-
PDF
- Split View
-
Views
-
Cite
Cite
Irene Campi, Simona Censi, Flavia Prodam, Luisa Petrone, Giulia Brigante, Tommaso Porcelli, Rosaria Maddalena Ruggeri, Maria Cristina Vigone, Giuditta Rurale, Serafino Lio, Carla Pelusi, Luca Persani, Increased cardiovascular morbidity and reduced life expectancy in a large Italian cohort of patients with resistance to thyroid hormone β (RTHβ), European Journal of Endocrinology, Volume 191, Issue 4, October 2024, Pages 407–415, https://doi.org/10.1093/ejendo/lvae117
Close - Share Icon Share
Abstract
Decreased survival and higher cardiovascular morbidity have been recently reported in a UK cohort of 61 RTHβ patients, but there is no evidence from other countries.
Retrospective cohort study from an historical group of 284 Italian RTHβ patients, diagnosed between 1984 and 2023.
We collected data on diagnosis of 284 cases and longitudinal data of 249 RTHβ who carried heterozygous pathogenic variants in the THRB gene. We studied how thyroid function and recognized risk factors for cardiovascular disease, such as hypertension and diabetes, affected overall mortality and major cardiovascular events.
The cumulative prevalence of sinus/supraventricular tachycardia and atrial fibrillation was 40% and 18%, respectively. FT4 values 57% higher than the upper limit of normal were associated with premature cardiovascular manifestations. Major cardiovascular events (MACEs) occurred in RTHβ patients at a median age (IQR) of 59.4 years (50.4-66.4) and early mortality resulted in a mean of 11 years of life lost. While at univariable analysis hypertension, dyslipidemia, high fasting glucose/diabetes were also associated with MACEs, at multivariable analysis only age at diagnosis, increased fT4 levels, and male gender remained significantly associated with MACEs and age at diagnosis and higher fT4 levels with mortality. Previous thyroidectomy or radioiodine therapy had no statistically significant effect in the prevention of major cardiovascular events or all-cause mortality.
These data should raise the general awareness on the cardiovascular risk and prompt a proactive cardiovascular monitoring in RTHβ, especially in men and those with fT4 levels above 30 pmol/L.
RTHβ patients with heterozygous THRB gene variants have an elevated risk of cardiovascular events and early mortality, which is comparable to that of primary hyperthyroidism. Gender, comorbidities such as hypertension and diabetes and increased fT4 levels, influenced their cardiovascular risk.
This risk has hitherto been neglected, based on the premise that the majority of RTH patients do not require therapy.
Further pharmacological trials are required to fully evaluate the effects of betablockers or thyroid hormone analogues such as triiodothyroacetic acid (TRIAC) for primary prevention. In the meanwhile, preventive lifestyle and pharmaceutical interventions aimed at controlling hypertension, metabolic disorders, and preventing overweight are recommended.
Introduction
Overt primary hyperthyroidism has been associated with an increased risk of CVDs, particularly in the elderly.1 Hyperthyroid patients have a greater risk of all-cause mortality, major cardiovascular events (MACEs), and heart failure as compared to euthyroid controls.2
Furthermore, several large studies have recently shown that in euthyroid individuals, fT4 levels in the upper quartile of the normal range were related to all-cause mortality,3,4 decreased life expectancy,4,5 incident CVDs,4 sudden cardiac death,6 and atrial fibrillation.7
Resistance to thyroid hormone beta (RTHβ) is characterized by central hyperthyroidism with non-suppressed TSH and serum fT4 and fT3 levels comparable to primary hyperthyroidism. This biochemical trait is produced by a decreased pituitary sensitivity to thyroid hormone (TH) due to dominant negative loss-of-function mutations in the THRB gene, encoding for the TH receptor (TR) beta (TRβ). As a result, tissues such as bone, skeletal muscle, and the cardiovascular system are chronically exposed to high TH via the normally functioning alpha isoform (TRα1).
Based on these premises, one could predict RTHβ to be related to increased cardiovascular risk and mortality when compared to the general population.
Previous studies have shown that while some systolic and diastolic parameters (heart rate, stroke volume, cardiac output, diastolic filling, maximal aortic flow velocity) are compatible with cardiac hyperthyroidism, others (left ventricle ejection and shortening fractions, left ventricle systolic diameter and wall thickness) are suggestive of euthyroidism8 or even subclinical hypothyroidism (systemic vascular resistance, arterial stiffness, and intima-media thickness).9-11 In addition, impaired insulin sensitivity and dyslipidemia have been also documented.8-12 Nevertheless, the lack of prospective data, as well as the heterogeneity of these monocentric populations, limited these studies. Therefore, most of the recommendation for follow-up and treatment are inconsistent. Whereas antithyroid medications, radioiodine, and total thyroidectomy are unquestionably useful in primary hyperthyroidism, these treatments are generally contraindicated in RTHβ. Furthermore, the two most recommended treatment, betablockers, and triiodothyroacetic acid (TRIAC) have never been evaluated in controlled studies.
Okosieme et al13 recently reported decreased survival and greater cardiovascular morbidity in 61 RTHβ patients compared to controls, highlighting the need for additional research to determine the optimal therapeutic approach to lower this risk. Here, we present data on mortality and long-term cardiovascular outcomes of 284 Italian RTHβ patients, which extend previous evidence and highlight additional factors influencing the cardiovascular risk in this condition.
Materials and methods
We included all the patients submitted to molecular analysis for central hyperthyroidism in three collaborating centers (Milan, Novara, and Padua) between 1984 and 2023 and we studied all 284 RTHβ cases carrying mutations in the THRB gene. The patients originate from 13 out of 20 Italian regions and represent a large sample of the population with RTHβ diagnosis in this country.
Long-term data were available from 155 (55%) patients followed prospectively for a median (IQR) of 11.7 years (5.9-22) in 8 collaborating center (Milan, Novara, Padua, Oderzo, Bologna, Modena, Florence, Naples, and Messina). Follow-up data were retrieved from Hospital Databases or from the general practitioners.
The all-cause mortality of 94 patients (33%) was derived from electronic regional registries reporting the status of the health service cards and the reasons for their inactivation (date of death or transfer). These databases are active since 1994, thus these information could not be retrieved in 35 patients (12%) who were not registered before 1994 (Figure 1).
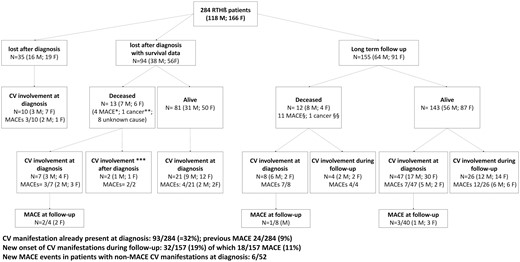
Selection of study population. RTHβ = resistance to thyroid hormone β; * myocardial ischemia (2 f; 1 m) and cerebrovascular event (1 m); ** breast cancer (1 f); *** one m and one f had MACE after diagnosis, which was the cause of death (data referred by affected relatives on follow-up); § cerebrovascular events: 3 m and 1 f; myocardial ischemia 2 m and 3 f; heart failure 2 m; §§ glioblastoma.
Life expectancy and mortality rate adjusted for age, gender, and year of the background Italian population by 1984 to 2023 were freely available on the website of the Italian National Institute of Statistic (ISTAT, http://dati.istat.it/).
Major adverse cardiac events (MACEs) were defined as a composite of cardiovascular death, acute myocardial infarction, heart failure, new cardiac arrhythmia (atrial fibrillation), angina, and stroke.14 According with the criteria for prediabetes established by the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) in 2010, high fasting glucose was defined as fasting blood glucose between 100 and 125 mg/dL (5.6 to 6.9 mmol/L). For the statistical analyses, we analyzed the glucometabolic dysfunctions as a composite of diabetes (N = 12) and high fasting glucose (N = 11).
