-
PDF
- Split View
-
Views
-
Cite
Cite
Shaan Khurshid, Lu-Chen Weng, Mostafa A Al-Alusi, Jennifer L Halford, Julian S Haimovich, Emelia J Benjamin, Ludovic Trinquart, Patrick T Ellinor, David D McManus, Steven A Lubitz, Accelerometer-derived physical activity and risk of atrial fibrillation, European Heart Journal, Volume 42, Issue 25, 1 July 2021, Pages 2472–2483, https://doi.org/10.1093/eurheartj/ehab250
Close - Share Icon Share
Abstract
Physical activity may be an important modifiable risk factor for atrial fibrillation (AF), but associations have been variable and generally based on self-reported activity.
We analysed 93 669 participants of the UK Biobank prospective cohort study without prevalent AF who wore a wrist-based accelerometer for 1 week. We categorized whether measured activity met the standard recommendations of the European Society of Cardiology, American Heart Association, and World Health Organization [moderate-to-vigorous physical activity (MVPA) ≥150 min/week]. We tested associations between guideline-adherent activity and incident AF (primary) and stroke (secondary) using Cox proportional hazards models adjusted for age, sex, and each component of the Cohorts for Heart and Aging Research in Genomic Epidemiology AF (CHARGE-AF) risk score. We also assessed correlation between accelerometer-derived and self-reported activity. The mean age was 62 ± 8 years and 57% were women. Over a median of 5.2 years, 2338 incident AF events occurred. In multivariable adjusted models, guideline-adherent activity was associated with lower risks of AF [hazard ratio (HR) 0.82, 95% confidence interval (CI) 0.75–0.89; incidence 3.5/1000 person-years, 95% CI 3.3–3.8 vs. 6.5/1000 person-years, 95% CI 6.1–6.8] and stroke (HR 0.76, 95% CI 0.64–0.90; incidence 1.0/1000 person-years, 95% CI 0.9–1.1 vs. 1.8/1000 person-years, 95% CI 1.6–2.0). Correlation between accelerometer-derived and self-reported MVPA was weak (Spearman r = 0.16, 95% CI 0.16–0.17). Self-reported activity was not associated with incident AF or stroke.
Greater accelerometer-derived physical activity is associated with lower risks of AF and stroke. Future preventive efforts to reduce AF risk may be most effective when targeting adherence to objective activity thresholds.
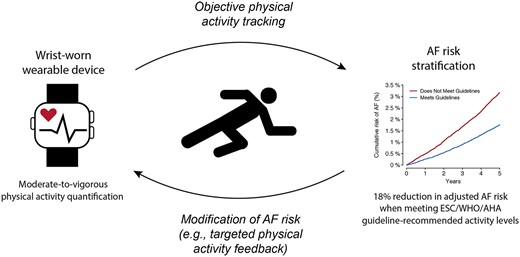
Wrist-worn accelerometer-derived moderate-to-vigorous physical activity levels meeting standard guideline recommendations is associated with lower risk of incident atrial fibrillation after adjustment for clinical risk factors. Wearable sensors may enable both objective assessment of physical activity and modification of AF risk through targeted feedback.
 Listen to the audio abstract of this contribution.
Listen to the audio abstract of this contribution.
See page 2484 for the editorial comment on this article (doi: 10.1093/eurheartj/ehab243)
Identification of modifiable risk factors for atrial fibrillation (AF) is a clinical imperative given the public health burden attributable to the arrhythmia.1 Physical activity has long been implicated as an AF risk factor, but relations between activity and AF have been variable and based largely on self-report. Most population-based studies have observed lower AF risk with exercise.2 , 3 Yet benefits may be limited to those participating in vigorous activity,4 and may be confounded by body mass index.5 Conversely, vigorous and endurance athletes may have an increased likelihood of developing AF.6
Previous work has quantified physical activity using questionnaires,7 , 8 which result in overestimation9 and correlate only modestly with energy expenditure.10 In contrast, wearable accelerometers provide an objective and reproducible activity measure.11 A precise understanding of the relations between accelerometer-derived activity and incident AF may therefore clarify the association between exercise and AF risk. Moreover, since consumer wearables are increasingly equipped with accelerometers, understanding the relationship between accelerometer-derived physical activity and AF risk may have implications for utilizing such devices to assess and modify risk for AF and related morbidity, including stroke.
In this study, we analysed accelerometer data from nearly 100 000 individuals in the UK Biobank prospective cohort study. We assessed associations between physical activity and incident AF and stroke, hypothesizing that individuals meeting guideline-based activity recommendations12–14 would have a lower risk of incident AF and stroke after adjustment for AF risk factors.
Methods
Study population
The UK Biobank is a prospective cohort of 502 629 participants enrolled 2006–2010.15 Briefly, 9.2 million individuals aged 40–69 years living within 25 miles of 22 assessment centres in the UK were invited, and 5.4% participated in the baseline assessment. Questionnaires and physical measures were collected at recruitment, and all participants are followed for outcomes through linkage to national health-related datasets. All participants provided written informed consent. The UK Biobank was approved by the UK Biobank Research Ethical Committee (reference # 11/NW/0382). Use of UK Biobank data (application 17488) was approved by the local Mass General Brigham Institutional Review Board.
Accelerometer-derived physical activity
Between February 2013 and December 2015, 236 519 UK Biobank participants were invited to wear a wrist-worn accelerometer for 1 week, of whom 106 053 agreed to participate and 103 695 submitted data (characteristics of individuals declining invitation were similar to those accepting, Supplementary material online, Table S1).11 Participants were sent an Axivity AX3 (Newcastle upon Tyne, UK) wrist-worn triaxial accelerometer. The sensor captured acceleration over 7 days at 100 Hz with dynamic range of ±8 g.
As described previously, acceleration signals were calibrated to gravity.11 Sample data were combined into 5 s epochs representing the average vector magnitude. Non-wear time was identified as consecutive stationary episodes ≥60 min in which all three axes had standard deviation <13.0 mg.16 Epochs representing non-wear time were imputed based on the average of similar time-of-day vector magnitude and intensity distribution data points on different days. We excluded individuals with insufficient wear time to support imputation (<72 h of wear time or no wear data in each 1 h period of the 24 h cycle), and whose signals were insufficient for calibration.11
Accelerometer-derived summary data included overall mean acceleration, a validated surrogate for global physical activity.16 , 17 As performed previously, we quantified moderate-to-vigorous physical activity (MVPA), defined as the sum of 5 s epochs where mean acceleration was ≥100 mg, a threshold that corresponds to activity of at least moderate intensity using wrist-worn accelerometers.18–21 We extracted MVPA in bouts (5-min periods where ≥80% of epochs met the MVPA threshold) in order to reduce the likelihood of misclassifying artefact as MVPA.19 , 20 In secondary analyses, we summed MVPA without restricting to bouts with minimum duration, and utilized an alternative threshold of >430 mg to quantify vigorous physical activity.18 For each individual, we determined whether MVPA levels met the standard recommendations of the European Society of Cardiology,14 American Heart Association,13 and World Health Organization12 (‘ESC/AHA/WHO standard’, ≥150 min/week), and the extended recommendation of the WHO for additional benefit (‘WHO extended’, ≥300 min/week).12 For participants contributing a full week of accelerometry, we tabulated the number of days individuals exceeded average daily MVPA corresponding to ESC/AHA/WHO standard (≥25 min/day) and WHO extended (≥45 min/day).
Self-reported physical activity
Self-reported physical activity data were obtained using the short-form international physical activity questionnaire (IPAQ).22 As performed previously, we converted time spent performing activity (walking, moderate, vigorous) into metabolic equivalent-min/week.23 We additionally categorized whether self-reported activity met ESC/AHA/WHO standard and WHO extended. In secondary analyses focusing on vigorous activity, we utilized data from a separate questionnaire reporting on the frequency of participation in strenuous sport.
Clinical atrial fibrillation risk
Clinical AF risk factors were defined based on inclusion in the Cohorts for Heart and Aging Research in Genomic Epidemiology AF (CHARGE-AF) score, a validated AF prediction instrument.24 Although not included in CHARGE-AF, we additionally included sex as an AF risk factor given previous associations with AF.1
Age, sex, height, weight, and blood pressure were obtained from the study visit most closely preceding accelerometry. Race was collected using self-report data and categorized as White vs. other race, in accordance with the CHARGE-AF definition.25 Tobacco and alcohol use were ascertained using standardized questionnaires. Alcohol use was classified as standard drinks (14 g alcohol) per week.13 The Townsend Deprivation Index26 was measured as a surrogate for socioeconomic deprivation. Antiplatelet and anticoagulant medication use was ascertained through self-report at an assessment visit as well as prescription information extracted from linked general practitioner electronic health record data. Remaining clinical factors were defined using self-report and diagnosis codes (updated through 31 March 2020). Clinical factor definitions are listed in Supplementary material online, Table S2. CHARGE-AF covariates and weights are shown in Supplementary material online, Table S3. Individuals with insufficient anthropometric or blood pressure data to calculate CHARGE-AF were excluded.
