-
PDF
- Split View
-
Views
-
Cite
Cite
Maria D’Souza, Dorte Nielsen, Inge Marie Svane, Kasper Iversen, Peter Vibe Rasmussen, Christian Madelaire, Emil Fosbøl, Lars Køber, Finn Gustafsson, Charlotte Andersson, Gunnar Gislason, Christian Torp-Pedersen, Morten Schou, The risk of cardiac events in patients receiving immune checkpoint inhibitors: a nationwide Danish study, European Heart Journal, Volume 42, Issue 16, 21 April 2021, Pages 1621–1631, https://doi.org/10.1093/eurheartj/ehaa884
Close - Share Icon Share
Abstract
The study aimed to estimate the risk of cardiac events in immune checkpoint inhibitor (ICI)-treated patients with lung cancer or malignant melanoma.
The study included consecutive patients with lung cancer or malignant melanoma in 2011–17 nationwide in Denmark. The main composite outcome was cardiac events (arrhythmia, peri- or myocarditis, heart failure) or cardiovascular death. Absolute risks were estimated and the association of ICI and cardiac events was analysed in multivariable Cox models. We included 25 573 patients with lung cancer. Of these, 743 were treated with programmed cell death-1 inhibitor (PD1i) and their 1-year absolute risk of cardiac events was 9.7% [95% confidence interval (CI) 6.8–12.5]. Of the 13 568 patients with malignant melanoma, 145 had PD1i and 212 had cytotoxic T-lymphocyte-associated protein-4 inhibitor (CTLA-4i) treatment. Their 1-year risks were 6.6% (1.8–11.3) and 7.5% (3.7–11.3). The hazard rates of cardiac events were higher in patients with vs. without ICI treatment. Within 6 months from 1st ICI administration, the hazard ratios were 2.14 (95% CI 1.50–3.05) in patients with lung cancer and 4.30 (1.38–13.42) and 4.93 (2.45–9.94) in patients with malignant melanoma with PD1i and CTLA-4i, respectively. After 6 months, HRs were 2.26 (1.27–4.02) for patients with lung cancer and 3.48 (1.91–6.35) for patients with malignant melanoma and CTLA-4i.
Among patients with lung cancer and malignant melanoma, ICI treated had increased rates of cardiac events. The absolute risks were higher in these data compared with previous pharmacovigilance studies (e.g. 1.8% peri-/myocarditis 1-year risk).
See page 1632 for the editorial comment on this article (doi: 10.1093/eurheartj/ehaa959)
Introduction
In the last decade, immune checkpoint inhibitors (ICI) have revolutionized the therapeutic landscape of many cancers including malignant melanoma and lung cancer.1–4 ICI have shown to significantly improve prognosis but may induce immune-related side effects of grades I–II (mild to moderate) in various organs in up to 40% of the patients, while grades III–IV (severe to potentially life-threatening) side effects are seen in ∼2% depending on drug type.5–7 Among the known immune-related side effects are pneumonitis, dermatitis, colitis, hepatitis, nephritis, and endocrine toxicities.8–12 Case studies and pharmacovigilance data suggest that the immune-related cardiac side effects are mainly affecting cardiac conduction and myocyte function, which may result in arrhythmias, peri- or myocarditis, heart failure, and sudden cardiac arrest.13–21 However, the risk of cardiac side effects outside these selected populations is widely uninvestigated.22 , 23
Herein, we report the incidence of cardiac events of arrhythmia, peri- or myocarditis, heart failure, and cardiovascular death following treatment with ICI in nationwide cohorts of patients with lung cancer or malignant melanoma. We further evaluated the association of ICI treatment (vs. no treatment) with the risk of developing cardiac events in patients with lung cancer and malignant melanoma.
Methods
Data sources
In Denmark, all citizens have a unique personal identification number assigned at birth or immigration. The identification number is registered at all contacts with the Danish authorities including all provided healthcare services. This system enables cross-linkage between different nationwide administrative Danish registries. The present study was based on data from four of these registries: (i) the Danish Civil Registration System (personal identification numbers, emigration/immigration status and date of birth), (ii) the Danish National Patient Register (diagnoses and dates from all admissions and outpatient contacts in Danish Hospitals since 1978), (iii) the Danish Register of Medicinal Products (data on all redeemed prescriptions from Danish Pharmacies), and (iv) the Danish Register of Causes of Death (date and cause of death).24–27 As the data in this study were based on anonymized administrative data protected by laws governed by the Danish Data Protection Agency, scientists are not allowed to report patient numbers or event numbers <3 or aggregated results based on <3 observations. This level of report is to ensure anonymity to the persons registered in the databases. To comply with this rule, many table entries in this manuscript are marked with not available (NA).
Study population and immune checkpoint inhibitor exposure
The study was designed as a retrospective cohort study including all consecutive patients with incident lung cancer or malignant melanoma from 2011 to 2017 nationwide in Denmark. The study period began with the introduction of ICI treatment in 2011 and ended in 2017, which was the latest year possible for the data sources used. The patients were followed from the date of cancer diagnosis and until development of a cardiac outcome, death, emigration, or at study end at 31 December 2017.
Patients were considered exposed to ICI at the date of 1st administration of ICI. The patients were identified via procedure codes for the ICI administrations (Supplementary material online, Table S1 ). This method has been validated recently for chemotherapy in colorectal cancer with a high overall positive predictive value (PPV) of 0.91 [95% confidence interval (CI) 0.90–0.92].28 Treatment with ICI was categorized as either treatment with cytotoxic T-lymphocyte-associated protein-4 inhibitor (CTLA-4i, ipilimumab) or programmed cell death-1 inhibitors (PD1i, pembrolizumab or nivolumab).
Outcomes
The main study outcome was a combined endpoint comprising cardiac disease defined as admission (hospitalizations or outpatient contacts) with arrhythmia (both tachy- and brady-arrhythmias), myocarditis or pericarditis, heart failure or cardiovascular death. Secondary endpoints were the components of the combined outcome analysed separately. Most of these outcomes have been validated with overall high PPVs.25 , 29 The definitions based on international classification of diseases (ICD)-10 codes are found in Supplementary material online, Table S1.
Cancer type, comorbidity, and pharmacotherapy
Comorbidity was defined from registered diagnoses codes (hospitalizations or outpatient visits) within 5 years before index. Concomitant pharmacotherapy was defined as reimbursed prescriptions with the specific drug according to ATC code within 6 months before study start. The definitions based on ICD-10 and ATC codes are supplied in Supplementary material online, Table S1.
Statistics
Baseline characteristics were summarized in frequencies and percentages for dichotomous variables and in medians with percentile 25–75 for continuous variables. χ2 tests and Kruskal–Wallis tests were used for comparisons across patient groups. The level for statistical significance was set at 5%, two sided.
In the analyses of absolute risk of outcomes, only the 1st outcome was registered. Hence, recurrent events were not analysed. In the analysis of each outcome, only patients who did not have this outcome at the time of the ICI administration were included. For example, in the analysis of absolute risk of heart failure, only patients without previous heart failure were included.
