-
PDF
- Split View
-
Views
-
Cite
Cite
Rachel Eikelboom, Richard P Whitlock, Raveen Muzaffar, Renato D Lopes, Deborah Siegal, Sam Schulman, Emilie P Belley-Côté, Direct oral anticoagulants versus vitamin K antagonists in the first 3 months after bioprosthetic valve replacement: a systematic review and meta-analysis, European Journal of Cardio-Thoracic Surgery, Volume 63, Issue 4, April 2023, ezad110, https://doi.org/10.1093/ejcts/ezad110
Close - Share Icon Share
Abstract
We conducted a systematic review and meta-analysis of randomized controlled trials comparing direct oral anticoagulants (DOACs) to vitamin K antagonists (VKAs) in the first 90 days after bioprosthetic valve implantation.
We systematically searched Embase, Medline and CENTRAL. We screened titles, abstracts and full texts, extracted data and assessed the risk of bias in duplicate. We pooled data using the Mantel–Haenzel method and random effects modelling. We conducted subgroup analyses based on the type of valve (transcatheter versus surgical) and timing of initiation of anticoagulation (<7 vs >7 days after valve implantation). We assessed the certainty of evidence using the Grading of Recommendations, Assessments, Development and Evaluation approach.
We included 4 studies of 2284 patients with a median follow-up of 12 months. Two studies examined transcatheter valves (1877/2284 = 83%) and 2 examined surgical valves (407/2284 = 17%). We found no statistically significant differences between DOACs and VKAs with regard to thrombosis, bleeding, death or subclinical valve thrombosis. However, there was a subgroup trend towards more bleeding with DOACs when initiated within 7 days of valve implantation.
In the existing randomized literature on DOACs versus VKAs in the first 90 days after bioprosthetic valve implantation, there appears to be no difference with regard to thrombosis, bleeding or death. Interpretation of the data is limited by small numbers of events and wide confidence intervals. Future studies should focus on surgical valves and should include long-term follow-up to assess any potential impact of randomized therapy on valve durability.
INTRODUCTION
More than 40 million people worldwide have aortic or mitral valve disease, and >200 000 valve replacements are performed every year in North America, mostly with bioprosthetic valves [1]. The risk of valve-related thrombosis and thromboembolism is highest in the first 90 days after valve implantation, and many patients are treated with an oral anticoagulant during this time period. In addition, ∼30–50% of patients undergoing bioprosthetic aortic or mitral valve replacement have an indication for long-term anticoagulation, most commonly atrial fibrillation [2–5]. Direct oral anticoagulants (DOACs) are used in the majority of patients with atrial fibrillation [6], reflecting randomized evidence demonstrating that in patients with atrial fibrillation, DOACs reduce the risk of stroke or systemic embolism by 19%, including a 50% reduction in the risk of intracranial haemorrhage compared to vitamin K antagonists (VKAs) [7]. However, uncertainty about the efficacy of DOACs for the prevention of bioprosthetic valve-related thrombosis and thromboembolism remains, particularly in the first 90 days after valve implantation. Furthermore, there are concerns about the early use of DOACs after valve implantation due to the risk of periprocedural bleeding given that DOACs have a rapid onset of effect and their reversal agents are not widely available.
We conducted a systematic review and meta-analysis of randomized controlled trials comparing DOACs to VKAs in patients with recent (<90 days) bioprosthetic valve replacement and an indication for oral anticoagulation.
PATIENTS AND METHODS
We conducted our review in accordance with a pre-specified protocol (PROSPERO registration number CRD42022328463).
Search strategy and selection criteria
We searched Embase, Medline and CENTRAL from 1 January 2007 until 9 November 2022. We created our search strategy (detailed in Supplementary Material, Table S1) with the assistance of an academic librarian. We restricted our search to publications from 2007 or later because the first clinical trial of DOACs was published in 2007. We checked references of relevant articles and reviewed the list of included studies with researchers with relevant expertise.
We included randomized controlled trials comparing DOACs to VKAs within 90 days of bioprosthetic surgical valve replacement or transcatheter aortic valve replacement (TAVR). We included any DOAC (dabigatran, rivaroxaban, apixaban or edoxaban) compared to any VKA, with or without antiplatelet therapy.
