-
PDF
- Split View
-
Views
-
Cite
Cite
Juliane Lippert, Gabrielle Smith, Silke Appenzeller, Laura-Sophie Landwehr, Alessandro Prete, Sonja Steinhauer, Miriam Asia, Hanna Urlaub, Yasir S Elhassan, Stefan Kircher, Wiebke Arlt, Martin Fassnacht, Barbara Altieri, Cristina L Ronchi, Circulating cell-free DNA-based biomarkers for prognostication and disease monitoring in adrenocortical carcinoma, European Journal of Endocrinology, Volume 190, Issue 3, March 2024, Pages 234–247, https://doi.org/10.1093/ejendo/lvae022
Close - Share Icon Share
Abstract
Adrenocortical carcinoma (ACC) is a rare aggressive cancer with heterogeneous behaviour. Disease surveillance relies on frequent imaging, which comes with significant radiation exposure. The aim of the study was to investigate the role of circulating cell-free DNA (ccfDNA)-related biomarkers (BMs) for prognostication and monitoring of ACC.
We investigated 34 patients with ACC and 23 healthy subjects (HSs) as controls. Circulating cell-free DNA was extracted by commercial kits and ccfDNA concentrations were quantified by fluorimeter (BM1). Targeted sequencing was performed using a customized panel of 27 ACC-specific genes. Leucocyte DNA was used to discriminate somatic variants (BM2), while tumour DNA was sequenced in 22/34 cases for comparison. Serial ccfDNA samples were collected during follow-up in 19 ACC patients (median period 9 months) and analysed in relationship with standard radiological imaging.
Circulating cell-free DNA concentrations were higher in ACC than HS (mean ± SD, 1.15 ± 1.56 vs 0.05 ± 0.05 ng/µL, P < .0001), 96% of them being above the cut-off of 0.146 ng/µL (mean HS + 2 SD, positive BM1). At ccfDNA sequencing, 47% of ACC showed at least 1 somatic mutation (positive BM2). A combined ccfDNA-BM score was strongly associated with both progression-free and overall survival (hazard ratio [HR] = 2.63; 95% CI, 1.13-6.13; P = .010, and HR = 5.98; 95% CI, 2.29-15.6; P = .0001, respectively). During disease monitoring, positive BM2 showed the best specificity (100%) and sensitivity (67%) to detect ACC recurrence or progress compared with BM1.
ccfDNA-related BMs are frequently detected in ACC patients and represent a promising, minimally invasive tool to predict clinical outcome and complement surveillance imaging. Our findings will be validated in a larger cohort of ACCs with long-term follow-up.
Adrenocortical carcinoma (ACC) is a rare and generally highly aggressive cancer. Despite recent developments, there are still critical unmet clinical needs for patients with ACC. In fact, no markers are available that can predict clinical outcomes at the time of the diagnosis. Moreover, follow-up requires frequent imaging that results in increased radiation exposure and cannot always answer diagnostic questions. In this study, we have developed a method for the evaluation of specific alterations in small fragments of genetic information (= DNA) released from tumour cells into the blood. By correlating these alterations with clinical data and standard radiological imaging, we have demonstrated that this approach can identify markers that could help to better predict the clinical course of ACC patients and recognize disease relapses and/or progression.
Introduction
Adrenocortical carcinoma (ACC) is a rare malignancy with a generally poor but heterogeneous prognosis.1 Five-year survival rates range from 13% to 80% depending on the European Network for the Study of Adrenocortical Tumors (ENSAT) tumour staging, resection status, and Ki67 index.1,2 However, currently available clinical and histopathological factors cannot always reliably distinguish patients with favourable from those with worse prognosis.3 Moreover, disease recurrences after resection of the primary tumour are frequent even in lower-risk ENSAT stages, and effective pharmacological therapies for advanced stages are lacking. Therefore, close disease monitoring is essential to allow timely management but relies on frequent radiological imaging,2 which causes relevant not only radiation exposure for patients but also significant costs for the health systems.
Liquid biopsy, ie, the analysis of tumour material obtained in a minimally invasive manner by sampling of blood or other body fluids, is being increasingly proposed in oncology for molecular profiling, detection of residual disease, and monitoring of disease evolution.4,5 Decades ago, it was demonstrated that plasma from cancer patients contains higher concentrations of circulating cell-free DNA (ccfDNA) than those from healthy individuals6 assuming that at least a part of ccfDNA originates from cancer cells.7-9 As a consequence, even if the origin of ccfDNA cannot be definitively determined, elevated levels of short DNA fragments may be a good marker for the detection of tumour DNA (T-DNA) in blood10,11 and used to monitor tumour evolution and response to therapy.12,13 Furthermore, tumour-associated genetic alterations, such as single nucleotide variants (SNVs), can be detected in ccfDNA.14,15 Sequencing of ccfDNA presents important advantages compared with sequencing of tumour-derived DNA. Firstly, it holds the potential of detecting all the alterations contained in the tumour, while single tissue samples provide only a limited characterization of the molecular signature.16-19 This is particularly relevant for heterogeneous cancer types, such as ACC. Secondly, serial blood samples are compatible with dynamic and minimally invasive cancer surveillance.17 Circulating cell-free DNA analysis has been also proposed as a potential prognostic tool. The presence of genetic variants in tumour-specific genes at the ccfDNA level has been associated with worse clinical outcomes and suggested as a predictive marker of response to therapy in multiple cancer types.20-22 More recently, elevated total ccfDNA concentrations have also been reported as a simple and cheap marker of shorter survival in patients with different cancers.23, 24 Finally, sequencing of ccfDNA can be used to identify key treatment targets, both at the time of diagnosis and in case of tumour progression or recurrence.22
Only 2 previous studies performed ccfDNA analysis in patients with ACC.25, 26 However, these included small case cohorts (only 17 patients in these 2 studies combined) and used heterogeneous techniques for both ccfDNA isolation and sequencing, and their findings cannot be considered conclusive. Moreover, there is only 1 case report providing serial targeted ccfDNA analysis for tumour monitoring to date.27
The aim of the present pilot study was to investigate ccfDNA-based biomarkers (BMs) in a larger, well-characterized cohort of patients with ACC and their potential role both as prognostic factors (AIM 1) and as tools for the detection of tumour recurrence or progression (AIM 2).
Material and methods
Patient cohort and study design
In the present study, we investigated consecutive patients older than 18 years examined in 2 tertiary referral centres between 2019 and 2021 (Figure 1). Inclusion criteria were (1) patients with adrenal masses suspicious for ACC according to current guidelines,2,3 (2) fully available clinical, biochemical, and radiological data at the time of diagnosis, and (3) final diagnosis of ACC based on current guidelines.2,3 These included histopathological confirmation of ACC for patients that underwent adrenal surgery or biopsies, or large, radiologically suspicious adrenal masses associated with severe biochemical and/or clinical steroid excess. Patients who were diagnosed with benign or malignant adrenocortical lesions other than ACC after workup were excluded from the study. Other exclusion criteria included a diagnosis of other active concomitant cancers and severe alterations in liver or kidney functions. After consideration of inclusion and exclusion criteria, the final cohort comprised a total of 34 patients with primary ACC that served for the evaluation of ccfDNA-BMs for prognostic classification of ACC (AIM 1).
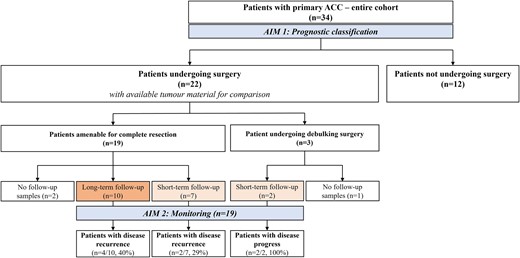
Flowchart of the patient cohort with primary ACC.
ACC, adrenocortical carcinoma; BM1, ccfDNA-based biomarker 1 (ccfDNA concentrations); BM2, ccfDNA-based biomarker 2 (somatic variants detected at ccfDNA level); long-term follow-up, at least 9 months after surgery; short-term follow-up, at least 3 months after surgery.
Peripheral blood samples were collected before surgery (baseline) in all participants with ACC. Blood samples were also collected from 23, as far as known, healthy subjects (HSs) recruited among university staff that served as controls for the baseline ccfDNA concentration analysis.
For a subgroup of patients with ACC, blood samples were additionally collected during standard follow-up visits after primary surgery (see details below and Figure 1) for the evaluation of the role of ccfDNA-based BMs as a monitoring tool (AIM 2).
The study is compliant with the Declaration of Helsinki. The study protocol was approved by both local ethics committees (#88/11 at the University Hospital of Wuerzburg; HBRC 11/606 and PrimeAct study REC 20/NW/0207 at the University of Birmingham). Written informed consent was obtained from all subjects.
Clinical, histopathological, and radiological data
Patient's age at diagnosis, symptoms at presentation (related to autonomous steroid secretion or mass effect), and initial ENSAT tumour stage were collected for all patients. Ki67 index and resection (R) status were recorded only for the 22 patients who underwent adrenalectomy (Figure 1) and used to calculate the S-GRAS score as previously published.28 In 4 additional cases, the Ki67 index was available from adrenal biopsies. A total of 15 patients received adjuvant treatment with mitotane after primary surgery according to current guidelines.2
Periodical surveillance imaging, ie, by thorax–abdomen–pelvis computed tomography scan with contrast, was performed every 3 months as per current guidelines.2 The occurrence of disease recurrence or progression as well as the total tumour burden was evaluated at baseline and periodical radiology scans as the sum of all measurable target lesions (in accordance with RECIST v1.1) by expert radiologists. The number and localization of eventual manifestations of disease recurrence were also recorded.
Overall survival (OS) was defined as the time from primary tumour resection or diagnosis to death. Progression-free survival (PFS) was defined as the time from diagnosis to the first radiological evidence of disease progression. Disease status and survival information were updated up to June 2023.
Sample processing and ccfDNA isolation
We have established a systematic and homogeneous pipeline for sample collection and processing in both our centres aiming to obtain reliable findings using clinically applicable techniques. In brief, 10-20 mL of blood were collected in EDTA tubes and kept on ice until centrifuged (within 2-3 h of blood collection) for 10 min at room temperature and 800 rpm. After centrifugation, plasma was transferred to clean centrifugation tubes without disturbing the buffy coat and centrifuged for another 10 min at 4 °C and 13.000 rpm. Plasma was transferred to a fresh centrifugation tube without disturbing the pellet and stored at −80 °C until analysis. Circulating cell-free DNA was isolated from 2-6 mL of plasma with the QIAamp MinElute ccfDNA Kit (Qiagen, Hilden, Germany) or the Cell3™ Xtract kit (Nonacus, Birmingham, UK) according to manufacturers’ instructions. We chose these commercially available kits according to their characteristics of suitability in clinical routine (including costings, time requirements and complexity of protocols, the necessity for additional equipment, and the amount of usable plasma). To confirm similarities between the 2 chosen kits, we compared the ccfDNA concentrations obtained in a representative subgroup of samples by isolating the same volume of plasma (1 mL) from the same samples. Hereby, we could demonstrate that the ccfDNA concentrations were superimposable between the Nonacus and the Qiagen kit (n = 6, 0.434 ± 0.203 vs 0.364 ± 0.182 ng/µL, P = .24).