Absolute risks were estimated at 6 months and 1 year after initial administration of PD1i or CTLA-4i in the ICI-treated patients using the Aalen–Johansen estimator incorporating the competing risk of non-cardiovascular death.
Multivariable Cox regression models were used for analysing the association of ICI treatment with the rate of cardiac events. The ICI exposure was time-updated, ensuring that the patients contributed with risk time as unexposed from the cancer diagnosis until 1st administration of ICI and with risk time as exposed from the ICI date until end of Follow-up. The control group was unexposed patients without ICI treatment. The models were adjusted for time since cancer diagnosis, age and sex. Linearity was tested and could be assumed for continuous variables (age) and interaction was tested for the categorical variable sex.
The statistical analyses were performed using SAS Software version 9.4 (SAS Institute Inc.) and R: A language and environment for statistical computing (version R-3.6.1).30
Ethics
As the study was based on anonymous data from the Danish nationwide administrative registers, no permission was needed from the local ethics committee and the study complied with the Declaration of Helsinki. The study was approved by the Danish Data Protection Agency (Project number: 2007-58-0015).
Results
Baseline characteristics
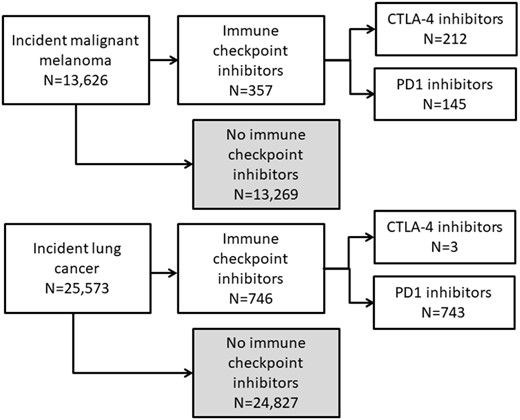
We included 25 573 patients with incident lung cancer and 13 626 with incident malignant melanoma diagnosed in 2011–17 nationwide in Denmark (Figure 1). The patients with lung cancer had a median age of 71 years (p25–p75 64–77) at diagnosis and 50.5% were men (Table 1). The most frequent comorbidities included hypertension (37.2%), chronic obstructive pulmonary disease (18.6%), and diabetes mellitus (13.2%). The patients with malignant melanoma had a median age of 60 years (p25–p75: 46–71) and 45.6% were men. Hypertension (21.6%) and diabetes mellitus (6.6%) were the most frequent comorbidities in this patient group.
Baseline characteristics of patients at the time of diagnosis of lung cancer or malignant melanoma
| . | Lung cancer . | Malignant melanoma . |
|---|---|---|
| n | 25573 | 13626 |
| Age, median (p25–p75) | 71 (64–77) | 60 (46–71) |
| Men, n (%) | 12918 (50.5) | 6222 (45.7) |
| Baseline comorbidity, n (%) | ||
| Myocardial infarction | 1949 (7.6) | 371 (2.7) |
| Heart failure | 1894 (7.4) | 341 (2.5) |
| Myocarditis | 239 (0.9) | 41 (0.3) |
| Arrhythmia | 3529 (13.8) | 1052 (7.7) |
| Peripheral arterial disease | 1016 (4.0) | 105 (0.8) |
| Diabetes mellitus | 3368 (13.2) | 899 (6.6) |
| Hypertension | 9511 (37.2) | 2934 (21.5) |
| Chronic kidney disease | 815 (3.2) | 209 (1.5) |
| Alcohol abuse | 1250 (4.9) | 173 (1.3) |
| Chronic liver disease | 433 (1.7) | 89 (0.7) |
| Chronic obstructive pulmonary disease | 4758 (18.6) | 302 (2.2) |
| Pharmacotherapy, n (%) | ||
| Calcium channel blockers | 5563 (21.8) | 1703 (12.5) |
| Beta blockers | 5699 (22.3) | 1573 (11.5) |
| Non loop diuretics | 6650 (26.0) | 2379 (17.5) |
| Loop diuretics | 3623 (14.2) | 650 (4.8) |
| RAS-inhibitors | 8658 (33.9) | 3060 (22.5) |
| Vitamin K antagonists | 1460 (5.7) | 441 (3.2) |
| Non vitamin K antagonists | 798 (3.1) | 194 (1.4) |
| ADP-inhibitors | 1831 (7.2) | 337 (2.5) |
| Aspirin | 6797 (26.6) | 1486 (10.9) |
| Statins | 8974 (35.1) | 2466 (18.1) |
| . | Lung cancer . | Malignant melanoma . |
|---|---|---|
| n | 25573 | 13626 |
| Age, median (p25–p75) | 71 (64–77) | 60 (46–71) |
| Men, n (%) | 12918 (50.5) | 6222 (45.7) |
| Baseline comorbidity, n (%) | ||
| Myocardial infarction | 1949 (7.6) | 371 (2.7) |
| Heart failure | 1894 (7.4) | 341 (2.5) |
| Myocarditis | 239 (0.9) | 41 (0.3) |
| Arrhythmia | 3529 (13.8) | 1052 (7.7) |
| Peripheral arterial disease | 1016 (4.0) | 105 (0.8) |
| Diabetes mellitus | 3368 (13.2) | 899 (6.6) |
| Hypertension | 9511 (37.2) | 2934 (21.5) |
| Chronic kidney disease | 815 (3.2) | 209 (1.5) |
| Alcohol abuse | 1250 (4.9) | 173 (1.3) |
| Chronic liver disease | 433 (1.7) | 89 (0.7) |
| Chronic obstructive pulmonary disease | 4758 (18.6) | 302 (2.2) |
| Pharmacotherapy, n (%) | ||
| Calcium channel blockers | 5563 (21.8) | 1703 (12.5) |
| Beta blockers | 5699 (22.3) | 1573 (11.5) |
| Non loop diuretics | 6650 (26.0) | 2379 (17.5) |
| Loop diuretics | 3623 (14.2) | 650 (4.8) |
| RAS-inhibitors | 8658 (33.9) | 3060 (22.5) |
| Vitamin K antagonists | 1460 (5.7) | 441 (3.2) |
| Non vitamin K antagonists | 798 (3.1) | 194 (1.4) |
| ADP-inhibitors | 1831 (7.2) | 337 (2.5) |
| Aspirin | 6797 (26.6) | 1486 (10.9) |
| Statins | 8974 (35.1) | 2466 (18.1) |
ADP, adenosine diphosphate; RAS, renin-angiotensin system.