Our outcomes of interest were thrombotic events (stroke, systemic embolism and valve thrombosis), major bleeding events, death, aortic valve reintervention and subclinical valve thrombosis [defined as hypoattenuated leaflet thickening, with or without reduced leaflet motion, diagnosed by computed tomography (CT)].
Two reviewers (Rachel Eikelboom and Raveen Muzaffar) independently screened titles and abstracts of identified studies using a standardized form. If a study’s title or abstract suggested it might be relevant, then the same reviewers independently assessed the full text for inclusion, resolving disagreements by discussion. We did not require a third reviewer for resolution.
Data abstraction and analysis
Two review authors (Rachel Eikelboom and Raveen Muzaffar) independently abstracted data about study design, sample size, baseline demographics and outcomes into a standardized Microsoft Excel spreadsheet.
We assessed risk of bias using the revised Cochrane Risk of Bias tool for randomized trials [8]. We resolved disagreements by discussion without requiring a third reviewer.
We used the Mantel–Haenszel method and random effects modelling to generate pooled risk ratios for all outcomes. We performed analyses in DataParty (DataParty Inc., Hamilton, ON, Canada; available at https://dataparty.ca). In cases of zero events, we imputed a value of 0.4 in the zero cell to allow for statistical analysis.
To evaluate heterogeneity, we used visual inspection of forest plots and computed Chi2 tests (statistical significance set at P < 0.10) and the I2 statistic (0–40% = might not be important, 30–60% = moderate, 50–90% = substantial, 75–100% = considerable).
We conducted a pre-specified subgroup analysis based on whether studies randomized patients within 7 days of valve replacement. We also conducted a post hoc subgroup analysis comparing transcatheter and surgical valves. We assessed for subgroup differences by performing a test for interaction and reporting the P-value. We also performed an exploratory sensitivity analysis using fixed effect modelling. In the event of significant heterogeneity in the pooled results, we also planned to investigate the effect of different duration of follow-up.
We used the Grading of Recommendations Assessment, Development and Evaluation [9] approach to summarize the certainty of evidence on an outcome-by-outcome basis as high, moderate, low or very low. Grading of Recommendations Assessment, Development and Evaluation involves assessing the certainty of evidence according to the following domains: risk of bias, indirectness, inconsistency, imprecision and publication bias. Two authors (Rachel Eikelboom and Raveen Muzaffar) determined certainty of evidence by discussion and consensus.
RESULTS
From 1127 citations, we found 4 eligible randomized controlled trials that included 2284 patients (Supplementary Material, Fig. S1).
Characteristics of included studies
The 4 studies that met inclusion criteria were published between 2016 and 2021. They all had a prospective randomized open blinded end point design. The studies included 2284 patients with the following types of valves: transcatheter aortic valve replacement (2 studies [10, 11], 1877 participants, 83%), surgical bioprosthetic mitral valve replacement (1 study [12], 191 participants, 8%) and surgical bioprosthetic aortic or mitral valve replacement (1 study [13], 218 participants, 10%). Studies compared apixaban [10], edoxaban [11, 13] or rivaroxaban [12] to VKAs with a therapeutic range of international normalized ratio of 2–3. The Anti-Thrombotic Strategy to Lower All Cardiovascular and Neurologic Ischaemic and Hemorrhagic Events after Trans-Aortic Valve Implantation for Aortic Stenosis (ATLANTIS) trial [10] included patients with and without an indication for oral anticoagulation, from which we included the subset of patients with an indication for oral anticoagulation. Oral anticoagulation was initiated between 12 h and 90 days after valve implantation. Although the Rivaroxaban for Valvular Heart Disease and Atrial Fibrillation (RIVER) trial [12] randomized patients any time after valve implantation, we only included the subset of patients who were randomized within 90 days of surgery. Follow-up duration ranged from 90 days to 18 months (median 12 months), and loss to follow-up was minimal (0.5–2.5% in individual studies). Study characteristics are summarized in Table 1.
