-
PDF
- Split View
-
Views
-
Cite
Cite
Jeske van Diemen, Petra Verdonk, Alaide Chieffo, Evelyn Regar, Fina Mauri, Vijay Kunadian, Garima Sharma, Roxana Mehran, Yolande Appelman, The importance of achieving sex- and gender-based equity in clinical trials: a call to action, European Heart Journal, Volume 42, Issue 31, 14 August 2021, Pages 2990–2994, https://doi.org/10.1093/eurheartj/ehab457
Close - Share Icon Share
Cardiovascular disease (CVD) is the leading cause of death in men and women worldwide.1 Although there have been significant advances in reducing CVD-related morbidity and mortality in both sexes, current guideline-directed therapies are based on data that predominantly include male patients.2 Consequently, in CVD management, female patients might currently be treated equally (the same), however, they are not treated based on equity (i.e. on their health needs).
It is believed that women are underrepresented because of a lower prevalence of CVD compared with men. However, even after correction of sex-specific prevalence, trials concerning coronary artery disease and heart failure continue to report a proportionally low percentage of female participants.3 , 4
While addressing CVD outcomes and interventions through a sex and gendered lens, it is important to recognize that these terms are often used interchangeably, though they differ in many ways. Gender is a social construct and refers to societal tasks, roles, and characteristics assigned to men and women, whereas sex is assigned at birth and refers to the underlying biological aspects of being male or female. Although sex and gender are not synonyms, they both are intertwined and for the purpose of this manuscript we will refer to gender in our inferences. Improving our understanding of sex- and gender-specific differences in the pathophysiology of CVD and their impact on CVD-related outcomes is the fastest and most sustainable way to improve health care, reduce research waste, and achieve health equity. Including women in clinical trials is therefore imperative. In this article, we discuss the current understanding regarding the motivators (e.g. stimuli to participate such as personal values, or financial compensation), facilitators (e.g. aids in participation such as transportation), and barriers (e.g. child and elderly care responsibilities) that collectively contribute to less enrolment and continuation of women in clinical trials and aim to construct a helpful guide on how to increase the participation of women and achieve a sex and gender balance in clinical trials.
Factors associated with underrepresentation of women in clinical trials
While examining the underrepresentation of women in clinical trials, it is important to discuss the lower participation rate of women in cardiovascular clinical trials and base the central question around why women enrol at lower rates and are more likely to drop out of trials once enrolled. Needless to say, multiple opportunities exist for a patient to fall out of the enrolment pathway, and several of these opportunities can likely be influenced by both patient-related and trial site-related factors.
To better understand the factors that impact underrepresentation of women in clinical trials, we performed an extensive literature search (as detailed in the Appendix) on articles that addressed the challenges in enrolment—motivators, facilitators, and barriers to the enrolment and continuation of women in clinical trials. We had initially aimed at a systematic review but there was a paucity of data to conduct an extensive review. We found only six articles that report on motivators, facilitators, and barriers for women to participate in cardiovascular medical trials.5–10 Of these, three studies were conducted in the USA,5–7 two in Canada,9 , 10 and one in Sweden,8 and included 846 men and 1122 women. In general for both men and women, motivators included the possibility of access to a better and more continuous care, and altruistic values such as the desire to promote science.8 , 9
Interestingly, one study performed in Sweden found that financial motivators such as reimbursements to enrol in trials played only a minor role for both men and women in their decisions whether or not to participate in research.8 None of the studies reported on possible facilitators to improve participation rates in female participants. In terms of barriers, both male and female participants reported time constraints, apprehension towards being in a clinical trial with an experimental design or therapy, or the potential of an unfavourable outcome and risk of harm.11
Women declined more often to participate because they perceived a higher risk of harm from trial participation than men.5 Furthermore, women more often than men reported transportation problems as a reason to decline trial participation.9 A high socioeconomic position (SEP) was associated with an increased willingness to participate among women.5 , 12 While this is a facilitator for higher SEP women to be enrolled in more clinical trials, this could potentially render women with lower SEP invisible or make them be even more underrepresented, thereby adding to the persistent disparity and inequity. Lastly, women who benefit from better representation and therapeutic benefit the most—i.e. women with a lower SEP—still remain grossly underrepresented. Interestingly, a facilitator for men to enrol in clinical trials was the opportunity of receiving helpful advice on health and lifestyle.8
It is worth noting that two studies did not find any sex and gender differences in motivators.6 , 11 However, one of these studies was not primarily designed to investigate sex- or gender-related differences.11 Furthermore, motivators, facilitators, and barriers are likely to differ per country and across health care systems. Researchers demonstrated that economic motivators were less important in Sweden, where health care is universally covered.8 Likewise, studies marking travel burden as a barrier to participate were conducted in Canada9 and the USA.7
Recommendations to improve representation of women in clinical trials
The solutions to improve the representation of women are multipronged and require a commitment from multiple stakeholders and society at large. While the details are beyond the purview of this paper, we listed the broad pillars of interventions below.