To reduce variability among the several immunoassays employed, fT4 and fT3 levels were expressed as a fold increase of the upper limit of normal (ULN).
For statistical purposes, the closest data before the outcome events were used for individuals with cardiovascular involvement, while the most recent were used for those without manifestations (Table 1). The reported treatments were prescribed at any time during follow-up, but always before the outcome (Table 1)
Incidence and characteristics of the cardiovascular manifestations found in RTHß patients.
| . | Values . |
|---|---|
| Male/female (% female) | 118/166 (58.5% female) |
| Age (years) at diagnosis, median (IQR) | 30 (15-47) |
| Length (years) of the follow-up, median (IQR) | 2.9 (0-13.6) |
| Inheritance (maternal/paternal/sporadic/unknown), N | 82/48/51/103 |
| Involved codon, N (cluster 1/2/3/codon 383) | 116/115/45/8 |
| Ablative treatments (thyroidectomy/131I), Nc | 35/11 |
| Anti-thyroid treatment before diagnosis, N of patients (%) | 47/222 (21%) |
| fT4/ULN, mean ± SDd | 1.7 ± 0.5 |
| fT3/ULN, mean ± SDd | 1.6 ± 0.5 |
| TSH mU/L, mean ± SD; median (IQR)d | 3.1 ± 5.1; 2.2 (1.3-3.5) |
| Goiter, N of patients (%) | 142/219 (65%) |
| Associated AITD, N of patients (%) | 52/218 (24%) |
| Graves’ disease | 9 (4.2%) |
| TPO or Tg-Ab positive antibodies and typical US pattern | 31 (14%) |
| Autoimmune primary hypothyroidism | 12 (5.5%) |
| TRIAC treatmentc | 20/185 (11%) |
| Betablockersc | 59/187 (32%) |
| Levothyroxinec | 47/256 (18%) |
| CV manifestation, N of patients (%) | 125/196 (64%) |
| Age at the onset of CV symptoms, median years (IQR) | 42.1 (27.3-57.2) |
| Sinus tachycardia, N of patients (%) | 79 (40%) |
| Valve defects, N of patients (%) | 5 (2.5%) |
| MACE, N of patients (%) | 48 (24%) |
| Age at the time of MACE, median years (IQR) | 59.4 (50.4-66.4) |
| N and type of events | |
| Cardiovascular death | 3 |
| Acute myocardial infarction and angina | 12 |
| Stroke | 10 |
| Heart failure | 12 |
| New cardiac arrhythmia | 35 |
| Overall mortality, N (%) | 25/249 (10%) |
| Age at death, median years (IQR) | 74.5 (66.8-80.5) |
| Life expectancy, median years (IQR)a | 83 (79-84.5) |
| Lost year of life, mean ± SD, median (IQR)b | 11.2 ± 12.3, 7 (2-17) |
| Causes of death | |
| MACE, N of patients (%) | 15 (60%) |
| Cardiovascular death | 3 |
| Acute myocardial infarction | 5 |
| Stroke | 5 |
| Heart failure | 2 |
| Cancer, N of patients (%) | 2 (8%) |
| unknown cause, N of patients (%) | 8 (32%) |
| Patients still alive, N of patients (%) | 224/249 (90%) |
| Age of patients alive, median (IQR) | 45 (29-58.7) |
| Patients who have reached or surpassed the life expectancy of the Italian population, N (%) | 6/249 (2.4%) |
| Risk factors | |
| Hypertension, N of patients (%) | 54/210 (26%) |
| Diabetes/high fasting glucose (>5.55 mmol/L), N of patients (%) | 23/212 (11%) |
| Obesity | 17/209 (8.1%) |
| Dyslipidemia/cholesterol-lowering drugs | 68/191 (36%) |
| Years of diagnosis (N of patients) | |
| 1984-1994 | 54 |
| 1995-2005 | 86 |
| 2006-2016 | 99 |
| 2017-2024 | 45 |
| . | Values . |
|---|---|
| Male/female (% female) | 118/166 (58.5% female) |
| Age (years) at diagnosis, median (IQR) | 30 (15-47) |
| Length (years) of the follow-up, median (IQR) | 2.9 (0-13.6) |
| Inheritance (maternal/paternal/sporadic/unknown), N | 82/48/51/103 |
| Involved codon, N (cluster 1/2/3/codon 383) | 116/115/45/8 |
| Ablative treatments (thyroidectomy/131I), Nc | 35/11 |
| Anti-thyroid treatment before diagnosis, N of patients (%) | 47/222 (21%) |
| fT4/ULN, mean ± SDd | 1.7 ± 0.5 |
| fT3/ULN, mean ± SDd | 1.6 ± 0.5 |
| TSH mU/L, mean ± SD; median (IQR)d | 3.1 ± 5.1; 2.2 (1.3-3.5) |
| Goiter, N of patients (%) | 142/219 (65%) |
| Associated AITD, N of patients (%) | 52/218 (24%) |
| Graves’ disease | 9 (4.2%) |
| TPO or Tg-Ab positive antibodies and typical US pattern | 31 (14%) |
| Autoimmune primary hypothyroidism | 12 (5.5%) |
| TRIAC treatmentc | 20/185 (11%) |
| Betablockersc | 59/187 (32%) |
| Levothyroxinec | 47/256 (18%) |
| CV manifestation, N of patients (%) | 125/196 (64%) |
| Age at the onset of CV symptoms, median years (IQR) | 42.1 (27.3-57.2) |
| Sinus tachycardia, N of patients (%) | 79 (40%) |
| Valve defects, N of patients (%) | 5 (2.5%) |
| MACE, N of patients (%) | 48 (24%) |
| Age at the time of MACE, median years (IQR) | 59.4 (50.4-66.4) |
| N and type of events | |
| Cardiovascular death | 3 |
| Acute myocardial infarction and angina | 12 |
| Stroke | 10 |
| Heart failure | 12 |
| New cardiac arrhythmia | 35 |
| Overall mortality, N (%) | 25/249 (10%) |
| Age at death, median years (IQR) | 74.5 (66.8-80.5) |
| Life expectancy, median years (IQR)a | 83 (79-84.5) |
| Lost year of life, mean ± SD, median (IQR)b | 11.2 ± 12.3, 7 (2-17) |
| Causes of death | |
| MACE, N of patients (%) | 15 (60%) |
| Cardiovascular death | 3 |
| Acute myocardial infarction | 5 |
| Stroke | 5 |
| Heart failure | 2 |
| Cancer, N of patients (%) | 2 (8%) |
| unknown cause, N of patients (%) | 8 (32%) |
| Patients still alive, N of patients (%) | 224/249 (90%) |
| Age of patients alive, median (IQR) | 45 (29-58.7) |
| Patients who have reached or surpassed the life expectancy of the Italian population, N (%) | 6/249 (2.4%) |
| Risk factors | |
| Hypertension, N of patients (%) | 54/210 (26%) |
| Diabetes/high fasting glucose (>5.55 mmol/L), N of patients (%) | 23/212 (11%) |
| Obesity | 17/209 (8.1%) |
| Dyslipidemia/cholesterol-lowering drugs | 68/191 (36%) |
| Years of diagnosis (N of patients) | |
| 1984-1994 | 54 |
| 1995-2005 | 86 |
| 2006-2016 | 99 |
| 2017-2024 | 45 |
astratified population life expectancy obtained by public dataset of the Italian National Institute for Statistics (ISTAT, http://dati.istat.it/)life expectancy for age, sex, and year of diagnosis.
blost years of life of deceased patients were calculated as the difference between the stratified-population life expectancy and the age of death.
ctreatments started at any time during patients’ life, but always analysed before the outcomes studied in this cohort (MACE and death).
dthe thyroid function tests reported in this table are the closest to the MACE or the last available for those without cardiovascular involvement.
