (15.0) | 4340 (36.2) | <0.001 |
| Cardiac glycosides | 59 (0.5) | 319 (2.7) | <0.001 |
| Class III antiarrhythmics | 41 (0.4) | 305 (2.5) | <0.001 |
| Any antiarrhythmic therapy | 128 (1.1) | 743 (6.2) | <0.001 |
| Calcium Channel Blocker | 672 (5.7) | 1406 (11.7) | <0.001 |
| Antidepressant drugs | 1571 (13.2) | 1906 (15.9) | <0.001 |
| Anticonvulsive drugs | 549 (4.6) | 633 (5.3) | 0.02 |
| Pulmonary arterial hypertension medication | 19 (0.2) | 84 (0.7) | <0.001 |
| Vitamin-K antagonists | 420 (3.5) | 1683 (14.0) | <0.001 |
| Novel oral anticoagulants | 183 (1.5) | 651 (5.4) | <0.001 |
| Antiplatelet drugs | 429 (3.6) | 1357 (11.3) | <0.001 |
| Variable . | Primary care physician follow-up . | Cardiology follow-up . | P-value . |
|---|---|---|---|
| Patients, n | 11 872 | 12 007 | |
| Age (Q1–Q3) | 40 (28–55) years | 46 (31–59) years | <0.001 |
| Female sex | 6784 (57.1) | 6381 (53.1) | <0.001 |
| Complexity (simple/moderate/high %) | 73.7%/20.5%/5.8% | 65.0%/25.0%/10.0% | <0.001 |
| Comorbidities, n (%): | |||
| Myocardial infarction | 148 (1.3) | 447 (3.7) | <0.001 |
| Heart failure | 557 (4.7) | 2264 (18.9) | <0.001 |
| Cardiac arrhythmias | 1934 (16.3) | 5110 (42.6) | <0.001 |
| Arterial hypertension | 4012 (33.8) | 6418 (53.5) | <0.001 |
| Renal failure | 544 (4.6) | 1133 (9.4) | <0.001 |
| Hepatic failure | 1354 (11.4) | 1745 (14.5) | <0.001 |
| Stroke or intracranial bleeding | 830 (6.7) | 1409 (11.7) | <0.001 |
| Diabetes | 945 (8.0) | 1276 (10.6) | <0.001 |
| Obesity | 2111 (17.8) | 2562 (21.3) | <0.001 |
| Nicotine abuse | 1585 (13.4) | 1938 (16.1) | <0.001 |
| Alcohol abuse | 510 (4.3) | 514 (4.5) | 0.95 |
| Pre-existing cardiac pacemaker | 40 (0.3) | 383 (3.2) | <0.001 |
| Pre-existing cardioverter defibrillator (ICD) | 14 (0.1) | 181 (1.5) | <0.001 |
| Co-existing medication, n (%): | |||
| ACE-Inhibitors or ARBs | 2137 (18.0) | 4254 (35.4) | <0.001 |
| Diuretic medication | 749 (6.3) | 2099 (17.5) | <0.001 |
| Beta-blockers | 1781 (15.0) | 4340 (36.2) | <0.001 |
| Cardiac glycosides | 59 (0.5) | 319 (2.7) | <0.001 |
| Class III antiarrhythmics | 41 (0.4) | 305 (2.5) | <0.001 |
| Any antiarrhythmic therapy | 128 (1.1) | 743 (6.2) | <0.001 |
| Calcium Channel Blocker | 672 (5.7) | 1406 (11.7) | <0.001 |
| Antidepressant drugs | 1571 (13.2) | 1906 (15.9) | <0.001 |
| Anticonvulsive drugs | 549 (4.6) | 633 (5.3) | 0.02 |
| Pulmonary arterial hypertension medication | 19 (0.2) | 84 (0.7) | <0.001 |
| Vitamin-K antagonists | 420 (3.5) | 1683 (14.0) | <0.001 |
| Novel oral anticoagulants | 183 (1.5) | 651 (5.4) | <0.001 |
| Antiplatelet drugs | 429 (3.6) | 1357 (11.3) | <0.001 |
Details on the definition of co-morbidities or medication are presented in the Supplementmentray material online. P-values refer to comparisons between the two groups. Statistically noticeable P-values are in bold.
