-
PDF
- Split View
-
Views
-
Cite
Cite
Thierry Le Tourneau, Solena Le Scouarnec, Caroline Cueff, Daniel Bernstein, Jan J J Aalberts, Simon Lecointe, Jean Mérot, Jonathan A Bernstein, Toon Oomen, Christian Dina, Matilde Karakachoff, Hubert Desal, Ousama Al Habash, Francesca N Delling, Romain Capoulade, Albert J H Suurmeijer, David Milan, Russell A Norris, Roger Markwald, Elena Aikawa, Susan A Slaugenhaupt, Xavier Jeunemaitre, Albert Hagège, Jean-Christian Roussel, Jean-Noël Trochu, Robert A Levine, Florence Kyndt, Vincent Probst, Hervé Le Marec, Jean-Jacques Schott, New insights into mitral valve dystrophy: a Filamin-A genotype–phenotype and outcome study, European Heart Journal, Volume 39, Issue 15, 14 April 2018, Pages 1269–1277, https://doi.org/10.1093/eurheartj/ehx505
Close - Share Icon Share
Abstract
Filamin-A (FLNA) was identified as the first gene of non-syndromic mitral valve dystrophy (FLNA-MVD). We aimed to assess the phenotype of FLNA-MVD and its impact on prognosis.
We investigated the disease in 246 subjects (72 mutated) from four FLNA-MVD families harbouring three different FLNA mutations. Phenotype was characterized by a comprehensive echocardiography focusing on mitral valve apparatus in comparison with control relatives. In this X-linked disease valves lesions were severe in men and moderate in women. Most men had classical features of mitral valve prolapse (MVP), but without chordal rupture. By contrast to regular MVP, mitral leaflet motion was clearly restricted in diastole and papillary muscles position was closer to mitral annulus. Valvular abnormalities were similar in the four families, in adults and young patients from early childhood suggestive of a developmental disease. In addition, mitral valve lesions worsened over time as encountered in degenerative conditions. Polyvalvular involvement was frequent in males and non-diagnostic forms frequent in females. Overall survival was moderately impaired in men (P = 0.011). Cardiac surgery rate (mainly valvular) was increased (33.3 ± 9.8 vs. 5.0 ± 4.9%, P < 0.0001; hazard ratio 10.5 [95% confidence interval: 2.9–37.9]) owing mainly to a lifetime increased risk in men (76.8 ± 14.1 vs. 9.1 ± 8.7%, P < 0.0001).
FLNA-MVD is a developmental and degenerative disease with complex phenotypic expression which can influence patient management. FLNA-MVD has unique features with both MVP and paradoxical restricted motion in diastole, sub-valvular mitral apparatus impairment and polyvalvular lesions in males. FLNA-MVD conveys a substantial lifetime risk of valve surgery in men.
Introduction
Mitral valve prolapse (MVP) is a common valve disorder which affects 2–3% of the population and is a major cause of valve surgery.1 Non-syndromic MVP, the most common form of the disease, encompasses different phenotypes from Fibro-elastic deficiency to classical Barlow disease.2–5 Familial inheritance of MVP has been largely recognized.1 , 6 , 7 Autosomal dominant transmission is the usual inheritance mode2 , 8 but an X-linked recessive form of mitral valve dystrophy (MVD) has been first encountered.6 , 9 The recent identification of genetic defects associated with either MVP or MVD allows starting to decipher genotype–phenotype relationship.7 , 10 , 11 The X-linked form [Filamin-A (FLNA)-MVD] was associated to a mutation in the FLNA (p.Pro637Gln, FLNA-P637Q) gene (OMIM 314400).7 Three other FLNA defects were subsequently identified.7 , 12 , 13 Filamin-A is a widely expressed hub protein involved in cardiac development, particularly in leaflets during fetal valve morphogenesis. Filamin-A molecular and cellular alterations leading to MVD impair the organization of cell actin network, cell spreading and migration capacities, the interaction with small-GTPase and therefore the response to mechanical stress.14
Due to the absence of gene identification until recently, the overall spectrum of either MVP or MVD including atypical features has been poorly characterized. In particular, and despite first attempts,15 phenotype and outcome characteristics of FLNA-MVD, which presents unique features compared with Barlow disease and Fibro-elastic deficiency, remained to be explored. Hence, this FLNA-MVD study was designed (i) to assess the genotype-phenotype relationship and (ii) to examine the outcome of patients.
Methods
The present study is based on a retrospective series of 246 subjects, from four FLNA-MVD families harbouring three different FLNA missense mutations, in whom we ascertained the FLNA genetic status. Echocardiography was carried out in 162 relatives. The study complies with the Declaration of Helsinki, the French guidelines for genetic research and was approved by local ethic committees. Written informed consent was obtained.
Phenotypic study
Patients and relatives from the four families (Families 1, 2, 3, and 4) were enrolled (see Supplementary material online, Figure S1). The genotype–phenotype FLNA study comprised all relatives of the four families in whom we obtained an echocardiography. Echocardiography was available for 162 subjects including 65 with FLNA mutations (90% of known mutated patients). Subjects not carrying the mutation served as controls (n = 97). A comprehensive clinical examination was carried out.