| Study . | Valve type . | Inclusion criteria . | Key exclusion criteria . | Intervention . | Comparison . | Timing of initiation of anticoagulation . | Mean follow-up duration . | Sample size . |
|---|---|---|---|---|---|---|---|---|
| TAVR |
|
|
| VKA INR 2–3 |
| 12 months | 451 |
|
|
|
| Rivaroxaban 20 mg daily. Dose reduced to 15 mg daily in patients with creatinine clearance 30–49 ml per 1.73 m2 of body-surface area | VKA INR 2–3 |
| 12 months | 189 |
| Surgical aortic or mitral valve |
|
| Edoxaban 60 mg daily. Dose reduced to 30 mg daily in patients with creatinine clearance 30–50 ml/min, body weight <60 kg or in patients receiving concomitant treatment with potent P-glycoprotein inhibitors | VKA INR 2–3 |
| 3 months | 218 |
| TAVR |
|
| Edoxaban 60 mg daily. Dose reduced to 30 mg daily in patients with creatinine clearance (Cockcroft–Gault formula) of 15–50 ml/min, body weight <60 kg or less and the use of certain P-glycoprotein inhibitors | VKA INR 2–3 |
| 18 months | 1416 |
| Study . | Valve type . | Inclusion criteria . | Key exclusion criteria . | Intervention . | Comparison . | Timing of initiation of anticoagulation . | Mean follow-up duration . | Sample size . |
|---|---|---|---|---|---|---|---|---|
| TAVR |
|
|
| VKA INR 2–3 |
| 12 months | 451 |
|
|
|
| Rivaroxaban 20 mg daily. Dose reduced to 15 mg daily in patients with creatinine clearance 30–49 ml per 1.73 m2 of body-surface area | VKA INR 2–3 |
| 12 months | 189 |
| Surgical aortic or mitral valve |
|
| Edoxaban 60 mg daily. Dose reduced to 30 mg daily in patients with creatinine clearance 30–50 ml/min, body weight <60 kg or in patients receiving concomitant treatment with potent P-glycoprotein inhibitors | VKA INR 2–3 |
| 3 months | 218 |
| TAVR |
|
| Edoxaban 60 mg daily. Dose reduced to 30 mg daily in patients with creatinine clearance (Cockcroft–Gault formula) of 15–50 ml/min, body weight <60 kg or less and the use of certain P-glycoprotein inhibitors | VKA INR 2–3 |
| 18 months | 1416 |
INR: international normalized ratio; IQR: interquartile range; MI: myocardial infarction; OAC: oral anticoagulation; VKA: vitamin K antagonist.
| Study . | Valve type . | Inclusion criteria . | Key exclusion criteria . | Intervention . | Comparison . | Timing of initiation of anticoagulation . | Mean follow-up duration . | Sample size . |
|---|---|---|---|---|---|---|---|---|
| TAVR |
|
|
| VKA INR 2–3 |
| 12 months | 451 |
|
|
|
| Rivaroxaban 20 mg daily. Dose reduced to 15 mg daily in patients with creatinine clearance 30–49 ml per 1.73 m2 of body-surface area | VKA INR 2–3 |
| 12 months | 189 |
| Surgical aortic or mitral valve |
|
| Edoxaban 60 mg daily. Dose reduced to 30 mg daily in patients with creatinine clearance 30–50 ml/min, body weight <60 kg or in patients receiving concomitant treatment with potent P-glycoprotein inhibitors | VKA INR 2–3 |
| 3 months | 218 |
| TAVR |
|
| Edoxaban 60 mg daily. Dose reduced to 30 mg daily in patients with creatinine clearance (Cockcroft–Gault formula) of 15–50 ml/min, body weight <60 kg or less and the use of certain P-glycoprotein inhibitors | VKA INR 2–3 |
| 18 months | 1416 |
| Study . | Valve type . | Inclusion criteria . | Key exclusion criteria . | Intervention . | Comparison . | Timing of initiation of anticoagulation . | Mean follow-up duration . | Sample size . |
|---|---|---|---|---|---|---|---|---|
| TAVR |
|
|
| VKA INR 2–3 |
| 12 months | 451 |
|
|
|
| Rivaroxaban 20 mg daily. Dose reduced to 15 mg daily in patients with creatinine clearance 30–49 ml per 1.73 m2 of body-surface area | VKA INR 2–3 |
| 12 months | 189 |
| Surgical aortic or mitral valve |
|
| Edoxaban 60 mg daily. Dose reduced to 30 mg daily in patients with creatinine clearance 30–50 ml/min, body weight <60 kg or in patients receiving concomitant treatment with potent P-glycoprotein inhibitors | VKA INR 2–3 |
| 3 months | 218 |
| TAVR |
|
| Edoxaban 60 mg daily. Dose reduced to 30 mg daily in patients with creatinine clearance (Cockcroft–Gault formula) of 15–50 ml/min, body weight <60 kg or less and the use of certain P-glycoprotein inhibitors | VKA INR 2–3 |
| 18 months | 1416 |
INR: international normalized ratio; IQR: interquartile range; MI: myocardial infarction; OAC: oral anticoagulation; VKA: vitamin K antagonist.