Outcomes
The primary outcome was incident AF. We considered atrial flutter equivalent to AF. Given the importance of stroke as a contributor to AF-related morbidity, we assessed incident stroke as a secondary outcome. AF and stroke were defined using previously validated definitions comprising self-reported diagnoses, diagnostic/procedural codes linked to hospital encounters, and death records27 , 28 (Supplementary material online, Table S2). Self-reported diagnoses were used only to ascertain the presence of AF and stroke at baseline (i.e. for exclusion from incident analyses). Given the potential misclassification of ischaemic vs. haemorrhagic stroke mechanisms using diagnosis codes29 and the fact that most strokes are ischaemic, we included all strokes in our primary analysis. We performed sensitivity analyses using strokes classified as ischaemic events based on diagnosis codes.
Statistical analysis
We assessed physical activity distributions in the sample. Given possible non-linear effects of physical activity on incident AF,30 we inspected linear and spline fits of the association between AF hazard and (i) mean acceleration, as well as (ii) MVPA min/week, in models adjusted for sex and each component of CHARGE-AF. The relation between overall mean acceleration and AF was approximately linear, while the association between MVPA and AF suggested possible non-linearity (Supplementary material online, Figure S1). Therefore, we analysed mean acceleration as a continuous variable in our models, and MVPA was categorized into deciles. For survival analyses, follow-up began at accelerometer completion and ended at AF (AF models), stroke (stroke models), or the earlier of death or last follow-up (Supplementary material online, Methods). Death was treated as a censoring event in the primary models given that cumulative mortality in the overall sample was low [5-year mortality 1.9%, 95% confidence interval (CI) 1.8–2.0]; however, in secondary analyses we fit subdistribution hazards models with death treated as a competing risk.31 Individuals with the outcome at the time of accelerometer wear were excluded from incident analyses.
To assess for associations between accelerometer-derived physical activity and incident AF, we fit Cox proportional hazards models with incident AF as the outcome and accelerometer-derived physical activity as the exposure. The primary exposure definition was MVPA exceeding ESC/AHA/WHO standard recommendations. We additionally classified activity as MVPA exceeding the WHO extended recommendation, mutually exclusive guideline-based categories (below ESC/AHA/WHO standard, exceeding ESC/AHA/WHO standard but below WHO extended, exceeding WHO extended), days meeting guideline-based average daily MVPA levels, overall mean acceleration, and decile of weekly MVPA (Supplementary material online, Figure S2). The proportional hazards assumption was assessed using Schoenfeld residuals. Non-proportional hazards (observed only for age and sex) were modelled by including interactions with strata of survival time. Within 5025 (5.4%) individuals contributing <1 week of accelerometry, we extrapolated observed MVPA to 7 days since physical activity thresholds are defined by week. We assessed models adjusted for age and sex, and models adjusted for sex and each component of the CHARGE-AF score (which includes age). We plotted the crude cumulative risk of AF stratified by activity level, tabulated incidence rates within strata, and calculated incidence rate ratios (IRRs). To generate adjusted risk curves, we plotted cumulative risk estimates from AF risk-adjusted Cox models assuming the mean CHARGE-AF score and stratifying by sex (since male sex was consistently associated with greater risk of AF in adjusted models). We then fit similar models assessing incident stroke.
We examined relations between self-reported physical activity and AF and stroke. Given non-normality, we assessed the Spearman correlation between self-reported vs. accelerometer-derived MVPA. We quantified level of agreement between achievement of guideline-recommended levels using kappa statistics and assessed significance of disagreement using McNemar’s test. We fit Cox models analogous to those described above using self-reported activity as the exposure. Given delay between IPAQ administration and accelerometry [median time from IPAQ to accelerometry (quartile-1, quartile-3): 5.5 years (4.6, 6.3)], we performed sensitivity analyses restricted to individuals contributing to follow-up IPAQ data [2.2 years (1.8, 2.5)].
We performed multiple secondary analyses, including fitting adjusted models using alternative accelerometer variables as the exposures of interest, such as the coefficient of variation in mean acceleration (e.g. a measure of overall variability in activity over the course of the week), and weekly minutes of vigorous physical activity. All secondary and sensitivity analyses are described in detail in the Supplementary material online, Methods. Analyses were performed using R v3.632 (packages: ‘survival’, ‘data.table’, and ‘prodlim’). We considered two-sided P-values <0.05 statistically significant.
Results
Sample characteristics
Of 103 695 participants contributing accelerometer data, we excluded 7000 for insufficient wear time or failure of calibration, 281 with inadequate data for clinical AF risk estimation, and 2745 with prevalent AF, withdrawn consent, or absence of follow-up data, resulting in 93 669 individuals in the primary analysis (Supplementary material online, Figure S3). The mean age was 62 ± 8 years and 57% were female. Other baseline characteristics are listed in Table 1. Characteristics of excluded individuals are shown in Supplementary material online, Table S4.
| Baseline characteristic . | Low activity tertilea (n = 31 202) . | Medium activity tertilea (n = 31 237) . | High activity tertilea (n = 31 230) . | Overall (n = 93 669) . |
|---|---|---|---|---|
| Age (years) | 64.5 ± 7.4 | 62.3 ± 7.7 | 59.9 ± 7.7 | 62.2 ± 7.8 |
| Female sex | 16 280 (52.2) | 18 370 (58.8) | 18 720 (59.9) | 53 370 (57.0) |
| Race | ||||
| White | 30 271 (97.0) | 30 206 (96.7) | 29 979 (96.0) | 90 456 (96.6) |
| Asian | 343 (1.1) | 364 (1.2) | 397 (1.3) | 1104 (1.2) |
| Black | 199 (0.6) | 250 (0.8) | 350 (1.1) | 799 (0.9) |
| Mixed | 141 (0.5) | 169 (0.5) | 211 (0.7) | 521 (0.6) |
| Others/unknown | 248 (0.8) | 248 (0.8) | 293 (0.9) | 789 (0.8) |
| Smoker | ||||
| Current | 2612 (8.4) | 1959 (6.3) | 1779 (5.7) | 6350 (6.8) |