IQR interquartile range. MACEs (major cardiovascular events) is a composite of cardiovascular death, acute myocardial infarction, heart failure, new cardiac arrhythmia (atrial fibrillation), angina, and stroke.
Incidence and characteristics of the cardiovascular manifestations found in RTHß patients.
| . | Values . |
|---|---|
| Male/female (% female) | 118/166 (58.5% female) |
| Age (years) at diagnosis, median (IQR) | 30 (15-47) |
| Length (years) of the follow-up, median (IQR) | 2.9 (0-13.6) |
| Inheritance (maternal/paternal/sporadic/unknown), N | 82/48/51/103 |
| Involved codon, N (cluster 1/2/3/codon 383) | 116/115/45/8 |
| Ablative treatments (thyroidectomy/131I), Nc | 35/11 |
| Anti-thyroid treatment before diagnosis, N of patients (%) | 47/222 (21%) |
| fT4/ULN, mean ± SDd | 1.7 ± 0.5 |
| fT3/ULN, mean ± SDd | 1.6 ± 0.5 |
| TSH mU/L, mean ± SD; median (IQR)d | 3.1 ± 5.1; 2.2 (1.3-3.5) |
| Goiter, N of patients (%) | 142/219 (65%) |
| Associated AITD, N of patients (%) | 52/218 (24%) |
| Graves’ disease | 9 (4.2%) |
| TPO or Tg-Ab positive antibodies and typical US pattern | 31 (14%) |
| Autoimmune primary hypothyroidism | 12 (5.5%) |
| TRIAC treatmentc | 20/185 (11%) |
| Betablockersc | 59/187 (32%) |
| Levothyroxinec | 47/256 (18%) |
| CV manifestation, N of patients (%) | 125/196 (64%) |
| Age at the onset of CV symptoms, median years (IQR) | 42.1 (27.3-57.2) |
| Sinus tachycardia, N of patients (%) | 79 (40%) |
| Valve defects, N of patients (%) | 5 (2.5%) |
| MACE, N of patients (%) | 48 (24%) |
| Age at the time of MACE, median years (IQR) | 59.4 (50.4-66.4) |
| N and type of events | |
| Cardiovascular death | 3 |
| Acute myocardial infarction and angina | 12 |
| Stroke | 10 |
| Heart failure | 12 |
| New cardiac arrhythmia | 35 |
| Overall mortality, N (%) | 25/249 (10%) |
| Age at death, median years (IQR) | 74.5 (66.8-80.5) |
| Life expectancy, median years (IQR)a | 83 (79-84.5) |
| Lost year of life, mean ± SD, median (IQR)b | 11.2 ± 12.3, 7 (2-17) |
| Causes of death | |
| MACE, N of patients (%) | 15 (60%) |
| Cardiovascular death | 3 |
| Acute myocardial infarction | 5 |
| Stroke | 5 |
| Heart failure | 2 |
| Cancer, N of patients (%) | 2 (8%) |
| unknown cause, N of patients (%) | 8 (32%) |
| Patients still alive, N of patients (%) | 224/249 (90%) |
| Age of patients alive, median (IQR) | 45 (29-58.7) |
| Patients who have reached or surpassed the life expectancy of the Italian population, N (%) | 6/249 (2.4%) |
| Risk factors | |
| Hypertension, N of patients (%) | 54/210 (26%) |
| Diabetes/high fasting glucose (>5.55 mmol/L), N of patients (%) | 23/212 (11%) |
| Obesity | 17/209 (8.1%) |
| Dyslipidemia/cholesterol-lowering drugs | 68/191 (36%) |
| Years of diagnosis (N of patients) | |
| 1984-1994 | 54 |
| 1995-2005 | 86 |
| 2006-2016 | 99 |
| 2017-2024 | 45 |
| . | Values . |
|---|---|
| Male/female (% female) | 118/166 (58.5% female) |
| Age (years) at diagnosis, median (IQR) | 30 (15-47) |
| Length (years) of the follow-up, median (IQR) | 2.9 (0-13.6) |
| Inheritance (maternal/paternal/sporadic/unknown), N | 82/48/51/103 |
| Involved codon, N (cluster 1/2/3/codon 383) | 116/115/45/8 |
| Ablative treatments (thyroidectomy/131I), Nc | 35/11 |
| Anti-thyroid treatment before diagnosis, N of patients (%) | 47/222 (21%) |
| fT4/ULN, mean ± SDd | 1.7 ± 0.5 |
| fT3/ULN, mean ± SDd | 1.6 ± 0.5 |
| TSH mU/L, mean ± SD; median (IQR)d | 3.1 ± 5.1; 2.2 (1.3-3.5) |
| Goiter, N of patients (%) | 142/219 (65%) |
| Associated AITD, N of patients (%) | 52/218 (24%) |
| Graves’ disease | 9 (4.2%) |
| TPO or Tg-Ab positive antibodies and typical US pattern | 31 (14%) |
| Autoimmune primary hypothyroidism | 12 (5.5%) |
| TRIAC treatmentc | 20/185 (11%) |
| Betablockersc | 59/187 (32%) |
| Levothyroxinec | 47/256 (18%) |
| CV manifestation, N of patients (%) | 125/196 (64%) |
| Age at the onset of CV symptoms, median years (IQR) | 42.1 (27.3-57.2) |
| Sinus tachycardia, N of patients (%) | 79 (40%) |
| Valve defects, N of patients (%) | 5 (2.5%) |
| MACE, N of patients (%) | 48 (24%) |
| Age at the time of MACE, median years (IQR) | 59.4 (50.4-66.4) |
| N and type of events | |
| Cardiovascular death | 3 |
| Acute myocardial infarction and angina | 12 |
| Stroke | 10 |
| Heart failure | 12 |
| New cardiac arrhythmia | 35 |
| Overall mortality, N (%) | 25/249 (10%) |
| Age at death, median years (IQR) | 74.5 (66.8-80.5) |
| Life expectancy, median years (IQR)a | 83 (79-84.5) |
| Lost year of life, mean ± SD, median (IQR)b | 11.2 ± 12.3, 7 (2-17) |
| Causes of death | |
| MACE, N of patients (%) | 15 (60%) |
| Cardiovascular death | 3 |
| Acute myocardial infarction | 5 |
| Stroke | 5 |
| Heart failure | 2 |
| Cancer, N of patients (%) | 2 (8%) |
| unknown cause, N of patients (%) | 8 (32%) |
| Patients still alive, N of patients (%) | 224/249 (90%) |
| Age of patients alive, median (IQR) | 45 (29-58.7) |
| Patients who have reached or surpassed the life expectancy of the Italian population, N (%) | 6/249 (2.4%) |
| Risk factors | |
| Hypertension, N of patients (%) | 54/210 (26%) |
| Diabetes/high fasting glucose (>5.55 mmol/L), N of patients (%) | 23/212 (11%) |
| Obesity | 17/209 (8.1%) |
| Dyslipidemia/cholesterol-lowering drugs | 68/191 (36%) |
| Years of diagnosis (N of patients) | |
| 1984-1994 | 54 |
| 1995-2005 | 86 |
| 2006-2016 | 99 |
| 2017-2024 | 45 |
astratified population life expectancy obtained by public dataset of the Italian National Institute for Statistics (ISTAT, http://dati.istat.it/)life expectancy for age, sex, and year of diagnosis.
blost years of life of deceased patients were calculated as the difference between the stratified-population life expectancy and the age of death.
ctreatments started at any time during patients’ life, but always analysed before the outcomes studied in this cohort (MACE and death).
dthe thyroid function tests reported in this table are the closest to the MACE or the last available for those without cardiovascular involvement.