Role of scientific journals and peer-reviewed media outlets
Overall, a simple and swift solution would be for all scientific journals to require authors to address sex and gender differences to publish—cardiovascular—manuscripts.13 Note that such mandatory measures usually do not prompt the aspired change in mind-set, but may lead to a ‘tick the box’ approach. Hence, to fully transform the research ethos towards more inclusiveness, we advocate for a multi-level approach. First, we need research to determine sex and gender differences in motivators, facilitators, and barriers to participate in cardiovascular research because the few studies conducted so far are all relatively small survey-based cohorts, leaving the rationale behind participating or declining participation in cardiovascular clinical research a black box. However, we need such knowledge to develop interventions that may boost the participation of women in trials. Second, scientific journals need to have focused issues on sex and gendered data in CVD and this will facilitate more research in this realm. Lastly, volunteer science organizations can work with medical journals in improving the knowledge of female patients on this issue, so when the opportunity to participate in a clinical trial arises, there is more awareness and willingness on their end to participate.
Improvement in randomized controlled clinical trial design
Frameworks that are designed to integrate health equity considerations into the design of randomized trials should be implemented in research. For example, PROGRESS Plus (Place of residence, Race, Occupation, Gender, Religion, Education, Socio-economic status, Social capital and “Plus” that includes other context specific factors) provides a useful framework to review multiple and intersecting social determinants of health in research design.14 However, while such frameworks tell us how to integrate female participants in research, they do not inform us how to recruit them to participate in trials in the first place.
Increase diversity of the research team
One way to increase the participatory rate of women in trials is to ensure a diverse research team.15 A recent study demonstrated appalling numbers. Of the clinical trial results published in JAMA, The Lancet, and New England Journal of Medicine from 1 January 2014 to 31 December 2018, women constituted only 10.1% of clinical trial leadership committees.16 A diverse workforce is better capable of understanding diverse participant populations and, hence, tailoring research products better to participants. Moreover, the inclusion of diversity within research teams may provide a source of more inclusivity, such as participatory research in which patients are included in the development of research questions, designs, analysis, and authoring of studies. For instance, in the Netherlands, ‘Harteraad’ is a patients’ organization for people with CVD and mediates in involving so-called ‘end-users’ or stakeholders in the design and conduction of cardiovascular trials. Stakeholders can play a key role in asking research questions, tailoring recruitment messages, developing and reviewing recruitment materials, but also facilitating improvement of—recruitment—materials with appeal to a broad audience.
Developing sex and gendered educational curricula in medicine
Designing and implementing sex- and gender diversity-responsive education is needed to prepare medical students, residents, and researchers for a diverse patient population in the future. Examples of additional training include the online courses developed by the ‘Sex and Gender Health Collaborative’. Incorporating these important curricula at early stages of medical education can have a ripple effect downstream.17 A summary of the above proposed course of action is depicted in Graphical abstract.