| Former | 11 580 (37.1) | 11 056 (35.4) | 10 777 (34.5) | 33 416 (35.7) |
| Never | 17 010 (54.5) | 18 222 (58.3) | 18 674 (59.8) | 53 906 (57.5) |
| Alcohol—drinks per week [quartile 1, quartile 3] | 8.7 [4, 16] | 9.3 [4, 16] | 9.3 [4, 16] | 9.3 [4, 16] |
| Townsend deprivation index | −1.6 ± 2.9 | −1.8 ± 2.8 | −1.7 ± 2.8 | −1.7 ± 2.8 |
| Antiplatelet use | 5866 (18.8) | 3922 (12.6) | 2797 (9.0) | 12 585 (13.4) |
| Oral anticoagulant use | 371 (1.2) | 202 (0.6) | 149 (0.5) | 722 (0.8) |
| Height (cm) | 169.4 ± 9.2 | 168.7 ± 9.0 | 168.6 ± 8.9 | 168.9 ± 9.1 |
| Weight (kg) | 80.8 ± 16.3 | 75.9 ± 14.5 | 72.4 ± 13.6 | 76.3 ± 15.2 |
| Body mass index (kg/m2) | 28.1 ± 5.0 | 26.6 ± 4.2 | 25.4 ± 3.8 | 26.7 ± 4.5 |
| Systolic blood pressure (mmHg) | 139 ± 18 | 137 ± 18 | 134 ± 18 | 137 ± 18 |
| Diastolic blood pressure (mmHg) | 83 ± 10 | 82 ± 10 | 80 ± 10 | 82 ± 10 |
| Anti-hypertensive medication use | 7716 (24.7) | 4844 (15.6) | 3052 (9.8) | 15 612 (16.7) |
| Diabetes | 1777 (5.7) | 736 (2.4) | 417 (1.3) | 2930 (3.1) |
| Heart failure | 283 (0.9) | 132 (0.4) | 58 (0.2) | 473 (0.5) |
| Myocardial infarction | 1030 (3.3) | 529 (1.7) | 282 (0.9) | 1841 (2.0) |
| Sleep apnoea | 483 (1.5) | 258 (0.8) | 181 (0.6) | 922 (1.0) |
| CHARGE-AF | 12.5 ± 0.9 | 12.2 ± 0.9 | 11.8 ± 0.9 | 12.2 ± 1.0 |
| Wear time (days) | 7 [7, 7] | 7 [7, 7] | 7 [7, 7] | 7 [7, 7] |
| Overall acceleration (mg) | 20.0 ± 3.1 | 27.2 ± 1.8 | 37.2 ± 6.9 | 28.1 ± 8.3 |
| Activity energy expenditure (MET-min/week)b | 0.35 ± 0.06 | 0.49 ± 0.03 | 0.67 ± 0.12 | 0.50 ± 0.16 |
| MVPA (min/week) | 55 [20, 110] | 130 [70, 205] | 275 [175, 405] | 135 [60, 250] |
| Meeting ESC/AHA/WHO standard activity recommendationc | 4872 (15.6) | 13 499 (43.2) | 25 442 (81.5) | 43 813 (46.8) |
| Meeting WHO extended activity recommendationd | 540 (1.7) | 3074 (9.8) | 13 933 (44.6) | 17 547 (18.7) |
| Baseline characteristic . | Low activity tertilea (n = 31 202) . | Medium activity tertilea (n = 31 237) . | High activity tertilea (n = 31 230) . | Overall (n = 93 669) . |
|---|---|---|---|---|
| Age (years) | 64.5 ± 7.4 | 62.3 ± 7.7 | 59.9 ± 7.7 | 62.2 ± 7.8 |
| Female sex | 16 280 (52.2) | 18 370 (58.8) | 18 720 (59.9) | 53 370 (57.0) |
| Race | ||||
| White | 30 271 (97.0) | 30 206 (96.7) | 29 979 (96.0) | 90 456 (96.6) |
| Asian | 343 (1.1) | 364 (1.2) | 397 (1.3) | 1104 (1.2) |
| Black | 199 (0.6) | 250 (0.8) | 350 (1.1) | 799 (0.9) |
| Mixed | 141 (0.5) | 169 (0.5) | 211 (0.7) | 521 (0.6) |
| Others/unknown | 248 (0.8) | 248 (0.8) | 293 (0.9) | 789 (0.8) |
| Smoker | ||||
| Current | 2612 (8.4) | 1959 (6.3) | 1779 (5.7) | 6350 (6.8) |
| Former | 11 580 (37.1) | 11 056 (35.4) | 10 777 (34.5) | 33 416 (35.7) |
| Never | 17 010 (54.5) | 18 222 (58.3) | 18 674 (59.8) | 53 906 (57.5) |
| Alcohol—drinks per week [quartile 1, quartile 3] | 8.7 [4, 16] | 9.3 [4, 16] | 9.3 [4, 16] | 9.3 [4, 16] |
| Townsend deprivation index | −1.6 ± 2.9 | −1.8 ± 2.8 | −1.7 ± 2.8 | −1.7 ± 2.8 |
| Antiplatelet use | 5866 (18.8) | 3922 (12.6) | 2797 (9.0) | 12 585 (13.4) |
| Oral anticoagulant use | 371 (1.2) | 202 (0.6) | 149 (0.5) | 722 (0.8) |
| Height (cm) | 169.4 ± 9.2 | 168.7 ± 9.0 | 168.6 ± 8.9 | 168.9 ± 9.1 |
| Weight (kg) | 80.8 ± 16.3 | 75.9 ± 14.5 | 72.4 ± 13.6 | 76.3 ± 15.2 |
| Body mass index (kg/m2) | 28.1 ± 5.0 | 26.6 ± 4.2 | 25.4 ± 3.8 | 26.7 ± 4.5 |
| Systolic blood pressure (mmHg) | 139 ± 18 | 137 ± 18 | 134 ± 18 | 137 ± 18 |
| Diastolic blood pressure (mmHg) | 83 ± 10 | 82 ± 10 | 80 ± 10 | 82 ± 10 |
| Anti-hypertensive medication use | 7716 (24.7) | 4844 (15.6) | 3052 (9.8) | 15 612 (16.7) |
| Diabetes | 1777 (5.7) | 736 (2.4) | 417 (1.3) | 2930 (3.1) |
| Heart failure | 283 (0.9) | 132 (0.4) | 58 (0.2) | 473 (0.5) |
| Myocardial infarction | 1030 (3.3) | 529 (1.7) | 282 (0.9) | 1841 (2.0) |
| Sleep apnoea | 483 (1.5) | 258 (0.8) | 181 (0.6) | 922 (1.0) |
| CHARGE-AF | 12.5 ± 0.9 | 12.2 ± 0.9 | 11.8 ± 0.9 | 12.2 ± 1.0 |
| Wear time (days) | 7 [7, 7] | 7 [7, 7] | 7 [7, 7] | 7 [7, 7] |
| Overall acceleration (mg) | 20.0 ± 3.1 | 27.2 ± 1.8 | 37.2 ± 6.9 | 28.1 ± 8.3 |
| Activity energy expenditure (MET-min/week)b | 0.35 ± 0.06 | 0.49 ± 0.03 | 0.67 ± 0.12 | 0.50 ± 0.16 |
| MVPA (min/week) | 55 [20, 110] | 130 [70, 205] | 275 [175, 405] | 135 [60, 250] |
| Meeting ESC/AHA/WHO standard activity recommendationc | 4872 (15.6) | 13 499 (43.2) | 25 442 (81.5) | 43 813 (46.8) |
| Meeting WHO extended activity recommendationd | 540 (1.7) | 3074 (9.8) | 13 933 (44.6) | 17 547 (18.7) |
Values are given as mean ± standard deviation, n (%), or median [quartile 1, quartile 3].
AF, atrial fibrillation; AHA, American Heart Association; ESC, European Society of Cardiology; MET, metabolic equivalent; MVPA, moderate-to-vigorous physical activity; WHO, World Health Organization.
Activity groups stratified by tertiles of overall mean acceleration.
Refers to average energy expenditure beyond the expenditure associated with the resting state. Estimated using the formula: where value = mean overall acceleration (mg).33
Defined as 150 min of moderate intensity or greater activity per week.11–13
Defined as 300 min of moderate intensity or greater activity per week.11
| Baseline characteristic . | Low activity tertilea (n = 31 202) . | Medium activity tertilea (n = 31 237) . | High activity tertilea (n = 31 230) . | Overall (n = 93 669) . |
|---|---|---|---|---|
| Age (years) | 64.5 ± 7.4 | 62.3 ± 7.7 | 59.9 ± 7.7 | 62.2 ± 7.8 |
| Female sex | 16 280 (52.2) | 18 370 (58.8) | 18 720 (59.9) | 53 370 (57.0) |
| Race | ||||
| White | 30 271 (97.0) | 30 206 (96.7) | 29 979 (96.0) | 90 456 (96.6) |
| Asian | 343 (1.1) | 364 (1.2) | 397 (1.3) | 1104 (1.2) |
| Black | 199 (0.6) | 250 (0.8) | 350 (1.1) | 799 (0.9) |
| Mixed | 141 (0.5) | 169 (0.5) | 211 (0.7) | 521 (0.6) |
| Others/unknown | 248 (0.8) | 248 (0.8) | 293 (0.9) | 789 (0.8) |
| Smoker | ||||
| Current | 2612 (8.4) | 1959 (6.3) | 1779 (5.7) | 6350 (6.8) |
| Former | 11 580 (37.1) | 11 056 (35.4) | 10 777 (34.5) | 33 416 (35.7) |
| Never | 17 010 (54.5) | 18 222 (58.3) | 18 674 (59.8) | 53 906 (57.5) |
| Alcohol—drinks per week [quartile 1, quartile 3] | 8.7 [4, 16] | 9.3 [4, 16] | 9.3 [4, 16] | 9.3 [4, 16] |
| Townsend deprivation index | −1.6 ± 2.9 | −1.8 ± 2.8 | −1.7 ± 2.8 | −1.7 ± 2.8 |
| Antiplatelet use | 5866 (18.8) | 3922 (12.6) | 2797 (9.0) | 12 585 (13.4) |
| Oral anticoagulant use | 371 (1.2) | 202 (0.6) | 149 (0.5) | 722 (0.8) |
| Height (cm) | 169.4 ± 9.2 | 168.7 ± 9.0 | 168.6 ± 8.9 | 168.9 ± 9.1 |
| Weight (kg) | 80.8 ± 16.3 | 75.9 ± 14.5 | 72.4 ± 13.6 | 76.3 ± 15.2 |