Overview over the study population stratified by primary care physician and cardiology follow-up groups
| Variable . | Primary care physician follow-up . | Cardiology follow-up . | P-value . |
|---|---|---|---|
| Patients, n | 11 872 | 12 007 | |
| Age (Q1–Q3) | 40 (28–55) years | 46 (31–59) years | <0.001 |
| Female sex | 6784 (57.1) | 6381 (53.1) | <0.001 |
| Complexity (simple/moderate/high %) | 73.7%/20.5%/5.8% | 65.0%/25.0%/10.0% | <0.001 |
| Comorbidities, n (%): | |||
| Myocardial infarction | 148 (1.3) | 447 (3.7) | <0.001 |
| Heart failure | 557 (4.7) | 2264 (18.9) | <0.001 |
| Cardiac arrhythmias | 1934 (16.3) | 5110 (42.6) | <0.001 |
| Arterial hypertension | 4012 (33.8) | 6418 (53.5) | <0.001 |
| Renal failure | 544 (4.6) | 1133 (9.4) | <0.001 |
| Hepatic failure | 1354 (11.4) | 1745 (14.5) | <0.001 |
| Stroke or intracranial bleeding | 830 (6.7) | 1409 (11.7) | <0.001 |
| Diabetes | 945 (8.0) | 1276 (10.6) | <0.001 |
| Obesity | 2111 (17.8) | 2562 (21.3) | <0.001 |
| Nicotine abuse | 1585 (13.4) | 1938 (16.1) | <0.001 |
| Alcohol abuse | 510 (4.3) | 514 (4.5) | 0.95 |
| Pre-existing cardiac pacemaker | 40 (0.3) | 383 (3.2) | <0.001 |
| Pre-existing cardioverter defibrillator (ICD) | 14 (0.1) | 181 (1.5) | <0.001 |
| Co-existing medication, n (%): | |||
| ACE-Inhibitors or ARBs | 2137 (18.0) | 4254 (35.4) | <0.001 |
| Diuretic medication | 749 (6.3) | 2099 (17.5) | <0.001 |
| Beta-blockers | 1781 (15.0) | 4340 (36.2) | <0.001 |
| Cardiac glycosides | 59 (0.5) | 319 (2.7) | <0.001 |
| Class III antiarrhythmics | 41 (0.4) | 305 (2.5) | <0.001 |
| Any antiarrhythmic therapy | 128 (1.1) | 743 (6.2) | <0.001 |
| Calcium Channel Blocker | 672 (5.7) | 1406 (11.7) | <0.001 |
| Antidepressant drugs | 1571 (13.2) | 1906 (15.9) | <0.001 |
| Anticonvulsive drugs | 549 (4.6) | 633 (5.3) | 0.02 |
| Pulmonary arterial hypertension medication | 19 (0.2) | 84 (0.7) | <0.001 |
| Vitamin-K antagonists | 420 (3.5) | 1683 (14.0) | <0.001 |
| Novel oral anticoagulants | 183 (1.5) | 651 (5.4) | <0.001 |
| Antiplatelet drugs | 429 (3.6) | 1357 (11.3) | <0.001 |
| Variable . | Primary care physician follow-up . | Cardiology follow-up . | P-value . |
|---|---|---|---|
| Patients, n | 11 872 | 12 007 | |
| Age (Q1–Q3) | 40 (28–55) years | 46 (31–59) years | <0.001 |
| Female sex | 6784 (57.1) | 6381 (53.1) | <0.001 |
| Complexity (simple/moderate/high %) | 73.7%/20.5%/5.8% | 65.0%/25.0%/10.0% | <0.001 |
| Comorbidities, n (%): | |||
| Myocardial infarction | 148 (1.3) | 447 (3.7) | <0.001 |
| Heart failure | 557 (4.7) | 2264 (18.9) | <0.001 |