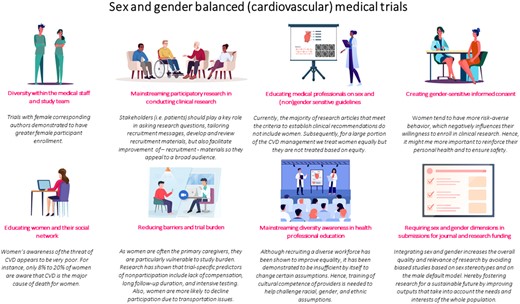
Recommendations for sex and gender balanced (cardiovascular) medical trials.
Improving access to clinical trial sites
Improving access to the centres participating in trials and other logistics such as onsite childcare or transportation can support women. In addition, improving the level of comfort and the overall clinical trial experience from informed consent to the use of biological specimens might help women feel more at ease. Deeper insights into how women make the informed decisions and who do they rely on are important as well. Women make decisions differently from men, which means that the same enrolment process may yield different enrolment rates by sex and gender. There are some data that women may take more time to gather to make an informed decision, they may require more sources of input and need to rely on the opinions of close friends, family members and external parties.18
In conclusion, these are challenging times where a sense of urgency to establish sex and gender equality is increasing. It appears, however, that barriers for women to participate in cardiovascular clinical trials are not yet lifted. Overall, women need extra reassurance of their significant value to participate in a clinical research setting and support to participate including solutions for mobility problems. The factors behind underrepresentation are likely to differ per country, regions and across cultures and health care systems, as there is no ‘one size fits all’ solution. By implementing the right frameworks for trial designs, including more women in the leadership of clinical trial committees and inviting female patients to participate in the discussion of the design, researchers will be more likely to overcome some of the barriers mentioned above. In the future, patients will hopefully no longer be viewed as a means to an end to complete a trial, but rather as equal partners in participatory research. Such involvement will not only help to improve patients’ recruitment and retention in trials, but most importantly help to achieve more societally relevant knowledge related to both women and men. We must continue to strive for more health equity that begins with improved representation of women in generating our scientific evidence. This is a call to action.
Funding
G.S. is supported by the Blumenthal Scholarship in Preventive Cardiology.
Statement of contribution
J.J.K.D. was involved with initial idea of the narrative review, conducted the literature research, and has written both the initial and final draft of the manuscript. P.V. was involved with initial idea of the narrative review, as well as the initial and final draft of the manuscript and has approved the final draft of the manuscript. A.C. was involved with the initial and final draft of the manuscript and has approved the final draft of the manuscript. E.R. was involved with the initial and final draft of the manuscript and has approved the final draft of the manuscript. J.M. was involved with the initial and final draft of the manuscript and has approved the final draft of the manuscript. V.K. involved with the initial and final draft of the manuscript and has approved the final draft of the manuscript. G.S. was involved with the initial and final draft of the manuscript and has approved the final draft of the manuscript. R.M. was involved with the initial and final draft of the manuscript and has approved the final draft of the manuscript. Y.A. was involved with initial idea of the narrative review, as well as the initial and final draft of the manuscript, and has approved the final draft of the manuscript.
Conflict of interest: none declared.
Appendix
(('cardiovascular disease'/exp OR ‘cardiovascular system’/exp OR ‘cardiovascular agent’/exp OR ‘cardiology’/exp) AND ‘clinical study’/exp AND ((participat* OR ‘take part’ OR enroll* OR recruit*) NEAR/4 (barrier* OR motivation* OR reason* OR refus* OR declin* OR accept* OR rationale)):ti, ab, kw) NOT (('cardiovascular disease'/exp OR ‘cardiovascular system’/exp OR ‘cardiovascular agent’/exp OR ‘cardiology’/exp) AND ‘clinical trial (topic)’/exp AND ((participat* OR ‘take part’ OR enroll* OR recruit*) NEAR/4 (barrier* OR motivation* OR reason* OR refus* OR declin* OR accept* OR rationale)):ti
References