In Family 1, of French origin, the phenotypic study comprised 151 relatives with or without FLNA-P637Q mutation. Family 2, presenting a de novo FLNA-H743P mutation, comprised three relatives. In Family 3 (G288R mutation), of European origin, which was previously published,12 echocardiograms were available for two male children. Finally, echocardiography was carried out in six patients in Family 4 (G288R mutation) previously published.13 In this family, histological analysis of one mitral leaflet segment obtained during MV repair was available. Mutations (Figure 1) are localized in the N-terminal part of the protein (NP_001104026).7
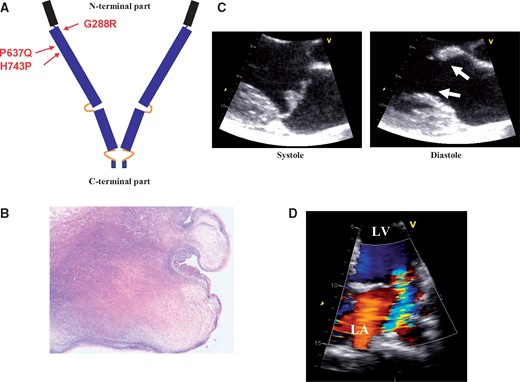
(A) Filamin-A protein with the C-terminal and N-terminal parts, and the three missense mutations localization, (B) microphotograph of a mitral leaflet piece (FLNA-G288R, Family 4, resection during mitral valve repair) showing a nodular thickened valve with myxomatous degeneration (colloidal iron, original magnification × 20), (C) typical aspect in echocardiography of the paradoxical association of mitral valve prolapse (systole) and restrictive motion in diastole (doming, arrows) in a P637Q patient in the parasternal long-axis view, (D) with moderate MR in the apical view.
Echocardiography
Echocardiograms were carried out according to the current recommendations16–18 using a commercially available ultrasound system (Vivid, GE Medical Systems, Milwaukee, WI, USA in most cases). Mitral valve (MV) apparatus was comprehensively assessed (see Supplementary Material online).
Follow-up
The outcome study was conducted from birth to 2016 and comprised the 246 relatives (72 mutated, 174 non-mutated). Total follow-up was 42 ± 22 years, ranging from 5 months (birth in 2016) to 87 years. The end points were overall survival and cardiac surgery rates in the whole population and after stratification according to gender.
Statistical analysis
Results are expressed as mean ± standard deviation, median (25–75th percentiles) or number (percentage). Comparisons between groups were performed with Student’s t-tests, or χ 2 tests, as appropriate. We analysed the association of mutation status with all the trait while accounting for familial correlation using variance decomposition. We used SOLAR software which simultaneously estimated the heritability of the trait, based on the phenotype and the kinship coefficient and the effect of the mutation status. The significance of the mutation effect was evaluated through a likelihood ratio test. Mitral valve measurement associations with age were tested with linear and non-linear regression and the best fit was retained. Overall survival and cardiac surgery rates were calculated by the Kaplan–Meier method and were compared with the Log-rank test. The association of FLNA mutation to end points used the Cox proportional hazards method. A two-tailed P-value ≤ 0.05 was considered significant. Statistical analyses were performed with SPSS software version 13 (SPSS, Chicago, IL, USA) and R version 3.3.2.
Results
Baseline characteristics of the 162 relatives enrolled in the phenotypic study are shown in Table 1. Clinical examination found only benign joint hypermobility syndrome and narrow palate in some individuals. No patient had pectus excavatum, severe scoliosis or severe myopia (see Supplementary Material online). Neurological status was normal in all patients, and brain magnetic resonance imaging carried out in severe FLNA-MVD (n = 8) were normal ruling out periventricular heterotopia (data not shown).
Baseline characteristics of FLNA-MVD patients (FLNA+) and control relatives (FLNA−) from four families in the genotype–phenotype study at the time of echocardiography
| . | FLNA + (n = 65) . | FLNA– (n = 97) . | P-value . |