Baseline characteristics of patients at the time of diagnosis of lung cancer or malignant melanoma
| . | Lung cancer . | Malignant melanoma . |
|---|---|---|
| n | 25573 | 13626 |
| Age, median (p25–p75) | 71 (64–77) | 60 (46–71) |
| Men, n (%) | 12918 (50.5) | 6222 (45.7) |
| Baseline comorbidity, n (%) | ||
| Myocardial infarction | 1949 (7.6) | 371 (2.7) |
| Heart failure | 1894 (7.4) | 341 (2.5) |
| Myocarditis | 239 (0.9) | 41 (0.3) |
| Arrhythmia | 3529 (13.8) | 1052 (7.7) |
| Peripheral arterial disease | 1016 (4.0) | 105 (0.8) |
| Diabetes mellitus | 3368 (13.2) | 899 (6.6) |
| Hypertension | 9511 (37.2) | 2934 (21.5) |
| Chronic kidney disease | 815 (3.2) | 209 (1.5) |
| Alcohol abuse | 1250 (4.9) | 173 (1.3) |
| Chronic liver disease | 433 (1.7) | 89 (0.7) |
| Chronic obstructive pulmonary disease | 4758 (18.6) | 302 (2.2) |
| Pharmacotherapy, n (%) | ||
| Calcium channel blockers | 5563 (21.8) | 1703 (12.5) |
| Beta blockers | 5699 (22.3) | 1573 (11.5) |
| Non loop diuretics | 6650 (26.0) | 2379 (17.5) |
| Loop diuretics | 3623 (14.2) | 650 (4.8) |
| RAS-inhibitors | 8658 (33.9) | 3060 (22.5) |
| Vitamin K antagonists | 1460 (5.7) | 441 (3.2) |
| Non vitamin K antagonists | 798 (3.1) | 194 (1.4) |
| ADP-inhibitors | 1831 (7.2) | 337 (2.5) |
| Aspirin | 6797 (26.6) | 1486 (10.9) |
| Statins | 8974 (35.1) | 2466 (18.1) |
| . | Lung cancer . | Malignant melanoma . |
|---|---|---|
| n | 25573 | 13626 |
| Age, median (p25–p75) | 71 (64–77) | 60 (46–71) |
| Men, n (%) | 12918 (50.5) | 6222 (45.7) |
| Baseline comorbidity, n (%) | ||
| Myocardial infarction | 1949 (7.6) | 371 (2.7) |
| Heart failure | 1894 (7.4) | 341 (2.5) |
| Myocarditis | 239 (0.9) | 41 (0.3) |
| Arrhythmia | 3529 (13.8) | 1052 (7.7) |
| Peripheral arterial disease | 1016 (4.0) | 105 (0.8) |
| Diabetes mellitus | 3368 (13.2) | 899 (6.6) |
| Hypertension | 9511 (37.2) | 2934 (21.5) |
| Chronic kidney disease | 815 (3.2) | 209 (1.5) |
| Alcohol abuse | 1250 (4.9) | 173 (1.3) |
| Chronic liver disease | 433 (1.7) | 89 (0.7) |
| Chronic obstructive pulmonary disease | 4758 (18.6) | 302 (2.2) |
| Pharmacotherapy, n (%) | ||
| Calcium channel blockers | 5563 (21.8) | 1703 (12.5) |
| Beta blockers | 5699 (22.3) | 1573 (11.5) |
| Non loop diuretics | 6650 (26.0) | 2379 (17.5) |
| Loop diuretics | 3623 (14.2) | 650 (4.8) |
| RAS-inhibitors | 8658 (33.9) | 3060 (22.5) |
| Vitamin K antagonists | 1460 (5.7) | 441 (3.2) |
| Non vitamin K antagonists | 798 (3.1) | 194 (1.4) |
| ADP-inhibitors | 1831 (7.2) | 337 (2.5) |
| Aspirin | 6797 (26.6) | 1486 (10.9) |
| Statins | 8974 (35.1) | 2466 (18.1) |
ADP, adenosine diphosphate; RAS, renin-angiotensin system.
During follow-up, 743 patients with lung cancer were treated with PD1i. Among the patients with malignant melanoma, 145 were treated with PD1i and 212 with CTLA-4i. The median age was 67 (p25–p75: 60–72), 62 (p25–p75: 51–74) and 67 (p25–p75: 60–72) years and the proportion of male sex was 52.6%, 59.3%, and 58.0% in the three treatment groups, respectively. The proportion of patients registered with comorbidity within 5 years was lower for patients with lung cancer treated with PD1i at the time of initial PD1i administration compared with all patients with lung cancer at the time of cancer diagnosis for hypertension (25.4%), chronic obstructive lung disease (13.7%), and diabetes mellitus (12.2%). Patients with malignant melanoma had higher frequencies of hypertension and diabetes mellitus within 5 years of 1st PD1i administration (26.9% and 11.0%) and 1st CTLA-4i administration (25.0% and 10.4%) compared with all patients with malignant melanoma at the time of cancer diagnosis (Table 2).
Baseline characteristics of patients at the time of initial administration of immune checkpoint inhibitor
| . | Lung cancer and PD1i . | Malignant melanoma and CTLA-4i . | Malignant melanoma and PD1i . |
|---|---|---|---|
| N | 743 | 212 | 145 |
| Age, median (p25–p75) | 67 (60–72) | 64 (54–70) | 62 (51–74) |
| Men, n (%) | 391 (52.6) | 123 (58.0) | 86 (59.3) |
| Comorbidity at ICI administration, n (%) | |||
| Myocardial infarction | 40 (5.4) | 10 (4.7) | <3 |
| Heart failure | 29 (3.9) | 5 (2.4) | <3 |
| Myocarditis | 14 (1.9) | <3 | <3 |
| Arrhythmia | 104 (14.0) | 20 (9.4) | 13 (9.0) |
| Peripheral arterial disease | 14 (1.9) | 3 (1.4) | <3 |
| Diabetes mellitus | 91 (12.2) | 22 (10.4) | 16 (11.0) |
| Hypertension | 189 (25.4) | 53 (25.0) | 39 (26.9) |
| Chronic kidney disease | 17 (2.3) | 3 (1.4) | 3 (2.1) |
| Alcohol abuse | 11 (1.5) | 12 (5.7) | 4 (2.8) |
| Chronic liver disease | 4 (0.5) | <3 | 5 (3.4) |
| Chronic obstructive pulmonary disease | 102 (13.7) | 7 (3.3) | <3 |
| Pharmacotherapy at ICI administration, n (%) | |||
| Calcium channel blockers | 114 (15.3) | 33 (15.6) | 24 (16.6) |
| Beta blockers | 150 (20.2) | 38 (17.9) | 13 (9.0) |
| Non loop diuretics | 145 (19.5) | 39 (18.4) | 38 (26.2) |
| Loop diuretics | 71 (9.6) | 12 (5.7) | 7 (4.8) |
| RAS-inhibitors | 189 (25.4) | 57 (26.9) | 41 (28.3) |
| Vitamin K antagonists | 33 (4.4) | 4 (1.9) | 3 (2.1) |
| Non vitamin K antagonists | 40 (5.4) | 3 (1.4) | 4 (2.8) |