| Body mass index (kg/m2) | 28.1 ± 5.0 | 26.6 ± 4.2 | 25.4 ± 3.8 | 26.7 ± 4.5 |
| Systolic blood pressure (mmHg) | 139 ± 18 | 137 ± 18 | 134 ± 18 | 137 ± 18 |
| Diastolic blood pressure (mmHg) | 83 ± 10 | 82 ± 10 | 80 ± 10 | 82 ± 10 |
| Anti-hypertensive medication use | 7716 (24.7) | 4844 (15.6) | 3052 (9.8) | 15 612 (16.7) |
| Diabetes | 1777 (5.7) | 736 (2.4) | 417 (1.3) | 2930 (3.1) |
| Heart failure | 283 (0.9) | 132 (0.4) | 58 (0.2) | 473 (0.5) |
| Myocardial infarction | 1030 (3.3) | 529 (1.7) | 282 (0.9) | 1841 (2.0) |
| Sleep apnoea | 483 (1.5) | 258 (0.8) | 181 (0.6) | 922 (1.0) |
| CHARGE-AF | 12.5 ± 0.9 | 12.2 ± 0.9 | 11.8 ± 0.9 | 12.2 ± 1.0 |
| Wear time (days) | 7 [7, 7] | 7 [7, 7] | 7 [7, 7] | 7 [7, 7] |
| Overall acceleration (mg) | 20.0 ± 3.1 | 27.2 ± 1.8 | 37.2 ± 6.9 | 28.1 ± 8.3 |
| Activity energy expenditure (MET-min/week)b | 0.35 ± 0.06 | 0.49 ± 0.03 | 0.67 ± 0.12 | 0.50 ± 0.16 |
| MVPA (min/week) | 55 [20, 110] | 130 [70, 205] | 275 [175, 405] | 135 [60, 250] |
| Meeting ESC/AHA/WHO standard activity recommendationc | 4872 (15.6) | 13 499 (43.2) | 25 442 (81.5) | 43 813 (46.8) |
| Meeting WHO extended activity recommendationd | 540 (1.7) | 3074 (9.8) | 13 933 (44.6) | 17 547 (18.7) |
| Baseline characteristic . | Low activity tertilea (n = 31 202) . | Medium activity tertilea (n = 31 237) . | High activity tertilea (n = 31 230) . | Overall (n = 93 669) . |
|---|---|---|---|---|
| Age (years) | 64.5 ± 7.4 | 62.3 ± 7.7 | 59.9 ± 7.7 | 62.2 ± 7.8 |
| Female sex | 16 280 (52.2) | 18 370 (58.8) | 18 720 (59.9) | 53 370 (57.0) |
| Race | ||||
| White | 30 271 (97.0) | 30 206 (96.7) | 29 979 (96.0) | 90 456 (96.6) |
| Asian | 343 (1.1) | 364 (1.2) | 397 (1.3) | 1104 (1.2) |
| Black | 199 (0.6) | 250 (0.8) | 350 (1.1) | 799 (0.9) |
| Mixed | 141 (0.5) | 169 (0.5) | 211 (0.7) | 521 (0.6) |
| Others/unknown | 248 (0.8) | 248 (0.8) | 293 (0.9) | 789 (0.8) |
| Smoker | ||||
| Current | 2612 (8.4) | 1959 (6.3) | 1779 (5.7) | 6350 (6.8) |
| Former | 11 580 (37.1) | 11 056 (35.4) | 10 777 (34.5) | 33 416 (35.7) |
| Never | 17 010 (54.5) | 18 222 (58.3) | 18 674 (59.8) | 53 906 (57.5) |
| Alcohol—drinks per week [quartile 1, quartile 3] | 8.7 [4, 16] | 9.3 [4, 16] | 9.3 [4, 16] | 9.3 [4, 16] |
| Townsend deprivation index | −1.6 ± 2.9 | −1.8 ± 2.8 | −1.7 ± 2.8 | −1.7 ± 2.8 |
| Antiplatelet use | 5866 (18.8) | 3922 (12.6) | 2797 (9.0) | 12 585 (13.4) |
| Oral anticoagulant use | 371 (1.2) | 202 (0.6) | 149 (0.5) | 722 (0.8) |
| Height (cm) | 169.4 ± 9.2 | 168.7 ± 9.0 | 168.6 ± 8.9 | 168.9 ± 9.1 |
| Weight (kg) | 80.8 ± 16.3 | 75.9 ± 14.5 | 72.4 ± 13.6 | 76.3 ± 15.2 |
| Body mass index (kg/m2) | 28.1 ± 5.0 | 26.6 ± 4.2 | 25.4 ± 3.8 | 26.7 ± 4.5 |
| Systolic blood pressure (mmHg) | 139 ± 18 | 137 ± 18 | 134 ± 18 | 137 ± 18 |
| Diastolic blood pressure (mmHg) | 83 ± 10 | 82 ± 10 | 80 ± 10 | 82 ± 10 |
| Anti-hypertensive medication use | 7716 (24.7) | 4844 (15.6) | 3052 (9.8) | 15 612 (16.7) |
| Diabetes | 1777 (5.7) | 736 (2.4) | 417 (1.3) | 2930 (3.1) |
| Heart failure | 283 (0.9) | 132 (0.4) | 58 (0.2) | 473 (0.5) |
| Myocardial infarction | 1030 (3.3) | 529 (1.7) | 282 (0.9) | 1841 (2.0) |
| Sleep apnoea | 483 (1.5) | 258 (0.8) | 181 (0.6) | 922 (1.0) |
| CHARGE-AF | 12.5 ± 0.9 | 12.2 ± 0.9 | 11.8 ± 0.9 | 12.2 ± 1.0 |
| Wear time (days) | 7 [7, 7] | 7 [7, 7] | 7 [7, 7] | 7 [7, 7] |
| Overall acceleration (mg) | 20.0 ± 3.1 | 27.2 ± 1.8 | 37.2 ± 6.9 | 28.1 ± 8.3 |
| Activity energy expenditure (MET-min/week)b | 0.35 ± 0.06 | 0.49 ± 0.03 | 0.67 ± 0.12 | 0.50 ± 0.16 |
| MVPA (min/week) | 55 [20, 110] | 130 [70, 205] | 275 [175, 405] | 135 [60, 250] |
| Meeting ESC/AHA/WHO standard activity recommendationc | 4872 (15.6) | 13 499 (43.2) | 25 442 (81.5) | 43 813 (46.8) |
| Meeting WHO extended activity recommendationd | 540 (1.7) | 3074 (9.8) | 13 933 (44.6) | 17 547 (18.7) |
Values are given as mean ± standard deviation, n (%), or median [quartile 1, quartile 3].
AF, atrial fibrillation; AHA, American Heart Association; ESC, European Society of Cardiology; MET, metabolic equivalent; MVPA, moderate-to-vigorous physical activity; WHO, World Health Organization.
Activity groups stratified by tertiles of overall mean acceleration.
Refers to average energy expenditure beyond the expenditure associated with the resting state. Estimated using the formula: where value = mean overall acceleration (mg).33
Defined as 150 min of moderate intensity or greater activity per week.11–13
Defined as 300 min of moderate intensity or greater activity per week.11
The distribution of accelerometer-derived physical activity is shown in Supplementary material online, Figure S4. Using accelerometer-derived MVPA, 43 813 (46.8%) participants met ESC/AHA/WHO standard recommendations, and 17 547 (18.7%) met WHO extended recommendations. The number of days individuals met guideline-recommended MVPA levels is shown in Supplementary material online, Figure S5. Using self-reported MVPA among 93 579 individuals providing IPAQ data, 48 445 (51.8%) met ESC/AHA/WHO standard, and 29 897 (31.9%) met WHO extended recommendations (Supplementary material online, Table S5 and Supplementary material online, Figure S6).
Atrial fibrillation associations
In total, 2338 individuals developed incident AF over a median of 5.2 (quartile-1: 4.6, quartile-3: 5.8) years of follow-up spanning August 2013–March 2020 [overall cumulative risk of AF 3.6% (95% CI 3.3–4.0); incidence rate 5.0/1000 person-years (95% CI 4.8–5.2)]. In models adjusted for sex and each component of CHARGE-AF, activity exceeding the ESC/AHA/WHO standard recommendation [hazard ratio (HR) 0.82 (95% CI 0.75–0.89)] and increasing MVPA decile [HR 0.96 per one decile increase (95% CI 0.94–0.97)] were associated with lower risk of AF (P < 0.01 for both, Table 2). The crude cumulative risk of AF stratified by adherence to ESC/AHA/WHO standard and WHO extended recommendations is shown in Figure 1. When assessing MVPA deciles as independent categories using adjusted models, AF risk decreased until decile seven (175–220 min/week), after which it remained approximately similar (Figure 2, Supplementary material online, Figure S1, and Supplementary material online, Table S6). Mean acceleration was not associated with AF in adjusted models [HR 0.97 per one standard deviation increase (95% CI 0.92–1.02), P = 0.25]. AF risk was similar among individuals meeting the WHO extended recommendation as compared to individuals meeting the ESC/AHA/WHO standard recommendation only [HR 1.14 (95% CI 0.98–1.31), P = 0.09, Supplementary material online, Tables S7 and S8].