|---|---|---|---|
| Age, years | 35 ± 22 | 37 ± 20 | 0.55 |
| Age ≥16 years, n (%) | 49 (75) | 82 (85) | 0.21 |
| Male, n (%) | 25 (38) | 57 (59) | 0.02 |
| Heart rate, beat/min | 76 ± 16 | 71 ± 13 | 0.03 |
| Systolic blood pressure, mmHg | 122 ± 17 | 128 ± 17 | 0.03 |
| Hypertension, n (%) | 5 (7.7) | 10 (10.3) | 0.77 |
| Diabetes, n (%) | 3 (4.6) | 1 (1) | 0.36 |
| Dyslipidaemia, n (%) | 8 (12.3) | 7 (7.2) | 0.41 |
| Tobacco, n (%) | 14 (21.5) | 10 (10.3) | 0.08 |
| Filamin-A mutation | |||
| P637Q | 55 | — | — |
| H743P | 2 | — | — |
| G288R | 8 | — | — |
| . | FLNA + (n = 65) . | FLNA– (n = 97) . | P-value . |
|---|---|---|---|
| Age, years | 35 ± 22 | 37 ± 20 | 0.55 |
| Age ≥16 years, n (%) | 49 (75) | 82 (85) | 0.21 |
| Male, n (%) | 25 (38) | 57 (59) | 0.02 |
| Heart rate, beat/min | 76 ± 16 | 71 ± 13 | 0.03 |
| Systolic blood pressure, mmHg | 122 ± 17 | 128 ± 17 | 0.03 |
| Hypertension, n (%) | 5 (7.7) | 10 (10.3) | 0.77 |
| Diabetes, n (%) | 3 (4.6) | 1 (1) | 0.36 |
| Dyslipidaemia, n (%) | 8 (12.3) | 7 (7.2) | 0.41 |
| Tobacco, n (%) | 14 (21.5) | 10 (10.3) | 0.08 |
| Filamin-A mutation | |||
| P637Q | 55 | — | — |
| H743P | 2 | — | — |
| G288R | 8 | — | — |
Baseline characteristics of FLNA-MVD patients (FLNA+) and control relatives (FLNA−) from four families in the genotype–phenotype study at the time of echocardiography
| . | FLNA + (n = 65) . | FLNA– (n = 97) . | P-value . |
|---|---|---|---|
| Age, years | 35 ± 22 | 37 ± 20 | 0.55 |
| Age ≥16 years, n (%) | 49 (75) | 82 (85) | 0.21 |
| Male, n (%) | 25 (38) | 57 (59) | 0.02 |
| Heart rate, beat/min | 76 ± 16 | 71 ± 13 | 0.03 |
| Systolic blood pressure, mmHg | 122 ± 17 | 128 ± 17 | 0.03 |
| Hypertension, n (%) | 5 (7.7) | 10 (10.3) | 0.77 |
| Diabetes, n (%) | 3 (4.6) | 1 (1) | 0.36 |
| Dyslipidaemia, n (%) | 8 (12.3) | 7 (7.2) | 0.41 |
| Tobacco, n (%) | 14 (21.5) | 10 (10.3) | 0.08 |
| Filamin-A mutation | |||
| P637Q | 55 | — | — |
| H743P | 2 | — | — |
| G288R | 8 | — | — |
| . | FLNA + (n = 65) . | FLNA– (n = 97) . | P-value . |
|---|---|---|---|
| Age, years | 35 ± 22 | 37 ± 20 | 0.55 |
| Age ≥16 years, n (%) | 49 (75) | 82 (85) | 0.21 |
| Male, n (%) | 25 (38) | 57 (59) | 0.02 |
| Heart rate, beat/min | 76 ± 16 | 71 ± 13 | 0.03 |
| Systolic blood pressure, mmHg | 122 ± 17 | 128 ± 17 | 0.03 |
| Hypertension, n (%) | 5 (7.7) | 10 (10.3) | 0.77 |
| Diabetes, n (%) | 3 (4.6) | 1 (1) | 0.36 |
| Dyslipidaemia, n (%) | 8 (12.3) | 7 (7.2) | 0.41 |
| Tobacco, n (%) | 14 (21.5) | 10 (10.3) | 0.08 |
| Filamin-A mutation | |||
| P637Q | 55 | — | — |
| H743P | 2 | — | — |
| G288R | 8 | — | — |
Mitral valve in FLNA-MVD patients
The main quantitative characteristics of MV apparatus, restricted to adult patients for ensuring a reliable comparative analysis, are shown in Table 2. Figure 1 shows a typical but moderate aspect of FLNA-MVD with mitral regurgitation (MR). Figure 2 displays a schematic of MV apparatus in controls (A) and FLNA patients (B and C). Men had a severe phenotype characterized by elongation of anterior (AL) and posterior (PL) leaflets. Both leaflets were thickened, billowing or prolapsing, redundant and frequently curled at their tip. By contrast, MV lesions were mild to moderate in women as expected in an X-linked disease. Only one woman (FLNA-H743P) had a severe phenotype owing to an extremely skewed X-chromosome inactivation (see Supplementary Material online). Mitral valve prolapse was found in 26 adult patients (53%), was more frequent in men (70%) compared with women (44%), and involved only the AL (10%), the PL (22%), or both leaflets (20%). In addition, minimal systolic displacement, a prodromal form of MVP, was found in six adults (12%), five women (16%), and one man (6%). The MV apparatus was similar to adult one in younger patients from early childhood. Histological analysis of a mitral leaflet segment demonstrated myxomatous degeneration (Figure 1).
