-
PDF
- Split View
-
Views
-
Cite
Cite
Alida L P Caforio, Myocarditis: endomyocardial biopsy and circulating anti-heart autoantibodies are key to diagnosis and personalized etiology-directed treatment, European Heart Journal, Volume 42, Issue 16, 21 April 2021, Pages 1618–1620, https://doi.org/10.1093/eurheartj/ehab024
Close - Share Icon Share
The editorial refers to ‘Myocarditis-associated necrotizing coronary vasculitis: incidence, cause and outcome’†, by A. Frustaci et al., on page 1609.
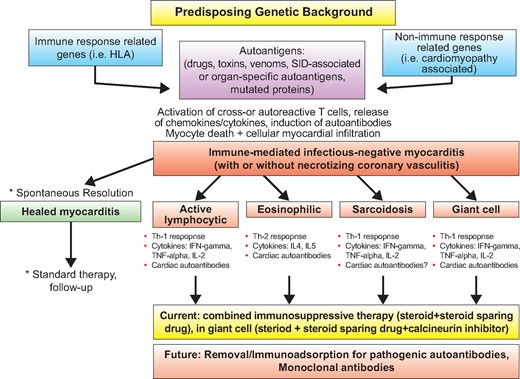
Biopsy and cardiac autoantibody driven tailored therapy in infectious negative immune-mediated myocarditis.
William Osler stated, back in 1892, in his Internal Medicine book: ‘‘There are three phases to treatment: diagnosis, diagnosis and diagnosis’’.1 This sentence is clearly applicable today, since optimal, personalized management in a clinical syndrome is based upon etiopathogenetic diagnosis. Myocarditis is still considered a rare and poorly understood condition, a diagnostic challenge for the cardiologist, and an orphan disease without a specific cure. However, myocarditis is not rare, is a syndrome with multiple presentations, often mimicking other non-inflammatory cardiac diseases. Prognosis is variable, ranging from spontaneous resolution to progressive heart failure, dilated cardiomyopathy, death or heart transplantation2. Key advances in diagnosis were the development of the endomyocardial biopsy technique using the King’s bioptome by Richardson, and the consensus histopathological classification and definition of myocarditis by endomyocardial biopsy, known as the Dallas criteria3. Neu et al. published the first seminal evidence suggesting the involvement of autoimmunity to cardiac self-antigens in a mouse model of myosin-induced autoimmune myocarditis4. Then, several groups in the late 80 s and early 90 s reported the presence of circulating anti-heart autoantibodies (AHA) against myosin as well as other self-antigens, in keeping with the hypothesis of autoimmunity having a major role in patients with acute and chronic myocarditis or dilated cardiomyopathy and their asymptomatic at-risk family members2. Cooper et al.5 described the efficacy of combined immunosuppressive therapy in a rare but previously lethal form of non-infectious autoimmune myocarditis, e.g., giant cell myocarditis, that currently, if a biopsy-proven diagnosis is achieved early, is curable by immunosuppression2. However, the multicenter Myocarditis Treatment Trial (MTT), designed to prove the efficacy of a 6-month immunosuppressive therapy in lymphocytic myocarditis of unspecified etiopathogenesis (e.g., viral vs autoimmune) showed no significant effect on survival, although the study was not powered to detect differences in survival6. The MTT results strongly discouraged cardiologists in the next decades on the use of endomyocardial biopsy to detect and treat myocarditis. However, researchers developed new diagnostic tools to be added to standard histology, in particular immunohistochemistry, to increase sensitivity of endomyocardial biopsy and characterize the number and type of infiltrating inflammatory cells, and molecular detection of genomic material of infectious agents mainly by polymerase chain reaction (PCR), to diagnose infectious, particularly viral myocarditis2; the use of such tools on endomyocardial biopsy was endorsed in the 1995 WHO classification3. Frustaci et al had a leading role in the resurrection of endomyocardial biopsy and of immunosuppressive therapy in myocarditis, pointing out, with the prospective TIMIC trial7 that only biopsy-proven virus-negative immune-mediated myocarditis may benefit from immunosuppression. In the current issue, Frustaci et al.8 add another very valuable piece of information to the myocarditis field.
Necrotizing coronary vasculitis (NCV) is a rare entity that may be associated to myocarditis, but incidence, cause and response to therapy is unreported. Among their cohort of 1916 patients with biopsy-proven myocarditis, 30 had NCV. Endomyocardial samples were retrospectively investigated with immunohistochemistry for Toll like Receptor 4 (TLR4) and real-time PCR for viral genomes. Serum samples were processed for AHA, IL-1β, IL-6, IL-8, TNF-α. Identification of an immunologic pathway (including virus-negativity, TLR4- and AHA-positivity) was followed by immunosuppression. The myocarditis-NCV cohort was followed for 6-months with 2 D-echo and/or cardiac magnetic resonance (CMR) and compared to 60 myocarditis patients and 30 controls. Increase in LVEF ≥10% was classified as response to therapy. Control endomyocardial biopsy followed the end of treatment.