Circulating cell-free DNA was then eluted in 40 µL of dH20 and stored at −20 °C until further processing.
ccfDNA analysis
ccfDNA concentration (BM1)
Circulating cell-free DNA concentrations were determined with a Quantus™ Fluorometer (Promega, Fitchburg, United States) according to the manufacturer's instructions. Different volumes of plasma taken for ccfDNA isolation were considered for the designation of the final ccfDNA concentration in a sample. A quality control (QC) for the desired fragment length of the ccfDNA (150-200 bp) was performed on a Bioanalyzer with Agilent High Sensitivity DNA Kit or with the TapeStation High Sensitivity 1000D system (both Agilent, Santa Clara, United States). All ccfDNA samples included in the analysis showed good quality in means of fragment length and no contamination with high molecular weight material. Representative examples of QC by TapeStation in both ACC and HS samples are shown in Figure S1. According to the QC analysis, we also calculated the calibrated ccfDNA concentrations (based on the percentage of concentrations at 100-250 bps) in a subgroup of 18 samples (including 14 patients with ACC and 4 HS). Here, we could observe a very good correlation between total and calibrated concentrations (F = 73.3, R = 0.906, P < .0001; Figure S2A and B). We therefore decided to use the total ccfDNA concentrations for all samples (ie, baseline and follow-ups).
ccfDNA sequencing for identification of somatic mutations (BM2)
All 34 baseline ccfDNA samples were sequenced. Longitudinal samples collected during follow-up were sequenced for 19 cases of patients who underwent adrenalectomy. In brief, ccfDNA samples were enriched with a customized gene panel, ie, Cell3™ Target Custom NGS Panel (Nonacus), according to the manufacturer's instructions. The Cell3™ Target is a target enrichment system for converting any type of DNA into libraries for next-generation sequencing. It uses error suppression technology to ensure confident calling of all mutations down to 0.1% variant allele frequency (VAF) and is ideal for rare variant detection in liquid biopsies (www.nonacus.com). The custom panel included 27 genes known to be associated with ACC29-31 (Table S1). These included 8 genes that are currently classified as drug targetable at different levels in the OncoKb database (www.oncokb.org), ie, TP53, KDM6A, EGFR, FGFR3, ATM, BRCA2, NF1, and PTCH1. The protocol included an end-repair and A-tailing step before adapter ligation at the beginning. After a pre-capture, PCR samples were pooled and target regions were hybridized and therefore enriched with biotin-labelled probes. Unique molecular identifiers were used to reduce the background noise created by PCR and sequencing errors and enable mutation calling of VAF down to 0.1%, especially important when deploying the ultra-deep sequencing necessary for the analysis of cfDNA. After another amplification step via post-capture PCR and quality check, libraries were ready for sequencing. Paired-end sequencing was performed on a NextSeq500 with NextSeq 500/550 Mid Output Kit v2.5 (150 Cycles) or on a NextSeq2000 with NextSeq 1000/2000 P2 Reagents (200 Cycles) v3 for estimated 13 Million reads per sample (Illumina, San Diego CA, United States).
To reliably classify ccfDNA variants as somatic or germline, reference germline DNA was isolated from matched peripheral blood samples using the NucleoSpin Blood L Kit (Macherey-Nagel, Bethlehem, PA, United States) according to the manufacturer's instructions. Library preparation of germline DNA was also conducted with the Cell3™ Target Custom NGS Panel (Nonacus) following the same protocol as for ccfDNA enrichment except of an initial fragmentation step. For those 12 patients where no tumour material was available for sequencing, we analysed genomic DNA from blood for the variants found in ccfDNA via Sanger sequencing.
Tumour tissue DNA isolation and sequencing
Matched formalin-fixed paraffin-embedded (FFPE) tumour tissues were available for sequencing in the 22 patients who underwent adrenalectomy. Tumour localization was annotated by an expert pathologist, and tumour cell content was assessed in a representative FFPE slide by haematoxylin–eosin staining before DNA isolation. Tumour cell content reached a high fraction (median 90%, range 60-95). DNA was isolated from tumour material using the GeneRead DNA FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer's instructions and as previously described.29 Library preparation of tumour was also conducted with the Cell3™ Target Custom NGS Panel (Nonacus) following the same protocol as for ccfDNA enrichment except for an initial fragmentation step.
Sequencing data analysis
Bcl2fastq de-multiplexing was performed as described in the Nonacus user manual (Cell3 ™ Target: Data Analysis Guidelines, Protocol Guide v1.0). Consensus BAM file preparation was also conducted according to the manufacturer's instructions using NonacusTools v1.0. Additionally, QC of the sequencing reads was carried out with FastQC v0.11.3 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), and read statistics were calculated using in-house scripts. Variant calling was performed with GensearchNGS (Phenosystems SA, Braine le Chateau, Belgium) for sequencing data from DNA isolated from tumour and blood, as well as ccfDNA. Called variants in tumour samples were compared with variants detected in germline DNA and further filtered for variants with minor allele frequency < 0.02, VAF > 0.2 for T-DNA and >0.01 for ccfDNA, coverage > 100, variant balance > 0.2, and variant type worse than synonymous. Detected variants were classified with the use of prediction tools32, 33 and databases, such as COSMIC,34 ClinVar,35 and cBioPortal.36 For the final analysis, only variants classified as uncertain, likely pathogenic and pathogenic, were considered. In cases where variants detected in ccfDNA were not detected in the corresponding T-DNA or vice versa, we manually searched for potential variants within the genomic positions (ie, beyond the given threshold of VAF or coverage).
ccfDNA-based BM definition
BM1 (quantitative analysis) was defined as positive when the total ccfDNA concentrations were above the cut-off derived from HS, ie, 0.146 ng/µL (mean HS + 2 SD). BM1 was defined as very high when the total ccfDNA concentrations were above the arbitrary cut-off of 1 ng/µL.
BM2 (genomic qualitative analysis) was defined as positive when at least 1 somatic variant was detected at targeted NGS at the ccfDNA levels.
Circulating cell-free DNA-based BM score was calculated as follows: baseline BM1 (negative = 0, positive = 1, very high = 2) + baseline BM2 (no variants = 0, one variant = 1, more than one variant = 2) for a minimum of 0 and a maximum of 4 points.
Statistical analysis
Data are shown as mean ± SD or median and range, as appropriate. Non-parametric Mann–Whitney U test and Fisher or χ2 tests were used to compare baseline continuous and dichotomic data, respectively. Non-parametric Kruskal–Wallis test was used to compare multiple variables, followed by the Bonferroni post hoc test. Correlations between 2 continuous variables were investigated by linear regression. Comparison between total and calibrated ccfDNA concentrations was additionally performed by the Bland–Altman test. Kaplan–Meier plots were used to investigate the proportional hazards assumption and to display the unadjusted survival curves for survival outcomes. Hazard ratio (HR), 95% CI, and P-values calculated by log-rank test (Mantel–Cox) were reported for each survival outcome (OS and PFS). Moreover, multivariable Cox survival models were fitted for OS and PFS, including variables significant at univariable analysis and available for all patients. Statistical analysis was performed using SPSS (version 9, IBM Deutschland GmbH, Ehningen, Germany) or GraphPad Prism (version 25, GraphPad Software, Boston, United States). P-value < .05 was considered statistically significant.
Results
Characteristics of the study cohort
We included a total of 34 patients with primary ACC in place (11M/23F, median age 55.5 years, range 23-83) and followed up in one of the two participating centres (Wuerzburg, Germany, or Birmingham, United Kingdom). The control group included 23 HS (9 M/14 F, median age 35 years, range 23-62). An overview of the demographic, clinical, and histopathological characteristics at the time of diagnosis and the results of the ccfDNA analysis are shown in Table 1.