Mitral valve apparatus in FLNA-MVD men and women (≥16 years old) compared with controls
| . | FLNA + men . | FLNA − men . | P-value . | FLNA + women . | FLNA − women . | P-value . |
|---|---|---|---|---|---|---|
| Mitral annulus diameter, mm/m2 | 21 ± 2 | 17 ± 2 | 0.12 | 19 ± 2** | 18 ± 2 | 0.9 |
| Mitral orifice area, cm2/m2 | 1.5 ± 0.4 | 1.8 ± 0.4 | 0.03 | 1.6 ± 0.3 | 1.7 ± 0.5 | 0.37 |
| Leaflet length | ||||||
| Anterior leaflet length, mm/m2 | 13.5 ± 2.1 | 11.5 ± 1.4 | 0.0004 | 12.7 ± 2.0 | 12.0 ± 1.4 | 0.81 |
| Posterior leaflet length, mm/m2 | 8.8 ± 1.5 | 6.2 ± 1.3 | <0.0001 | 6.6 ± 1.1* | 5.9 ± 1.2 | 0.07 |
| Anterior/posterior leaflet length ratio | 1.5 ± 0.3 | 1.9 ± 0.3 | 0.0002 | 1.9 ± 0.4 | 2.1 ± 0.7 | 0.17 |
| Leaflet thickness | ||||||
| Tip anterior leaflet thickness, mm/m2 | 2.1 ± 0.6 | 1.1 ± 0.2 | <0.0001 | 1.5 ± 0.4** | 1.2 ± 0.3 | 0.08 |
| Tip posterior leaflet thickness, mm/m2 | 2.0 ± 0.5 | 1.1 ± 0.3 | <0.0001 | 1.4 ± 0.4* | 1.1 ± 0.3 | 0.001 |
| Leaflet position to annulus line | ||||||
| Anterior leaflet position, mm/m2 | −0.8 ± 1.5 | 1.9 ± 0.7 | 0.01 | 0.5 ± 1.5** | 1.6 ± 0.9 | 0.16 |
| Posterior leaflet position, mm/m2 | −0.9 ± 1.5 | 1.8 ± 1.1 | <0.0001 | −0.1 ± 1.9 | 1.7 ± 0.7 | 0.008 |
| Anterior leaflet prolapse, % | 9 (53) | 0 | — | 6 (19) | 0 | — |
| Posterior leaflet prolapse, % | 11 (65) | 0 | — | 9 (28) | 0 | — |
| Subvalvular apparatus | ||||||
| Anterior chordal length, mm/m2 | 11.5 ± 2.2 | 11.8 ± 1.6 | 0.55 | 10.2 ± 2.2** | 12.1 ± 2.0 | 0.02 |
| Posterior chordal length, mm/m2 | 10.8 ± 2.2 | 12.4 ± 2.1 | 0.10 | 10.6 ± 1.9 | 12.2 ± 2.2 | 0.009 |
| Anterior PM tip/LV length, % | 27 ± 4% | 32 ± 4% | 0.0002 | 27 ± 3% | 31 ± 5% | 0.02 |
| Posterior PM tip/LV length, % | 25 ± 4% | 33 ± 5% | <0.0001 | 28 ± 4% | 32 ± 4% | 0.0009 |
| . | FLNA + men . | FLNA − men . | P-value . | FLNA + women . | FLNA − women . | P-value . |
|---|---|---|---|---|---|---|
| Mitral annulus diameter, mm/m2 | 21 ± 2 | 17 ± 2 | 0.12 | 19 ± 2** | 18 ± 2 | 0.9 |
| Mitral orifice area, cm2/m2 | 1.5 ± 0.4 | 1.8 ± 0.4 | 0.03 | 1.6 ± 0.3 | 1.7 ± 0.5 | 0.37 |
| Leaflet length | ||||||
| Anterior leaflet length, mm/m2 | 13.5 ± 2.1 | 11.5 ± 1.4 | 0.0004 | 12.7 ± 2.0 | 12.0 ± 1.4 | 0.81 |
| Posterior leaflet length, mm/m2 | 8.8 ± 1.5 | 6.2 ± 1.3 | <0.0001 | 6.6 ± 1.1* | 5.9 ± 1.2 | 0.07 |
| Anterior/posterior leaflet length ratio | 1.5 ± 0.3 | 1.9 ± 0.3 | 0.0002 | 1.9 ± 0.4 | 2.1 ± 0.7 | 0.17 |
| Leaflet thickness | ||||||
| Tip anterior leaflet thickness, mm/m2 | 2.1 ± 0.6 | 1.1 ± 0.2 | <0.0001 | 1.5 ± 0.4** | 1.2 ± 0.3 | 0.08 |
| Tip posterior leaflet thickness, mm/m2 | 2.0 ± 0.5 | 1.1 ± 0.3 | <0.0001 | 1.4 ± 0.4* | 1.1 ± 0.3 | 0.001 |
| Leaflet position to annulus line | ||||||
| Anterior leaflet position, mm/m2 | −0.8 ± 1.5 | 1.9 ± 0.7 | 0.01 | 0.5 ± 1.5** | 1.6 ± 0.9 | 0.16 |
| Posterior leaflet position, mm/m2 | −0.9 ± 1.5 | 1.8 ± 1.1 | <0.0001 | −0.1 ± 1.9 | 1.7 ± 0.7 | 0.008 |
| Anterior leaflet prolapse, % | 9 (53) | 0 | — | 6 (19) | 0 | — |
| Posterior leaflet prolapse, % | 11 (65) | 0 | — | 9 (28) | 0 | — |
| Subvalvular apparatus | ||||||
| Anterior chordal length, mm/m2 | 11.5 ± 2.2 | 11.8 ± 1.6 | 0.55 | 10.2 ± 2.2** | 12.1 ± 2.0 | 0.02 |
| Posterior chordal length, mm/m2 | 10.8 ± 2.2 | 12.4 ± 2.1 | 0.10 | 10.6 ± 1.9 | 12.2 ± 2.2 | 0.009 |
| Anterior PM tip/LV length, % | 27 ± 4% | 32 ± 4% | 0.0002 | 27 ± 3% | 31 ± 5% | 0.02 |
| Posterior PM tip/LV length, % | 25 ± 4% | 33 ± 5% | <0.0001 | 28 ± 4% | 32 ± 4% | 0.0009 |
Data are indexed to body surface area.
LV, left ventricle; PM, papillary muscle.
P < 0.0001,
P < 0.05, for comparison between men and women.