| ADP-inhibitors | 42 (5.7) | 13 (6.1) | 8 (5.5) |
| Aspirin | 147 (19.8) | 25 (11.8) | 15 (10.3) |
| Statins | 194 (26.1) | 45 (21.2) | 23 (15.9) |
| . | Lung cancer and PD1i . | Malignant melanoma and CTLA-4i . | Malignant melanoma and PD1i . |
|---|---|---|---|
| N | 743 | 212 | 145 |
| Age, median (p25–p75) | 67 (60–72) | 64 (54–70) | 62 (51–74) |
| Men, n (%) | 391 (52.6) | 123 (58.0) | 86 (59.3) |
| Comorbidity at ICI administration, n (%) | |||
| Myocardial infarction | 40 (5.4) | 10 (4.7) | <3 |
| Heart failure | 29 (3.9) | 5 (2.4) | <3 |
| Myocarditis | 14 (1.9) | <3 | <3 |
| Arrhythmia | 104 (14.0) | 20 (9.4) | 13 (9.0) |
| Peripheral arterial disease | 14 (1.9) | 3 (1.4) | <3 |
| Diabetes mellitus | 91 (12.2) | 22 (10.4) | 16 (11.0) |
| Hypertension | 189 (25.4) | 53 (25.0) | 39 (26.9) |
| Chronic kidney disease | 17 (2.3) | 3 (1.4) | 3 (2.1) |
| Alcohol abuse | 11 (1.5) | 12 (5.7) | 4 (2.8) |
| Chronic liver disease | 4 (0.5) | <3 | 5 (3.4) |
| Chronic obstructive pulmonary disease | 102 (13.7) | 7 (3.3) | <3 |
| Pharmacotherapy at ICI administration, n (%) | |||
| Calcium channel blockers | 114 (15.3) | 33 (15.6) | 24 (16.6) |
| Beta blockers | 150 (20.2) | 38 (17.9) | 13 (9.0) |
| Non loop diuretics | 145 (19.5) | 39 (18.4) | 38 (26.2) |
| Loop diuretics | 71 (9.6) | 12 (5.7) | 7 (4.8) |
| RAS-inhibitors | 189 (25.4) | 57 (26.9) | 41 (28.3) |
| Vitamin K antagonists | 33 (4.4) | 4 (1.9) | 3 (2.1) |
| Non vitamin K antagonists | 40 (5.4) | 3 (1.4) | 4 (2.8) |
| ADP-inhibitors | 42 (5.7) | 13 (6.1) | 8 (5.5) |
| Aspirin | 147 (19.8) | 25 (11.8) | 15 (10.3) |
| Statins | 194 (26.1) | 45 (21.2) | 23 (15.9) |
ADP, adenosine diphosphate; CTLA-4i, cytotoxic T-lymphocyte-associated protein-4 inhibitor, PD1i, programmed cell death 1 inhibitor; RAS, renin-angiotensin system.
Baseline characteristics of patients at the time of initial administration of immune checkpoint inhibitor
| . | Lung cancer and PD1i . | Malignant melanoma and CTLA-4i . | Malignant melanoma and PD1i . |
|---|---|---|---|
| N | 743 | 212 | 145 |
| Age, median (p25–p75) | 67 (60–72) | 64 (54–70) | 62 (51–74) |
| Men, n (%) | 391 (52.6) | 123 (58.0) | 86 (59.3) |
| Comorbidity at ICI administration, n (%) | |||
| Myocardial infarction | 40 (5.4) | 10 (4.7) | <3 |
| Heart failure | 29 (3.9) | 5 (2.4) | <3 |
| Myocarditis | 14 (1.9) | <3 | <3 |
| Arrhythmia | 104 (14.0) | 20 (9.4) | 13 (9.0) |
| Peripheral arterial disease | 14 (1.9) | 3 (1.4) | <3 |
| Diabetes mellitus | 91 (12.2) | 22 (10.4) | 16 (11.0) |
| Hypertension | 189 (25.4) | 53 (25.0) | 39 (26.9) |
| Chronic kidney disease | 17 (2.3) | 3 (1.4) | 3 (2.1) |
| Alcohol abuse | 11 (1.5) | 12 (5.7) | 4 (2.8) |
| Chronic liver disease | 4 (0.5) | <3 | 5 (3.4) |
| Chronic obstructive pulmonary disease | 102 (13.7) | 7 (3.3) | <3 |
| Pharmacotherapy at ICI administration, n (%) | |||
| Calcium channel blockers | 114 (15.3) | 33 (15.6) | 24 (16.6) |
| Beta blockers | 150 (20.2) | 38 (17.9) | 13 (9.0) |
| Non loop diuretics | 145 (19.5) | 39 (18.4) | 38 (26.2) |
| Loop diuretics | 71 (9.6) | 12 (5.7) | 7 (4.8) |
| RAS-inhibitors | 189 (25.4) | 57 (26.9) | 41 (28.3) |
| Vitamin K antagonists | 33 (4.4) | 4 (1.9) | 3 (2.1) |
| Non vitamin K antagonists | 40 (5.4) | 3 (1.4) | 4 (2.8) |
| ADP-inhibitors | 42 (5.7) | 13 (6.1) | 8 (5.5) |
| Aspirin | 147 (19.8) | 25 (11.8) | 15 (10.3) |
| Statins | 194 (26.1) | 45 (21.2) | 23 (15.9) |
| . | Lung cancer and PD1i . | Malignant melanoma and CTLA-4i . | Malignant melanoma and PD1i . |
|---|---|---|---|
| N | 743 | 212 | 145 |
| Age, median (p25–p75) | 67 (60–72) | 64 (54–70) | 62 (51–74) |
| Men, n (%) | 391 (52.6) | 123 (58.0) | 86 (59.3) |
| Comorbidity at ICI administration, n (%) | |||
| Myocardial infarction | 40 (5.4) | 10 (4.7) | <3 |
| Heart failure | 29 (3.9) | 5 (2.4) | <3 |
| Myocarditis | 14 (1.9) | <3 | <3 |
| Arrhythmia | 104 (14.0) | 20 (9.4) | 13 (9.0) |
| Peripheral arterial disease | 14 (1.9) | 3 (1.4) | <3 |
| Diabetes mellitus | 91 (12.2) | 22 (10.4) | 16 (11.0) |
| Hypertension | 189 (25.4) | 53 (25.0) | 39 (26.9) |
| Chronic kidney disease | 17 (2.3) | 3 (1.4) | 3 (2.1) |
| Alcohol abuse | 11 (1.5) | 12 (5.7) | 4 (2.8) |
| Chronic liver disease | 4 (0.5) | <3 | 5 (3.4) |
| Chronic obstructive pulmonary disease | 102 (13.7) | 7 (3.3) | <3 |
| Pharmacotherapy at ICI administration, n (%) | |||
| Calcium channel blockers | 114 (15.3) | 33 (15.6) | 24 (16.6) |
| Beta blockers | 150 (20.2) | 38 (17.9) | 13 (9.0) |
| Non loop diuretics | 145 (19.5) | 39 (18.4) | 38 (26.2) |
| Loop diuretics | 71 (9.6) | 12 (5.7) | 7 (4.8) |
| RAS-inhibitors | 189 (25.4) | 57 (26.9) | 41 (28.3) |
| Vitamin K antagonists | 33 (4.4) | 4 (1.9) | 3 (2.1) |
| Non vitamin K antagonists | 40 (5.4) | 3 (1.4) | 4 (2.8) |
| ADP-inhibitors | 42 (5.7) | 13 (6.1) | 8 (5.5) |
| Aspirin | 147 (19.8) | 25 (11.8) | 15 (10.3) |
| Statins | 194 (26.1) | 45 (21.2) | 23 (15.9) |
ADP, adenosine diphosphate; CTLA-4i, cytotoxic T-lymphocyte-associated protein-4 inhibitor, PD1i, programmed cell death 1 inhibitor; RAS, renin-angiotensin system.