Demographic, clinical, and histopathological characteristics of 34 patients with primary ACC and results of ccfDNA analysis.
| Patient-ID . | Sex/age . | Symptoms . | ENSAT tumour stage . | Metastasis at diagnosis . | ccfDNA levels (ng/µL of plasma) . | ccfDNA sequencing - Gene name . | Surgery . | R status . | Ki67 index . | S-GRAS score . | Available Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC-P1 | F/55 | Yes | 4 | Liver, lung, LN | 0.195 | CTNNB1(NM_001098209.1) | No | NA | NA | NA | No |
| ACC-P2 | M/83 | Yes | 3 | None | 0.301 | No variants identified | Yes | 0 | 9 | 3 | Long |
| ACC-P3 | M/43 | Yes | 3 | None | Yes | MEN1 (NM_130799.2) ZNRF3 (NM_001206998.1) | Yes | 0 | 30 | 4 | Long |
| ACC-P4 | M/71 | No | 3 | None | 0.173 | No variants identified | Yes | 0 | 40 | 4 | Short |
| ACC-P5 | F/23 | Yes | 4 | Lung | 1.935 | No variants identified | Yes | 2 | 40 | 8 | Short |
| ACC-P6 | M/36 | Yes | 2 | None | 0.151 | No variants identified | Yes | 0 | 3 | 1 | Long |
| ACC-P7 | F/68 | Yes | 4 | Liver | 2.325 | No variants identified | Yes | 2 | 30 | 9 | No |
| ACC-P8 | F/74 | No | 3 | None | 1.120 | MEN1 (NM_130799.2) ZNRF3 (NM_001206998.1) | Yes | 1 | 40 | 6 | Short |
| ACC-P9 | M/37 | Yes | 4 | Liver, lung | 0.735 | TP53 (NM_000546.5) | No | NA | NA | NA | No |
| ACC-P10 | F/54 | Yes | 4 | Lung | 2.700 | ZNRF3 (NM_001206998.1) | No | NA | NA | NA | No |
| ACC-P11 | M/64 | Yes | 4 | Liver | 4.350 | CTNNB1 (NM_001098209.1) DAXX (NM_001141970.1 RB1 (NM_000321.2) TP53 (NM_000546.5) | No | NA | NA | NA | No |
| ACC-P12 | F/73 | Yes | 4 | Liver | 6.000 | KMT2D (NM_003482.3) | No | NA | NA | NA | No |
| ACC-P13 | M/57 | Yes | 4 | Liver | 0.401 | KMT2D (NM_003482.3) | No | NA | NA | NA | No |
| ACC-P14 | F/36 | Yes | 3 | None | 0.193 | No variants identified | Yes | 1 | 30 | 6 | Short |
| ACC-P15 | F/57 | Yes | 3 | None | 0.890 | No variants identified | Yes | 1 | 10 | 6 | Long |
| ACC-P16 | M/50 | No | 2 | None | 0.062 | NF1 (NM_000267.3)b | Yes | 0 | 17 | 1 | Short |
| ACC-P17 | F/65 | Yes | 3 | None | 0.385 | GNAS (NM_000516.5) | Yes | 1 | 25 | 7 | Short |
| ACC-P18 | F/60 | Yes | 4 | Lung, LN | 5.500 | CTNNB1 (NM_001098209.1) ATM (NM_000051.3)b | No | NA | NA | NA | No |
| ACC-P21 | F/56 | No | 2 | None | 0.251 | No variants identified | Yes | 0 | 30 | 3 | Long |
| ACC-P22 | F/50 | No | 3 | None | 0.247 | No variants identified | Yes | 0 | 18 | 3 | Long |
| ACC-P23 | F/27 | Yes | 3 | None | 0.189 | No variants identified | Yes | 0 | 40 | 4 | No |
| ACC-P24 | M/31 | Yes | 2 | None | 0.163 | No variants identified | Yes | 0 | 12 | 2 | Long |
| ACC-P25 | F/56 | No | 3 | None | 0.454 | No variants identified | Yes | 0 | 5 | 2 | Long |
| ACC-P26 | F/39 | No | 2 | None | 0.185 | CTNNB1 (NM_001098209.1) | Yes | 0 | 40 | 2 | Short |
| ACC-P27 | M/64 | No | 1 | None | 0.114 | No variants identified | Yes | 0 | 23 | 3 | No |
| ACC-P28 | F/35 | Yes | 4 | Lung | 1.473 | TERT (NM_198253.2) (NM_002734.4) | Yes | 2 | 80 | 8 | Short |
| ACC-P29 | F/56 | Yes | 3 | None | 0.302 | No variants identified | Yes | 0 | 12 | 4 | Short |
| ACC-P30 | F/53 | Yes | 2 | None | 0.368 | APC (NM_000038.5) | Yes | 0 | 30 | 4 | Long |
| ACC-P31 | F/51 | Yes | 2 | None | 1.380 | ATM (NM_000051.3)b | Yes | 0 | 22 | 4 | Long |
| ACC-P32 | F/26 | Yes | 4 | Lung | 0.414 | No variants identified | No | NA | 19a | NA | No |
| ACC-P33 | M/48 | Yes | 4 | Liver, lung | 0.846 | No variants identified | No | NA | NA | NA | No |
| ACC-P34 | F/62 | Yes | 4 | Liver | 0.781 | No variants identified | No | NA | 20a | NA | No |
| ACC-P35 | F/58 | Yes | 4 | Liver | 5.758 | No variants identified | No | NA | 75a | NA | No |
| ACC-P36 | F/59 | Yes | 4 | Liver, lung and LN | 2.800 | MEN1 (NM_130799.2) | No | NA | 20a | NA | No |
| Patient-ID . | Sex/age . | Symptoms . | ENSAT tumour stage . | Metastasis at diagnosis . | ccfDNA levels (ng/µL of plasma) . | ccfDNA sequencing - Gene name . | Surgery . | R status . | Ki67 index . | S-GRAS score . | Available Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC-P1 | F/55 | Yes | 4 | Liver, lung, LN | 0.195 | CTNNB1(NM_001098209.1) | No | NA | NA | NA | No |
| ACC-P2 | M/83 | Yes | 3 | None | 0.301 | No variants identified | Yes | 0 | 9 | 3 | Long |
| ACC-P3 | M/43 | Yes | 3 | None | Yes | MEN1 (NM_130799.2) ZNRF3 (NM_001206998.1) | Yes | 0 | 30 | 4 | Long |
| ACC-P4 | M/71 | No | 3 | None | 0.173 | No variants identified | Yes | 0 | 40 | 4 | Short |
| ACC-P5 | F/23 | Yes | 4 | Lung | 1.935 | No variants identified | Yes | 2 | 40 | 8 | Short |
| ACC-P6 | M/36 | Yes | 2 | None | 0.151 | No variants identified | Yes | 0 | 3 | 1 | Long |
| ACC-P7 | F/68 | Yes | 4 | Liver | 2.325 | No variants identified | Yes | 2 | 30 | 9 | No |
| ACC-P8 | F/74 | No | 3 | None | 1.120 | MEN1 (NM_130799.2) ZNRF3 (NM_001206998.1) | Yes | 1 | 40 | 6 | Short |
| ACC-P9 | M/37 | Yes | 4 | Liver, lung | 0.735 | TP53 (NM_000546.5) | No | NA | NA | NA | No |
| ACC-P10 | F/54 | Yes | 4 | Lung | 2.700 | ZNRF3 (NM_001206998.1) | No | NA | NA | NA | No |
| ACC-P11 | M/64 | Yes | 4 | Liver | 4.350 | CTNNB1 (NM_001098209.1) DAXX (NM_001141970.1 RB1 (NM_000321.2) TP53 (NM_000546.5) | No | NA | NA | NA | No |
| ACC-P12 | F/73 | Yes | 4 | Liver | 6.000 | KMT2D (NM_003482.3) | No | NA | NA | NA | No |
| ACC-P13 | M/57 | Yes | 4 | Liver | 0.401 | KMT2D (NM_003482.3) | No | NA | NA | NA | No |
| ACC-P14 | F/36 | Yes | 3 | None | 0.193 | No variants identified | Yes | 1 | 30 | 6 | Short |
| ACC-P15 | F/57 | Yes | 3 | None | 0.890 | No variants identified | Yes | 1 | 10 | 6 | Long |
| ACC-P16 | M/50 | No | 2 | None | 0.062 | NF1 (NM_000267.3)b | Yes | 0 | 17 | 1 | Short |
| ACC-P17 | F/65 | Yes | 3 | None | 0.385 | GNAS (NM_000516.5) | Yes | 1 | 25 | 7 | Short |
| ACC-P18 | F/60 | Yes | 4 | Lung, LN | 5.500 | CTNNB1 (NM_001098209.1) ATM (NM_000051.3)b | No | NA | NA | NA | No |
| ACC-P21 | F/56 | No | 2 | None | 0.251 | No variants identified | Yes | 0 | 30 | 3 | Long |
| ACC-P22 | F/50 | No | 3 | None | 0.247 | No variants identified | Yes | 0 | 18 | 3 | Long |
| ACC-P23 | F/27 | Yes | 3 | None | 0.189 | No variants identified | Yes | 0 | 40 | 4 | No |
| ACC-P24 | M/31 | Yes | 2 | None | 0.163 | No variants identified | Yes | 0 | 12 | 2 | Long |
| ACC-P25 | F/56 | No | 3 | None | 0.454 | No variants identified | Yes | 0 | 5 | 2 | Long |
| ACC-P26 | F/39 | No | 2 | None | 0.185 | CTNNB1 (NM_001098209.1) | Yes | 0 | 40 | 2 | Short |
| ACC-P27 | M/64 | No | 1 | None | 0.114 | No variants identified | Yes | 0 | 23 | 3 | No |
| ACC-P28 | F/35 | Yes | 4 | Lung | 1.473 | TERT (NM_198253.2) (NM_002734.4) | Yes | 2 | 80 | 8 | Short |
| ACC-P29 | F/56 | Yes | 3 | None | 0.302 | No variants identified | Yes | 0 | 12 | 4 | Short |
| ACC-P30 | F/53 | Yes | 2 | None | 0.368 | APC (NM_000038.5) | Yes | 0 | 30 | 4 | Long |
| ACC-P31 | F/51 | Yes | 2 | None | 1.380 | ATM (NM_000051.3)b | Yes | 0 | 22 | 4 | Long |
| ACC-P32 | F/26 | Yes | 4 | Lung | 0.414 | No variants identified | No | NA | 19a | NA | No |
| ACC-P33 | M/48 | Yes | 4 | Liver, lung | 0.846 | No variants identified | No | NA | NA | NA | No |
| ACC-P34 | F/62 | Yes | 4 | Liver | 0.781 | No variants identified | No | NA | 20a | NA | No |
| ACC-P35 | F/58 | Yes | 4 | Liver | 5.758 | No variants identified | No | NA | 75a | NA | No |
| ACC-P36 | F/59 | Yes | 4 | Liver, lung and LN | 2.800 | MEN1 (NM_130799.2) | No | NA | 20a | NA | No |
R status = resection status of primary tumour, S-GRAS score calculated as previously published (Elhassan YS et al., Eur J Endocrinol 2021, DOI: 10.1530/EJE-21-0510). Long follow-up: at least 9 months. Short follow-up: at least 3 months.
Abbreviations: ACC, adrenocortical carcinoma; ccfDNA, circulating cell-free DNA; ENSAT, European Network for the Study of Adrenocortical Tumors; F, female; LN, lymph node; M, male; NA, not applicable.
aki67 index available from adrenal tumour biopsy.
bKnown drug targetable genes according to OncoKb database.
Demographic, clinical, and histopathological characteristics of 34 patients with primary ACC and results of ccfDNA analysis.