Mitral valve apparatus in FLNA-MVD men and women (≥16 years old) compared with controls
| . | FLNA + men . | FLNA − men . | P-value . | FLNA + women . | FLNA − women . | P-value . |
|---|---|---|---|---|---|---|
| Mitral annulus diameter, mm/m2 | 21 ± 2 | 17 ± 2 | 0.12 | 19 ± 2** | 18 ± 2 | 0.9 |
| Mitral orifice area, cm2/m2 | 1.5 ± 0.4 | 1.8 ± 0.4 | 0.03 | 1.6 ± 0.3 | 1.7 ± 0.5 | 0.37 |
| Leaflet length | ||||||
| Anterior leaflet length, mm/m2 | 13.5 ± 2.1 | 11.5 ± 1.4 | 0.0004 | 12.7 ± 2.0 | 12.0 ± 1.4 | 0.81 |
| Posterior leaflet length, mm/m2 | 8.8 ± 1.5 | 6.2 ± 1.3 | <0.0001 | 6.6 ± 1.1* | 5.9 ± 1.2 | 0.07 |
| Anterior/posterior leaflet length ratio | 1.5 ± 0.3 | 1.9 ± 0.3 | 0.0002 | 1.9 ± 0.4 | 2.1 ± 0.7 | 0.17 |
| Leaflet thickness | ||||||
| Tip anterior leaflet thickness, mm/m2 | 2.1 ± 0.6 | 1.1 ± 0.2 | <0.0001 | 1.5 ± 0.4** | 1.2 ± 0.3 | 0.08 |
| Tip posterior leaflet thickness, mm/m2 | 2.0 ± 0.5 | 1.1 ± 0.3 | <0.0001 | 1.4 ± 0.4* | 1.1 ± 0.3 | 0.001 |
| Leaflet position to annulus line | ||||||
| Anterior leaflet position, mm/m2 | −0.8 ± 1.5 | 1.9 ± 0.7 | 0.01 | 0.5 ± 1.5** | 1.6 ± 0.9 | 0.16 |
| Posterior leaflet position, mm/m2 | −0.9 ± 1.5 | 1.8 ± 1.1 | <0.0001 | −0.1 ± 1.9 | 1.7 ± 0.7 | 0.008 |
| Anterior leaflet prolapse, % | 9 (53) | 0 | — | 6 (19) | 0 | — |
| Posterior leaflet prolapse, % | 11 (65) | 0 | — | 9 (28) | 0 | — |
| Subvalvular apparatus | ||||||
| Anterior chordal length, mm/m2 | 11.5 ± 2.2 | 11.8 ± 1.6 | 0.55 | 10.2 ± 2.2** | 12.1 ± 2.0 | 0.02 |
| Posterior chordal length, mm/m2 | 10.8 ± 2.2 | 12.4 ± 2.1 | 0.10 | 10.6 ± 1.9 | 12.2 ± 2.2 | 0.009 |
| Anterior PM tip/LV length, % | 27 ± 4% | 32 ± 4% | 0.0002 | 27 ± 3% | 31 ± 5% | 0.02 |
| Posterior PM tip/LV length, % | 25 ± 4% | 33 ± 5% | <0.0001 | 28 ± 4% | 32 ± 4% | 0.0009 |
| . | FLNA + men . | FLNA − men . | P-value . | FLNA + women . | FLNA − women . | P-value . |
|---|---|---|---|---|---|---|
| Mitral annulus diameter, mm/m2 | 21 ± 2 | 17 ± 2 | 0.12 | 19 ± 2** | 18 ± 2 | 0.9 |
| Mitral orifice area, cm2/m2 | 1.5 ± 0.4 | 1.8 ± 0.4 | 0.03 | 1.6 ± 0.3 | 1.7 ± 0.5 | 0.37 |
| Leaflet length | ||||||
| Anterior leaflet length, mm/m2 | 13.5 ± 2.1 | 11.5 ± 1.4 | 0.0004 | 12.7 ± 2.0 | 12.0 ± 1.4 | 0.81 |
| Posterior leaflet length, mm/m2 | 8.8 ± 1.5 | 6.2 ± 1.3 | <0.0001 | 6.6 ± 1.1* | 5.9 ± 1.2 | 0.07 |
| Anterior/posterior leaflet length ratio | 1.5 ± 0.3 | 1.9 ± 0.3 | 0.0002 | 1.9 ± 0.4 | 2.1 ± 0.7 | 0.17 |
| Leaflet thickness | ||||||
| Tip anterior leaflet thickness, mm/m2 | 2.1 ± 0.6 | 1.1 ± 0.2 | <0.0001 | 1.5 ± 0.4** | 1.2 ± 0.3 | 0.08 |
| Tip posterior leaflet thickness, mm/m2 | 2.0 ± 0.5 | 1.1 ± 0.3 | <0.0001 | 1.4 ± 0.4* | 1.1 ± 0.3 | 0.001 |
| Leaflet position to annulus line | ||||||
| Anterior leaflet position, mm/m2 | −0.8 ± 1.5 | 1.9 ± 0.7 | 0.01 | 0.5 ± 1.5** | 1.6 ± 0.9 | 0.16 |
| Posterior leaflet position, mm/m2 | −0.9 ± 1.5 | 1.8 ± 1.1 | <0.0001 | −0.1 ± 1.9 | 1.7 ± 0.7 | 0.008 |
| Anterior leaflet prolapse, % | 9 (53) | 0 | — | 6 (19) | 0 | — |
| Posterior leaflet prolapse, % | 11 (65) | 0 | — | 9 (28) | 0 | — |
| Subvalvular apparatus | ||||||
| Anterior chordal length, mm/m2 | 11.5 ± 2.2 | 11.8 ± 1.6 | 0.55 | 10.2 ± 2.2** | 12.1 ± 2.0 | 0.02 |
| Posterior chordal length, mm/m2 | 10.8 ± 2.2 | 12.4 ± 2.1 | 0.10 | 10.6 ± 1.9 | 12.2 ± 2.2 | 0.009 |
| Anterior PM tip/LV length, % | 27 ± 4% | 32 ± 4% | 0.0002 | 27 ± 3% | 31 ± 5% | 0.02 |
| Posterior PM tip/LV length, % | 25 ± 4% | 33 ± 5% | <0.0001 | 28 ± 4% | 32 ± 4% | 0.0009 |
Data are indexed to body surface area.
LV, left ventricle; PM, papillary muscle.
P < 0.0001,
P < 0.05, for comparison between men and women.