Absolute risks
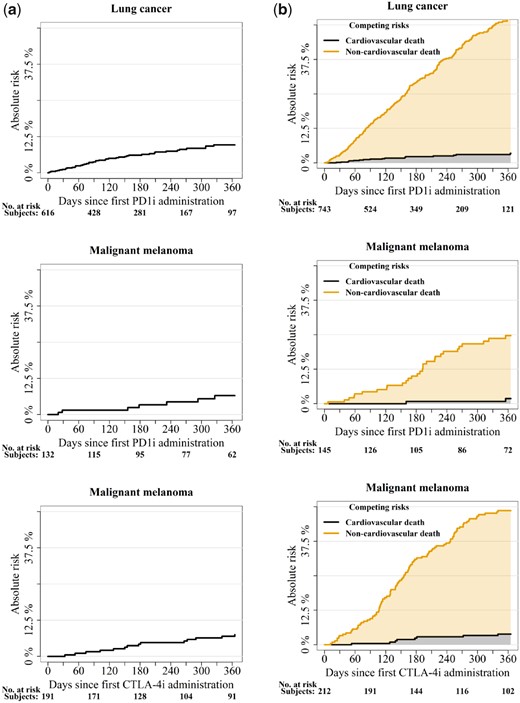
The absolute risks of developing cardiac outcomes at 6 months and 1 year after 1st ICI administration according to cancer and ICI drug type are shown in Table 3 and Figure 2A.
(A) One-year absolute risk of a cardiac event. (B) One-year absolute risk of cardiovascular and non-cardiovascular death.
Absolute risks of cardiac outcomes at 6 months and 1 year after initial administration of immune checkpoint inhibitor
| . | Events at 6 months . | Absolute risk at 6 months . | Events at 1 year . | Absolute risk at 1 year . |
|---|---|---|---|---|
| Lung cancer and PD1i | ||||
| Cardiac event | 32 | 6.1 (4.0–8.2) | 43 | 9.7 (6.8–12.5) |
| Cardiac arrhythmia | 17 | 3.1 (1.6–4.5) | 26 | 5.8 (3.5–8.1) |
| Myocarditis or pericarditis | 11 | 1.8 (0.7–2.8) | 11 | 1.8 (0.7–2.8) |
| Heart failure | 6 | 1.0 (0.2–1.7) | 11 | 2.5 (1.0–4.0) |
| Cardiovascular death | 14 | 2.3 (1.1–3.5) | 18 | 3.4 (1.8–5.1) |
| Non-cardiovascular death | 257 | 27.2 (23.6–30.8) | 400 | 50.3 (45.4–55.3) |
| Malignant melanoma and PD1i | ||||
| Cardiac event | 4 | 3.4 (0.1–6.7) | 7 | 6.6 (1.8–11.3) |
| Cardiac arrhythmia | 4 | 3.4 (0.1–6.7) | 7 | 6.6 (1.8–11.3) |
| Myocarditis or pericarditis | <3 | NA | <3 | NA |
| Heart failure | <3 | NA | <3 | NA |
| Cardiovascular death | <3 | NA | <3 | NA |
| Non-cardiovascular death | 16 | 10.0 (4.8–15.2) | 36 | 22.8 (15.2–30.4) |
| Malignant melanoma and CTLA-4i | ||||
| Cardiac event | 9 | 4.8 (1.7–7.8) | 14 | 7.5 (3.7–11.3) |
| Cardiac arrhythmia | 4 | 2.1 (0.1–4.1) | 6 | 3.2 (0.7–5.7) |
| Myocarditis or pericarditis | <3 | NA | <3 | NA |
| Heart failure | <3 | NA | <3 | NA |
| Cardiovascular death | 6 | 2.9 (0.6–5.1) | 8 | 3.9 (1.2–6.5) |
| Non-cardiovascular death | 60 | 27.2 (23.6–30.8) | 97 | 50.3 (45.4–55.3) |
| . | Events at 6 months . | Absolute risk at 6 months . | Events at 1 year . | Absolute risk at 1 year . |
|---|---|---|---|---|
| Lung cancer and PD1i | ||||
| Cardiac event | 32 | 6.1 (4.0–8.2) | 43 | 9.7 (6.8–12.5) |
| Cardiac arrhythmia | 17 | 3.1 (1.6–4.5) | 26 | 5.8 (3.5–8.1) |
| Myocarditis or pericarditis | 11 | 1.8 (0.7–2.8) | 11 | 1.8 (0.7–2.8) |
| Heart failure | 6 | 1.0 (0.2–1.7) | 11 | 2.5 (1.0–4.0) |
| Cardiovascular death | 14 | 2.3 (1.1–3.5) | 18 | 3.4 (1.8–5.1) |
| Non-cardiovascular death | 257 | 27.2 (23.6–30.8) | 400 | 50.3 (45.4–55.3) |
| Malignant melanoma and PD1i | ||||
| Cardiac event | 4 | 3.4 (0.1–6.7) | 7 | 6.6 (1.8–11.3) |
| Cardiac arrhythmia | 4 | 3.4 (0.1–6.7) | 7 | 6.6 (1.8–11.3) |
| Myocarditis or pericarditis | <3 | NA | <3 | NA |
| Heart failure | <3 | NA | <3 | NA |
| Cardiovascular death | <3 | NA | <3 | NA |
| Non-cardiovascular death | 16 | 10.0 (4.8–15.2) | 36 | 22.8 (15.2–30.4) |
| Malignant melanoma and CTLA-4i | ||||
| Cardiac event | 9 | 4.8 (1.7–7.8) | 14 | 7.5 (3.7–11.3) |
| Cardiac arrhythmia | 4 | 2.1 (0.1–4.1) | 6 | 3.2 (0.7–5.7) |
| Myocarditis or pericarditis | <3 | NA | <3 | NA |
| Heart failure | <3 | NA | <3 | NA |
| Cardiovascular death | 6 | 2.9 (0.6–5.1) | 8 | 3.9 (1.2–6.5) |
| Non-cardiovascular death | 60 | 27.2 (23.6–30.8) | 97 | 50.3 (45.4–55.3) |
CTLA-4i, cytotoxic T-lymphocyte-associated protein-4 inhibitor; PD1i, programmed cell death 1 inhibitor.