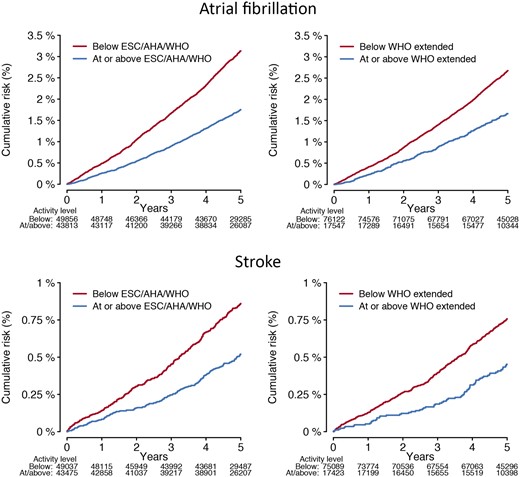
Cumulative risks of atrial fibrillation and stroke stratified by adherence to physical activity recommendations. Depicted is the cumulative risk of atrial fibrillation (upper panels) and stroke (lower panels) stratified by accelerometer-derived physical activity levels meeting European Society of Cardiology,14 American Heart Association,13 and World Health Organization standard thresholds (≥150 min of moderate-to-vigorous physical activity per week, left panels),12 and the World Health Organization threshold for additional health benefits (≥300 min of moderate-to-vigorous physical activity per week, right panels).12
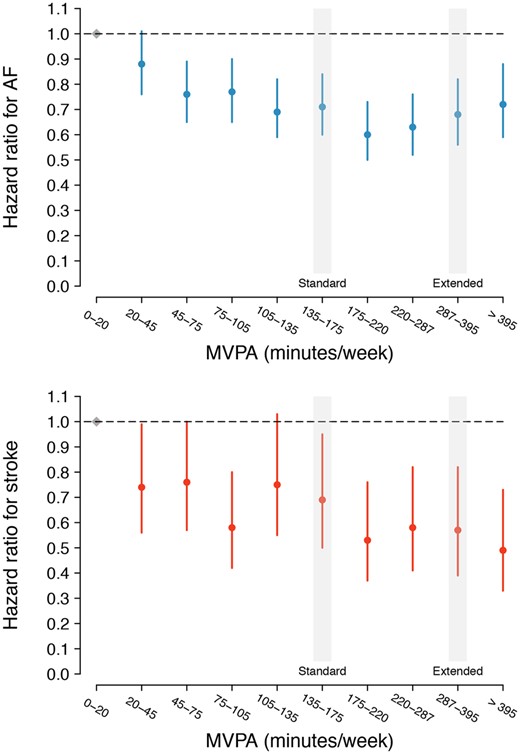
Adjusted hazard for atrial fibrillation and stroke stratified by decile of moderate-to-vigorous physical activity. Depicted are multivariable adjusted hazard ratios (and 95% confidence intervals) for atrial fibrillation (top panel) and stroke (bottom panel) according to increasing decile of accelerometer-derived moderate-to-vigorous physical activity minutes per week. In each plot, the first decile (0–20 min of moderate-to-vigorous physical activity per week) is the reference, depicted in grey. The moderate-to-vigorous physical activity range corresponding to the depicted decile is shown on the x-axis. The horizontal hashed line depicts a hazard ratio of one (i.e. equal hazard to decile one). The deciles containing the thresholds corresponding to the European Society of Cardiology,14 American Heart Association,13 and World Health Organization standard thresholds (≥150 min of moderate-to-vigorous physical activity per week, ‘Standard’),12 and the World Health Organization threshold for additional health benefits (≥300 min of moderate-to-vigorous physical activity per week, ‘Extended’)12 are indicated on each plot with text and a grey shadow.
Multivariable associations between physical activity and incident atrial fibrillation and stroke
| Activity measure . | Adjusted for age and sex . | Adjusted for CHARGE-AF componentsa and sex . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | C-statistic (95% CI) . | P . | Hazard ratio (95% CI) . | C-statistic (95% CI) . | P . | |
| Incident AF (n = 93 669) | ||||||
| Meets ESC/AHA/WHO standard recommendationb | 0.69 (0.63–0.75) | 0.743 (0.733–0.752) | <0.01 | 0.82 (0.75–0.89) | 0.762 (0.752–0.771) | <0.01 |
| Meets WHO extended recommendationc | 0.80 (0.71–0.91) | 0.739 (0.730–0.749) | <0.01 | 0.96 (0.85–1.09) | 0.761 (0.751–0.770) | 0.53 |
| Overall acceleration (per 1 SD increase) | 0.85 (0.81–0.90) | 0.741 (0.732–0.751) | <0.01 | 0.97 (0.92–1.02) | 0.761 (0.752–0.770) | 0.25 |
| Decile of weekly MVPAd (per 1 decile increase) | 0.92 (0.91–0.93) | 0.746 (0.736–0.755) | <0.01 | 0.95 (0.94–0.97) | 0.762 (0.753–0.771) | <0.01 |
| N = 2338 AF events; median follow-up 5.2 years (quartile 1: 4.6, quartile 3: 5.8) | ||||||
| Incident stroke (n = 92 512) | ||||||
| Meets ESC/AHA/WHO standard recommendationb | 0.69 (0.58–0.82) | 0.716 (0.697–0.734) | <0.01 | 0.76 (0.64–0.90) | 0.734 (0.715–0.754) | <0.01 |
| Meets WHO extended recommendationc | 0.70 (0.55–0.90) | 0.712 (0.694–0.731) | <0.01 | 0.78 (0.61–0.99) | 0.733 (0.713–0.753) | 0.04 |
| Overall acceleration (per 1 SD increase) | 0.77 (0.70–0.85) | 0.717 (0.698–0.736) | <0.01 | 0.82 (0.75–0.91) | 0.734 (0.715–0.754) | <0.01 |
| Decile of weekly MVPAd (per 1 decile increase) | 0.92 (0.89–0.95) | 0.719 (0.700–0.737) | <0.01 | 0.94 (0.91–0.97) | 0.736 (0.716–0.755) | <0.01 |
| N = 645 stroke events; median follow-up 5.2 years (quartile 1: 4.7, quartile 3: 5.8) | ||||||
| Activity measure . | Adjusted for age and sex . | Adjusted for CHARGE-AF componentsa and sex . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | C-statistic (95% CI) . | P . | Hazard ratio (95% CI) . | C-statistic (95% CI) . | P . | |
| Incident AF (n = 93 669) | ||||||
| Meets ESC/AHA/WHO standard recommendationb | 0.69 (0.63–0.75) | 0.743 (0.733–0.752) | <0.01 | 0.82 (0.75–0.89) | 0.762 (0.752–0.771) | <0.01 |
| Meets WHO extended recommendationc | 0.80 (0.71–0.91) | 0.739 (0.730–0.749) | <0.01 | 0.96 (0.85–1.09) | 0.761 (0.751–0.770) | 0.53 |
| Overall acceleration (per 1 SD increase) | 0.85 (0.81–0.90) | 0.741 (0.732–0.751) | <0.01 | 0.97 (0.92–1.02) | 0.761 (0.752–0.770) | 0.25 |
| Decile of weekly MVPAd (per 1 decile increase) | 0.92 (0.91–0.93) | 0.746 (0.736–0.755) | <0.01 | 0.95 (0.94–0.97) | 0.762 (0.753–0.771) | <0.01 |
| N = 2338 AF events; median follow-up 5.2 years (quartile 1: 4.6, quartile 3: 5.8) | ||||||
| Incident stroke (n = 92 512) | ||||||
| Meets ESC/AHA/WHO standard recommendationb | 0.69 (0.58–0.82) | 0.716 (0.697–0.734) | <0.01 | 0.76 (0.64–0.90) | 0.734 (0.715–0.754) | <0.01 |
| Meets WHO extended recommendationc | 0.70 (0.55–0.90) | 0.712 (0.694–0.731) | <0.01 | 0.78 (0.61–0.99) | 0.733 (0.713–0.753) | 0.04 |
| Overall acceleration (per 1 SD increase) | 0.77 (0.70–0.85) | 0.717 (0.698–0.736) | <0.01 | 0.82 (0.75–0.91) | 0.734 (0.715–0.754) | <0.01 |
| Decile of weekly MVPAd (per 1 decile increase) | 0.92 (0.89–0.95) | 0.719 (0.700–0.737) | <0.01 | 0.94 (0.91–0.97) | 0.736 (0.716–0.755) | <0.01 |
| N = 645 stroke events; median follow-up 5.2 years (quartile 1: 4.7, quartile 3: 5.8) | ||||||
AHA, American Heart Association; AF, atrial fibrillation; ESC, European Society of Cardiology; CI, confidence interval; MVPA, moderate-to-vigorous physical activity; WHO, World Health Organization.
CHARGE-AF components include age, race, smoking, height, weight, systolic blood pressure, diastolic blood pressure, anti-hypertensive medication use, diabetes, heart failure, and myocardial infarction.
Defined as 150 min of moderate intensity or greater activity per week.11–13
Defined as 300 min of moderate intensity or greater activity per week.11
Decile cut-offs (min): 0–20, 20–45, 45–75, 75–105, 105–135, 135–175, 175–220, 220–287, 287–395, and >395.