| Cardiac arrhythmias | 1934 (16.3) | 5110 (42.6) | <0.001 |
| Arterial hypertension | 4012 (33.8) | 6418 (53.5) | <0.001 |
| Renal failure | 544 (4.6) | 1133 (9.4) | <0.001 |
| Hepatic failure | 1354 (11.4) | 1745 (14.5) | <0.001 |
| Stroke or intracranial bleeding | 830 (6.7) | 1409 (11.7) | <0.001 |
| Diabetes | 945 (8.0) | 1276 (10.6) | <0.001 |
| Obesity | 2111 (17.8) | 2562 (21.3) | <0.001 |
| Nicotine abuse | 1585 (13.4) | 1938 (16.1) | <0.001 |
| Alcohol abuse | 510 (4.3) | 514 (4.5) | 0.95 |
| Pre-existing cardiac pacemaker | 40 (0.3) | 383 (3.2) | <0.001 |
| Pre-existing cardioverter defibrillator (ICD) | 14 (0.1) | 181 (1.5) | <0.001 |
| Co-existing medication, n (%): | |||
| ACE-Inhibitors or ARBs | 2137 (18.0) | 4254 (35.4) | <0.001 |
| Diuretic medication | 749 (6.3) | 2099 (17.5) | <0.001 |
| Beta-blockers | 1781 (15.0) | 4340 (36.2) | <0.001 |
| Cardiac glycosides | 59 (0.5) | 319 (2.7) | <0.001 |
| Class III antiarrhythmics | 41 (0.4) | 305 (2.5) | <0.001 |
| Any antiarrhythmic therapy | 128 (1.1) | 743 (6.2) | <0.001 |
| Calcium Channel Blocker | 672 (5.7) | 1406 (11.7) | <0.001 |
| Antidepressant drugs | 1571 (13.2) | 1906 (15.9) | <0.001 |
| Anticonvulsive drugs | 549 (4.6) | 633 (5.3) | 0.02 |
| Pulmonary arterial hypertension medication | 19 (0.2) | 84 (0.7) | <0.001 |
| Vitamin-K antagonists | 420 (3.5) | 1683 (14.0) | <0.001 |
| Novel oral anticoagulants | 183 (1.5) | 651 (5.4) | <0.001 |
| Antiplatelet drugs | 429 (3.6) | 1357 (11.3) | <0.001 |
Details on the definition of co-morbidities or medication are presented in the Supplementmentray material online. P-values refer to comparisons between the two groups. Statistically noticeable P-values are in bold.
During a cumulative observation period of 69 414 years after the allocation period, corresponding to an average follow-up time of 2.88 years/patient, 524 patients died, and 2517 patients experienced a major adverse event. The leading cause of non-lethal major adverse events were emergency cardiac or neurology admission (n = 1831), neurological complications (n = 501), survived resuscitation (n = 371), acute myocardial infarction (n = 246), and heart transplantation or implantation of a cardiac assist device (n = 11). Both mortality and major adverse event rates were highest in patients with severe lesion complexity (4.3% mortality and 15.6% major adverse events) compared to patients with moderate complexity (2.4% mortality and 10.6% major adverse events) and mild complexity (1.9% mortality and 9.8% major adverse events), respectively.