| Patient-ID . | Sex/age . | Symptoms . | ENSAT tumour stage . | Metastasis at diagnosis . | ccfDNA levels (ng/µL of plasma) . | ccfDNA sequencing - Gene name . | Surgery . | R status . | Ki67 index . | S-GRAS score . | Available Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC-P1 | F/55 | Yes | 4 | Liver, lung, LN | 0.195 | CTNNB1(NM_001098209.1) | No | NA | NA | NA | No |
| ACC-P2 | M/83 | Yes | 3 | None | 0.301 | No variants identified | Yes | 0 | 9 | 3 | Long |
| ACC-P3 | M/43 | Yes | 3 | None | Yes | MEN1 (NM_130799.2) ZNRF3 (NM_001206998.1) | Yes | 0 | 30 | 4 | Long |
| ACC-P4 | M/71 | No | 3 | None | 0.173 | No variants identified | Yes | 0 | 40 | 4 | Short |
| ACC-P5 | F/23 | Yes | 4 | Lung | 1.935 | No variants identified | Yes | 2 | 40 | 8 | Short |
| ACC-P6 | M/36 | Yes | 2 | None | 0.151 | No variants identified | Yes | 0 | 3 | 1 | Long |
| ACC-P7 | F/68 | Yes | 4 | Liver | 2.325 | No variants identified | Yes | 2 | 30 | 9 | No |
| ACC-P8 | F/74 | No | 3 | None | 1.120 | MEN1 (NM_130799.2) ZNRF3 (NM_001206998.1) | Yes | 1 | 40 | 6 | Short |
| ACC-P9 | M/37 | Yes | 4 | Liver, lung | 0.735 | TP53 (NM_000546.5) | No | NA | NA | NA | No |
| ACC-P10 | F/54 | Yes | 4 | Lung | 2.700 | ZNRF3 (NM_001206998.1) | No | NA | NA | NA | No |
| ACC-P11 | M/64 | Yes | 4 | Liver | 4.350 | CTNNB1 (NM_001098209.1) DAXX (NM_001141970.1 RB1 (NM_000321.2) TP53 (NM_000546.5) | No | NA | NA | NA | No |
| ACC-P12 | F/73 | Yes | 4 | Liver | 6.000 | KMT2D (NM_003482.3) | No | NA | NA | NA | No |
| ACC-P13 | M/57 | Yes | 4 | Liver | 0.401 | KMT2D (NM_003482.3) | No | NA | NA | NA | No |
| ACC-P14 | F/36 | Yes | 3 | None | 0.193 | No variants identified | Yes | 1 | 30 | 6 | Short |
| ACC-P15 | F/57 | Yes | 3 | None | 0.890 | No variants identified | Yes | 1 | 10 | 6 | Long |
| ACC-P16 | M/50 | No | 2 | None | 0.062 | NF1 (NM_000267.3)b | Yes | 0 | 17 | 1 | Short |
| ACC-P17 | F/65 | Yes | 3 | None | 0.385 | GNAS (NM_000516.5) | Yes | 1 | 25 | 7 | Short |
| ACC-P18 | F/60 | Yes | 4 | Lung, LN | 5.500 | CTNNB1 (NM_001098209.1) ATM (NM_000051.3)b | No | NA | NA | NA | No |
| ACC-P21 | F/56 | No | 2 | None | 0.251 | No variants identified | Yes | 0 | 30 | 3 | Long |
| ACC-P22 | F/50 | No | 3 | None | 0.247 | No variants identified | Yes | 0 | 18 | 3 | Long |
| ACC-P23 | F/27 | Yes | 3 | None | 0.189 | No variants identified | Yes | 0 | 40 | 4 | No |
| ACC-P24 | M/31 | Yes | 2 | None | 0.163 | No variants identified | Yes | 0 | 12 | 2 | Long |
| ACC-P25 | F/56 | No | 3 | None | 0.454 | No variants identified | Yes | 0 | 5 | 2 | Long |
| ACC-P26 | F/39 | No | 2 | None | 0.185 | CTNNB1 (NM_001098209.1) | Yes | 0 | 40 | 2 | Short |
| ACC-P27 | M/64 | No | 1 | None | 0.114 | No variants identified | Yes | 0 | 23 | 3 | No |
| ACC-P28 | F/35 | Yes | 4 | Lung | 1.473 | TERT (NM_198253.2) (NM_002734.4) | Yes | 2 | 80 | 8 | Short |
| ACC-P29 | F/56 | Yes | 3 | None | 0.302 | No variants identified | Yes | 0 | 12 | 4 | Short |
| ACC-P30 | F/53 | Yes | 2 | None | 0.368 | APC (NM_000038.5) | Yes | 0 | 30 | 4 | Long |
| ACC-P31 | F/51 | Yes | 2 | None | 1.380 | ATM (NM_000051.3)b | Yes | 0 | 22 | 4 | Long |
| ACC-P32 | F/26 | Yes | 4 | Lung | 0.414 | No variants identified | No | NA | 19a | NA | No |
| ACC-P33 | M/48 | Yes | 4 | Liver, lung | 0.846 | No variants identified | No | NA | NA | NA | No |
| ACC-P34 | F/62 | Yes | 4 | Liver | 0.781 | No variants identified | No | NA | 20a | NA | No |
| ACC-P35 | F/58 | Yes | 4 | Liver | 5.758 | No variants identified | No | NA | 75a | NA | No |
| ACC-P36 | F/59 | Yes | 4 | Liver, lung and LN | 2.800 | MEN1 (NM_130799.2) | No | NA | 20a | NA | No |
| Patient-ID . | Sex/age . | Symptoms . | ENSAT tumour stage . | Metastasis at diagnosis . | ccfDNA levels (ng/µL of plasma) . | ccfDNA sequencing - Gene name . | Surgery . | R status . | Ki67 index . | S-GRAS score . | Available Follow-up . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACC-P1 | F/55 | Yes | 4 | Liver, lung, LN | 0.195 | CTNNB1(NM_001098209.1) | No | NA | NA | NA | No |
| ACC-P2 | M/83 | Yes | 3 | None | 0.301 | No variants identified | Yes | 0 | 9 | 3 | Long |
| ACC-P3 | M/43 | Yes | 3 | None | Yes | MEN1 (NM_130799.2) ZNRF3 (NM_001206998.1) | Yes | 0 | 30 | 4 | Long |
| ACC-P4 | M/71 | No | 3 | None | 0.173 | No variants identified | Yes | 0 | 40 | 4 | Short |
| ACC-P5 | F/23 | Yes | 4 | Lung | 1.935 | No variants identified | Yes | 2 | 40 | 8 | Short |
| ACC-P6 | M/36 | Yes | 2 | None | 0.151 | No variants identified | Yes | 0 | 3 | 1 | Long |
| ACC-P7 | F/68 | Yes | 4 | Liver | 2.325 | No variants identified | Yes | 2 | 30 | 9 | No |
| ACC-P8 | F/74 | No | 3 | None | 1.120 | MEN1 (NM_130799.2) ZNRF3 (NM_001206998.1) | Yes | 1 | 40 | 6 | Short |
| ACC-P9 | M/37 | Yes | 4 | Liver, lung | 0.735 | TP53 (NM_000546.5) | No | NA | NA | NA | No |
| ACC-P10 | F/54 | Yes | 4 | Lung | 2.700 | ZNRF3 (NM_001206998.1) | No | NA | NA | NA | No |
| ACC-P11 | M/64 | Yes | 4 | Liver | 4.350 | CTNNB1 (NM_001098209.1) DAXX (NM_001141970.1 RB1 (NM_000321.2) TP53 (NM_000546.5) | No | NA | NA | NA | No |
| ACC-P12 | F/73 | Yes | 4 | Liver | 6.000 | KMT2D (NM_003482.3) | No | NA | NA | NA | No |
| ACC-P13 | M/57 | Yes | 4 | Liver | 0.401 | KMT2D (NM_003482.3) | No | NA | NA | NA | No |
| ACC-P14 | F/36 | Yes | 3 | None | 0.193 | No variants identified | Yes | 1 | 30 | 6 | Short |
| ACC-P15 | F/57 | Yes | 3 | None | 0.890 | No variants identified | Yes | 1 | 10 | 6 | Long |
| ACC-P16 | M/50 | No | 2 | None | 0.062 | NF1 (NM_000267.3)b | Yes | 0 | 17 | 1 | Short |
| ACC-P17 | F/65 | Yes | 3 | None | 0.385 | GNAS (NM_000516.5) | Yes | 1 | 25 | 7 | Short |
| ACC-P18 | F/60 | Yes | 4 | Lung, LN | 5.500 | CTNNB1 (NM_001098209.1) ATM (NM_000051.3)b | No | NA | NA | NA | No |
| ACC-P21 | F/56 | No | 2 | None | 0.251 | No variants identified | Yes | 0 | 30 | 3 | Long |
| ACC-P22 | F/50 | No | 3 | None | 0.247 | No variants identified | Yes | 0 | 18 | 3 | Long |
| ACC-P23 | F/27 | Yes | 3 | None | 0.189 | No variants identified | Yes | 0 | 40 | 4 | No |
| ACC-P24 | M/31 | Yes | 2 | None | 0.163 | No variants identified | Yes | 0 | 12 | 2 | Long |
| ACC-P25 | F/56 | No | 3 | None | 0.454 | No variants identified | Yes | 0 | 5 | 2 | Long |
| ACC-P26 | F/39 | No | 2 | None | 0.185 | CTNNB1 (NM_001098209.1) | Yes | 0 | 40 | 2 | Short |
| ACC-P27 | M/64 | No | 1 | None | 0.114 | No variants identified | Yes | 0 | 23 | 3 | No |
| ACC-P28 | F/35 | Yes | 4 | Lung | 1.473 | TERT (NM_198253.2) (NM_002734.4) | Yes | 2 | 80 | 8 | Short |
| ACC-P29 | F/56 | Yes | 3 | None | 0.302 | No variants identified | Yes | 0 | 12 | 4 | Short |
| ACC-P30 | F/53 | Yes | 2 | None | 0.368 | APC (NM_000038.5) | Yes | 0 | 30 | 4 | Long |
| ACC-P31 | F/51 | Yes | 2 | None | 1.380 | ATM (NM_000051.3)b | Yes | 0 | 22 | 4 | Long |
| ACC-P32 | F/26 | Yes | 4 | Lung | 0.414 | No variants identified | No | NA | 19a | NA | No |
| ACC-P33 | M/48 | Yes | 4 | Liver, lung | 0.846 | No variants identified | No | NA | NA | NA | No |
| ACC-P34 | F/62 | Yes | 4 | Liver | 0.781 | No variants identified | No | NA | 20a | NA | No |
| ACC-P35 | F/58 | Yes | 4 | Liver | 5.758 | No variants identified | No | NA | 75a | NA | No |
| ACC-P36 | F/59 | Yes | 4 | Liver, lung and LN | 2.800 | MEN1 (NM_130799.2) | No | NA | 20a | NA | No |
R status = resection status of primary tumour, S-GRAS score calculated as previously published (Elhassan YS et al., Eur J Endocrinol 2021, DOI: 10.1530/EJE-21-0510). Long follow-up: at least 9 months. Short follow-up: at least 3 months.
Abbreviations: ACC, adrenocortical carcinoma; ccfDNA, circulating cell-free DNA; ENSAT, European Network for the Study of Adrenocortical Tumors; F, female; LN, lymph node; M, male; NA, not applicable.
aki67 index available from adrenal tumour biopsy.
bKnown drug targetable genes according to OncoKb database.
A complete flowchart showing the final ACC cohort and available follow-ups is shown in Figure 1. In brief, a total of 22 patients underwent adrenalectomy (three of whom with debulking purposes) and had available tumour tissue material. The remaining 12 patients did not undergo surgery due to the presence of metastatic disease at the time of diagnosis or non-operable primary tumours. Nineteen patients who underwent surgery were also tested during post-surgical follow-up for monitoring purposes: 9 of them for at least 3 months (short term) and 10 for at least 9 months (long term).
Relationship between ccfDNA concentrations (BM1) and clinical parameters
Patients with ACC had higher total ccfDNA concentrations than HS (1.15 ± 1.56 vs 0.050 ± 0.048 ng/µL, P < .0001; Figure 2A). Overall, 96% of ACC were positive for BM1, while 32% of ACC cases showed very high ccfDNA concentrations, ie, >1 ng/µL (Figure 2B). Higher ccfDNA levels were associated with larger tumour burden and more aggressive disease. In fact, patients with advanced stage ACC—ie, non-amenable for complete surgical resection—presented a higher frequency of very high ccfDNA concentrations (11% vs 60%, respectively, P < .001; Figure S3). Moreover, patients with ENSAT stage 4 had higher ccfDNA concentrations (2.41 ± 2.06 ng/µL) compared with patients with stage 3 (0.41 ± 0.31 ng/µL, P = .0117) or 1-2 (0.33 ± 0.43 ng/µL, P = .0004) (Figure 2C). Finally, ccfDNA levels correlated positively with both the Ki67 index (n = 22, P = .0034, R = 0.57; Figure 2D) and the number of distant metastases (n = 30, P = .021, R = 0.42). There was no significant correlation between the ccfDNA concentrations and age or presence of symptoms at diagnosis.
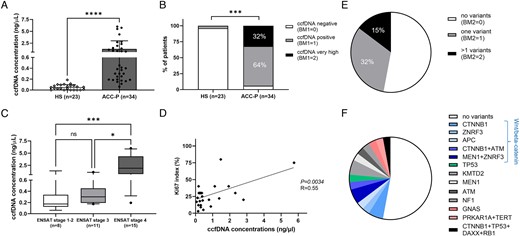
Baseline total ccfDNA concentrations and somatic variants detected in ccfDNA samples by targeted next-generation sequencing from 34 patients with primary adrenocortical carcinoma (ACC-p). (A) Comparison of ccfDNA concentrations between ACC and 23 HSs; (B) comparison of ccfDNA concentrations between ACC and HS as follows: ccfDNA positive if levels above the cut-off of 0.146 ng/µL, ccfDNA very high levels if above 1 ng/µL; (C) relationship between ccfDNA concentrations and ENSAT tumour stage in ACC. Statistics by Kruskal–Wallis test followed by the Bonferroni post hoc test; (D) correlation between ccfDNA concentrations and Ki67 proliferation index in ACC. Statistics by linear regression; (E) pie chart showing the proportion of cases with one or more than 1 somatic variant in ACC-specific genes; (F) pie chart showing the proportion of samples with individual somatic variants (ie, gene names). ACC, adrenocortical carcinoma; BM, biomarker; ccfDNA, circulating cell-free DNA; ENSAT, European Network for the Study of Adrenocortical Tumors; HS, healthy subject.