Absolute risks of cardiac outcomes at 6 months and 1 year after initial administration of immune checkpoint inhibitor
| . | Events at 6 months . | Absolute risk at 6 months . | Events at 1 year . | Absolute risk at 1 year . |
|---|---|---|---|---|
| Lung cancer and PD1i | ||||
| Cardiac event | 32 | 6.1 (4.0–8.2) | 43 | 9.7 (6.8–12.5) |
| Cardiac arrhythmia | 17 | 3.1 (1.6–4.5) | 26 | 5.8 (3.5–8.1) |
| Myocarditis or pericarditis | 11 | 1.8 (0.7–2.8) | 11 | 1.8 (0.7–2.8) |
| Heart failure | 6 | 1.0 (0.2–1.7) | 11 | 2.5 (1.0–4.0) |
| Cardiovascular death | 14 | 2.3 (1.1–3.5) | 18 | 3.4 (1.8–5.1) |
| Non-cardiovascular death | 257 | 27.2 (23.6–30.8) | 400 | 50.3 (45.4–55.3) |
| Malignant melanoma and PD1i | ||||
| Cardiac event | 4 | 3.4 (0.1–6.7) | 7 | 6.6 (1.8–11.3) |
| Cardiac arrhythmia | 4 | 3.4 (0.1–6.7) | 7 | 6.6 (1.8–11.3) |
| Myocarditis or pericarditis | <3 | NA | <3 | NA |
| Heart failure | <3 | NA | <3 | NA |
| Cardiovascular death | <3 | NA | <3 | NA |
| Non-cardiovascular death | 16 | 10.0 (4.8–15.2) | 36 | 22.8 (15.2–30.4) |
| Malignant melanoma and CTLA-4i | ||||
| Cardiac event | 9 | 4.8 (1.7–7.8) | 14 | 7.5 (3.7–11.3) |
| Cardiac arrhythmia | 4 | 2.1 (0.1–4.1) | 6 | 3.2 (0.7–5.7) |
| Myocarditis or pericarditis | <3 | NA | <3 | NA |
| Heart failure | <3 | NA | <3 | NA |
| Cardiovascular death | 6 | 2.9 (0.6–5.1) | 8 | 3.9 (1.2–6.5) |
| Non-cardiovascular death | 60 | 27.2 (23.6–30.8) | 97 | 50.3 (45.4–55.3) |
| . | Events at 6 months . | Absolute risk at 6 months . | Events at 1 year . | Absolute risk at 1 year . |
|---|---|---|---|---|
| Lung cancer and PD1i | ||||
| Cardiac event | 32 | 6.1 (4.0–8.2) | 43 | 9.7 (6.8–12.5) |
| Cardiac arrhythmia | 17 | 3.1 (1.6–4.5) | 26 | 5.8 (3.5–8.1) |
| Myocarditis or pericarditis | 11 | 1.8 (0.7–2.8) | 11 | 1.8 (0.7–2.8) |
| Heart failure | 6 | 1.0 (0.2–1.7) | 11 | 2.5 (1.0–4.0) |
| Cardiovascular death | 14 | 2.3 (1.1–3.5) | 18 | 3.4 (1.8–5.1) |
| Non-cardiovascular death | 257 | 27.2 (23.6–30.8) | 400 | 50.3 (45.4–55.3) |
| Malignant melanoma and PD1i | ||||
| Cardiac event | 4 | 3.4 (0.1–6.7) | 7 | 6.6 (1.8–11.3) |
| Cardiac arrhythmia | 4 | 3.4 (0.1–6.7) | 7 | 6.6 (1.8–11.3) |
| Myocarditis or pericarditis | <3 | NA | <3 | NA |
| Heart failure | <3 | NA | <3 | NA |
| Cardiovascular death | <3 | NA | <3 | NA |
| Non-cardiovascular death | 16 | 10.0 (4.8–15.2) | 36 | 22.8 (15.2–30.4) |
| Malignant melanoma and CTLA-4i | ||||
| Cardiac event | 9 | 4.8 (1.7–7.8) | 14 | 7.5 (3.7–11.3) |
| Cardiac arrhythmia | 4 | 2.1 (0.1–4.1) | 6 | 3.2 (0.7–5.7) |
| Myocarditis or pericarditis | <3 | NA | <3 | NA |
| Heart failure | <3 | NA | <3 | NA |
| Cardiovascular death | 6 | 2.9 (0.6–5.1) | 8 | 3.9 (1.2–6.5) |
| Non-cardiovascular death | 60 | 27.2 (23.6–30.8) | 97 | 50.3 (45.4–55.3) |
CTLA-4i, cytotoxic T-lymphocyte-associated protein-4 inhibitor; PD1i, programmed cell death 1 inhibitor.
To ensure anonymity to persons registered in the databases used for this study, aggregated results should be based on minimum three observations. Due to this general rule, it was not possible to report the absolute 1-year risks of peri- or myocarditis and heart failure in patients with malignant melanoma or the risk of cardiovascular death in patients with malignant melanoma and PD1i.
The median follow-up time from 1st administration of the drug was 164 days (p25–p75: 72–276, minimum 1, maximum 765) for the lung cancer patients treated with PD1i and 326 days (p25–p75: 168–589, minimum 7, maximum 2413) and 315 days (p25–p75: 150–678, minimum 12, maximum 1997) for the patients with malignant melanoma treated with PD1i and CTLA-4i, respectively. Table 4 shows the median, minimum, and maximum time to event from 1st administration of the drug for the patients experiencing events.