The weighted mean patient age was 79 years, and 49% (1111/2284) were female. Patients with TAVR were older on average than patients with surgical valve replacement (82 vs 64 years). Overall, 84% (1911/2284) of patients had hypertension, 32% (732/2284) had diabetes and 15% (340/2284) had a previous stroke.
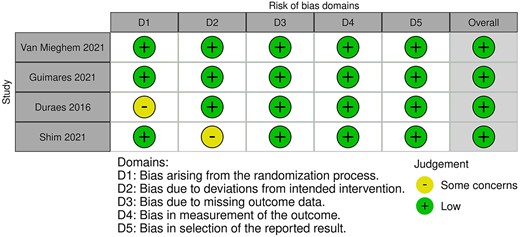
Risk of bias was low for all included studies, as summarized in Fig. 1.
Thrombosis
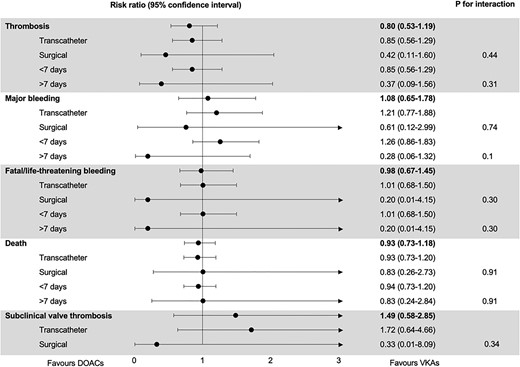
All studies provided data on stroke, 1 study provided data on systemic embolism and 2 studies provided data on valve thrombosis, over a median follow-up of 12 months. Patients taking DOACs experienced 40 thrombotic events (4%) and patients taking VKAs experienced 50 thrombotic events (4%), resulting in a risk ratio of 0.81 [95% confidence interval (CI), 0.54–1.22; Fig. 2]. In absolute terms, DOACs were associated with 8 fewer thrombotic events per 1000 patients than VKAs (95% CI, from 20 fewer to 10 more). Neither transcatheter versus surgical valves (P for interaction 0.44) nor randomization within versus >7 days after valve implantation (P for interaction 0.31) were effect modifiers. Subgroup estimates are summarized in Fig. 3. A sensitivity analysis using fixed effects yielded almost identical results [relative risk (RR) 0.81, 95% CI 0.54–1.21].
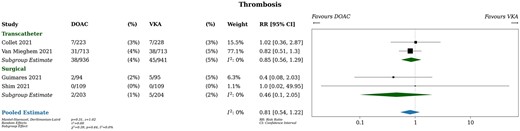
Thrombosis. DOAC: direct oral anticoagulant; RR: relative risk; VKA: vitamin K antagonist.
Subgroup analysis. DOAC: direct oral anticoagulant; RR: relative risk; VKA: vitamin K antagonist.
Heterogeneity was likely unimportant based on visual inspection of the forest plot and statistical tests (I2 = 0%). The certainty of the evidence was judged to be moderate due to wide CIs which include both clinically meaningful and clinically unimportant differences. The summary of findings table presents further details on assessments of the certainty of the evidence for all outcomes (Supplementary Material, Table S2).