Relationship between somatic variants at ccfDNA (BM2) and clinical parameters
At ccfDNA sequencing, 47% of ACC showed at least 1 somatic mutation (positive BM2) and 15% at least 2 mutations (Figure 2E and F and Table 1). The most frequently altered genes included CTNNB1 (12%), ZNRF3 (9%), MEN1 (9%), TP53 (6%), ATM (6%), and KMT2D (6%). As expected, the majority of the altered genes belonged to the Wnt/β-catenin pathway (ie, CTNNB1, ZNRF3, APC) followed by those linked to chromatin remodelling (Figure 2F and Table S1). The VAF was overall comprised between 1.0% and 30.5%. The type of detected genetic variants and the corresponding VAFs are reported in Table 1 and Table S2.
Interestingly, patients with advanced stage ACC—ie, non-amenable for complete surgical resection (n = 15)—presented a higher frequency of one or more than 1 somatic variant at baseline ccfDNA compared with those with early stages, ie, 40% and 20% vs 26% and 11%, respectively (P = .0052, Table 1 and Figure S3). In this subgroup of more aggressive cases, the most frequent alterations were observed in CTNNB1 (n = 3), TP53 (n = 2), and KMT2D (n = 2). Of note, three of these variants affected known drug targetable genes (9% of the total): two patients presented missense mutations in ATM and one presented a missense mutation in NF1.
Comparison between somatic variants in ccfDNA and T-DNA
We compared the ccfDNA mutational status with available corresponding primary T-DNA in 22 patients. These perfectly matched in 68% of cases (Table 2 and Table S2).
Comparison between targeted next-generation sequencing in matched T-DNA and ccfDNA in 22 ACC-P.
| . | . | T-DNA sequencing . | ccfDNA sequencing . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Sample- ID . | ENSAT tumour stage . | Gene name . | Variant . | VAF (%) . | Gene name . | Variant . | VAF (%) . | Correspondence between T-DNA and ccfDNA . |
| ACC-P2 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P3 | 3 | MEN1 (NM_130799.2) | p.Tyr351a | 74.80 | MEN1 (NM_130799.2) | p.Tyr351a | 8.2 | Yes |
| ZNFR3 (NM_001206998.1) | p.Cys333a | 3.9 | Only in ccfDNA | |||||
| ACC-P4 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P5 | 4 | No variants identified | No variants identified | Yes | ||||
| ACC-P6 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P7 | 4 | No variants identified | No variants identified | Yes | ||||
| ACC-P8 | 3 | MEN1 (NM_130799.2) ZNFR3 (NM_001206998.1) | p.Arg460a p.Phe474Argfs*95 | 84.4 74.8 | MEN1 (NM_130799.2) ZNFR3 (NM_001206998.1) | p.Arg460a p.Phe474Argfs*95 | 2.6 1.9 | Yes Yes |
| ACC-P14 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P15 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P16 | 2 | No variants identified | NF1 (NM_000267.3)a | p.Leu2735Met | 7.0 | Only in ccfDNA | ||
| ACC-P17 | 3 | No variants identified | GNAS (NM_000516.5) | p.Arg265= | 3.3 | Only in ccfDNA | ||
| ACC-P21 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P22 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P23 | 3 | APC (NM_000038.5) | p.Arg876a | 66.0 | No variants identified | Only in T-DNA | ||
| ACC-P24 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P25 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P26 | 2 | CTNNB1 (NM_001098209.1) | p.Ser45Ala | 74.0 | CTNNB1 (NM_001098209.1) | p.Ser45Ala | 4.5 | Yes Only in T-DNA |
| MEN1 (NM_130799.2) | p.Asp180_Trp183del | 20.9 | ||||||
| ACC-P27 | 1 | NF1 (NM_000267.3) TP53 (NM_000546.59 NOTCH1 (NM_017617.5) | p.Arg2343Gln p.Ala159Val p.Arg2104His | 26.4 24.6 20.6 | No variants identified | Only in T-DNA Only in T-DNA Only in T-DNA | ||
| ACC-P28 | 4 | PRKAR1A (NM_002734.49 TERT (NM_198253.2) | c.Glu55a p.Leu234Phe | 76.6 28.0 | PRKAR1A (NM_002734.49 TERT (NM_198253.2) | p.Glu55a p.Leu234Phe | 10.9 26.5 | Yes |
| ACC-P29 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P30 | 2 | No variants identified | APC (NM_000038.5) | p.Asn32Ile | 30.5 | Only in ccfDNA | ||
| ACC-P31 | 2 | ATM (NM_000051.3)a | p.Arg337Cys | 88.6 | ATM (NM_000051.3)a | p.Arg337Cys | 9.9 | Yes |
| . | . | T-DNA sequencing . | ccfDNA sequencing . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Sample- ID . | ENSAT tumour stage . | Gene name . | Variant . | VAF (%) . | Gene name . | Variant . | VAF (%) . | Correspondence between T-DNA and ccfDNA . |
| ACC-P2 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P3 | 3 | MEN1 (NM_130799.2) | p.Tyr351a | 74.80 | MEN1 (NM_130799.2) | p.Tyr351a | 8.2 | Yes |
| ZNFR3 (NM_001206998.1) | p.Cys333a | 3.9 | Only in ccfDNA | |||||
| ACC-P4 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P5 | 4 | No variants identified | No variants identified | Yes | ||||
| ACC-P6 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P7 | 4 | No variants identified | No variants identified | Yes | ||||
| ACC-P8 | 3 | MEN1 (NM_130799.2) ZNFR3 (NM_001206998.1) | p.Arg460a p.Phe474Argfs*95 | 84.4 74.8 | MEN1 (NM_130799.2) ZNFR3 (NM_001206998.1) | p.Arg460a p.Phe474Argfs*95 | 2.6 1.9 | Yes Yes |
| ACC-P14 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P15 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P16 | 2 | No variants identified | NF1 (NM_000267.3)a | p.Leu2735Met | 7.0 | Only in ccfDNA | ||
| ACC-P17 | 3 | No variants identified | GNAS (NM_000516.5) | p.Arg265= | 3.3 | Only in ccfDNA | ||
| ACC-P21 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P22 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P23 | 3 | APC (NM_000038.5) | p.Arg876a | 66.0 | No variants identified | Only in T-DNA | ||
| ACC-P24 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P25 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P26 | 2 | CTNNB1 (NM_001098209.1) | p.Ser45Ala | 74.0 | CTNNB1 (NM_001098209.1) | p.Ser45Ala | 4.5 | Yes Only in T-DNA |
| MEN1 (NM_130799.2) | p.Asp180_Trp183del | 20.9 | ||||||
| ACC-P27 | 1 | NF1 (NM_000267.3) TP53 (NM_000546.59 NOTCH1 (NM_017617.5) | p.Arg2343Gln p.Ala159Val p.Arg2104His | 26.4 24.6 20.6 | No variants identified | Only in T-DNA Only in T-DNA Only in T-DNA | ||
| ACC-P28 | 4 | PRKAR1A (NM_002734.49 TERT (NM_198253.2) | c.Glu55a p.Leu234Phe | 76.6 28.0 | PRKAR1A (NM_002734.49 TERT (NM_198253.2) | p.Glu55a p.Leu234Phe | 10.9 26.5 | Yes |
| ACC-P29 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P30 | 2 | No variants identified | APC (NM_000038.5) | p.Asn32Ile | 30.5 | Only in ccfDNA | ||
| ACC-P31 | 2 | ATM (NM_000051.3)a | p.Arg337Cys | 88.6 | ATM (NM_000051.3)a | p.Arg337Cys | 9.9 | Yes |
Somatic variants detected in both ccfDNA and T-DNA are in bold.
Abbreviations: ACC-P, primary adrenocortical carcinoma; ccfDNA, circulating cell-free DNA; ENSAT, European Network for the Study of Adrenocortical Tumors; T-DNA, tumour DNA; VAF, variant allele frequency.
aKnown drug targetable genes according to OncoKb database.
Comparison between targeted next-generation sequencing in matched T-DNA and ccfDNA in 22 ACC-P.
| . | . | T-DNA sequencing . | ccfDNA sequencing . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Sample- ID . | ENSAT tumour stage . | Gene name . | Variant . | VAF (%) . | Gene name . | Variant . | VAF (%) . | Correspondence between T-DNA and ccfDNA . |
| ACC-P2 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P3 | 3 | MEN1 (NM_130799.2) | p.Tyr351a | 74.80 | MEN1 (NM_130799.2) | p.Tyr351a | 8.2 | Yes |
| ZNFR3 (NM_001206998.1) | p.Cys333a | 3.9 | Only in ccfDNA | |||||
| ACC-P4 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P5 | 4 | No variants identified | No variants identified | Yes | ||||
| ACC-P6 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P7 | 4 | No variants identified | No variants identified | Yes | ||||
| ACC-P8 | 3 | MEN1 (NM_130799.2) ZNFR3 (NM_001206998.1) | p.Arg460a p.Phe474Argfs*95 | 84.4 74.8 | MEN1 (NM_130799.2) ZNFR3 (NM_001206998.1) | p.Arg460a p.Phe474Argfs*95 | 2.6 1.9 | Yes Yes |
| ACC-P14 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P15 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P16 | 2 | No variants identified | NF1 (NM_000267.3)a | p.Leu2735Met | 7.0 | Only in ccfDNA | ||
| ACC-P17 | 3 | No variants identified | GNAS (NM_000516.5) | p.Arg265= | 3.3 | Only in ccfDNA | ||
| ACC-P21 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P22 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P23 | 3 | APC (NM_000038.5) | p.Arg876a | 66.0 | No variants identified | Only in T-DNA | ||
| ACC-P24 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P25 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P26 | 2 | CTNNB1 (NM_001098209.1) | p.Ser45Ala | 74.0 | CTNNB1 (NM_001098209.1) | p.Ser45Ala | 4.5 | Yes Only in T-DNA |
| MEN1 (NM_130799.2) | p.Asp180_Trp183del | 20.9 | ||||||
| ACC-P27 | 1 | NF1 (NM_000267.3) TP53 (NM_000546.59 NOTCH1 (NM_017617.5) | p.Arg2343Gln p.Ala159Val p.Arg2104His | 26.4 24.6 20.6 | No variants identified | Only in T-DNA Only in T-DNA Only in T-DNA | ||
| ACC-P28 | 4 | PRKAR1A (NM_002734.49 TERT (NM_198253.2) | c.Glu55a p.Leu234Phe | 76.6 28.0 | PRKAR1A (NM_002734.49 TERT (NM_198253.2) | p.Glu55a p.Leu234Phe | 10.9 26.5 | Yes |
| ACC-P29 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P30 | 2 | No variants identified | APC (NM_000038.5) | p.Asn32Ile | 30.5 | Only in ccfDNA | ||
| ACC-P31 | 2 | ATM (NM_000051.3)a | p.Arg337Cys | 88.6 | ATM (NM_000051.3)a | p.Arg337Cys | 9.9 | Yes |
| . | . | T-DNA sequencing . | ccfDNA sequencing . | . | ||||
|---|---|---|---|---|---|---|---|---|
| Sample- ID . | ENSAT tumour stage . | Gene name . | Variant . | VAF (%) . | Gene name . | Variant . | VAF (%) . | Correspondence between T-DNA and ccfDNA . |
| ACC-P2 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P3 | 3 | MEN1 (NM_130799.2) | p.Tyr351a | 74.80 | MEN1 (NM_130799.2) | p.Tyr351a | 8.2 | Yes |
| ZNFR3 (NM_001206998.1) | p.Cys333a | 3.9 | Only in ccfDNA | |||||
| ACC-P4 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P5 | 4 | No variants identified | No variants identified | Yes | ||||
| ACC-P6 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P7 | 4 | No variants identified | No variants identified | Yes | ||||
| ACC-P8 | 3 | MEN1 (NM_130799.2) ZNFR3 (NM_001206998.1) | p.Arg460a p.Phe474Argfs*95 | 84.4 74.8 | MEN1 (NM_130799.2) ZNFR3 (NM_001206998.1) | p.Arg460a p.Phe474Argfs*95 | 2.6 1.9 | Yes Yes |
| ACC-P14 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P15 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P16 | 2 | No variants identified | NF1 (NM_000267.3)a | p.Leu2735Met | 7.0 | Only in ccfDNA | ||
| ACC-P17 | 3 | No variants identified | GNAS (NM_000516.5) | p.Arg265= | 3.3 | Only in ccfDNA | ||
| ACC-P21 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P22 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P23 | 3 | APC (NM_000038.5) | p.Arg876a | 66.0 | No variants identified | Only in T-DNA | ||
| ACC-P24 | 2 | No variants identified | No variants identified | Yes | ||||
| ACC-P25 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P26 | 2 | CTNNB1 (NM_001098209.1) | p.Ser45Ala | 74.0 | CTNNB1 (NM_001098209.1) | p.Ser45Ala | 4.5 | Yes Only in T-DNA |
| MEN1 (NM_130799.2) | p.Asp180_Trp183del | 20.9 | ||||||
| ACC-P27 | 1 | NF1 (NM_000267.3) TP53 (NM_000546.59 NOTCH1 (NM_017617.5) | p.Arg2343Gln p.Ala159Val p.Arg2104His | 26.4 24.6 20.6 | No variants identified | Only in T-DNA Only in T-DNA Only in T-DNA | ||
| ACC-P28 | 4 | PRKAR1A (NM_002734.49 TERT (NM_198253.2) | c.Glu55a p.Leu234Phe | 76.6 28.0 | PRKAR1A (NM_002734.49 TERT (NM_198253.2) | p.Glu55a p.Leu234Phe | 10.9 26.5 | Yes |
| ACC-P29 | 3 | No variants identified | No variants identified | Yes | ||||
| ACC-P30 | 2 | No variants identified | APC (NM_000038.5) | p.Asn32Ile | 30.5 | Only in ccfDNA | ||
| ACC-P31 | 2 | ATM (NM_000051.3)a | p.Arg337Cys | 88.6 | ATM (NM_000051.3)a | p.Arg337Cys | 9.9 | Yes |
Somatic variants detected in both ccfDNA and T-DNA are in bold.