The results of the unadjusted univariate Cox proportional hazard analysis comparing the effect of cardiology follow-up vs. mere PCP follow-up in the entire cohort as well as various sub cohorts are presented in Supplementary material online, Table S2. After comprehensive multivariable and propensity score adjustment, cardiology follow-up was associated with a significantly lower risk of death [hazard ratio (HR) 0.81, 95% CI 0.67–0.98, P = 0.03] and major adverse events (HR 0.85; 95% CI 0.78–0.92, P < 0.001) compared to patients only followed by PCPs. These results were confirmed both on multivariable adjustment without propensity scores (HR 0.81, 95% CI 0.67–0.98; P = 0.03 and HR 0.86, 95% CI 0.79–0.93; P < 0.001, for mortality and the combined endpoint, respectively) as well as propensity score only adjustment (HR 0.78, 95% CI 0.65–0.95; P = 0.015 and HR 0.84, 95% CI 0.77–0.92; P < 0.001, for mortality and the combined endpoint, respectively). In pre-specified subgroup analyses, cardiology follow-up was associated with a significantly lower risk of death specifically in females (HR 0.65; P = 0.005), patients over 45 years of age (HR 0.76; P = 0.01) as well as patients with moderate or high complexity underlying heart defects (HR 0.73; P = 0.048). For the composite endpoint of death or severe adverse events, multivariate propensity score-adjusted subgroup analyses revealed a significantly reduced risk in males (HR 0.82; P = 0.001), females (HR 0.88; P = 0.046), patients over the age of 45 years (HR 0.77; P < 0.001), patients with simple underlying heart defects (HR 0.85; P = 0.003) as well as those with moderate or severe complexity cardiac defects (HR 0.81; P = 0.007). The results of the stratified subgroup analyses are presented in Table 2. Figure 1 shows the survival and freedom from event curves for the two endpoints based on the results of the multivariable propensity score adjusted Cox proportional hazards analysis.
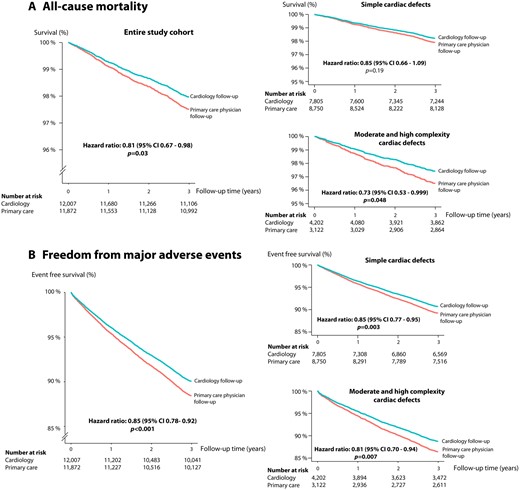
Freedom from death as well as freedom from death/major adverse events in the entire cohort as well as specifically in patients with simple or moderate/high complexity cardiac defects based on the results of the multivariable propensity score adjusted Cox proportional hazards analysis.
Results of the multivariable propensity score adjusted analysis comparing primary care physician vs. cardiology follow-up for the primary (all-cause mortality) and secondary (composite of death or major adverse events) endpoint
| . | Comparison cardiology vs primary physician follow-up . | |||
|---|---|---|---|---|
| Number of events . | Hazard ratio . | 95% CI . | P-value . | |
| All-cause mortality | ||||
| Overall cohort (n = 23 879) | 520 | 0.81 | 0.67–0.98 | 0.03 |
| Subgroup analyses | ||||
| Male sex (n = 10 714) | 314 | 0.96 | 0.74–1.24 | 0.75 |
| Female sex (n = 13 165) | 206 | 0.65 | 0.48–0.88 | 0.005 |
| Age group <45 years (n = 12 326) | 91 | 0.93 | 0.58–1.5 | 0.77 |
| Age group 45–70 years (n = 11 553) | 429 | 0.76 | 0.62–0.94 | 0.01 |
| Moderate or high complexity cardiac lesion (n = 7324) | 210 | 0.73 | 0.53–0.997 | 0.048 |