| . | Events during full follow-up . | Median (IQR) . | Min. . | Max. . |
|---|---|---|---|---|
| Lung cancer and PD1i | ||||
| Cardiac event | 44 | 93 (50–191) | 2 | 455 |
| Arrhythmia | 27 | 133 (35–208) | 2 | 455 |
| Heart failure | 12 | 194 (62–290) | 23 | 376 |
| Peri- or myocarditis | 11 | 75 (53–93) | 34 | 149 |
| Cardiovascular death | 18 | 101 (48–159) | 23 | 364 |
| Malignant melanoma and PD1i | ||||
| Cardiac event | 9 | 232 (156–326) | 20 | 469 |
| Arrhythmia | 7 | 178 (28–293) | 20 | 326 |
| Heart failure | < 3 | NA | NA | NA |
| Peri- or myocarditis | < 3 | NA | NA | NA |
| Cardiovascular death | 4 | 392 (258–449) | 160 | 469 |
| Malignant melanoma and CTLA-4i | ||||
| Cardiac event | 19 | 266 (128–398) | 33 | 1037 |
| Arrhythmia | 8 | 211 (126–368) | 74 | 416 |
| Heart failure | < 3 | NA | NA | NA |
| Peri- or myocarditis | < 3 | NA | NA | NA |
| Cardiovascular death | 12 | 226 (141–384) | 53 | 1037 |
| . | Events during full follow-up . | Median (IQR) . | Min. . | Max. . |
|---|---|---|---|---|
| Lung cancer and PD1i | ||||
| Cardiac event | 44 | 93 (50–191) | 2 | 455 |
| Arrhythmia | 27 | 133 (35–208) | 2 | 455 |
| Heart failure | 12 | 194 (62–290) | 23 | 376 |
| Peri- or myocarditis | 11 | 75 (53–93) | 34 | 149 |
| Cardiovascular death | 18 | 101 (48–159) | 23 | 364 |
| Malignant melanoma and PD1i | ||||
| Cardiac event | 9 | 232 (156–326) | 20 | 469 |
| Arrhythmia | 7 | 178 (28–293) | 20 | 326 |
| Heart failure | < 3 | NA | NA | NA |
| Peri- or myocarditis | < 3 | NA | NA | NA |
| Cardiovascular death | 4 | 392 (258–449) | 160 | 469 |
| Malignant melanoma and CTLA-4i | ||||
| Cardiac event | 19 | 266 (128–398) | 33 | 1037 |
| Arrhythmia | 8 | 211 (126–368) | 74 | 416 |
| Heart failure | < 3 | NA | NA | NA |
| Peri- or myocarditis | < 3 | NA | NA | NA |
| Cardiovascular death | 12 | 226 (141–384) | 53 | 1037 |
| . | Events during full follow-up . | Median (IQR) . | Min. . | Max. . |
|---|---|---|---|---|
| Lung cancer and PD1i | ||||
| Cardiac event | 44 | 93 (50–191) | 2 | 455 |
| Arrhythmia | 27 | 133 (35–208) | 2 | 455 |
| Heart failure | 12 | 194 (62–290) | 23 | 376 |
| Peri- or myocarditis | 11 | 75 (53–93) | 34 | 149 |
| Cardiovascular death | 18 | 101 (48–159) | 23 | 364 |
| Malignant melanoma and PD1i | ||||
| Cardiac event | 9 | 232 (156–326) | 20 | 469 |
| Arrhythmia | 7 | 178 (28–293) | 20 | 326 |
| Heart failure | < 3 | NA | NA | NA |
| Peri- or myocarditis | < 3 | NA | NA | NA |
| Cardiovascular death | 4 | 392 (258–449) | 160 | 469 |
| Malignant melanoma and CTLA-4i | ||||
| Cardiac event | 19 | 266 (128–398) | 33 | 1037 |
| Arrhythmia | 8 | 211 (126–368) | 74 | 416 |
| Heart failure | < 3 | NA | NA | NA |
| Peri- or myocarditis | < 3 | NA | NA | NA |
| Cardiovascular death | 12 | 226 (141–384) | 53 | 1037 |
| . | Events during full follow-up . | Median (IQR) . | Min. . | Max. . |
|---|---|---|---|---|
| Lung cancer and PD1i | ||||
| Cardiac event | 44 | 93 (50–191) | 2 | 455 |
| Arrhythmia | 27 | 133 (35–208) | 2 | 455 |
| Heart failure | 12 | 194 (62–290) | 23 | 376 |
| Peri- or myocarditis | 11 | 75 (53–93) | 34 | 149 |
| Cardiovascular death | 18 | 101 (48–159) | 23 | 364 |
| Malignant melanoma and PD1i | ||||
| Cardiac event | 9 | 232 (156–326) | 20 | 469 |
| Arrhythmia | 7 | 178 (28–293) | 20 | 326 |
| Heart failure | < 3 | NA | NA | NA |
| Peri- or myocarditis | < 3 | NA | NA | NA |
| Cardiovascular death | 4 | 392 (258–449) | 160 | 469 |
| Malignant melanoma and CTLA-4i | ||||
| Cardiac event | 19 | 266 (128–398) | 33 | 1037 |
| Arrhythmia | 8 | 211 (126–368) | 74 | 416 |
| Heart failure | < 3 | NA | NA | NA |
| Peri- or myocarditis | < 3 | NA | NA | NA |
| Cardiovascular death | 12 | 226 (141–384) | 53 | 1037 |
The 1-year non-cardiovascular mortality was substantial in patients with lung cancer and PD1i [50.3% (95% CI 45.4–55.3)] and in patients with malignant melanoma treated with PD1i [22.8% (15.2–30.4)] and CTLA-4i [44.8% (38.0–51.5)] (Figure 2B).
Relative rates
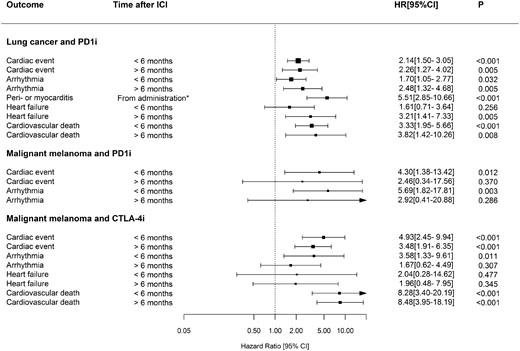
First time administration of ICI was associated with the rate of cardiac events. The relative rates of cardiac events, arrhythmia, peri- or myocarditis, heart failure, and cardiovascular death comparing patients with and without ICI treatment are displayed in Figure 3.
Hazard ratios of cardiac outcomes from multivariable time-updated Cox regression analyses.
Patients with lung cancer receiving PD1i-treatment had increased rates of cardiac events compared with patients not receiving PD1i at <6 months (hazard ratio (HR) 2.14, 95% CI 1.50–3.05) and >6 months (HR 2.26, 95% CI 1.27–4.02) after 1st PD1i-administration (Figure 3). Among patients with malignant melanoma, patients receiving PD1i had increased cardiac event rates in the 1st 6 months following PD1i 1st administration (HR 4.30, 95% CI 1.38–13.42), but not after 6 months (HR 2.46, 95% CI 0.34–17.56) compared with patients not receiving PD1i. Moreover, patients receiving CTLA-4i had increased cardiac event rates in the 1st 6 months (HR 4.93, 95% CI 2.45–9.94) and after 6 months (HR 3.48, 95% CI 1.91–6.35) compared with patients not receiving CTLA-4i.
Discussion
The study provided large-scale, real-world observational data on the risk of cardiac events associated with ICI treatment (Take home figure). Based on a complete nationwide cohort of patients with lung cancer or malignant melanoma, two main findings were established. First, the 1-year absolute risks of cardiac events were quantified in patients with lung cancer treated with PD1i and patients with malignant melanoma treated with PD1i or CTLA-4i, respectively. The risks were higher than risk estimates from previous pharmacovigilance studies. Second, patients receiving ICI had increased relative rates of cardiac events compared with patients not receiving ICI in hazard ratio analyses. Among patients with lung cancer and PD1i, the relative rates of the combined outcome of cardiac events, arrhythmia, peri- or myocarditis, heart failure, and cardiovascular death were increased and, in patients with malignant melanoma and PD1i or CTLA-4i, the rates of the combined outcome of cardiac events and arrhythmia were increased.
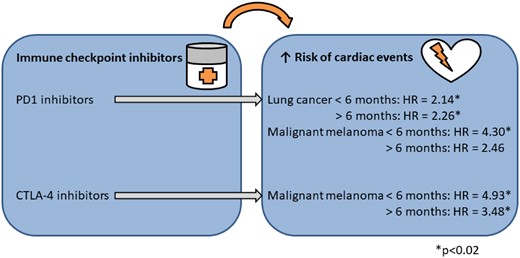
Immune checkpoint inhibitor treatment and the associated risks of cardiac events.