IQR interquartile range. MACEs (major cardiovascular events) is a composite of cardiovascular death, acute myocardial infarction, heart failure, new cardiac arrhythmia (atrial fibrillation), angina, and stroke.
Genetic investigations of the THRB gene were undertaken with prior, informed written consent. The study complies with the Declaration of Helsinki and was performed according to ethics committee approval of Istituto Auxologico Italiano, Milan (projects: PNRR-MR1-2022-12375726, THYcom and RTH2018, 05C821_2018).
Statistical analysis was performed with MedCalc software version 22.009 and with GraphPad Prism software version 5.1 for Windows.
Univariable analysis was used for testing the unadjusted association between variables and outcome. Variables with statistically significant association with the outcomes were included in a multivariable logistic regression model to determine odds ratios (OR) together with their 95% confidence intervals (CI). The predictive model was determined by enter selection using a P value = .05.
The Cochran-Armitage test for trend was performed to assess changes in the proportion of RTHß cases with cardiovascular manifestations before 55 years of age and MACEs, as a function of the year of diagnosis.
The receiver operating characteristic curve (ROC) was performed to calculate the best cut-off value for cardiovascular prediction.
Preliminary data of this study were presented at the 14th International Workshop on Resistance to Thyroid Hormone & Thyroid Hormone Action. Asilomar, Monterey, California, April 26 - 29, 2023.15
Results
General characteristics of the RTHβ patients included in the study
All patients had the typical central hyperthyroidism biochemical pattern (Table 1). The variants identified in this cohort covered all three hot spot clusters of the THRB gene (Figure 2). As previously reported,16 only in some RTHβ variants (the so-called type 1 mutations), the severity of central hyperthyroidism correlates with the T3 affinity measured in vitro, whereas this does not occur in type 2 mutations. This is suggestive of a certain phenotype-genotype correlation only in THRB variants affecting the T3 binding, but not in the type 2 with aberrant interaction with cofactors and weaker dominant negative effect. Consistently, also in this cohort, we found that serum fT4 and fT3 levels inversely correlated with the in vitro T3 binding affinity in the carriers of type 1 mutants (Spearman r −0.46; 95% CI −0.59 to −0.31 P < .0001 and r – 0.46; 95% CI −0.59 to −0.3 P < .0001), but not in those with type 2 mutants, (Spearman r, 0.06; 95% CI −0.24 to +0.37 P .67 and r 0.03; 95% CI −0.27 to +0.33 P .85).
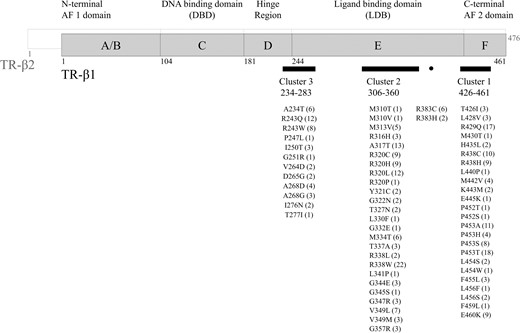
THRB variants found in the 284 Italian RTHβ patients the numbers in brackets represent the number of individuals harboring non-synonymous variants of THRB gene. * Denotes novel missense variants segregating with central hyperthyroidism in the affected families and located at the same codon as other known pathogenic mutations (p.P452R/L/S and p.L456S/W, respectively).
Thirty-five and eleven patients were submitted to total thyroidectomy and radioiodine, respectively.
To assess possible selection bias, we studied the baseline characteristics of the patients on follow-up and those lost to follow-up (Table S1). The male-to-female ratio, the age at diagnosis, the prevalence of risk factors for CV diseases (obesity, hypertension, diabetes and HFG, and dyslipidemia) as well as fT4 levels at baseline were not statistically different in the two groups. On the contrary, the different features leading to RTHβ diagnosis are not expected to cause a significant bias. Indeed, about 50% and 25% of the patients were diagnosed because of familiar screening or thyrotoxic features, respectively (Table 2). In addition, 40% of thyrotoxic patients, did not report subjective tachycardia at diagnosis and only in 3% of cases cardiac arrythmias was the presenting phenotype (Table 2). This is not surprising, as in Italy the routinary screening for thyroid dysfunctions is made with a reflex TSH strategy and the diagnosis of RTHβ is often delayed even in patients with severe cardiac manifestations.
Prevalence of MACE and CV manifestations according to the main features leading to RTHβ diagnosis.
| Features leading to RTHβ diagnosis . | N (%) . | CV manifestations at diagnosis N . | MACE events at diagnosis N . |
|---|---|---|---|
| Familial screening | 136 (47.9%) | 28 | 8 |
| Failure to thrive/ADHD | 27 (9.5%) | 2 | 0 |
| Incidental finding | 18 (6.3%) | 0 | 0 |
| Nodular goiter | 15 (5.3%) | 2 | 0 |
| Hypothyroidism refractory to treatment | 7 (2.5%) | 2 | 1 |
| Graves’ diseasea | 2 (0.7%) | 0 | 0 |
| Thyrotoxic features (tachycardia N = 37 or other features N = 33) | 70 (24.6%) | 50 | 6 |
| Cardiac arrhythmias N = 8 (atrial fibrillation, paroxysmal supraventricular tachycardia) or heart failure N = 1 | 9 (3.2%) | 9 | 9 |
| Total | 284 | 93 | 24 |
| Features leading to RTHβ diagnosis . | N (%) . | CV manifestations at diagnosis N . | MACE events at diagnosis N . |
|---|---|---|---|
| Familial screening | 136 (47.9%) | 28 | 8 |
| Failure to thrive/ADHD | 27 (9.5%) | 2 | 0 |
| Incidental finding | 18 (6.3%) | 0 | 0 |
| Nodular goiter | 15 (5.3%) | 2 | 0 |
| Hypothyroidism refractory to treatment | 7 (2.5%) | 2 | 1 |
| Graves’ diseasea | 2 (0.7%) | 0 | 0 |
| Thyrotoxic features (tachycardia N = 37 or other features N = 33) | 70 (24.6%) | 50 | 6 |
| Cardiac arrhythmias N = 8 (atrial fibrillation, paroxysmal supraventricular tachycardia) or heart failure N = 1 | 9 (3.2%) | 9 | 9 |
| Total | 284 | 93 | 24 |
aGraves’ disease patients had suppressed TSH at diagnosis and central hyperthyroidism become evident following thionamides treatment.
CV cardiovascular, ADHD attention deficit hyperactivity disorder.
Prevalence of MACE and CV manifestations according to the main features leading to RTHβ diagnosis.
| Features leading to RTHβ diagnosis . | N (%) . | CV manifestations at diagnosis N . | MACE events at diagnosis N . |
|---|---|---|---|
| Familial screening | 136 (47.9%) | 28 | 8 |
| Failure to thrive/ADHD | 27 (9.5%) | 2 | 0 |
| Incidental finding | 18 (6.3%) | 0 | 0 |
| Nodular goiter | 15 (5.3%) | 2 | 0 |
| Hypothyroidism refractory to treatment | 7 (2.5%) | 2 | 1 |
| Graves’ diseasea | 2 (0.7%) | 0 | 0 |
| Thyrotoxic features (tachycardia N = 37 or other features N = 33) | 70 (24.6%) | 50 | 6 |
| Cardiac arrhythmias N = 8 (atrial fibrillation, paroxysmal supraventricular tachycardia) or heart failure N = 1 | 9 (3.2%) | 9 | 9 |
| Total | 284 | 93 | 24 |
| Features leading to RTHβ diagnosis . | N (%) . | CV manifestations at diagnosis N . | MACE events at diagnosis N . |
|---|---|---|---|
| Familial screening | 136 (47.9%) | 28 | 8 |
| Failure to thrive/ADHD | 27 (9.5%) | 2 | 0 |
| Incidental finding | 18 (6.3%) | 0 | 0 |
| Nodular goiter | 15 (5.3%) | 2 | 0 |
| Hypothyroidism refractory to treatment | 7 (2.5%) | 2 | 1 |
| Graves’ diseasea | 2 (0.7%) | 0 | 0 |
| Thyrotoxic features (tachycardia N = 37 or other features N = 33) | 70 (24.6%) | 50 | 6 |
| Cardiac arrhythmias N = 8 (atrial fibrillation, paroxysmal supraventricular tachycardia) or heart failure N = 1 | 9 (3.2%) | 9 | 9 |
| Total | 284 | 93 | 24 |
aGraves’ disease patients had suppressed TSH at diagnosis and central hyperthyroidism become evident following thionamides treatment.