| Simple cardiac lesion (n = 16 555) | 310 | 0.85 | 0.66–1.09 | 0.19 |
| All-cause mortality or major adverse event | ||||
| Overall cohort (n = 23 879) | 2491 | 0.85 | 0.78–0.92 | <0.001 |
| Subgroup analyses | ||||
| Male sex (n = 10 714) | 1350 | 0.82 | 0.73–0.93 | 0.001 |
| Female sex (n = 13 165) | 1141 | 0.88 | 0.77–0.998 | 0.046 |
| Age group <45 years (n = 12 326) | 703 | 0.99 | 0.84–1.17 | 0.91 |
| Age group 45–70 years (n = 11 553) | 1788 | 0.77 | 0.70–0.85 | <0.001 |
| Moderate or high complexity cardiac lesion (n = 7324) | 872 | 0.81 | 0.70–0.94 | 0.007 |
| Simple cardiac lesion (n = 16 555) | 1619 | 0.85 | 0.77–0.95 | 0.003 |
| . | Comparison cardiology vs primary physician follow-up . | |||
|---|---|---|---|---|
| Number of events . | Hazard ratio . | 95% CI . | P-value . | |
| All-cause mortality | ||||
| Overall cohort (n = 23 879) | 520 | 0.81 | 0.67–0.98 | 0.03 |
| Subgroup analyses | ||||
| Male sex (n = 10 714) | 314 | 0.96 | 0.74–1.24 | 0.75 |
| Female sex (n = 13 165) | 206 | 0.65 | 0.48–0.88 | 0.005 |
| Age group <45 years (n = 12 326) | 91 | 0.93 | 0.58–1.5 | 0.77 |
| Age group 45–70 years (n = 11 553) | 429 | 0.76 | 0.62–0.94 | 0.01 |
| Moderate or high complexity cardiac lesion (n = 7324) | 210 | 0.73 | 0.53–0.997 | 0.048 |
| Simple cardiac lesion (n = 16 555) | 310 | 0.85 | 0.66–1.09 | 0.19 |
| All-cause mortality or major adverse event | ||||
| Overall cohort (n = 23 879) | 2491 | 0.85 | 0.78–0.92 | <0.001 |
| Subgroup analyses | ||||
| Male sex (n = 10 714) | 1350 | 0.82 | 0.73–0.93 | 0.001 |
| Female sex (n = 13 165) | 1141 | 0.88 | 0.77–0.998 | 0.046 |
| Age group <45 years (n = 12 326) | 703 | 0.99 | 0.84–1.17 | 0.91 |
| Age group 45–70 years (n = 11 553) | 1788 | 0.77 | 0.70–0.85 | <0.001 |
| Moderate or high complexity cardiac lesion (n = 7324) | 872 | 0.81 | 0.70–0.94 | 0.007 |
| Simple cardiac lesion (n = 16 555) | 1619 | 0.85 | 0.77–0.95 | 0.003 |
Data are provided for the overall cohort as well as for predefined subgroups of patients. A HR <1 indicates superior survival/freedom from adverse events for the group with cardiology follow-up. Details on the definition of the composite endpoint as well as variables included in the propensity score/multivariable analysis are given in the Methods section as well as Supplementary material online, Table S1. Text in bold identifies significant parameters.
Results of the multivariable propensity score adjusted analysis comparing primary care physician vs. cardiology follow-up for the primary (all-cause mortality) and secondary (composite of death or major adverse events) endpoint
| . | Comparison cardiology vs primary physician follow-up . | |||
|---|---|---|---|---|
| Number of events . | Hazard ratio . | 95% CI . | P-value . | |
| All-cause mortality | ||||
| Overall cohort (n = 23 879) | 520 | 0.81 | 0.67–0.98 | 0.03 |
| Subgroup analyses | ||||
| Male sex (n = 10 714) | 314 | 0.96 | 0.74–1.24 | 0.75 |
| Female sex (n = 13 165) | 206 | 0.65 | 0.48–0.88 | 0.005 |
| Age group <45 years (n = 12 326) | 91 | 0.93 | 0.58–1.5 | 0.77 |
| Age group 45–70 years (n = 11 553) | 429 | 0.76 | 0.62–0.94 | 0.01 |