Bleeding
All studies provided data on life-threatening, disabling or major bleeding over a median follow-up of 12 months. Patients taking DOACs experienced 125 bleeding events (11%) and patients taking VKAs experienced 100 bleeding events (9%), resulting in a risk ratio of 1.12 (95% CI, 0.67–1.9; Fig. 4). In absolute terms, patients taking DOACs had 11 more bleeding events per 1000 patients (95% CI, from 21 fewer to 79 more) compared to patients taking VKAs, without a significant subgroup difference between surgical and transcatheter valves (P for interaction 0.74). There was a trend towards more bleeding in patients taking DOACs when they were randomized within 7 days of valve implantation, and a trend towards more bleeding in patients taking VKAs when they were randomized >7 days after valve implantation (P for interaction 0.10). A sensitivity analysis using fixed effects yielded a similar RR but a 95% CI was much narrower (RR 1.26, 95% CI 0.98–1.61).

Life threatening, disabling or major bleeding. DOAC: direct oral anticoagulant; RR: relative risk; VKA: vitamin K antagonist.
Three studies provided data on fatal or life-threatening bleeding over a median follow-up of 14 months. Patients taking DOACs experienced 47 fatal or life-threatening bleeding events (4.5%) and patients taking VKAs experienced 49 events (4.9%), resulting in a risk ratio of 0.98 (95% CI, 0.67–1.45; Supplementary Material, Fig. S2). In absolute terms, patients taking DOACs had 1 fewer bleeding events per 1000 patients (95% CI, from 16 fewer to 21 more) compared to patients taking VKAs, without a significant subgroup difference between surgical and transcatheter valves (P for interaction 0.30) or randomization within versus >7 days after valve implantation (P for interaction 0.30).
Inconsistency was present based on visual inspection of the forest plot, which showed that individual studies had effect estimates in favour of both DOACs and VKAs, and statistical tests (I2 = 48%). Effect estimates were very imprecise, with large CIs that included both clinically meaningful and clinically unimportant differences. The certainty of the evidence was judged to be low.
Death
All studies provided data on mortality over a median follow-up of 12 months. Patients taking DOACs experienced 112 deaths (10%) and patients taking VKAs experienced 120 deaths (10%), resulting in a risk ratio of 0.94 (95% CI, 0.74–1.19; Fig. 5). In absolute terms, patients taking DOACs experienced 6 fewer deaths per 1000 patients (95% CI, from 27 fewer to 20 more) compared to patients taking VKAs. Neither transcatheter versus surgical valve (P for interaction 0.91) nor randomization within versus >7 days after valve implantation (P for interaction 0.91) were effect modifiers. A sensitivity analysis using fixed effects yielded identical results.
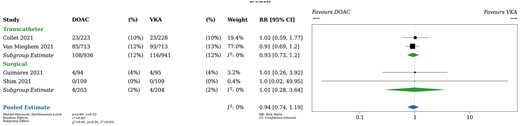
Death. DOAC: direct oral anticoagulant; RR: relative risk; VKA: vitamin K antagonist.
Heterogeneity was likely unimportant based on visual inspection of the forest plot and statistical tests (I2 = 0%). The certainty of the evidence was judged to be moderate due to wide Cis, which include both clinically meaningful and clinically unimportant differences.
Valve reintervention
No studies reported incidence of valve reintervention.
Subclinical valve thrombosis
Only 2 studies of the 4 included in this systematic review reported the incidence of subclinical valve thrombosis over a median follow-up of 7.5 months. Patients taking DOACs experienced 9 events of subclinical valve thrombosis (4%) patients taking VKAs experienced 7 events of subclinical valve thrombosis (3%), resulting in a risk ratio of 1.49 (95% CI, 0.58–3.85; Fig. 6). In absolute terms, patients taking DOACs experienced 16 more events of subclinical valve thrombosis per 1000 patients (95% CI, from 13 fewer to 92 more) compared to patients taking VKAs. Transcatheter versus surgical valve implantation was not an effect modifier (P for interaction 0.34). Both studies initiated therapy within 7 days of valve implantation. A sensitivity analysis using fixed effects yielded almost identical results (RR 1.43, 95% CI 0.57–3.59).