26 Myocarditis-NCV patients presented with heart failure; 4 with electrical instability. Cause of Myocarditis-NCV included infectious agents in a minority (10%) and immune-mediated causes in the remainder (rarely chest trauma; drug-hypersensitivity; hyper eosinophilic syndrome; primary autoimmune diseases in 33%, idiopathic 44%). AHA were positive in immune-mediated myocarditis-NCV and in virus-negative myocarditis patients; myocarditis-NCV patients with AHA positive status presented autoreactivity in vessel walls. TLR4 was overexpressed in immune-mediated and poorly detectable in viral myocarditis. Interleukin-1β was significantly higher in myocarditis-NCV than in myocarditis without NCV, the former presenting 24% in-hospital mortality compared with 1.5% of the latter. Immunosuppression induced improvement of cardiac function in 88% of myocarditis-NCV and 86% of virus-negative myocarditis patients without NCV. The study conclusion is that NCV is histologically detectable in 1.5% of myocarditis cases. NCV includes viral and immune-mediated causes, intra-hospital mortality is higher compared to the non NCV-cohort. The immunologic pathway with or without NCV is associated with beneficial response to immunosuppressive therapy. Thus, the new study from Frustaci et al8 supports previous studies suggesting that using serum AHA testing as well as histology, immunohistology and viral PCR on endomyocardial biopsy, it is nowadays possible to define distinct etiopathogenetic subsets of myocarditis, in particular infectious vs. immune-mediated, e.g., infection-negative forms2. This characterization is key to define who are the infection-negative cases in which immunosuppression and immunomodulation may be beneficial and is in keeping with the 2013 expert consensus paper of the European Society of Cardiology (ESC) Working Group on Myocardial and Pericardial Disease2. Conversely, immunosuppressive therapy and immunomodulation are contraindicated and may be detrimental in patients with active myocardial infection2. The efficacy of immunosuppression in biopsy-proven virus-negative myocarditis has also been recently reported in patients with arrhythmia presentation9 and in a metaanalysis10.
In the last years, cardiovascular magnetic resonance imaging (CMR) has been put forward as a noninvasive imaging tool in inflammatory heart muscle disease11. In the study by Frustaci et al8 CMR was unable to distinguish myocarditis with or without NCV, as well as viral from autoimmune myocarditis. These observations confirm that CMR does not replace endomyocardial biopsy, it is currently unable to differentiate between infectious and immune-mediated forms, but is valuable to refine the clinical suspicion of myocarditis and for noninvasive follow-up2. In the Frustaci’s study there were both viral and immune-mediated NCV cases, and only the association of negative viral PCR and positive AHA identified immune-mediated myocarditis, even in patients with associated systemic immune-mediated diseases (SIDs)8. Thus, these data are also in keeping with the 2017 expert consensus paper of ESC Working Group on Myocardial and Pericardial Disease on SIDs 12. Myocarditis in SIDs portends a negative prognosis, but if of infectious origin, due to opportunistic infections in patients with a background immunosuppression, it should be treated with a reduction of immunosuppression.12 Conversely, if myocarditis in SIDs is due to an immune-mediated process, it needs an upgrade of immunosuppression12. Thus, even in patients with SIDs, endomyocardial biopsy and AHA testing is key to reach an etiopathogenetic diagnosis and tailored treatment.12
Last but not least, recent evidence from different groups suggests that an immune-mediated pathogenesis may be a final common pathway of heart dysfunction and arrhythmia not only in organ-specific autoimmune myocarditis and in immune-mediated myocarditis associated with SIDs, but also in genetically determined cardiomyopathies with an inflammatory phenotype, particularly arrhythmogenic right ventricular cardiomyopathy13–14, dilated cardiomyopathy15 and in Brugada syndrome.16 These observations open the possibility that, in the context of a genetically susceptible background of both immune response and non-immune response related genes, mutated proteins may also act as autoantigens and trigger the autoimmune cascade, thus current and future immunosuppressive therapies may be used in a wider range of cardiomyopathies (Graphical abstract).
Therefore, Frustaci et al should be appreciated for their long-lasting pioneering work in the myocarditis field. Using endomyocardial biopsy, AHA and new refined tissue and serum biomarkers of immune-mediated pathogenesis, we will rapidly progress on new and effective tailored treatments for myocarditis.
Footnotes
† doi:10.1093/eurheartj/ehaa973.
Acknowledgements
A.L.P.C. acknowledges the support of Budget Integrato per la Ricerca dei Dipartimenti (BIRD, year 2019), Padova University, Padova, Italy (project Title: Myocarditis: genetic background, predictors of dismal prognosis and of response to immunosuppressive therapy.) and of the Italian Ministry of Health, Target Research, Rome, Italy, year 2019 (project Title: Biopsy-Proven Myocarditis: Genetic Background, Predictors of Dismal Prognosis and of Response To Immunosuppressive Therapy And Preclinical Evaluation of Innovative Immunomodulatory Therapies)
Conflict of interest: none declared.
References