| Moderate or high complexity cardiac lesion (n = 7324) | 210 | 0.73 | 0.53–0.997 | 0.048 |
| Simple cardiac lesion (n = 16 555) | 310 | 0.85 | 0.66–1.09 | 0.19 |
| All-cause mortality or major adverse event | ||||
| Overall cohort (n = 23 879) | 2491 | 0.85 | 0.78–0.92 | <0.001 |
| Subgroup analyses | ||||
| Male sex (n = 10 714) | 1350 | 0.82 | 0.73–0.93 | 0.001 |
| Female sex (n = 13 165) | 1141 | 0.88 | 0.77–0.998 | 0.046 |
| Age group <45 years (n = 12 326) | 703 | 0.99 | 0.84–1.17 | 0.91 |
| Age group 45–70 years (n = 11 553) | 1788 | 0.77 | 0.70–0.85 | <0.001 |
| Moderate or high complexity cardiac lesion (n = 7324) | 872 | 0.81 | 0.70–0.94 | 0.007 |
| Simple cardiac lesion (n = 16 555) | 1619 | 0.85 | 0.77–0.95 | 0.003 |
| . | Comparison cardiology vs primary physician follow-up . | |||
|---|---|---|---|---|
| Number of events . | Hazard ratio . | 95% CI . | P-value . | |
| All-cause mortality | ||||
| Overall cohort (n = 23 879) | 520 | 0.81 | 0.67–0.98 | 0.03 |
| Subgroup analyses | ||||
| Male sex (n = 10 714) | 314 | 0.96 | 0.74–1.24 | 0.75 |
| Female sex (n = 13 165) | 206 | 0.65 | 0.48–0.88 | 0.005 |
| Age group <45 years (n = 12 326) | 91 | 0.93 | 0.58–1.5 | 0.77 |
| Age group 45–70 years (n = 11 553) | 429 | 0.76 | 0.62–0.94 | 0.01 |
| Moderate or high complexity cardiac lesion (n = 7324) | 210 | 0.73 | 0.53–0.997 | 0.048 |
| Simple cardiac lesion (n = 16 555) | 310 | 0.85 | 0.66–1.09 | 0.19 |
| All-cause mortality or major adverse event | ||||
| Overall cohort (n = 23 879) | 2491 | 0.85 | 0.78–0.92 | <0.001 |
| Subgroup analyses | ||||
| Male sex (n = 10 714) | 1350 | 0.82 | 0.73–0.93 | 0.001 |
| Female sex (n = 13 165) | 1141 | 0.88 | 0.77–0.998 | 0.046 |
| Age group <45 years (n = 12 326) | 703 | 0.99 | 0.84–1.17 | 0.91 |
| Age group 45–70 years (n = 11 553) | 1788 | 0.77 | 0.70–0.85 | <0.001 |
| Moderate or high complexity cardiac lesion (n = 7324) | 872 | 0.81 | 0.70–0.94 | 0.007 |
| Simple cardiac lesion (n = 16 555) | 1619 | 0.85 | 0.77–0.95 | 0.003 |
Data are provided for the overall cohort as well as for predefined subgroups of patients. A HR <1 indicates superior survival/freedom from adverse events for the group with cardiology follow-up. Details on the definition of the composite endpoint as well as variables included in the propensity score/multivariable analysis are given in the Methods section as well as Supplementary material online, Table S1. Text in bold identifies significant parameters.
Discussion
Based on a large representative, nationwide dataset including over 20 000 ACHD patients we have found that—contrary to current guideline recommendations—over 50% of ACHD patients were not linked to regular cardiology care. Despite lacking contact to cardiac services, almost all patients had at least one contact with a PCP during the study period, suggesting that opportunities to refer patients to cardiac specialists were present but were missed by the respective family doctors. This is alarming as cardiology care provision was found to be indeed associated with superior survival and a lower rate of major cardiac and neurological complications in this population.