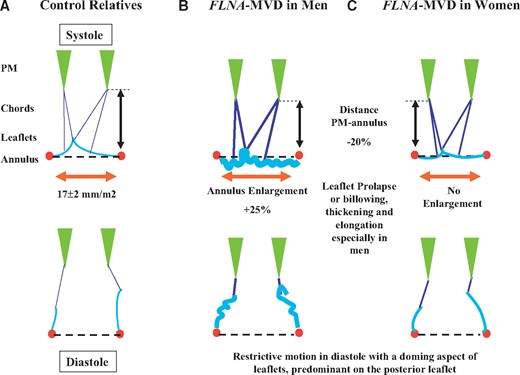
Schematic of MV apparatus morphology averaged in systole (top) and diastole (bottom) (A) in adult controls, (B) in FLNA-MVD (FLNA+) men and (C) in FLNA-MVD women. In men (B) mitral annulus is 25% larger, mitral leaflets are thickened, redundant, elongated, and prolapsed in systole (top). In addition the distance between papillary muscles tip and mitral annulus line (symbolized by the double black arrow) is reduced by 20%. In diastole (bottom), mitral leaflets motion is limited with a doming aspect. In women (C) MV apparatus changes are moderate. PM, papillary muscle; FLNA, Filamin-A; MVD, mitral valve dystrophy.
Mitral leaflets restrictive motion in diastole
The phenotype of MV disease associated also an unusual doming aspect or restrictive motion in diastole which predominates on the PL (Figures 1 and 2). This aspect was seen in 84% of patients of both genders and any age, and in the three different FLNA mutations. The PL restrictive motion was even the predominant feature of MV involvement in females mimicking with that regard a discrete rheumatic- or drug-induced lesion. Further, a mild-to-moderate degree of congenital MV stenosis with incomplete opening of one commissure was occasionally observed (see Supplementary material online, Figure S2).
Sub-valvular mitral valve apparatus
Mitral valve chordae were shortened (in women) and papillary muscles (PM) development and position were affected (Table 2). No chordal rupture was diagnosed in our series of patients but chordae were occasionally elongated and a few primary chordae were absent in one FLNA-P637Q man. Abnormal leaflet-like tissue replaced most chordae in one patient. In addition, chordae displayed uncommon ‘web’ morphology in a young patient suggestive of a severe impairment of chordae development. Both PM tips were closer to mitral annulus line as compared with controls. Moreover, PMs were absent or severely underdeveloped in two patients with P637Q and G288R mutations (see Supplementary material online, Figure S1A–1C).
Relation of mitral valve parameters to age in FLNA-MVD
The main MV parameters correlated strongly to age in FLNA patients, especially in males, in agreement with a degenerative process of the MV apparatus (Figure 3). We further stratified FLNA-MVD adult patients in two groups under and above 40 years old. Although younger adults (≤40 years old) were taller, PL length [19.6 (confidence interval (CI) 25–75%; 18.3–20.3) vs. 15.3 mm (13.8–16.5), P = 0.015] and tip thickness [5.3 (3.9–5.4) vs. 3.1 mm (2.9–3.2), P = 0.032] were greater in older FLNA mutated men. Mitral valve lesions were detected in childhood especially in males (at 2 months in the younger one), clearly suggesting that valves lesions are also developmental lesions. Indeed fetal echocardiography carried out in two of them diagnosed MVD before birth.
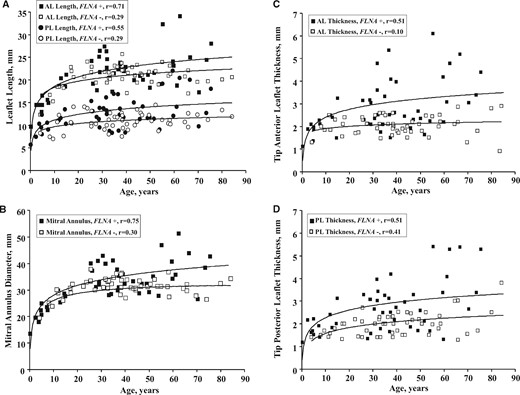
Relation of age to (A) anterior and posterior leaflets length, (B) mitral annulus diameter, and (C and D) leaflets thickness in patients of both genders, with (black squares or circles) or without (white squares or circles) FLNA mutations. Values of all MV parameters increase with age up to 16–20 years old and were eventually quite stable in controls. All parameters have greater values in FLNA-MVD patients at any adult age, with a divergence between the regression slope in FLNA-MVD patients and controls. AL, anterior leaflet; PL, posterior leaflet.
Mitral regurgitation
Valve regurgitation (see Supplementary material online, Table S1) was assessed in all FLNA-MVD patients (n = 65). Mitral regurgitation was moderate or severe in 10 of 25 (40%) male patients and in 9 of 17 (53%) adult men, but in only 3 out of 40 (8%) female patients (P < 0.0001). Mechanism of MR was prolapse and/or annulus enlargement in male while the mechanism was either prolapse or pseudo-prolapse in female patients with a restrictive motion of the PL.
Aortic, tricuspid, and pulmonary valves
Polyvalvular disease (see supplementary material online, Figure S3), defined as the involvement of at least two valves, was found in 23 (35%) of 65 FLNA patients, mainly in male patients (n = 20, 80%). Aortic, tricuspid, and pulmonary involvement translated into moderate billowing or prolapse and led to severe regurgitation of the aortic valve only. Some patients had thickened aortic cusps with restricted motion. Bicuspid aortic valves were identified in 5 (7.7%) patients and 1 (1%) control (P = 0.07). All valve lesions appeared more severe in FLNA-H743P and G288R patients as compared with FLNA-P637Q, but the limited number of H743P or G288R patients precluded any reliable comparison. In FLNA-H743P and G288R patients polyvalvular involvement was frequent (50%), mainly in the form of moderate billowing.