Multivariable associations between physical activity and incident atrial fibrillation and stroke
| Activity measure . | Adjusted for age and sex . | Adjusted for CHARGE-AF componentsa and sex . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | C-statistic (95% CI) . | P . | Hazard ratio (95% CI) . | C-statistic (95% CI) . | P . | |
| Incident AF (n = 93 669) | ||||||
| Meets ESC/AHA/WHO standard recommendationb | 0.69 (0.63–0.75) | 0.743 (0.733–0.752) | <0.01 | 0.82 (0.75–0.89) | 0.762 (0.752–0.771) | <0.01 |
| Meets WHO extended recommendationc | 0.80 (0.71–0.91) | 0.739 (0.730–0.749) | <0.01 | 0.96 (0.85–1.09) | 0.761 (0.751–0.770) | 0.53 |
| Overall acceleration (per 1 SD increase) | 0.85 (0.81–0.90) | 0.741 (0.732–0.751) | <0.01 | 0.97 (0.92–1.02) | 0.761 (0.752–0.770) | 0.25 |
| Decile of weekly MVPAd (per 1 decile increase) | 0.92 (0.91–0.93) | 0.746 (0.736–0.755) | <0.01 | 0.95 (0.94–0.97) | 0.762 (0.753–0.771) | <0.01 |
| N = 2338 AF events; median follow-up 5.2 years (quartile 1: 4.6, quartile 3: 5.8) | ||||||
| Incident stroke (n = 92 512) | ||||||
| Meets ESC/AHA/WHO standard recommendationb | 0.69 (0.58–0.82) | 0.716 (0.697–0.734) | <0.01 | 0.76 (0.64–0.90) | 0.734 (0.715–0.754) | <0.01 |
| Meets WHO extended recommendationc | 0.70 (0.55–0.90) | 0.712 (0.694–0.731) | <0.01 | 0.78 (0.61–0.99) | 0.733 (0.713–0.753) | 0.04 |
| Overall acceleration (per 1 SD increase) | 0.77 (0.70–0.85) | 0.717 (0.698–0.736) | <0.01 | 0.82 (0.75–0.91) | 0.734 (0.715–0.754) | <0.01 |
| Decile of weekly MVPAd (per 1 decile increase) | 0.92 (0.89–0.95) | 0.719 (0.700–0.737) | <0.01 | 0.94 (0.91–0.97) | 0.736 (0.716–0.755) | <0.01 |
| N = 645 stroke events; median follow-up 5.2 years (quartile 1: 4.7, quartile 3: 5.8) | ||||||
| Activity measure . | Adjusted for age and sex . | Adjusted for CHARGE-AF componentsa and sex . | ||||
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) . | C-statistic (95% CI) . | P . | Hazard ratio (95% CI) . | C-statistic (95% CI) . | P . | |
| Incident AF (n = 93 669) | ||||||
| Meets ESC/AHA/WHO standard recommendationb | 0.69 (0.63–0.75) | 0.743 (0.733–0.752) | <0.01 | 0.82 (0.75–0.89) | 0.762 (0.752–0.771) | <0.01 |
| Meets WHO extended recommendationc | 0.80 (0.71–0.91) | 0.739 (0.730–0.749) | <0.01 | 0.96 (0.85–1.09) | 0.761 (0.751–0.770) | 0.53 |
| Overall acceleration (per 1 SD increase) | 0.85 (0.81–0.90) | 0.741 (0.732–0.751) | <0.01 | 0.97 (0.92–1.02) | 0.761 (0.752–0.770) | 0.25 |
| Decile of weekly MVPAd (per 1 decile increase) | 0.92 (0.91–0.93) | 0.746 (0.736–0.755) | <0.01 | 0.95 (0.94–0.97) | 0.762 (0.753–0.771) | <0.01 |
| N = 2338 AF events; median follow-up 5.2 years (quartile 1: 4.6, quartile 3: 5.8) | ||||||
| Incident stroke (n = 92 512) | ||||||
| Meets ESC/AHA/WHO standard recommendationb | 0.69 (0.58–0.82) | 0.716 (0.697–0.734) | <0.01 | 0.76 (0.64–0.90) | 0.734 (0.715–0.754) | <0.01 |
| Meets WHO extended recommendationc | 0.70 (0.55–0.90) | 0.712 (0.694–0.731) | <0.01 | 0.78 (0.61–0.99) | 0.733 (0.713–0.753) | 0.04 |
| Overall acceleration (per 1 SD increase) | 0.77 (0.70–0.85) | 0.717 (0.698–0.736) | <0.01 | 0.82 (0.75–0.91) | 0.734 (0.715–0.754) | <0.01 |
| Decile of weekly MVPAd (per 1 decile increase) | 0.92 (0.89–0.95) | 0.719 (0.700–0.737) | <0.01 | 0.94 (0.91–0.97) | 0.736 (0.716–0.755) | <0.01 |
| N = 645 stroke events; median follow-up 5.2 years (quartile 1: 4.7, quartile 3: 5.8) | ||||||
AHA, American Heart Association; AF, atrial fibrillation; ESC, European Society of Cardiology; CI, confidence interval; MVPA, moderate-to-vigorous physical activity; WHO, World Health Organization.
CHARGE-AF components include age, race, smoking, height, weight, systolic blood pressure, diastolic blood pressure, anti-hypertensive medication use, diabetes, heart failure, and myocardial infarction.
Defined as 150 min of moderate intensity or greater activity per week.11–13
Defined as 300 min of moderate intensity or greater activity per week.11
Decile cut-offs (min): 0–20, 20–45, 45–75, 75–105, 105–135, 135–175, 175–220, 220–287, 287–395, and >395.
The IRR for AF was approximately half among individuals meeting the ESC/AHA/WHO standard recommendation [0.55 (95% CI 0.50–0.59)] and WHO extended recommendation [0.63 (95% CI 0.55–0.71)] vs. those not meeting the respective recommendations (Supplementary material online, Table S9). The crude cumulative risk of AF stratified by mutually exclusive guideline-based categories of physical activity and deciles of MVPA are shown in Supplementary material online, Figures S7 and S8, respectively. Sex-stratified adjusted curves demonstrated that AF risk was higher for men vs. women at a given activity level and are shown in Supplementary material online, Figures S9–S11.
The number of days individuals met guideline-recommended MVPA levels was also associated with lower risk of incident AF [HR 0.95 per day with ≥25 min (95% CI 0.93–0.97), P < 0.01; HR 0.95 per day with ≥45 min (95% CI 0.93–0.98), P < 0.01; Figure 3 and Supplementary material online, Table S10].
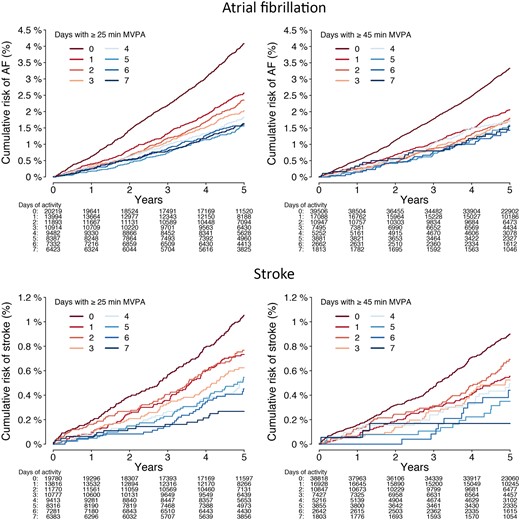
Cumulative risks of atrial fibrillation and stroke stratified by days meeting guideline-recommended moderate-to-vigorous physical activity levels. Depicted is the cumulative risk of atrial fibrillation (upper panels) and stroke (lower panels) stratified by the number of days individuals met the average daily moderate-to-vigorous physical activity levels corresponding to the European Society of Cardiology,14 American Heart Association,13 and World Health Organization standard thresholds (≥25 min/day, left panels),12 and the World Health Organization threshold for additional health benefits (≥45 min/day, right panels).12 Only individuals contributing a full week of accelerometer data are included (n = 88 644 for atrial fibrillation analyses; n = 87 536 for stroke analyses).
Correlation between accelerometer-derived and self-reported MVPA was weak [r = 0.16 (95% CI 0.16–0.17), P < 0.01, Figure 4] and agreement for meeting the ESC/AHA/WHO standard [raw agreement: 56.0%; kappa 0.12 (95% CI 0.12–0.13)] and WHO extended [64.8%; 0.09 (95% CI 0.08–0.10)] recommendations was poor (Supplementary material online, Tables S11 and S12). Although disagreement occurred in both directions, a greater proportion of individuals met standard guidelines by self-report data but did not meet them objectively (47%), as compared to individuals who met guidelines objectively based on accelerometer data but did not meet them according to self-report (42%, P < 0.01). Self-reported activity meeting guideline recommendations was not associated with decreased risk of AF or stroke (Supplementary material online, Tables S13 and S14).