The positive impact of centralizing care at tertiary ACHD centres has been documented based on the implementation of routine referral to specialized ACHD centres and following the introduction of national ACHD guidelines in Canada.6 More recently, Cordina et al.,14 using data from an Australian ACHD referral centre, reported that patients followed by ACHD-trained cardiologists were more likely to be treated according to guidelines compared with those under the care of general cardiologists. To our knowledge, no robust data on the impact of ambulatory general cardiology follow-up on morbidity and mortality are available in ACHD. Current guidelines recommend that all ACHD patients should consult an ACHD cardiologist at least once, with the majority of patients requiring regular follow-up and treatment by appropriately trained ACHD cardiac specialists.8 Alarmingly, 49% of patients with congenital defects were only seen by PCPs during a 3-year period in our study. This included 36% of patients with high and 45% of patients with moderate complexity underlying lesions. These percentages, thus, could serve as estimates for the proportion of patients’ lost to follow-up in the German system. These findings suggest that failure to implement the guidelines occurs at the lowest level of the medical system, namely at the PCP level as the patients’ first contact point for medical care. The current data thus illustrate that loss-to-follow-up and lapses in appropriate care for ACHD patients do not equate to lack of contact with the medical system per se but lack of vertical referral from PCPs to specialized cardiology services. A first direct consequence of our study is thus to increase PCP as well as patient awareness and encourage proactive and regular ACHD patient referral to outpatient cardiology services. While potentially particularly relevant for ACHD patients with more complex forms of congenital heart disease, we contend that PCPs should be encouraged to cooperate with cardiac services for all ACHD patients. This is due to the intricate nature of ACHD and the fact that anatomy cannot be readily separated from physiology in this setting.2 In addition, given the evidence provided, we suggest to primarily focus efforts on ensuring vertical referral from PCPs to local cardiologists rather than overburdening busy frontline family physicians with the decision to stratify ACHD patients into those requiring direct referral to specifically trained ACHD cardiologists or ACHD centres and those in whom referral to a general cardiologist might suffice. This is based on our observation that care provided by general cardiologists was highly intertwined with specialist ACHD services. In fact, over 50% of ACHD patients at tertiary centres also consulted a general office-based cardiologist during the study period, suggesting a close collaboration between these two sectors. Furthermore, while cardiology care was associated with a lower mortality rate only in patients with more severe heart defects, a lower major adverse event rate was also evident in patients with simple lesions compared to PCP care, emphasizing that all patients may benefit from specialist referral.
The results of the current study should not be misinterpreted as a carte blanche to provide general cardiology care to all ACHD patients without regular referral to specialized ACHD services. In fact, ACHD patients should not be managed in isolation and without involving specialized ACHD centres.2 , 8 Rather, it highlights the need for the establishment and integration of specifically ACHD-trained office-based colleagues and ACHD tertiary centres into the general cardiology landscape. We cannot exclude the possibility that individual non-ACHD-trained cardiologists may unnecessarily delay referral of patients to specialized centres, as highlighted by Cordina et al. previously.14 However, our study suggests that—at least in our studied setting—general cardiology and specialist ACHD services appear highly integrated and collaborative. Currently, 20 superregional, 3 regional ACHD units and 8 specialized ACHD practices have been accredited in Germany.19 In addition, around 350 paediatric or adult cardiologists have obtained training and specific accreditation for ACHD. Given the rapidly increasing number of ACHD patients, there remains a need for an increased specialized ACHD workforce and accessible services closer to patients’ homes. Compared to the total number of ambulatory general adult and paediatric cardiologists in Germany (over 2800 in the BARMER database), this number is still low. Therefore, raising awareness and enabling training of general cardiologists continues to remain of importance. Giving that most ACHD patients are co-managed by general cardiologists, the Cardiac Societies should be encouraged to intensify ACHD training for the general cardiology audience for example by giving ACHD-specific topics greater room at national and international meetings or within educational publications. In addition, patient information, education, and involvement are key to improve compliance with regular follow-up recommendations and allow patients to act as their own advocates when confronting inappropriate primary care decisions.20 , 21 Overall, appropriate patient advocacy encouraging and enabling patients to take ownership of their medical care through continuous patient education and involvement seems to be a key element in ensuring long-term adherence to high quality medical care. This process likely should be initiated early in life by paediatric cardiologists and paediatric centres as well as be combined with a structured transition and transfer process to adult care over time.
We contend that the magnitude of the mortality and morbidity reduction in the current study was highly clinically relevant. At 3 years of follow-up, an absolute risk difference in mortality of ∼0.9% was observed in ACHD patients with moderate or severe complexity cardiac defects under cardiology care compared to those with mere PCP follow-up. If confirmed in a prospective manner, this number would extrapolate to the potential to save one life per 100 ACHD patients over 3 years if appropriate care would be provided. Similarly, in the medium/severe complexity group, the absolute risk difference between groups was ∼2.3% for the composite endpoint after 3 years in favour of the cardiology care group.