Abbreviations: ACC-P, primary adrenocortical carcinoma; ccfDNA, circulating cell-free DNA; ENSAT, European Network for the Study of Adrenocortical Tumors; T-DNA, tumour DNA; VAF, variant allele frequency.
aKnown drug targetable genes according to OncoKb database.
In particular, in 12 out of 14 patients with no somatic variants at baseline ccfDNA that underwent adrenalectomy, T-DNA also showed also no detectable somatic variants. In 3 cases (13.6%), variants were only found in T-DNA (but not in ccfDNA), including one with APC variant (VAF 66.0%), one with MEN1 variant (VAF 20.9%), and one with variants in NF1 (VAF 26.4%), NOTCH1 (VAF 24.6%), and TP53 (VAF 20.6%) (Table 2).
Conversely, we observed somatic variants at the ccfDNA level that were not detected at T-DNA in 4 cases (18.2%), ie, affecting APC, GNAS, NF1, and ZNRF3 genes. Specifically, variants detected in ZNRF3 (VAF 3.9%) and NF1 (VAF 7.0%) are classified as pathogenic in the COSMIC database, while variants in APC (VAF 3.3%) and GNAS (VAF 30.5%) are reported as uncertain or not reported yet.
Relationship between ccfDNA-based BMs and clinical outcome (AIM 1)
We then investigated the role of ccfDNA-based BMs for prognostic classification (AIM 1). We first performed univariate survival analysis testing the prognostic role of ccfDNA concentrations (negative BM1, positive BM1, and very high BM1). BM1 was clearly associated with both PFS (HR = 3.45; 95% CI, 1.49-7.96; P = .0038 by log-rank test, median PFS 2 vs 15 months vs undefined; Figure 3A) and OS (HR = 6.28; 95% CI, 2.43-17.58; P = .0003, median survival 5 vs 45 months vs undefined; Figure 3B). Also, the presence of one or more somatic variants in baseline ccfDNA (positive BM2, one or more variants) was able to distinguish patients with unfavourable outcomes, ie, with a shorter PFS (2 vs 18 months vs undefined; HR = 1.89; 95% CI, 1.05-3.40; P = .0256 by log-rank test; Figure 3C) and OS (8 vs 9 vs 40 months; HR = 2.47; 95% CI, 1.33-4.56; P = .0058; Figure 3D).
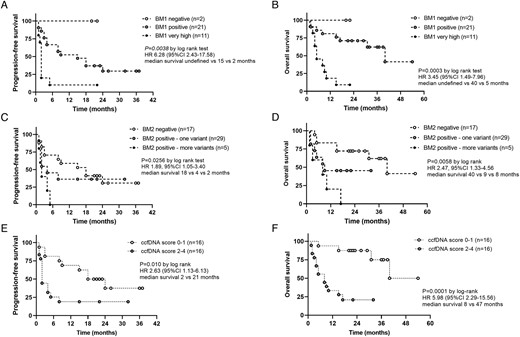
Prognostic role of ccfDNA-related biomarkers (AIM 1)—relationship between ccfDNA-related biomarkers (ie, BM1, BM2, and ccfDNA-based BM score) and clinical outcomes in 34 patients with adrenocortical carcinoma. A-C-E) Kaplan–Meier curves for PFS and (B-D-F) Kaplan–Meier curves for OS. Statistical analysis by log-rank test. ACC, adrenocortical carcinoma; BM, biomarker; BM1, total ccfDNA concentrations; BM2, somatic variants at ccfDNA; ccfDNA, circulating cell-free DNA; HR, hazard ratio; OS, overall survival; PFS, progression-free survival.
A ccfDNA-based BM score was then calculated starting from baseline ccfDNA-based BMs as described in “Materials and methods” for a minimum of 0 and a maximum of 4 points. This score was strongly associated with both PFS and OS at univariate analysis (HR = 2.63; 95% CI, 1.13-6.13; P = .010, and HR = 5.98, 95% CI, 2.29-15.6; P = .0001, respectively) (Figure 3E and F). Importantly, this prognostic role was confirmed at multivariable analysis including the ENSAT tumour stage (model 1—dichotomic variable: HR = 2.86, P = .061 for PFS and HR = 8.80, P = .004 for OS, respectively) (model 2—non-dichotomic variable: HR = 1.81, P = .009 for PFS and HR = 3.39, P < .001 for OS, respectively) (Table 3).
Univariable and multivariable survival analysis for PFS and OS in 34 patients with adrenocortical carcinomas.
| . | Univariable analysis . | Multivariable analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | PFS . | OS . | PFS . | OS . | ||||
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Parameter (n of patients if different from 34) | ||||||||
| Age (≤50 vs >50 years) | 0.88 (0.33-2.40) | .814 | 0.65 (0.28-1.51) | .314 | ||||
| Symptoms at diagnosis (yes vs no) | 2.44 (0.72-8.30) | .154 | .146 | |||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 6.02 (2.14-16.9) | <.001 | 9.49 (2.89-31.2) | <.001 |
| Resection status (n = 22) (R0 vs R1 vs R2) | 1.77 (1.06-2.95) | .030 | 2.59 (1.25-5.37) | .011 | ||||
| Ki67 index (n = 26) (<10 vs 10-19 vs ≥20) | 2.43 (0.92-6.42) | .074 | 5.54 (0.84-36.6) | .075 | ||||
| S-GRAS score (n = 22) (group 1 vs 2 vs 3 vs 4) | 2.14 (1.10-4.16) | .025 | 7.52 (1.83-30.9) | .005 | ||||
| ccfDNA-based BM1 (0 vs 1 vs 2) | 3.45 (1.49-7.96) | .004 | 6.28 (2.24-17.6) | <.001 | 2.01 (0.67-6.02) | .213 | 2.25 (0.68-7.39) | .183 |
| ccfDNA-based BM2 (0 vs 1 vs 2) | 1.89 (1.05-3.40) | .033 | 2.47 (1.33-4.56) | .004 | 1.71 (0.86-3.38) | .125 | 4.16 (1.86-9.31) | <.001 |
| ccfDNA-based scores—Model 1 | ||||||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 5.51 (2.08-14.6) | <.001 | 5.43 (2.12-13.9) | <.001 |
| ccfDNA-based BM score (positive vs negative) | 2.87 (1.20-6.90) | .018 | 8.24 (2.29-29.7) | .001 | 2.86 (0.95-7.54) | .061 | 8.80 (2.01-38.4) | .004 |
| ccfDNA-based scores—Model 2 | ||||||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 6.10 (2.19-16.9) | <.001 | 7.71 (2.82-21.1) | <.001 |
| ccfDNA-based BM score (0 vs 1 vs 2 vs 3 vs 4) | 1.80 (1.22-2.66) | .003 | 2.37 (1.51-3.71) | <.001 | 1.81 (1.15-2.81) | .009 | 3.39 (1.83-6.29) | <.001 |
| . | Univariable analysis . | Multivariable analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | PFS . | OS . | PFS . | OS . | ||||
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Parameter (n of patients if different from 34) | ||||||||
| Age (≤50 vs >50 years) | 0.88 (0.33-2.40) | .814 | 0.65 (0.28-1.51) | .314 | ||||
| Symptoms at diagnosis (yes vs no) | 2.44 (0.72-8.30) | .154 | .146 | |||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 6.02 (2.14-16.9) | <.001 | 9.49 (2.89-31.2) | <.001 |
| Resection status (n = 22) (R0 vs R1 vs R2) | 1.77 (1.06-2.95) | .030 | 2.59 (1.25-5.37) | .011 | ||||
| Ki67 index (n = 26) (<10 vs 10-19 vs ≥20) | 2.43 (0.92-6.42) | .074 | 5.54 (0.84-36.6) | .075 | ||||
| S-GRAS score (n = 22) (group 1 vs 2 vs 3 vs 4) | 2.14 (1.10-4.16) | .025 | 7.52 (1.83-30.9) | .005 | ||||
| ccfDNA-based BM1 (0 vs 1 vs 2) | 3.45 (1.49-7.96) | .004 | 6.28 (2.24-17.6) | <.001 | 2.01 (0.67-6.02) | .213 | 2.25 (0.68-7.39) | .183 |
| ccfDNA-based BM2 (0 vs 1 vs 2) | 1.89 (1.05-3.40) | .033 | 2.47 (1.33-4.56) | .004 | 1.71 (0.86-3.38) | .125 | 4.16 (1.86-9.31) | <.001 |
| ccfDNA-based scores—Model 1 | ||||||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 5.51 (2.08-14.6) | <.001 | 5.43 (2.12-13.9) | <.001 |
| ccfDNA-based BM score (positive vs negative) | 2.87 (1.20-6.90) | .018 | 8.24 (2.29-29.7) | .001 | 2.86 (0.95-7.54) | .061 | 8.80 (2.01-38.4) | .004 |
| ccfDNA-based scores—Model 2 | ||||||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 6.10 (2.19-16.9) | <.001 | 7.71 (2.82-21.1) | <.001 |
| ccfDNA-based BM score (0 vs 1 vs 2 vs 3 vs 4) | 1.80 (1.22-2.66) | .003 | 2.37 (1.51-3.71) | <.001 | 1.81 (1.15-2.81) | .009 | 3.39 (1.83-6.29) | <.001 |
S-GRAS score was calculated as previously published (Elhassan et al., Eur J Endocrinol 2021, DOI: 10.1530/EJE-21-0510). Significant variables available in all patients are in bold.