![Distribution of self-reported moderate-to-vigorous physical activity across deciles of accelerometer-derived moderate-to-vigorous physical activity. Depicted are distributions of self-reported moderate-to-vigorous physical activity (y-axis) across deciles of increasing accelerometer-derived moderate-to-vigorous physical activity (x-axis). Each boxplot represents a decile of accelerometer-derived moderate-to-vigorous physical activity and is located at the median accelerometer-derived moderate-to-vigorous physical activity within that decile. For each boxplot, the black bar denotes the median self-reported moderate-to-vigorous physical activity, the box represents the interquartile range, and the whiskers represent points beyond the interquartile range. The top plot depicts all 93 579 individuals contributing self-report data (median [quartile 1, quartile 3] years from questionnaire to accelerometer wear 5.5 [4.6, 6.3]), while the bottom plot depicts 8205 individuals contributing self-report data at a follow-up assessment (2.2 [1.8, 2.5]). Points greater than quartile 3 plus 1.5 times the interquartile range and points smaller than quartile 1 minus 1.5 times the interquartile range are not depicted.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/42/25/10.1093_eurheartj_ehab250/3/m_ehab250f4.jpeg?Expires=1750481565&Signature=HUVYwQAss8pVl3sBDS-EWw~FiobzP8Ulcp8J1y7PcDhxA1B8pbM9mvnWkVPzkBIHefubQzz6DcaIi6azTxfQyKM0a9JrxwMw1i35D-qAAU0A94n-R7VKLAM6b7DX2rxnBKZzhELAGBBd1d3sNZ5f23I57zBH7hITK-f8YOtm7VAunANKHsFQ2So348loNa2K~8svPubyitMq4HMi8Fjf89F3Bj7y4DxnYc3kqHVEnMSkhZfhiCEPeU5GGFT2Yi1kh1ygLYwV81VblbZ5jGSNW-L9rHKFu6tRG3dqSB3PhMrQcTcnQ9k3QZ1ZIlLpV6VIz~UrnHc0OQtzddfqkm2nSg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Distribution of self-reported moderate-to-vigorous physical activity across deciles of accelerometer-derived moderate-to-vigorous physical activity. Depicted are distributions of self-reported moderate-to-vigorous physical activity (y-axis) across deciles of increasing accelerometer-derived moderate-to-vigorous physical activity (x-axis). Each boxplot represents a decile of accelerometer-derived moderate-to-vigorous physical activity and is located at the median accelerometer-derived moderate-to-vigorous physical activity within that decile. For each boxplot, the black bar denotes the median self-reported moderate-to-vigorous physical activity, the box represents the interquartile range, and the whiskers represent points beyond the interquartile range. The top plot depicts all 93 579 individuals contributing self-report data (median [quartile 1, quartile 3] years from questionnaire to accelerometer wear 5.5 [4.6, 6.3]), while the bottom plot depicts 8205 individuals contributing self-report data at a follow-up assessment (2.2 [1.8, 2.5]). Points greater than quartile 3 plus 1.5 times the interquartile range and points smaller than quartile 1 minus 1.5 times the interquartile range are not depicted.
Stroke associations
A total of 645 participants developed incident stroke, of which 46 (7.1%) occurred following incident AF. The overall cumulative risk of stroke was 0.92% (95% CI 0.83–1.00) and the stroke incidence rate 1.4/1000 person-years (95% CI 1.3–1.5). In models adjusted for sex and each component of CHARGE-AF, activity meeting the ESC/AHA/WHO standard recommendation [HR 0.76 (95% CI 0.64–0.90), P < 0.01], activity meeting the ESC/AHA/WHO extended recommendation [HR 0.78 (95% CI 0.61–0.99), P = 0.04], mean acceleration [HR 0.82 per 1-SD (95% CI 0.75–0.91), P < 0.01], and increasing MVPA decile [HR 0.94 per one decile increase (95% CI 0.91–0.97), P < 0.01] were each associated with lower risk of stroke (Table 2). The crude cumulative risk of stroke stratified by adherence to ESC/AHA/WHO standard and WHO extended recommendations is shown in Figure 1. When assessing MVPA deciles as independent categories using adjusted models, stroke risk appeared to decrease uniformly across increasing deciles (Figure 2 and Supplementary material online, Table S6). Stroke risk was similar among individuals meeting the WHO extended recommendation as compared to individuals meeting only the ESC/AHA/WHO standard recommendation [HR 0.91 (95% CI 0.69–1.21), P = 0.51, Supplementary material online, Tables S7 and S8].
The IRR for stroke was approximately half among individuals exceeding the ESC/AHA/WHO standard recommendation [0.55 (95% CI 0.47–0.64)] and WHO extended recommendation [0.55 (95% CI 0.40–0.71)] vs. those not meeting the respective recommendations (Supplementary material online, Table S9). Crude cumulative risk of stroke stratified by activity level are shown in Figure 2 and Supplementary material online, Figures S7 and S8. Sex-stratified adjusted curves demonstrated that stroke risk was higher for men vs. women at a given activity level and are shown in Supplementary material online, Figures S12–S14.
The number of days individuals met guideline-recommended MVPA levels was also associated with lower risk of stroke [HR 0.91 per day with ≥25 min (95% CI 0.87–0.95), P < 0.01; HR 0.90 per day with ≥45 minutes (95% CI 0.85–0.95), P < 0.01, Supplementary material online, Table S10 and Figure 3].
Exploratory and sensitivity analyses
In exploratory sex and AF risk-adjusted analyses, greater vigorous physical activity was generally associated with a reduced risk of AF (HR 0.98 per one decile increase, 95% CI 0.96–0.99, P < 0.01), until the highest decile. Individuals in the highest decile of vigorous physical activity (>67 min/week) had a similar risk of AF as those in the lowest decile (HR 0.89, 95% CI 0.73–1.09, P = 0.25), and a higher risk than individuals in the 8th decile (28–40 min/week; HR 1.34, 95% CI 1.07–1.68, P = 0.01), who had the lowest adjusted AF risk overall (Supplementary material online, Figure S15). In contrast, greater vigorous physical activity was associated with a more uniform reduction in stroke risk (HR 0.94 per one decile increase, 95% CI 0.91–0.97, P < 0.01). Individuals in higher deciles of vigorous activity were more likely to report engagement in strenuous sports (chi-square trend P < 0.01), including 0.3% in the lowest decile vs. 2.1% in the highest decile (Supplementary material online, Figure S16).
Increasing intra-individual coefficient of variation in mean acceleration [HR 0.98 per 10% increase (95% CI 0.97–0.99), P < 0.01] was also associated with decreased AF risk. Associations between guideline-adherent activity and reduced AF and stroke risk persisted in analyses restricted to the 88 645 individuals contributing a full week of accelerometry, non-imputed data, MVPA defined without bout criteria, in models adjusted for body mass index (instead of height and weight), and in models additionally adjusted for alcohol use, sleep apnoea, Townsend Deprivation Index, antiplatelet use, and anticoagulant use (Supplementary material online, Tables S15–S18). Stroke association results were similar in models considering only events coded as ischaemic strokes (Supplementary material online, Table S19). Associations between meeting ESC/AHA/WHO standard recommendations and incident AF (subdistribution HR 0.82, 95% CI 0.75–0.90, P < 0.01) and stroke (subdistribution HR 0.77, 95% CI 0.65–0.92, P < 0.01) were also similar when death was modelled as a competing risk. Detailed results of the primary AF and stroke models including covariate strengths of association are shown in Supplementary material online, Tables S20 and S21. Exploration of models utilizing overall mean acceleration as the activity variable revealed that adjustment only for age, sex, and weight was sufficient to attenuate associations with incident AF [HR 0.96 (95% CI 0.91–1.01), P = 0.09].
Discussion
Using a unique resource of objectively measured physical activity data derived from wrist-worn accelerometers obtained in nearly 100 000 primarily middle-aged and older individuals over 7 days, we found that increased activity was associated with lower risk of incident AF. Individuals meeting guideline recommendations from the ESC,14 AHA,13 and WHO12 had approximately half the AF incidence compared to individuals not meeting activity recommendations. Accelerometer-derived physical activity was a far more robust indicator of AF risk than self-reported activity measured several years prior, which was not associated with disease risk. We observed similar associations between accelerometer-derived physical activity and incident stroke.
Our study extends prior work by quantifying associations between objective physical activity measured using wrist-worn accelerometers and incident AF. Previous studies have offered conflicting results regarding the association between activity and AF, with certain studies suggesting benefit,2 , 3 others showing no relationship after adjusting for confounders,5 and yet others demonstrating higher risk at either extreme.6 In contrast to self-reported activity, which is subject to recall bias9 and correlates modestly with measured energy expenditure,10 we leveraged prospectively collected accelerometer-derived physical activity in nearly 100 000 individuals to assess associations with incident AF and stroke. Consistent with limitations of self-reported activity, questionnaire-based activity levels correlated very weakly with objective physical activity measured several years later, and observed associations with AF and stroke were not apparent using self-reported activity measures alone.
Our results identify objectively quantified physical activity as an important modifiable AF risk factor. Using wrist-worn accelerometers in 5000 individuals, O’Neal et al.34 recently reported lower AF risk with increasing MVPA, but only a trend towards reduced risk with guideline-based levels of activity. Our findings demonstrate that activity in accordance with guidelines is indeed associated with substantially lower risks of incident AF and stroke, even after adjustment for AF risk factors. Previous studies by Elliott et al.7 and Said et al.35 in larger subsets of the UK Biobank have suggested greater self-reported activity may be beneficial for AF risk. However, similar to Tikkanen et al.,2 we did not observe an association between self-reported activity and incident AF after considering baseline clinical factors. Although contrasting findings may be related to differences in sample size and composition, as well as our adjustment for each component of the CHARGE-AF instrument, we note that the effect sizes observed in the studies reporting significant associations were modest. Therefore, especially since current clinical guidelines do not comment on how adherence to activity recommendations should be ascertained,13 , 14 our study provides important evidence that objectively measured activity is a more robust indicator of AF risk than self-reported activity. In addition to established benefits on weight and blood pressure, exercise may directly reduce AF risk through favourable effects on autonomic tone and atrial function.30 , 36 Of note, the association between overall mean acceleration and AF was attenuated after adjustment for AF risk (in particular, weight5), whereas MVPA—both overall and according to guideline thresholds—remained strongly associated with decreased AF risk in adjusted models, suggesting that activity of at least moderate intensity may be particularly important for AF risk.34 On balance, our findings suggest that efforts to prevent AF by promoting physical activity are warranted and should utilize objectively measured activity data when possible.