CV cardiovascular, ADHD attention deficit hyperactivity disorder.
Cardiovascular manifestations in RTHβ patients
One hundred twenty five RTHβ patients had a cardiovascular morbidity at diagnosis or during follow-up. The most frequent manifestation was sinus tachycardia (N = 79), followed by atrial fibrillation (N = 34) (Figure 1 and Table 1). Cerebrovascular and coronary ischemic events occurred in 22 patients, with fatal consequence in 13 cases (Table 1). The cumulative prevalence of CV manifestations and MACEs was 64% and 24%, respectively and MACEs occurred at a median age (IQR) of 59.4 years (50.4-66.4) in RTHβ (Table 1). In particular, MACEs occurred before the age of 55 years and 40 years, in 40% and 15% of cases, respectively.
Interestingly, while in the general population death rates for CVD decreased by 54% between 1990 and 2017,17 in the same period, the cumulative prevalence of CV manifestations and MACE in RTHβ did not significantly modify over time (Cochran-Armitage test for trend, P = .76 and P = .42, Figure S1, Panel A and B).
Twenty-five RTHβ patients died during follow-up at a median age of 74.5 years (Table 1). Stratified charts with life expectancy of the Italian reference population for age and years are available in the period 1980-2023. By comparing the survival of RTHβ with these charts, we found that life expectancy was reduced in RTHβ patients (median, IQR = 76.5, 66.8-80.5 years vs 83.0, 79-84.5 years, P = .0001, Mann Whitney test). We then calculated the lost year of life of the 25 deceased patients as the difference between the stratified-population life expectancy and the age of death, and we found a mean of 11.2 years of life lost in the RTHβ cohort. In particular, only 7 patients reached or surpassed their life expectancy. The remaining 18 patients died prematurely 5 to 37 years before the life expectancy (Table 1). MACEs were the most common cause of death (60%), however, the reason of death was unknown in 30% of the patients (Table 1).
Risk factors associated with cardiovascular involvement in RTHβ
When analyzing the unadjusted risk factors associated with MACEs, we found that hypertension, high fasting glucose/diabetes, dyslipidemia, fT4/ULN ratio, goiter, age at diagnosis, and male gender were associated with an increased risk. In contrast, no effects on MACEs risk were found for thyroidectomy, radioiodine treatment, levothyroxine treatment for iatrogenic/autoimmune hypothyroidism, thyroid autoimmunity, obesity, betablockers or TRIAC treatments, the type of mutant or location of the variants (Table 3A, on the left). At multivariable analyses only fT4/ULN ratio, age at diagnosis, and male gender retained the statistical significance (Table 3A, on the right).
| Risk factor for MACEs . | . | Univariable . | Multivariable (N = 128, 39 events) . | ||||
|---|---|---|---|---|---|---|---|
| N . | OR . | 95% CI . | P-value . | OR . | 95% CI . | P-value . | |
| A) Risk factors associated with MACE | |||||||
| Male gender | 178 | 2.34 | 1.19-4.41 | 0.02 | 6.86 | 1.97-23.84 | 0.003 |
| Age at diagnosis (years) | 178 | 1.08 | 1.05-1.15 | <0.0001 | 1.10 | 1.04-1.15 | <0.0001 |
| fT4/ULN | 156 | 2.21 | 1.02-4.74 | 0.04 | 5.12 | 1.40-18.74 | 0.01 |
| Diabetes/HFG | 170 | 2.85 | 1.12-7.27 | 0.03 | 1.59 | 0.29-8.60 | 0.59 |
| Hypertension | 168 | 5.11 | 2.45-10.63 | <0.0001 | 2.74 | 0.69-10.90 | 0.15 |
| Dyslipidemia/cholesterol-lowering drugs | 152 | 3.72 | 1.78-7.89 | 0.0005 | 0.95 | 0.24-3.61 | 0.94 |
| Obesity | 158 | 0.66 | 0.18-2.49 | 0.56 | 0.52 | 0.05-5.05 | 0.56 |
| Thyroid autoimmunity (positive TPO or Tg-Ab) and/or US pattern | 150 | 0.43 | 0.19-1.08 | 0.06 | |||
| Associated hypothyroidism (iatrogenic/autoimmune) | 173 | 1.34 | 0.28-3.05 | 0.49 | |||
| Goiter (simple or nodular) | 151 | 2.54 | 1.11-5.87 | 0.02 | |||
| Previous anti-thyroid drugs | 153 | 1.26 | 0.54-2.96 | 0.58 | |||
| Previous 131I | 170 | 3.21 | 0.85-11.50 | 0.08 | |||
| Previous thyroidectomy | 170 | 1.01 | 0.39-2.57 | 0.97 | |||
| TRIAC | 135 | 0.46 | 0.10-2.39 | 0.43 | |||
| Betablockers | 138 | 2.61 | 0.94-7.11 | 0.06 | |||
| Risk factor for MACEs . | . | Univariable . | Multivariable (N = 128, 39 events) . | ||||
|---|---|---|---|---|---|---|---|
| N . | OR . | 95% CI . | P-value . | OR . | 95% CI . | P-value . | |
| A) Risk factors associated with MACE | |||||||
| Male gender | 178 | 2.34 | 1.19-4.41 | 0.02 | 6.86 | 1.97-23.84 | 0.003 |
| Age at diagnosis (years) | 178 | 1.08 | 1.05-1.15 | <0.0001 | 1.10 | 1.04-1.15 | <0.0001 |
| fT4/ULN | 156 | 2.21 | 1.02-4.74 | 0.04 | 5.12 | 1.40-18.74 | 0.01 |
| Diabetes/HFG | 170 | 2.85 | 1.12-7.27 | 0.03 | 1.59 | 0.29-8.60 | 0.59 |
| Hypertension | 168 | 5.11 | 2.45-10.63 | <0.0001 | 2.74 | 0.69-10.90 | 0.15 |
| Dyslipidemia/cholesterol-lowering drugs | 152 | 3.72 | 1.78-7.89 | 0.0005 | 0.95 | 0.24-3.61 | 0.94 |
| Obesity | 158 | 0.66 | 0.18-2.49 | 0.56 | 0.52 | 0.05-5.05 | 0.56 |
| Thyroid autoimmunity (positive TPO or Tg-Ab) and/or US pattern | 150 | 0.43 | 0.19-1.08 | 0.06 | |||
| Associated hypothyroidism (iatrogenic/autoimmune) | 173 | 1.34 | 0.28-3.05 | 0.49 | |||