Abbreviations: BM, biomarker; BM1, total ccfDNA concentrations; BM2, somatic variants detected in ccfDNA; ccfDNA, circulating cell-free DNA; ENSAT, European Network for the Study of Adrenocortical Tumors; OS, overall survival; PFS, progression-free survival.
Univariable and multivariable survival analysis for PFS and OS in 34 patients with adrenocortical carcinomas.
| . | Univariable analysis . | Multivariable analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | PFS . | OS . | PFS . | OS . | ||||
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Parameter (n of patients if different from 34) | ||||||||
| Age (≤50 vs >50 years) | 0.88 (0.33-2.40) | .814 | 0.65 (0.28-1.51) | .314 | ||||
| Symptoms at diagnosis (yes vs no) | 2.44 (0.72-8.30) | .154 | .146 | |||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 6.02 (2.14-16.9) | <.001 | 9.49 (2.89-31.2) | <.001 |
| Resection status (n = 22) (R0 vs R1 vs R2) | 1.77 (1.06-2.95) | .030 | 2.59 (1.25-5.37) | .011 | ||||
| Ki67 index (n = 26) (<10 vs 10-19 vs ≥20) | 2.43 (0.92-6.42) | .074 | 5.54 (0.84-36.6) | .075 | ||||
| S-GRAS score (n = 22) (group 1 vs 2 vs 3 vs 4) | 2.14 (1.10-4.16) | .025 | 7.52 (1.83-30.9) | .005 | ||||
| ccfDNA-based BM1 (0 vs 1 vs 2) | 3.45 (1.49-7.96) | .004 | 6.28 (2.24-17.6) | <.001 | 2.01 (0.67-6.02) | .213 | 2.25 (0.68-7.39) | .183 |
| ccfDNA-based BM2 (0 vs 1 vs 2) | 1.89 (1.05-3.40) | .033 | 2.47 (1.33-4.56) | .004 | 1.71 (0.86-3.38) | .125 | 4.16 (1.86-9.31) | <.001 |
| ccfDNA-based scores—Model 1 | ||||||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 5.51 (2.08-14.6) | <.001 | 5.43 (2.12-13.9) | <.001 |
| ccfDNA-based BM score (positive vs negative) | 2.87 (1.20-6.90) | .018 | 8.24 (2.29-29.7) | .001 | 2.86 (0.95-7.54) | .061 | 8.80 (2.01-38.4) | .004 |
| ccfDNA-based scores—Model 2 | ||||||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 6.10 (2.19-16.9) | <.001 | 7.71 (2.82-21.1) | <.001 |
| ccfDNA-based BM score (0 vs 1 vs 2 vs 3 vs 4) | 1.80 (1.22-2.66) | .003 | 2.37 (1.51-3.71) | <.001 | 1.81 (1.15-2.81) | .009 | 3.39 (1.83-6.29) | <.001 |
| . | Univariable analysis . | Multivariable analysis . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | PFS . | OS . | PFS . | OS . | ||||
| . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . | HR (95% CI) . | P-value . |
| Parameter (n of patients if different from 34) | ||||||||
| Age (≤50 vs >50 years) | 0.88 (0.33-2.40) | .814 | 0.65 (0.28-1.51) | .314 | ||||
| Symptoms at diagnosis (yes vs no) | 2.44 (0.72-8.30) | .154 | .146 | |||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 6.02 (2.14-16.9) | <.001 | 9.49 (2.89-31.2) | <.001 |
| Resection status (n = 22) (R0 vs R1 vs R2) | 1.77 (1.06-2.95) | .030 | 2.59 (1.25-5.37) | .011 | ||||
| Ki67 index (n = 26) (<10 vs 10-19 vs ≥20) | 2.43 (0.92-6.42) | .074 | 5.54 (0.84-36.6) | .075 | ||||
| S-GRAS score (n = 22) (group 1 vs 2 vs 3 vs 4) | 2.14 (1.10-4.16) | .025 | 7.52 (1.83-30.9) | .005 | ||||
| ccfDNA-based BM1 (0 vs 1 vs 2) | 3.45 (1.49-7.96) | .004 | 6.28 (2.24-17.6) | <.001 | 2.01 (0.67-6.02) | .213 | 2.25 (0.68-7.39) | .183 |
| ccfDNA-based BM2 (0 vs 1 vs 2) | 1.89 (1.05-3.40) | .033 | 2.47 (1.33-4.56) | .004 | 1.71 (0.86-3.38) | .125 | 4.16 (1.86-9.31) | <.001 |
| ccfDNA-based scores—Model 1 | ||||||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 5.51 (2.08-14.6) | <.001 | 5.43 (2.12-13.9) | <.001 |
| ccfDNA-based BM score (positive vs negative) | 2.87 (1.20-6.90) | .018 | 8.24 (2.29-29.7) | .001 | 2.86 (0.95-7.54) | .061 | 8.80 (2.01-38.4) | .004 |
| ccfDNA-based scores—Model 2 | ||||||||
| ENSAT tumour stage (1-2 vs 3 vs 4) | 6.40 (2.34-17.5) | <.001 | 6.60 (2.51-17.3) | <.001 | 6.10 (2.19-16.9) | <.001 | 7.71 (2.82-21.1) | <.001 |
| ccfDNA-based BM score (0 vs 1 vs 2 vs 3 vs 4) | 1.80 (1.22-2.66) | .003 | 2.37 (1.51-3.71) | <.001 | 1.81 (1.15-2.81) | .009 | 3.39 (1.83-6.29) | <.001 |
S-GRAS score was calculated as previously published (Elhassan et al., Eur J Endocrinol 2021, DOI: 10.1530/EJE-21-0510). Significant variables available in all patients are in bold.
Abbreviations: BM, biomarker; BM1, total ccfDNA concentrations; BM2, somatic variants detected in ccfDNA; ccfDNA, circulating cell-free DNA; ENSAT, European Network for the Study of Adrenocortical Tumors; OS, overall survival; PFS, progression-free survival.
Role of ccfDNA-based BMs in disease monitoring (AIM 2)
Finally, we tested the potential role of ccfDNA-based BMs for longitudinal disease monitoring (AIM 2). Serial blood samples collected during standard follow-up visits after primary surgery were available for 19 patients (median duration 9 months, range: 3-12; Figure 1).
In two of the patients who underwent a debulking surgery due to severe steroid excess (ACC-P5 and ACC-P28), ccfDNA concentrations persisted at very high levels after surgery, which coincided with rapid disease progression. Importantly, in one case (ACC-P28), 2 somatic variants were detected at baseline and remained detectable at the 3-month follow-up analysis (PRKAR1A VAF from 10.9% to 4.9%, TERT VAF from 26.5% to 5.0%) when the radiological imaging showed enlarging liver metastases (Table S2 and Figure S4).
In 6 cases, patients presented with disease recurrences during surveillance, ie, at short-term follow-ups (n = 2 at 3 months and n = 1 at 6 months) or long-term follow-ups (n = 1 at 9 months and n = 1 at 12 months). In some cases, even if BM2 could not be used during monitoring, ccfDNA concentrations alone could mirror the trend of the radiological imaging, greatly increasing at the time of disease recurrence (representative example shown in Figure 4A). Two cases presented with somatic variants in MEN1 and ZNRF3 at baseline ccfDNA (ACC-P3 and ACC-P8; Figure S4). In one case, these persisted in the 3-month sample when the patient showed an early disease recurrence (ie, liver metastases). At the 6-month follow-up, the patient showed a mixed response to treatment with mitotane, but variants could not be detected due to low coverage. The same patient presented a rapid progression 9 months after surgery with a significant increase in the size of the liver lesions and multiple lung metastases (Figure 4B). Simultaneously, both the ccfDNA concentrations and the VAF % of both variants sharply increased (to 46.4% and 45.8%, respectively). In the other case, no variants were detected at the 3-month follow-up due to low coverage.

Monitoring role of ccfDNA-related biomarkers (AIM 2)—2 representative examples of longitudinal ccfDNA analysis from serial samples collected in patients with primary ACC that developed disease recurrence during follow-up after successful surgery. (A) patient with ACC with no detected somatic variants at both baseline ccfDNA and T-DNA. Disease recurrence was observed at surveillance imaging 12 months after adrenalectomy during adjuvant treatment with mitotane; (B) patient with ACC with 2 somatic variants at baseline ccfDNA. First surveillance imaging 3 months after adrenalectomy showed an early disease recurrence with evidence of liver metastases. An initial mixed response at mitotane was observed at 6- and 9-month follow-ups, followed by progressive disease with increase in size of liver metastases and new multiple lung metastases. This was followed by start of systemic chemotherapy with etoposide–doxorubicin–cisplatin. ACC-P, primary adrenocortical carcinoma; ccfDNA, circulating cell-free DNA; mixed, mixed response to treatment; PD, progressive disease; Rec, disease recurrence; Sx, surgery; T-DNA, tumour DNA; TF, tumour free; VAF, variant allele frequency.
Finally, 11 cases showed no evidence of disease recurrence at the last available CT TAP scan. The trend observed in mean total ccfDNA concentrations over time in this group is shown in Figure 5A. Of note, ccfDNA levels progressively decreased during surveillance, even if in very few cases remained slightly above the chosen cut-off (representative examples in Figure 5B and C). Among these cases, 5 presented somatic variants at baseline ccfDNA (Figure S4). Importantly, these variants were no more detectable neither at first post-surgical follow-up nor during further surveillance (representative examples are shown in Figure 5D-F).
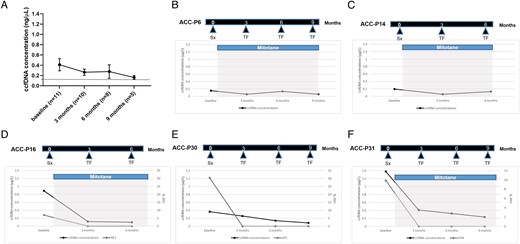
Monitoring role of ccfDNA-related biomarkers (AIM 2)—overview and 5 representative examples of longitudinal ccfDNA analysis from serial samples collected in patients with ACC that remained tumour free at radiological imaging during follow-up after successful primary surgery. (A) total ccfDNA concentrations prior to surgery and during follow-up (mean ± standard deviation); (B and C) patients without somatic variants at baseline ccfDNA; (D-F) patients with detected somatic variants at baseline ccfDNA. ACC-P, primary adrenocortical carcinoma; ccfDNA, circulating cell-free DNA; Sx, surgery; TF, tumour free; VAF, variant allele frequency.