Specifically, population-level efforts to reduce AF risk may be most effective if they aim to maximize the proportion of individuals objectively achieving guideline-adherent activity levels, rather than encouraging higher volume or intensity exercise among individuals who are already active. After adjusting for clinical AF risk factors, AF hazard appeared to decrease until the seventh decile of MVPA (i.e. 175–220 min of MVPA/week), after which point it remained relatively constant. Likewise, AF and stroke risk among individuals exceeding the more stringent WHO activity threshold (≥300 min MVPA/week) were similar compared to individuals exceeding the standard thresholds (≥150 min MVPA/week) only. Such findings support recent results suggesting that inactivity may be a causal risk factor for coronary heart disease.37 Consistent with prior observations,6 , 38–40 adjusted AF risk appeared lowest at intermediate levels of vigorous activity, with individuals in the lowest (0–3 min/week) and highest (>67 min/week) deciles of vigorous activity both having a roughly 30% increased risk of AF as compared to individuals in the 8th decile (i.e. 28–40 min/week). On balance, our findings support the recommendations of the 2020 ESC guidelines on AF management, which promote moderate levels of physical activity among individuals at risk for AF.41 Importantly, vigorous activity quantification using wrist-worn accelerometers is less well-established than classification of MVPA,18–21 and our window of objective observation was limited to 1 week. As a result, future work is warranted to clarify potential positive associations between especially vigorous or abundant objective physical activity and AF, especially among younger individuals.39
Our results highlight a potential opportunity to link AF diagnostic and preventive efforts (Graphical Abstract).42 The mAFA-II trial recently showed that a smartphone app enabling both an AF screening and risk mitigation pathway resulted in reduced AF-related hospitalizations.43 Since wrist-worn wearables capable of AF screening are becoming increasingly prevalent and are now commonly equipped with accelerometers, our results suggest that wearable sensors may have utility in both prevention and detection of AF.44 We acknowledge that different devices may be available for objectively measuring physical activity, each with different potential advantages and disadvantages. The wrist-worn accelerometer may be optimally suited for measuring activity in relation to AF risk given the increasing capability of consumer technology to detect possible AF via photoplethysmography and electrocardiography. Nevertheless, uncertainties and challenges remain with respect to identification of effective methods for encouraging health promotion via mobile technology, incorporation of wearable sensor data into clinical workflows, and uptake of wearable sensors by individuals most likely to benefit. Furthermore, more robust methods of classifying vigorous athletic activity may be important to define the relations between athletics and AF. Whether wearable devices will lead to improved health outcomes remains unknown and requires rigorous prospective evaluation.
Our study should be considered in the context of design. First, generalizability may be limited by geographic and ancestral specificity, as well as bias introduced by enrolment. Similarly, individuals who accepted the invitation to the accelerometry sub-study had slightly lower rates of cardiac comorbidity than UK Biobank participants who declined. However, the AF and stroke rates we observed in our sample are generally consistent with population-based estimates within similar age groups.45–47 Second, use of diagnostic and procedural codes27 to define AF introduces potential misclassification. Third, the duration of exposure measurement is limited and an individual’s activity habits may have been influenced by the awareness of being monitored. Nevertheless, the current study represents an analysis of a unique resource of objectively measured physical activity obtained at scale, and is consistent with previous reports associating objective short-term activity measurement with longitudinal outcomes.33 , 34 Fourth, we collapsed moderate and vigorous physical activity as MVPA in our primary analyses given multiple studies supporting the use of wrist-worn activity sensors to classify activity of at least moderate intensity.18–21 However, we did perform exploratory analyses in which we specifically quantified vigorous physical activity using a validated threshold.18 Fifth, accuracy of MVPA detection using wrist-worn accelerometers varies somewhat across studies and may be lower with certain activities (e.g. stationary cycling).48 , 49 Reassuringly, MVPA levels in our study are broadly consistent with estimates from national UK-based surveys,50 self-reported data from the same individuals, and past wrist-worn accelerometer-based studies.34 Furthermore, variability in continuous acceleration measurement (in contrast to proprietary step counts) across device brands is modest.21 Sixth, the IPAQ was administered roughly five years prior to accelerometry in most participants. As a result, more recent questionnaire data may have provided a more accurate self-reported activity estimate, although we note that the IPAQ demonstrates good short-term repeatability.22 Furthermore, we did not observe associations between self-reported activity and reduced risk of AF or stroke among the subset of individuals who provided follow-up IPAQ data, which more closely preceded accelerometer wear (i.e. within 3 years). Seventh, given observational design we cannot infer causality or eliminate residual confounding.
In summary, among nearly 100 000 middle-aged and older individuals within a prospective UK-based cohort, guideline-adherent objective activity levels quantified using wrist-based sensors worn over 7 days was associated with lower risks of incident AF and stroke after adjustment for AF risk factors. In contrast to accelerometer-derived activity, self-reported physical activity measured several years prior was not associated with reduced risks of AF or stroke. Future clinical guidelines should acknowledge the potential value of objectively assessing adherence to recommended activity levels. Wearable devices capable of objective activity monitoring, motivational messaging, and AF detection may offer an integrated mechanism for AF prevention and diagnosis.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by American Heart Association (AHA) 18SFRN34150007 (L.T.); National Institutes of Health (NIH) 2R01HL092577 and 1R01HL141434 01A1, AHA 18SFRN34110082, and Robert Wood Johnson 74624 (E.J.B.); NIH T32HL007208 (S.K.); NIH R38HL150212 (J.S.H.); NIH 1R01HL092577, K24HL105780, AHA 18SFRN34110082, and Foundation Leducq 14CVD01 (P.T.E.); Grant from Bayer AG to the Broad Institute focused on the genetics and therapeutics of cardiovascular diseases (P.T.E.); AHA 18SFRN34110082 (L.-C.W.); and NIH 1R01139731 and AHA 18SFRN34250007 (S.A.L.).
Conflict of interest: Starting 2020, E.J.B. is an uncompensated member for MyHeartLab Steering Committee, a PI-initiated study from Samsung to UCSF (PI: Jeffrey Olgin, MD). P.T.E. has served on advisory boards or consulted for Bayer AG, Quest Diagnostics, MyoKardia, and Novartis. S.A.L. receives sponsored research support from Bristol Myers Squibb/Pfizer, Bayer AG, Boehringer Ingelheim, and Fitbit, has consulted for Bristol Myers Squibb/Pfizer and Bayer AG, and participates in a research collaboration with IBM. D.D.M. receives research support from Bristol Myers Squibb/Pfizer, Boehringer Ingelheim, Philips Healthcare, Flexcon, Samsung, Apple Computer, and Fitbit and has consulted for Bristol Myers Squibb/Pfizer, Samsung, Philips, Flexcon, Boston Biomedical Associates, and Rose Consulting. The remaining authors report no disclosures. L.T. reports grants from American Heart Association, during the conduct of the study. P.T.E. reports grants and personal fees from Bayer AG, personal fees from Novartis, and personal fees from MyoKardia, during the conduct of the study. S.K. reports grants from National Institutes of Health, during the conduct of the study. D.D.M. reports grants and personal fees from Bristol Myers Squib, grants and personal fees from Pfizer, grants from Boeringher Ingelheim and Philips, grants and personal fees from Flexcon, personal fees from Samsung, non-financial support from Apple, non-financial support and other from Fitbit, and personal fees from Boston Biomedical Associates, outside the submitted work; he also serves on the Steering Committee of the GUARD-AF study (BMS/Pfizer) and on the Advisory Committee for the Fitbit Heart Study. E.J.B. reports grants from the National Institutes of Health and the American Heart Association, during the conduct of the study. L.-C.W. reports grants from American Heart Association, during the conduct of the study. S.A.L. reports grants from NIH, grants from AHA, grants and personal fees from BMS/Pfizer, grants from Boehringer Ingelheim, grants and personal fees from Bayer AG, grants from IBM, grants from Fitbit, and personal fees from Blackstone Life Sciences, outside the submitted work.
Data availability
UK Biobank data are publicly accessible for research use by application (https://www.ukbiobank.ac.uk/). The MVPA exposure variable used in the primary analyses will be returned to the UK Biobank for use by future researchers. The data processing scripts supporting the analyses described herein will be made available upon request to the corresponding author.
References
World Health Organization.
UK Biobank Outcome Adjudication Group. Definitions of Stroke for UK Biobank Phase 1 Outcomes Adjudication. http://biobank.ctsu.ox.ac.uk/crystal/crystal/docs/alg_outcome_stroke.pdf. Date accessed 12 August 2020.
R Core Team (
National Health Service. Health Survey for England;