| Goiter (simple or nodular) | 151 | 2.54 | 1.11-5.87 | 0.02 | |||
| Previous anti-thyroid drugs | 153 | 1.26 | 0.54-2.96 | 0.58 | |||
| Previous 131I | 170 | 3.21 | 0.85-11.50 | 0.08 | |||
| Previous thyroidectomy | 170 | 1.01 | 0.39-2.57 | 0.97 | |||
| TRIAC | 135 | 0.46 | 0.10-2.39 | 0.43 | |||
| Betablockers | 138 | 2.61 | 0.94-7.11 | 0.06 | |||
| Risk factor for mortality . | . | Univariable . | Multivariable (N = 141, 21 events) . | ||||
|---|---|---|---|---|---|---|---|
| N . | OR . | 95% CI . | P-value . | OR . | 95% CI . | P-value . | |
| B) Risk factors associated with mortality | |||||||
| Male gender | 249 | 2.36 | 1.02-5.49 | <0.05 | 1.78 | 0.47-6.58 | 0.39 |
| Age at diagnosis (years) | 249 | 1.08 | 1.05-1.15 | <0.0001 | 1.09 | 1.05-1.16 | 0.0003 |
| fT4/ULN | 211 | 2.36 | 1.05-5.36 | 0.04 | 8.84 | 1.90-44.01 | 0.008 |
| Diabetes/HFG | 189 | 1.73 | 0.53-5.65 | 0.36 | 0.76 | 0.27-4.56 | 0.77 |
| Hypertension | 187 | 4.01 | 1.61-10.00 | 0.03 | 2.71 | 0.58-12.66 | 0.20 |
| Dyslipidemia/cholesterol-lowering drugs | 183 | 3.30 | 1.32-8.23 | 0.01 | 0.94 | 0.21-4.23 | 0.94 |
| Obesity | NA | NA | |||||
| Atrial fibrillation | 210 | 7.93 | 3.07-20.58 | <0.0001 | 0.77 | 0.17-3.44 | 0.74 |
| Thyroid autoimmunity (positive TPO or Tg-Ab) and/or US pattern | 199 | 0.53 | 0.17-2.24 | 0.28 | |||
| Associated hypothyroidism (iatrogenic/autoimmune) | 239 | 0.63 | 0.21-2.22 | 0.47 | |||
| Goiter (simple or nodular) | 200 | 5.45 | 1.22-24.55 | 0.03 | |||
| Previous anti-thyroid drugs | 205 | 1.54 | 0.52-4.52 | 0.45 | |||
| Previous 131I | 211 | 1.28 | 0.15-10.80 | 0.82 | |||
| Previous thyroidectomy | 231 | 0.33 | 0.05-2.56 | 0.29 | |||
| TRIAC | 177 | 0.46 | 0.06-3.87 | 0.46 | |||
| Betablockers | 177 | 2.63 | 0.79-8.76 | 0.12 | |||
| Risk factor for mortality . | . | Univariable . | Multivariable (N = 141, 21 events) . | ||||
|---|---|---|---|---|---|---|---|
| N . | OR . | 95% CI . | P-value . | OR . | 95% CI . | P-value . | |
| B) Risk factors associated with mortality | |||||||
| Male gender | 249 | 2.36 | 1.02-5.49 | <0.05 | 1.78 | 0.47-6.58 | 0.39 |
| Age at diagnosis (years) | 249 | 1.08 | 1.05-1.15 | <0.0001 | 1.09 | 1.05-1.16 | 0.0003 |
| fT4/ULN | 211 | 2.36 | 1.05-5.36 | 0.04 | 8.84 | 1.90-44.01 | 0.008 |
| Diabetes/HFG | 189 | 1.73 | 0.53-5.65 | 0.36 | 0.76 | 0.27-4.56 | 0.77 |
| Hypertension | 187 | 4.01 | 1.61-10.00 | 0.03 | 2.71 | 0.58-12.66 | 0.20 |
| Dyslipidemia/cholesterol-lowering drugs | 183 | 3.30 | 1.32-8.23 | 0.01 | 0.94 | 0.21-4.23 | 0.94 |
| Obesity | NA | NA | |||||
| Atrial fibrillation | 210 | 7.93 | 3.07-20.58 | <0.0001 | 0.77 | 0.17-3.44 | 0.74 |
| Thyroid autoimmunity (positive TPO or Tg-Ab) and/or US pattern | 199 | 0.53 | 0.17-2.24 | 0.28 | |||
| Associated hypothyroidism (iatrogenic/autoimmune) | 239 | 0.63 | 0.21-2.22 | 0.47 | |||
| Goiter (simple or nodular) | 200 | 5.45 | 1.22-24.55 | 0.03 | |||
| Previous anti-thyroid drugs | 205 | 1.54 | 0.52-4.52 | 0.45 | |||
| Previous 131I | 211 | 1.28 | 0.15-10.80 | 0.82 | |||
| Previous thyroidectomy | 231 | 0.33 | 0.05-2.56 | 0.29 | |||
| TRIAC | 177 | 0.46 | 0.06-3.87 | 0.46 | |||
| Betablockers | 177 | 2.63 | 0.79-8.76 | 0.12 | |||
HFG high fasting glucose, 131I radioiodine treatment, ULN upper limit of normal, OR odds ratio.
| Risk factor for MACEs . | . | Univariable . | Multivariable (N = 128, 39 events) . | ||||
|---|---|---|---|---|---|---|---|
| N . | OR . | 95% CI . | P-value . | OR . | 95% CI . | P-value . | |
| A) Risk factors associated with MACE | |||||||
| Male gender | 178 | 2.34 | 1.19-4.41 | 0.02 | 6.86 | 1.97-23.84 | 0.003 |
| Age at diagnosis (years) | 178 | 1.08 | 1.05-1.15 | <0.0001 | 1.10 | 1.04-1.15 | <0.0001 |
| fT4/ULN | 156 | 2.21 | 1.02-4.74 | 0.04 | 5.12 | 1.40-18.74 | 0.01 |
| Diabetes/HFG | 170 | 2.85 | 1.12-7.27 | 0.03 | 1.59 | 0.29-8.60 | 0.59 |
| Hypertension | 168 | 5.11 | 2.45-10.63 | <0.0001 | 2.74 | 0.69-10.90 | 0.15 |
| Dyslipidemia/cholesterol-lowering drugs | 152 | 3.72 | 1.78-7.89 | 0.0005 | 0.95 | 0.24-3.61 | 0.94 |
| Obesity | 158 | 0.66 | 0.18-2.49 | 0.56 | 0.52 | 0.05-5.05 | 0.56 |
| Thyroid autoimmunity (positive TPO or Tg-Ab) and/or US pattern | 150 | 0.43 | 0.19-1.08 | 0.06 | |||
| Associated hypothyroidism (iatrogenic/autoimmune) | 173 | 1.34 | 0.28-3.05 | 0.49 | |||
| Goiter (simple or nodular) | 151 | 2.54 | 1.11-5.87 | 0.02 | |||
| Previous anti-thyroid drugs | 153 | 1.26 | 0.54-2.96 | 0.58 | |||
| Previous 131I | 170 | 3.21 | 0.85-11.50 | 0.08 | |||
| Previous thyroidectomy | 170 | 1.01 | 0.39-2.57 | 0.97 | |||
| TRIAC | 135 | 0.46 | 0.10-2.39 | 0.43 | |||
| Betablockers | 138 | 2.61 | 0.94-7.11 | 0.06 | |||
| Risk factor for MACEs . | . | Univariable . | Multivariable (N = 128, 39 events) . | ||||
|---|---|---|---|---|---|---|---|
| N . | OR . | 95% CI . | P-value . | OR . | 95% CI . | P-value . | |
| A) Risk factors associated with MACE | |||||||
| Male gender | 178 | 2.34 | 1.19-4.41 | 0.02 | 6.86 | 1.97-23.84 | 0.003 |
| Age at diagnosis (years) | 178 | 1.08 | 1.05-1.15 | <0.0001 | 1.10 | 1.04-1.15 | <0.0001 |
| fT4/ULN | 156 | 2.21 | 1.02-4.74 | 0.04 | 5.12 | 1.40-18.74 | 0.01 |
| Diabetes/HFG | 170 | 2.85 | 1.12-7.27 | 0.03 | 1.59 | 0.29-8.60 | 0.59 |