It has been suspected that most cardiac side effects occur early after treatment within the 1st week to months.11 , 13 , 14 , 19–21 , 31 , 32 Our results suggest that increased rates of cardiac events are also seen after the initial 6 months of the 1st administration. This prolonged increased risk may reflect that the data in this study were all hospitalizations not limited to adverse event registration in pharmacovigilance data. Pharmacovigilance data may be characterized by having a decreasing adverse event registration rate with time from drug initiation, whereas this effect should be absent in the administration data used in this study.
In the current study, we found increased relative rates of arrhythmia in both patients with lung cancer and PD1i and patients with malignant melanoma and PD1i and CTLA-4i. Our results consolidate findings from previous pharmacovigilance studies. One study investigated reported adverse events in ICI-treated patients compared with patients treated with any other type of drug and found association of supraventricular arrhythmias but not ventricular arrhythmias with ICI treatment.16 In addition, a post hoc analysis of adverse event reports from cross-trial data from 59 trials showed higher rates of arrhythmia.17 Moreover, we found that the arrhythmia risk was time dependent. Increased relative rates were seen in the 1st 6 months in all patients receiving ICI, but the relative rate was only increased after the initial 6 months in patients with lung cancer. The estimates in the patients with malignant melanoma were based on small cohorts (n = 212 and 145) and significantly increased rates could potentially be found if investigated in a larger patient material.
Moreover, we observed relatively increased rates of peri- or myocarditis in patients with lung cancer treated with PD1i. The association of ICI and myocarditis has been commented in previous case reports14 , 18 , 20 , 21 and investigated in a recent case–control study.13 In addition, the association is supported by results from pharmacovigilance studies finding increased risk of peri- or myocarditis after ICI treatment.16 , 17 Pharmacovigilance studies have suggested that ∼0.03–1% of treated patients reported myocarditis.12 , 14 , 16 , 17 , 31 , 33 , 34 In the current study, the 1 year risk of peri- or myocarditis was 1.8%, thus suggesting that the risk may be higher than previously estimated.
The relative rate of heart failure was only found to be increased in patients with lung cancer and PD1i after 6 months. Similar rates were found for patients with malignant melanoma and PD1i or CTLA-4i and for patients with lung cancer within the 1st 6 months. The similar relative rates were in line with previous studies that have analysed PD1i and CTLA-4i together.16 , 17 In patients with lung cancer and PD1i, the 1-year risk of heart failure was 2.5%. A previous pharmacovigilance study found a 0.71% proportion of reported adverse events of heart failure in ICI-treated patients in a 10-year observation period.16 Additionally, in a pooled analysis of 59 trials the incidence was found to be 0.53%.17The estimates are difficult to compare head to head, but the estimates from the current study suggest that heart failure risks may be higher in some patient groups than caught by pharmacovigilance studies.
The relative rates of cardiovascular death were increased in patients with lung cancer treated with PD1i and in patients with malignant melanoma treated with CTLA-4i compared with patients without ICI treatment. This was in contrast to previously published pharmacovigilance study results.16 , 17 In addition, a single-institution cohort study of patients with lung cancer found no association of ICI treatment and major cardiac events including cardiovascular death.35
The mechanisms behind the immune-related side effects are not fully elucidated and several hypotheses of different mechanisms exist including increased T-cell activity against antigens present in myocytes, increasing levels of autoantibodies and inflammatory cytokines, and enhanced complement-mediated inflammation.11 Moreover, studies of mice deficient in CTLA-4 and PD1 receptors have shown increased risks of a variety of cardiac diseases including myocarditis and heart failure, supporting the biological plausibility of myocardial damage associated with ICI treatment.36 , 37 In addition, myocardial damage has been observed in case studies of ICI-treated patients and is suspected of being the substrate for cardiac disease development in the treated patients.13 , 14
In conclusion, the absolute 1-year absolute risk of cardiac events was ≈7–10% in patients with lung cancer and malignant melanoma treated with ICI. The risk estimates were higher in these real-world data compared with previous pharmacovigilance studies. Moreover, ICI-treated patients had increased rates of cardiac events compared with patients not receiving ICI. The findings urge increased awareness of cardiac events in patients receiving ICI.
Strengths and limitations
The major strength of this study was the inclusion of a large cohort of consecutive patients treated with ICI from 2011 to 2017. The unique data on all patients and all hospital contacts regarding cardiac disease constituted the basis for novel insight into the risk of cardiotoxicity associated with ICI treatment in nationwide real-world patients with lung cancer and malignant melanoma.
This observational study was based on register data and importantly, treatment with ICI was not randomized. Our findings suggest a strong association between treatment with ICI and cardiac events, which was adjusted for important confounders such as age, sex, and time with cancer. However, we did not have information on smoking status, cancer stage, and other important clinical risk factors, which could confound or weaken the associations. During the study period, new indications for ICI treatments were continuously introduced. We adjusted for the year of cancer diagnosis in the time-updated Cox model and the hazard ratio estimates from this model compared patients with the same year of diagnosis, e.g. it compared the hazard rate in a patient diagnosed in 2012 with lung cancer and treated with ICI to a patient diagnosed in 2012 with lung cancer and not receiving ICI treatment.
The outcome definitions based on ICD-10 codes have been validated with high PPVs for heart failure (100, 95% CI 92.9–100), pericarditis (92, 85–96) and myocarditis (64, 52–74), and arrhythmias (similar definition to ours) (95, 89–98).25 , 29 Notably, the combined outcome of cardiac events and cardiovascular death has not been validated. However, as the outcome was based on the validated component outcomes, we expect the PPV to be similar to the individual PPVs.
This study was not optimal for analysing the risk of vascular events as drug-induced acceleration of atherosclerosis may take longer time than the median follow-up (164–326 days).
Finally, in the current study, 1st administration of ICI and the subsequent 1-year risk of cardiac side effects were investigated. The associations of cardiotoxicity with different intensities of treatment and different treatment combinations constitute important future research studies.38 As dual checkpoint blockade was introduced in 2017 and atezolizumab was introduced in 2016, the patient numbers were too low to base estimates on. However, as these treatments are increasingly frequently used, future studies based on data from 2017 and later will be relevant.
Supplementary material
Supplementary material is available at European Heart Journal online.
Data availability
The data used for the analyses in this study are available from Statistics Denmark but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available.
Funding
The Danish Heart Foundation (15-R99-A5858-22910) and The VELUX Foundation (000012057).
Conflict of interest: Dr M.D. reports grants from The Danish Heart Foundation and grants from The VELUX Foundation, during the conduct of the study. Dr F.G. reports personal fees from Abbott, personal fees from Orion Pharma, personal fees from Boehringer-Ingelheim, personal fees from Pfizer, personal fees from AstraZeneca, personal fees from Carmat, and personal fees from Novartis, outside the submitted work. Dr C.T.-P. reports grants from Bayer and grants from Novo Nordisk, outside the submitted work. Dr L.K. reports personal fees from Speakers honorarium for Novartis, AstraZeneca, and Boehringer, outside the submitted work. Dr D.N., Dr I.M.S., Dr K.I., Dr P.V.R., Dr C.M., Dr E.F., Dr L.K., Dr C.A., Dr G.G. and Dr M.S. declared no conflicts of interest.
References
R Core Team.