Due to the nature of the analysis and especially the fact that follow-up was limited and general cardiology and ACHD cardiology care are highly intertwined, we abstained from analysing the specific effect of specialized ACHD care on outcome in addition to having general cardiology care. This is also due to the fact that, given the nature of the analysis, we lack some more granular data, for example on ventricular function, to better risk stratify the population. In addition, the number of patients who died while being under ACHD specialist care over the 3-year follow-up period was limited (71 deaths), thus further limiting the statistical power of the analysis. The available data indicate that especially younger and more complex ACHD patients seemed to have been referred on to specialized services by office-based general cardiologists. We speculate that this—together with the highly connected nature of service delivery between general cardiologists and ACHD specialists—may explain why we could not statistically isolate the effect of specialist ACHD care on top of general cardiology follow-up. Supplementary material online, Table S3 gives and overviews over care delivery by ACHD specialists compared to general office-based cardiologists.
Strength of the current report
To the best of our knowledge, this is one of the largest cohorts providing a population-based real-world overview over the contemporary care situation for ACHD in a high-resource setting. The current report reveals the unmet needs in the follow-up and referral of ACHD patients to cardiology services by using a large robust, complete and representative administrative database. Unlike single centre reports, the database covers all insured individuals irrespective of tertiary centre follow-up, including also patients lost to cardiology follow-up. Furthermore, the longitudinal data available including complete information on all in- and outpatient contacts with the healthcare system as well as prescribed medication enabled us to adjust for varying baseline characteristics of the patients.
Limitations
This is a retrospective study and the employed administrative database was not primarily designed for research purposes. Specifically, the underlying ICD-10 codes were not designed specifically for congenital heart disease and do not fully account for important anatomic details in this context. Ideally a nomenclature system specifically designed for this patient cohort such as the International Paediatric and Congenital Cardiac Code system would be desirable to this end. Further registry-based studies using more detailed coding systems are suggested to further confirm the associations described here.22 Estimates of the percentage of patients lost to follow-up especially for ACHD patients with simple defects should be interpreted with caution as we chose a 3-year allocation period, which might be too short for some patients at the best end of the spectrum of simple ACHD. The results of the study should be interpreted within the context of the German healthcare system and apply mainly to this and similar devolved healthcare systems. We accept that the situation may be different in highly centralized systems such as the English NHS. We cannot exclude the possibility that PCPs did refer patients to cardiology services, but patients ignored the advice and failed to arrange for consultation appointments. Given the magnitude of the problem, however, it appears unlikely that 50% of patients would ignore the advice of their family physician and fail to arrange for appropriate appointments within a 3-year period. Due to the nature of the study, it is possible that unobserved differences between the PCP and cardiology follow-up groups existed at baseline and that these were not fully compensated despite the extensive multivariable and propensity score-based statistical adjustment. As general cardiology services and specialized ACHD care (including at tertiary centres) are highly interrelated, we refrained from assessing the benefit of isolated specialized ACHD care beyond that provided by general cardiologist with the ongoing support by ACHD services.
Conclusions
Despite clear guideline recommendations requesting regular cardiology follow-up of ACHD patients, the current study demonstrates that ∼50% of contemporary patients are not receiving appropriate cardiology care even in a country with well-established specialized ACHD care. Alarmingly, almost all patients were in contact with their respective PCP during the study period, suggesting that opportunities for cardiology referral existed but were missed. The fact that cardiology care was indeed associated with a significant clinically relevant survival benefit and a lower rate of major complications in this population reinforces the need for action. Apparently, major efforts are still required to alert PCPs and patients to appropriate ACHD care.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The study was conducted within the framework of the OptAHF project (Optimizing the care in ACHD; g-BA innovation fonds 2018). Research in the Department of Cardiology III, University Hospital Münster was supported by the Karla VÖLLM Stiftung, Krefeld, Germany.
Conflict of interest: none declared.
References