| Hypertension | 168 | 5.11 | 2.45-10.63 | <0.0001 | 2.74 | 0.69-10.90 | 0.15 |
| Dyslipidemia/cholesterol-lowering drugs | 152 | 3.72 | 1.78-7.89 | 0.0005 | 0.95 | 0.24-3.61 | 0.94 |
| Obesity | 158 | 0.66 | 0.18-2.49 | 0.56 | 0.52 | 0.05-5.05 | 0.56 |
| Thyroid autoimmunity (positive TPO or Tg-Ab) and/or US pattern | 150 | 0.43 | 0.19-1.08 | 0.06 | |||
| Associated hypothyroidism (iatrogenic/autoimmune) | 173 | 1.34 | 0.28-3.05 | 0.49 | |||
| Goiter (simple or nodular) | 151 | 2.54 | 1.11-5.87 | 0.02 | |||
| Previous anti-thyroid drugs | 153 | 1.26 | 0.54-2.96 | 0.58 | |||
| Previous 131I | 170 | 3.21 | 0.85-11.50 | 0.08 | |||
| Previous thyroidectomy | 170 | 1.01 | 0.39-2.57 | 0.97 | |||
| TRIAC | 135 | 0.46 | 0.10-2.39 | 0.43 | |||
| Betablockers | 138 | 2.61 | 0.94-7.11 | 0.06 | |||
| Risk factor for mortality . | . | Univariable . | Multivariable (N = 141, 21 events) . | ||||
|---|---|---|---|---|---|---|---|
| N . | OR . | 95% CI . | P-value . | OR . | 95% CI . | P-value . | |
| B) Risk factors associated with mortality | |||||||
| Male gender | 249 | 2.36 | 1.02-5.49 | <0.05 | 1.78 | 0.47-6.58 | 0.39 |
| Age at diagnosis (years) | 249 | 1.08 | 1.05-1.15 | <0.0001 | 1.09 | 1.05-1.16 | 0.0003 |
| fT4/ULN | 211 | 2.36 | 1.05-5.36 | 0.04 | 8.84 | 1.90-44.01 | 0.008 |
| Diabetes/HFG | 189 | 1.73 | 0.53-5.65 | 0.36 | 0.76 | 0.27-4.56 | 0.77 |
| Hypertension | 187 | 4.01 | 1.61-10.00 | 0.03 | 2.71 | 0.58-12.66 | 0.20 |
| Dyslipidemia/cholesterol-lowering drugs | 183 | 3.30 | 1.32-8.23 | 0.01 | 0.94 | 0.21-4.23 | 0.94 |
| Obesity | NA | NA | |||||
| Atrial fibrillation | 210 | 7.93 | 3.07-20.58 | <0.0001 | 0.77 | 0.17-3.44 | 0.74 |
| Thyroid autoimmunity (positive TPO or Tg-Ab) and/or US pattern | 199 | 0.53 | 0.17-2.24 | 0.28 | |||
| Associated hypothyroidism (iatrogenic/autoimmune) | 239 | 0.63 | 0.21-2.22 | 0.47 | |||
| Goiter (simple or nodular) | 200 | 5.45 | 1.22-24.55 | 0.03 | |||
| Previous anti-thyroid drugs | 205 | 1.54 | 0.52-4.52 | 0.45 | |||
| Previous 131I | 211 | 1.28 | 0.15-10.80 | 0.82 | |||
| Previous thyroidectomy | 231 | 0.33 | 0.05-2.56 | 0.29 | |||
| TRIAC | 177 | 0.46 | 0.06-3.87 | 0.46 | |||
| Betablockers | 177 | 2.63 | 0.79-8.76 | 0.12 | |||
| Risk factor for mortality . | . | Univariable . | Multivariable (N = 141, 21 events) . | ||||
|---|---|---|---|---|---|---|---|
| N . | OR . | 95% CI . | P-value . | OR . | 95% CI . | P-value . | |
| B) Risk factors associated with mortality | |||||||
| Male gender | 249 | 2.36 | 1.02-5.49 | <0.05 | 1.78 | 0.47-6.58 | 0.39 |
| Age at diagnosis (years) | 249 | 1.08 | 1.05-1.15 | <0.0001 | 1.09 | 1.05-1.16 | 0.0003 |
| fT4/ULN | 211 | 2.36 | 1.05-5.36 | 0.04 | 8.84 | 1.90-44.01 | 0.008 |
| Diabetes/HFG | 189 | 1.73 | 0.53-5.65 | 0.36 | 0.76 | 0.27-4.56 | 0.77 |
| Hypertension | 187 | 4.01 | 1.61-10.00 | 0.03 | 2.71 | 0.58-12.66 | 0.20 |
| Dyslipidemia/cholesterol-lowering drugs | 183 | 3.30 | 1.32-8.23 | 0.01 | 0.94 | 0.21-4.23 | 0.94 |
| Obesity | NA | NA | |||||
| Atrial fibrillation | 210 | 7.93 | 3.07-20.58 | <0.0001 | 0.77 | 0.17-3.44 | 0.74 |
| Thyroid autoimmunity (positive TPO or Tg-Ab) and/or US pattern | 199 | 0.53 | 0.17-2.24 | 0.28 | |||
| Associated hypothyroidism (iatrogenic/autoimmune) | 239 | 0.63 | 0.21-2.22 | 0.47 | |||
| Goiter (simple or nodular) | 200 | 5.45 | 1.22-24.55 | 0.03 | |||
| Previous anti-thyroid drugs | 205 | 1.54 | 0.52-4.52 | 0.45 | |||
| Previous 131I | 211 | 1.28 | 0.15-10.80 | 0.82 | |||
| Previous thyroidectomy | 231 | 0.33 | 0.05-2.56 | 0.29 | |||
| TRIAC | 177 | 0.46 | 0.06-3.87 | 0.46 | |||
| Betablockers | 177 | 2.63 | 0.79-8.76 | 0.12 | |||
HFG high fasting glucose, 131I radioiodine treatment, ULN upper limit of normal, OR odds ratio.
Regarding mortality, we found that fT4/ULN ratio, age at diagnosis, atrial fibrillation, hypertension, male gender, and goiter were associated with a mild increase of the risk, although at multivariable logistic analyses only fT4/ULN ratio and age at diagnosis were significantly associated with mortality (Table 3B, on the right). Interestingly, fT4/ULN was associated with MACE and mortality, with an OR of 5.1 and 8.8, respectively (Table 3A and 3B, on the right).
We also performed a sensitivity analysis, by including in the logistic model the age at the included covariate measurement, instead of the age at diagnosis, confirming these findings (Table S3).
Given the significant association of fT4/ULN with cardiovascular outcomes, we tried to identify a threshold associated with an increased risk. By ROC curve analysis, we found that fT4 values 57% higher than the ULN were significantly associated with premature cardiovascular manifestations, although with a low discrimination accuracy (AUC <0.7). The AUC of the curve improved when the analysis was restricted to patients with premature (<55 years) CV involvement (P = .005, AUC 0.675) (Figure 3).
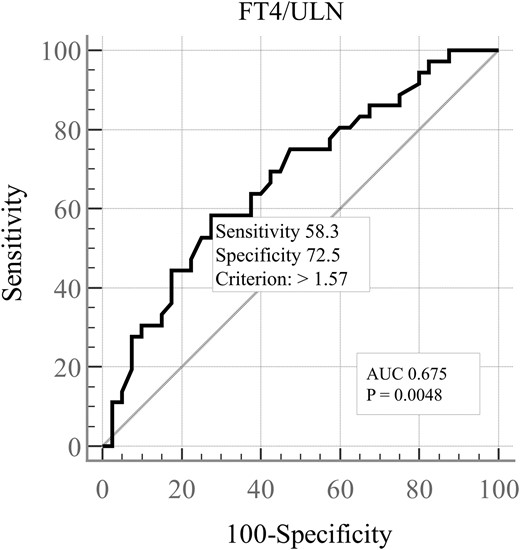
Receiver operating characteristic curve (ROC) for prediction of cardiovascular manifestations based on the fT4 serum levels. ULN upper limit of the normal range, AUC area under the curve.