Thoracic aorta
In adult patients, outflow tract diameter (12.2 ± 1.3 vs. 11.8 ± 1.0 mm/m2, P = 0.15) and ascending aorta size (15.3 ± 3.1 vs. 15.0 ± 2.4 mm/m2, P = 0.52) did not differ between FLNA patients and relatives but aortic sinus was slightly larger (17.3 ± 2.5 vs. 16.3 ± 1.9 mm/m2, P = 0.04). One FLNA patient died awaiting thoracic aorta aneurysm surgery, and another patient (75 year old) developed a 52 mm thoracic aorta dilatation, 24 years after bicuspid aortic valve surgery. Ascending aorta diameter was smaller than 35 mm in other FLNA patients but was found at 44 mm in one control relative.
Cardiac surgery and follow-up
Out of the 246 subjects (72 mutated), 14 patients underwent cardiac surgery. Cardiac surgery was performed in three control relatives and consisted in coronary artery bypass grafting (75-year-old man), myxoma resection (81-year-old woman), and MV replacement (69-year-old man). Cardiac surgery was performed in 11 out of 72 FLNA-MVD patients (9 men, 3–63 year old) and consisted in either mitral or aortic or both valves surgery. Mitral regurgitation was treated mainly by MV repair and annulus undersizing (replacement in one G288R patient).
Compared with controls (Figure 4), overall survival tended to decrease [80.6 ± 7.4 vs. 89.0 ± 5.2%, P = 0.13; (hazard ratio (HR) 2.0 (0.8–5.0), P = 0.15], owing to a decrease in male survival [55.9 ± 14.1 vs. 83.9 ± 8.8%, P = 0.011; HR 3.8 (1.3–11.3), P = 0.024]. Cardiac surgery rate was strongly increased in FLNA-MVD patients (33.3 ± 9.8 vs. 5.0 ± 4.9%, P < 0.0001) with a HR of 10.5 [95% CI (2.9–37.9), P < 0.0001], owing to a substantial lifetime increased risk of cardiac surgery (mainly valvular) in males [76.8 ± 14.1 vs. 9.1 ± 8.7%, P < 0.0001; HR 48 (6–385), P < 0.0001].
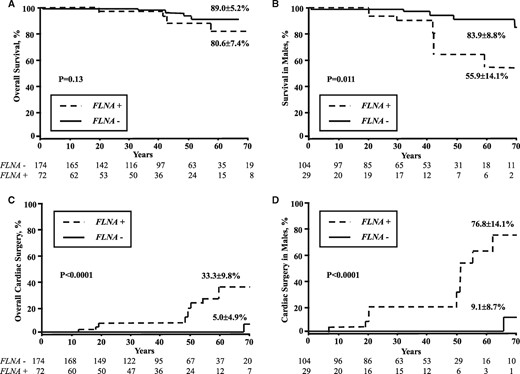
Life-time influence of FLNA-MVD (FLNA+) up to 70 years old on (A) overall survival, (B) survival in male patients, (C) overall cardiac surgery rate, and (D) cardiac surgery rate in male patients. The life-time risk of cardiac surgery depends essentially on the substantial increase in valve surgery in FLNA-MVD males.
Discussion
The identification of FLNA as the first gene of non-syndromic MVD with an X-linked inheritance, in a large family and in additional small families, provided the unique opportunity to start deciphering phenotype variability of MV abnormalities. Hence, the present study aimed to depict the full spectrum of valve defects as well as the outcome of FLNA-MVD.
FLNA-MVD is a developmental and degenerative disease
Comprehensive echocardiography demonstrated unique organic features of FLNA-MVD that may change the paradigm of the so called ‘degenerative’ MV diseases. Indeed, FLNA-MVD is both a developmental and degenerative pathology. Valves lesions were diagnosed during childhood and even during fetal life highlighting the congenital origin of the disease, in agreement with experimental data in mice.19 On the other hand, MV lesions worsened in older patients of our series. This result is in line with the worsening of MV lesions over time in unselected patients with non-FLNA-MVP20 or with Marfan syndrome.21
Mitral valve prolapse and mitral regurgitation in Filamin-A patients
Consistent with an X-linked disease, FLNA mutations lead to a more severe phenotype in men, who are haemizygous for the mutation, as compared with women. In male patients, MV leaflet prolapse and annulus alterations are close to non-FLNA-MVP.1 , 22 However, no chordal rupture was diagnosed and chordae were rather shortened. Although MVP was found approximately in 70% of male and 40% of female patients, non-diagnostic or prodromal MV lesions were frequent, given a partial or complete penetrance in >65% of mutation carriers. In addition, subtle MV alterations were detected in almost all mutation carriers which highlight the large spectrum of MV abnormalities encountered in FLNA-MVD and likely in classical aetiologies of MVP.2 Mitral regurgitation was a frequent complication, especially in male patients, and was accessible to MV repair in most cases.
Subvalvular apparatus alteration
Discrete congenital sub-valvular lesions in FLNA-MVD patients are highly suggestive of a developmental disorder involving the whole MV apparatus. Chordae shortening is uncommon in MVP,22 but is in agreement with the initial publication of Monteleone and Fagan6 and with experimental data in mice.19 In addition, we also identified defects in the development of PM which appeared displaced towards the annulus, underdeveloped or even absent. Little is known regarding PM and chordae development. Whereas leaflets and chordae originate from atrio-ventricular cushions, PM arise from the ventricular wall. The delamination process separates PM from myocardial wall while primary leaflet tissue is progressively sculpted to form individualized chordae arising from PM tip.23 The absence of FLNA in the myocardium suggests that PM defects observed in FLNA-MVD are likely the consequence of an alteration of the complex and misunderstood interplay between myocardium and valve cushions.