Overall, even in this relatively small cohort of cases with fully available data, ccfDNA-related BM1 showed a sensitivity of 100% and a specificity of 36% (n = 19), while BM2 showed a sensitivity of 100% and a specificity of 67% (n = 8).
Of note, we also sequenced longitudinal ccfDNA samples from 10 patients with no somatic variants detected in neither baseline ccfDNA nor T-DNA (ie, 3 short-term and 7 long-term). Among these cases, 4 developed a disease recurrence over the time (40% of the total). Interestingly, none of them showed any somatic variants at the serial ccfDNA samples (Table S3).
Discussion
In the present study, we performed a comprehensive ccfDNA analysis in prospectively collected samples from a large cohort of 34 patients with primary ACC. We could demonstrate that ccfDNA-based BMs can be detected in a noticeable proportion of patients and could be proposed for both prognostic classification and disease monitoring.
We first investigated the relationship between total ccfDNA concentrations (namely BM1) and clinical parameters at time of diagnosis. To this aim, we used a robust pipeline for sample collection and processing, as well as commercially available, ready-to-use, highly sensitive ccfDNA isolation kits. Circulating cell-free DNA levels were significantly higher in patients with ACC compared with HSs and correlated with the tumour burden and aggressiveness, as previously reported for other cancer types.37-39 Importantly, using an arbitrary cut-off based on levels observed in HSs, BM1 was considered positive in 96% of ACC. The proportion of circulating tumour DNA (ctDNA) in the background of overall ccfDNA has been historically reported as highly variable, ranging from 0.01% to 90%.9,16,40 In fact, many factors may influence the concentration of ctDNA, including tumour volume, localization and vascularization, hepatic and renal clearance, and anti-cancer treatments.41 In our patient cohort, all baseline samples were collected at the time of diagnosis, when patients were treatment naïve and had normal liver and kidney function, therefore excluding these potential interferences. We could also demonstrate that positive BM1 was strongly associated with the worst clinical outcome, both in terms of PFS and OS, in agreement with recent reports showing the potential prognostic role of ccfDNA concentrations alone.20 These findings are of particular interest considering that measuring ccfDNA levels is a minimally invasive, cheap, and straightforward technique that could easily be implemented in clinical practice to further improve prognostic classification of ACC.
The information about somatic genetic events detected at the ccfDNA level was shown to be of additional importance. Here, we used a library preparation procedure incorporating an error suppression technology to ensure confident calling of mutations down to 0.1% VAF. In fact, almost 50% of primary ACC presented at least 1 somatic variant at ccfDNA. This proportion of positive cases is higher than those described in previous studies on smaller cohorts of patients, ie, 20%-30%.25, 26 This could be at least in part due to (1) the use of a more homogeneous series of patients with primary ACC in place; (2) a robust pipeline for collection, processing, isolation, and measurement of ccfDNA; and (3) the utilization of a highly sensitive customized ACC-specific gene panel. Another recent study, using a different approach, ie, Guardant360 (Guardant Health, Inc., Redwood City, CA, United States) that allows to analyse not only SNV/indels but also gene fusions and copy number amplifications, identified alterations in ccfDNA in up to 80% of patients with ACC.42
In our cohort, somatic alterations detected in ccfDNA samples matched with available T-DNA in almost 70% of cases. Moreover, in about 20% of cases, variants in ACC-specific genes (ZNRF3, APC, GNAS, and NF1) were detected only in ccfDNA—but not in T-DNA. These findings further confirm the additional value and the potential clinical utility of ccfDNA sequencing in molecular profiling—compared with tissue sequencing.19,43-45 This is particularly relevant for highly heterogeneous cancers such as ACC. Of note, at ccfDNA sequencing, the most frequently affected pathway confirmed to be Wnt/β-catenin and chromatin remodelling—as previously reported in multiple pan-genomic or targeted studies on both snap-frozen and FFPE tumour samples.29-31 On the contrary, only a few variants were detected at the ccfDNA level affecting genes in the p53/Rb pathway.
The presence of somatic variants at ccfDNA showed to have prognostic value (AIM 1), being linked to a shorter progression-free and OS, in agreement with previous studies on other solid tumours .46-48 In fact, the ccfDNA-based BM score calculated by merging BM1 and BM2, was found to be strongly associated with clinical outcomes, remaining a significant, independent prognostic factor at multivariable analysis including the ENSAT tumour stage. This is of particular interest considering that ACC is a generally aggressive cancer with heterogeneous and difficult-to-predict clinical outcomes. Therefore, we could suggest that ccfDNA-based BM evaluated at the time of diagnosis could be used for improving the prognostic classification of patients with ACC.
It is important to mention that at least 8 genes contained in our customized panel of ACC-specific genes are classified as drug targetable. At ccfDNA sequencing, somatic variants were detected in one of these genes in three patients (9% of the total), ie, two presented missense mutations in ATM (targetable by PARP inhibitors) and one a missense mutation in NF1 (targetable by MEK inhibitors). Interestingly, in 2 cases, information about druggable genetic events could only be gained by analysing ccfDNA. In fact, in 1 case, the patient did not undergo surgery due to the presence of disseminated disease, and in the other case, T-DNA sequencing did not detect the presence of any variants. These findings further corroborate the potential clinical utility of ccfDNA analysis for molecular profiling and identification of targetable events in ACC,42 similar to what has been proposed for other cancer types.18,22
We also evaluated the potential role of ccfDNA analysis for disease monitoring (AIM 2) in serial samples of 19 patients who underwent standard follow-up visits.2 We observed a relatively good correspondence between the ccfDNA-based BMs and the radiological evidence of tumour manifestations. For instance, patients with advanced ACC who underwent debulking surgery presented very high levels of ccfDNA after surgery in agreement with rapid disease progression. When present at baseline, somatic variants remained detectable in most cases during surveillance and matched with the radiological disease progression. Moreover, among patients with disease recurrences, one presented with baseline ccfDNA somatic variants that persisted at the first post-surgical follow-up when the patient showed an early disease recurrence, while both the ccfDNA concentrations and VAF% sharply increased at the time of rapid disease progression. Finally, in patients with no evidence of recurrent disease, somatic variants were not detectable in ccfDNA neither at first post-surgical follow-up nor during further surveillance.
In the relatively small cohort of cases with fully available data, both BM1 and BM2 showed a very high sensitivity (both 100%), while BM2 showed a better specificity than BM1 (67% vs 36%). Therefore, we hypothesize that ccfDNA analysis could be useful to complement radiological surveillance for both the detection of early recurrences in patients with successfully resected ACC and monitoring of disease evolution and/or response to treatment in patients with advanced ACC, similar to what has been proposed for other solid tumours.49 However, further studies on larger cohorts of patients with longer follow-up periods are required to validate our findings.
Overall, ccfDNA analysis has evolved since its inception with improvements in the technologies and detection limits and represents a set of research tools that appear poised to enter routine clinical care.50, 51 As a matter of fact, an FDA-approved ctDNA assay, the Cobas epidermal growth factor receptor (EGFR) Mutation Test (Roche, Basel, Switzerland), is available to detect EGFR mutations and drive the use of EGFR tyrosine kinase inhibitor therapy in non-small cell lung cancer. Moreover, there are 2 CLIA-certified commercially available ctDNA platforms: the mentioned Guardant360 panel for the assessment of 73 cancer genes and the PlasmaSelect (Personal Genome Diagnostics, Inc., Baltimore, MD, United States) with a 64-gene panel. However, this approach is still quite expensive and not readily available, especially for rare cancer types.
Limitations of the study are represented by the relatively short period of follow-up (up to max. 12 months) and the lack of an evaluation of ccfDNA-related BMs in the early post-operative time point (ie, 6-8 weeks). These aspects were beyond the scope of this pilot study and will be the aim of a future long-term project. Moreover, in few cases, some follow-up visits (and therefore blood collections) have been missing due to the restrictions related to the COVID-19 pandemic that significantly reduced the face-to-face access to the health systems.
In conclusion, ccfDNA-related BMs are frequently detected in patients with ACC and may represent a promising, minimally invasive tool to predict early disease progression and complement imaging in disease surveillance. Further studies are, however, required before these BMs could be proposed for implementation in clinical practice.
Acknowledgements
The authors are grateful to Ms. Michaela Haaf for coordinating the clinical data record in Wuerzburg and to Antonia Lorey for their skilful technical assistance. This work has been carried out with the help of the Interdisciplinary Bank of Biomaterials and Data of the University Hospital of Wuerzburg and the Julius Maximilian University of Wuerzburg (IBDW). The implementation of the IBDW has been supported by the Federal Ministry for Education and Research (grant number FKZ: 01EY1102). We also thank the EU COST Action CA20122 Harmonization for supportive networking (www.goharmonisation.com).
Supplementary material
Supplementary material is available at European Journal of Endocrinology online.
Funding
This work has been supported by the Deutsche Krebshilfe (70113526 to M.F., C.L.R, and S.A.), the patient Association for Multiple Endocrine Neoplasia Disorders (AMEND) (ACC Research Award 2019 and 2021 to C.L.R.), and the European Reference Network on Rare Endocrine Conditions (Endo-ERN). A.P. receives support from the National Institute for Health and Care Research (NIHR) Birmingham Biomedical Research Centre at the University Hospitals Birmingham NHS Foundation Trust and the University of Birmingham (grant reference number NIHR203326), while C.L.R receives support from HRA Pharma Rare Disease (research grant). The funders of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care UK.
Authors’ contributions
Juliane Lippert (Conceptualization [equal], Formal analysis [equal], Investigation [equal], Methodology [equal], Project administration [equal], Writing—original draft [equal]), Gabrielle Smith (Formal analysis [equal], Investigation [equal[, Methodology [equal], Project administration [equal]), Silke Appenzeller (Data curation [equal], Formal analysis [equal], Funding acquisition [equal], Investigation [equal], Methodology [equal], Software [equal], Writing—review & editing [equal]), Laura-Sophie Landwehr (Investigation [equal], Methodology [equal[, Writing—review & editing [equal]), Alessandro Prete (Funding acquisition [equal], Investigation [equal], Methodology [equal], Writing—review & editing [equal]), Sonja Steinhauer (Investigation [equal], Methodology [equal], Project administration [equal]), Miriam Asia (Investigation [equal], Resources [equal]), Hanna Urlaub (Investigation [equal], Methodology [equal], Project administration [equal]), Yasir Elhassan (Resources [equal], Writing—review & editing [equal]), Stefan Kircher (Investigation [equal], Methodology [equal], Resources [equal]), Wiebke Arlt (Conceptualization [equal], Writing—review & editing [equal]), Martin Fassnacht (Conceptualization [equal], Funding acquisition [equal], Supervision [equal], Writing—review & editing [equal]), Barbara Altieri (Conceptualization [equal], Data curation [equal], Investigation [equal], Methodology [equal], Resources [equal], Supervision [equal], Writing—review & editing [equal]), and Cristina Ronchi (Conceptualization [equal], Data curation [equal], Formal analysis [equal], Funding acquisition [equal], Project administration [equal], Resources [equal], Supervision [equal], Writing—original draft [equal])
Conflict of interest: The authors declare no potential conflict of interest in relation to this work. Co-author W.A. is on the editorial board of EJE. They were not involved in the review or editorial process for this paper, on which they are listed as authors.