The impact of anti-thyroid drugs on MACE and mortality could not be assessed as all patients discontinued the thionamides following RTHβ diagnosis. However, we have studied the association with this treatment on some thyroid outcomes, and we found that methimazole was associated with a substantial risk of goiter, thyroid nodules, and the need for total thyroidectomy for compressive symptoms (Table S2).
Discussion
We studied a large Italian cohort of RTHβ patients carrying heterozygous THRB pathogenic variants and we found that RTHβ patients are at risk of premature MACEs and earlier mortality when compared to the reference population, similarly, to what was recently described in a smaller sample of Welsh patients.13 In addition, we provide new information on several risk factors that appear to play an important role in the cardiovascular predisposition of these patients.
In about 40% of RTHβ patients of this cohort, MACEs occurred earlier than 55 years of age and early mortality resulted in a mean of 11 years of life lost in RTHβ patients.18,19 Sixty-four patients at diagnosis and fourteen during follow-up (40%) had sinus/supraventricular tachycardia. Twenty-four cases at diagnosis and eleven during follow-up had atrial fibrillation (AF), leading to a prevalence of 18% which is instead 2.04% and 8.1% in the Italian population over 17 and 65 years, respectively.18,19
We observed that fasting hyperglycemia and diabetes, dyslipidemia, hypertension, and male gender were all associated with MACEs, similarly to what usually occurs in the general population. Interestingly, the unadjusted risk factors associated with mortality were male gender, hypertension, and previous episode of atrial fibrillation.
The increased CV risk of RTHβ patients likely depends upon the combination of metabolic disorders (dyslipidemia, hepatic steatosis, high fasting glucose) and excessive TH action on the cardiac function.8-12 Our study cannot distinguish the relative weight of these different risk factors, but at multivariable analysis the associations with metabolic characteristics were lost, while significant association remained only between age at diagnosis, increased fT4 levels, and gender with MACEs or age at diagnosis and higher fT4 levels with mortality. We then compared RTHβ patients with or without premature cardiac manifestations and found significantly higher fT4 levels (P = .005) in symptomatic versus asymptomatic patients. Consistently, we found that the fT4 values 57% higher than the ULN were associated with premature cardiovascular manifestations. Interestingly, this cut-off value was similar to the one identified by Okosieme et al (30 pmol/L)..13 The whole of these data indicates that chronic exposure of heart to high thyroid hormones favors the progression toward AF and MACEs. Nevertheless, neither TRIAC, nor an associated hypothyroidism, nor previous thyroidectomy nor radioiodine treatment had any statistically significant effect in reducing the risk of MACEs and mortality.
Thionamides were discontinued in all cases following the diagnosis of RTHβ, thus we have no data regarding a possible use of these drugs. Nevertheless, we show that previous chronic methimazole treatment was significantly associated with accelerated goiter growth and total thyroidectomy for compressive symptoms (Table S2)
Among the factors contributing to the increased cardiovascular risk, one could also consider the lower educational level and learning disabilities that are typical of RTHβ,20 but also represent well-known risk factors in the Italian population.17,21
Because of the assumption that most RTHβ patients do not require treatment, a considerable number of patients discontinued TRIAC (which is still available only as galenic formulation in Italy) and had limited investigations after diagnosis, and this may have contributed to the cardiovascular burden.
Moreover, our data indicate an insufficient awareness of RTHβ and its intrinsic morbidity among both patients and general practitioners. For example, less than half of the historical RTHβ patients applied for the rare disease certificate, giving right to a comprehensive exemption from health care charges in Italy.
The main limitation of our study is the lack of a control population to conduct a cohort study comparing individuals with and without RTHβ.13 This would be tough to accomplish because de-identified data from the general population are designed on a regional basis. Furthermore, the RTHβ registry, which was launched in 2017, is insufficient since we have evidence that several historical patients have not yet been recorded, thus matching between case and controls would be presently unfeasible. In addition, we have very limited information on smoking habit of these patients and occurrence of cardiovascular disease in the unaffected members of the family. Since TRIAC was administered only to 10% of the patients and in half of patients betablockers were given only after MACEs, we cannot give any information on the role of these treatments on primary cardiovascular prevention. The number of secondary MACEs was also insufficient to perform additional sub-analysis to study their role in secondary prevention. The lack of follow-up information in 129 patients and the inability to certify the existence in life of 35 patients have indeed reduced the power of this study.
In conclusion, we found that Italian RTHβ patients are at risk of MACEs and premature death.
These data should raise the general awareness on the cardiovascular risk and prompt a proactive cardiovascular monitoring in RTHβ, especially in males and in those with fT4 levels 57% higher than the ULN (about 30 pmol/L). Our data suggest that most of the risk factors associated with CVDs in RTHβ are the same of the general population. Thus, we believe that a healthy lifestyle and drugs aimed at controlling hypertension, metabolic disorders, and preventing overweight should be recommended in these patients.
Given the impact of increased fT4 levels on the prevalence of CVDs, further prospective randomized trials are needed to find the best pharmacological treatment options for RTHβ patients.
Acknowledgments
We thank Martina Romanisio and Valeria Cambria for technical assistance. We acknowledge the major contribution of Professor Paolo Beck-Peccoz, Dr. Deborah Mannavola, Dr Giorgio Radetti, to the data collection process along the years and we thank the contribution of several additional clinicians for the referral of Italian RTHβ patients to our group.
Supplementary material
Supplementary material is available at European Journal of Endocrinology online.
Funding
This work was funded by BIBLIOSAN.
Authors’ contributions
Irene Campi (Conceptualization [equal], Data curation [lead], Formal analysis [lead], Investigation [equal], Writing—original draft [lead], Writing—review & editing [supporting]), Simona Censi (Data curation [equal], Investigation [equal], Visualization [supporting], Writing—original draft [equal]), Flavia Prodam (Data curation [equal], Investigation [equal], Visualization [supporting], Writing—original draft [equal]), Luisa Petrone (Data curation [equal], Investigation [equal], Visualization [supporting], Writing—original draft [equal]), Giulia Brigante (Data curation [equal], Investigation [equal], Visualization [supporting], Writing—original draft [equal]), Tommaso Porcelli (Data curation [equal], Investigation [equal], Visualization [supporting], Writing—original draft [equal]), Maria Cristina Vigone (Data curation [equal], Investigation [equal], Visualization [supporting], Writing—original draft [equal]), Giuditta Rurale (Data curation [equal], Formal analysis [supporting], Investigation [equal], Visualization [supporting], Writing—original draft [equal], Writing—review & editing [supporting]), Serafino Lio (Data curation [equal], Investigation [equal], Visualization [supporting], Writing—original draft [equal]), Carla Pelusi (Data curation [equal], Investigation [equal], Visualization [supporting], Writing—original draft [equal]), Luca Persani (Conceptualization [lead], Funding acquisition [lead], Investigation [equal], Methodology [lead], Supervision [lead], Validation [equal], Visualization [equal], Writing—review & editing [lead]), and Rosaria Maddalena Ruggeri (Data curation [equal], Investigation [equal], Visualization [equal], Writing—original draft [equal])
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to privacy restrictions. Deidentified participant data are available from the corresponding author on reasonable request.
References
Author notes
Conflict of interest: No competing financial interests exist.