Restrictive mitral valve motion in diastole
Surprisingly, FLNA mutations elicit both a MVP and a paradoxical restrictive motion in diastole. Such dual motion alteration is unusual in MVP disease; it has been reported in overt or sub-clinical rheumatic fever,24 in advanced stages of Barlow disease where some chordae can be thickened and retracted,5 and in severe MR with tethering of the non-prolapsed leaflet secondary to LV dilatation.25 The mechanism of the restrictive motion in FLNA-MVD is not clearly established but seems to be the consequence of mitral leaflets thickening and stiffening, PM displacement with shortness of primary chordae,19 and incomplete commissural opening. This paradoxical association is a unique feature in MVP. Hence, FLNA-MVD can be regarded as a unique cause of MVP beside Barlow disease and Fibro-elastic deficiency.
Polyvalvular involvement
Polyvalvular prolapse is a classical finding in connective tissue disorders such as Marfan syndrome21 , 26 and in some aneuploidy syndromes.27 By contrast, polyvalvular involvement is rare in euploid patients without extra-cardiac anomalies. The association of tricuspid valve prolapse to MVP is not very common.28 In a large series of 400 patients with MVP,29 only 11 (3%) had multiple valve involvement (tricuspid and aortic). Most of them were male as in FLNA-MVD. Polyvalvular involvement is a frequent finding in FLNA-MVD, especially in male patients, suggesting a defect in valvulogenesis.7 , 12 , 13 Indeed, during heart morphogenesis, FLNA is expressed in all valvular cushions19 and likely explains multiple valves lesions. Finally, although the difference with control relatives did not reach significance, bicuspid aortic valve is a relatively frequent finding and might be a developmental consequence of FLNA mutations.12 This hypothesis is reinforced by the frequent finding of bicuspid valves in FLNA-mice (data not shown).
Influence of Filamin-A mutation on outcome
It is noteworthy that FLNA-MVD is associated with a substantial life-time risk of cardiac valve surgery, up to 75% in male patients at 70 years old, which appears largely higher than in Barlow disease (estimated around 5–15%)21 , 22 , 30 highlighting the unusual severity of this MV disease and the need for early diagnosis and close monitoring.
Limitations
Although we gathered echocardiography and outcome data from four different families with three different mutations, most FLNA-MVD subjects were relatives linked to a common ancestor born in the 18th century (P637Q family). Echocardiography data were available in only 162 (66%) subjects but included 90% of known mutated patients. In this exploratory study, multiple statistical comparisons were carried out so that some marginally significant differences can be due to chance.
Conclusion
FLNA-MVD is a unique X-linked disease with both developmental and degenerative alterations, involving the whole MV apparatus and other valves. FLNA-MVD presents specific characteristics that differ from the usual forms of MVP ( Take home figure). Beside classical features of MVP, leaflet motion is paradoxically restricted in diastole. Diagnosis of FLNA-MVD should therefore be sought in male patients with unusual features of MVP and can be an alternative aetiology for explaining restrictive valve lesions. Non-diagnostic or prodromal forms of MV disease are frequent. FLNA-MVD is associated with a substantial life-time risk of valve surgery, but MR can be generally cured by classical repair.

(A) Aspect of mitral valve apparatus with the paradoxical association of mitral valve prolapse (systole) and restrictive motion in diastole (doming, arrows) in FLNA-MVD, (B) myxomatous change of a mitral leaflet (colloidal iron), (C) degenerative aspect of FLNA-MVD with progressive lengthening of leaflets (AL, anterior leaflet; PL, posterior leaflet), and (D) substantial life-time risk of cardiac surgery in FLNA-MVD males.
The present study is the first to start deciphering the genotype–phenotype relationships in FLNA-MVD. Thanks to an international collaboration on FLNA-MVD we were able to characterize precisely the disease. Our work paves the way for further gene discovery and phenotype–genotype assessment in either MVP or MVD which appear more various than previously thought. Familial screening and individualized management adapted to phenotype and predicted outcome according to causal gene should reduce complications of either MVP or MVD in the future.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
We are indebted to Monique Dupas, Marie Marrec, and Guenola Coste (Institut du Thorax, Nantes, France) for their assistance in patient enrolment and familial screening, and to Aurélie Thollet for her assistance in MVP research organization.
Funding
Leducq Foundation Transatlantic Network of Excellence in Mitral Valve Disease (2008-2013, Paris, France); Fondation GenaVie (2010, Nantes, France); Fédération Française de Cardiologie (2011, Paris, France); French Ministry of Health ‘PHRC National 2007’ (n°20-17); and ‘PHRC-I 2012’ (API12/N/019, Paris, France). NIH: HL131546 (RAN), GM103444 (RAN), and HL127692 (RAN, DM, SS), K23HL116652 (FD), HL109506 (RAL, EA), HL128099 (RAL). Inserm Translational Research Grant (2012-2016, Paris, France) to T.L.T.
Conflict of interest: none declared.
References
Author notes
Hervé Le Marec and Jean-Jacques Schott contributed equally to the study.
See page 1278 for the editorial comment on this article (doi: 10.1093/eurheartj/ehx578)