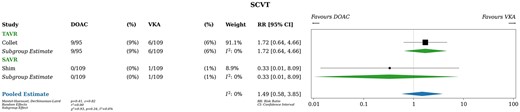
Subclinical valve thrombosis. DOAC: direct oral anticoagulant; RR: relative risk; VKA: vitamin K antagonist.
Heterogeneity was likely unimportant based on visual inspection of the forest plot and statistical tests (I2 = 0%). The certainty of the evidence was judged to be low due to wide Cis, which include both clinically meaningful and clinically unimportant differences.
DISCUSSION
In this systematic review and meta-analysis, 4 randomized trials of 2284 patients compared DOACs to VKAs in the first 3 months after bioprosthetic valve implantation. We found no statistically significant difference in the rates of thrombosis, bleeding or death over a median follow-up of 12 months. Point estimates of effect were not consistently in favour of either DOACs or VKAs; rather, for some outcomes they directionally favoured DOACs while for other outcomes they favoured VKAs. These results were consistent in transcatheter and surgical valves, and when randomized therapy was initiated <7 vs >7 days after valve implantation. However, there was a trend towards increased bleeding when DOACs were initiated within 7 days of valve implantation. The interpretation of our results is limited by the low numbers of included trials and the fact that most events come from a single trial.
The European Society of Cardiology (ESC) guidelines provide differing recommendations for patients with bioprosthetic valve replacement and an indication for anticoagulation (most commonly atrial fibrillation) based on time since valve implantation [14, 15]. Beyond 90 days after valve implantation, they recommend DOACs as first-line therapy (class 2A, level of evidence B). This recommendation reflects randomized trial data showing that DOACs reduce the risk of any stroke or systemic embolism by 19% patients with atrial fibrillation [7]. Within 90 days of valve implantation, the guidelines suggest that DOACs may be a reasonable alternative to VKAs in patients with bioprosthetic mitral valve replacement, based on the results of the RIVER [12] trial (class 2A, level of evidence B). However, they recommend VKAs in the first 90 days after bioprosthetic surgical aortic valve replacement (class 1, level of evidence C) and they do not make a specific recommendation for DOACs or VKAs for TAVR patients. The guidelines distinguish between patients with and without another indication for anticoagulation in addition to their bioprosthetic valve replacement. However, the ESC nor the American College of Cardiology/American Heart Association no longer provide specific recommendations based on the presence of additional thromboembolic risk factors (e.g. hypercoagulable state or left ventricular dysfunction) [14, 15].
There are practical reasons why DOACs may be preferred to VKAs in the first 90 days after valve implantation. While recovering from their valve implantation procedure, patients may not be able to drive, increasing the inconvenience of VKA monitoring. Changes to their other medications may introduce new drug–drug interactions with VKAs. Patients’ dietary intake may be inconsistent contributing to international normalized ratio variability. In addition, patients are less sensitive to warfarin in the first 3 months after surgical valve implantation resulting in a 50% increase in warfarin dose requirements [16]. DOACs avoid the need for dose adjustments and therapeutic monitoring and have far fewer drug–drug and food–drug interactions than VKAs.
However, in addition to sparse evidence demonstrating their noninferiority to VKAs in patients with bioprosthetic valves, there are safety concerns about the use of DOACs in the first 90 days after valve implantation. The Randomized, Phase II Study to Evaluate the Safety and Pharmacokinetics of Oral Dabigatran Etexilate in Patients after Heart Valve Replacement (RE-ALIGN) trial randomized patients with mechanical valve implantation to dabigatran versus warfarin 3–7 days after surgery [17]. Dabigatran was less effective than warfarin in preventing thrombus formation on mechanical valve surfaces and was associated with increased risk of valve thrombosis (5 events in the dabigatran arm vs 0 in the warfarin arm) and stroke (9 events in the dabigatran arm vs 0 in the warfarin arm). In addition, there were 7 major bleeds in the dabigatran arm and 2 in the warfarin arm (hazard ratio 1.76, 95% CI 0.37–8.46); all of the bleeds were intrapericardial.
The RE-ALIGN trial has important differences from the trials included in this systematic review. With regard to thrombosis, mechanical valves are more thrombogenic than bioprosthetic valves. The mechanisms are incompletely understood, but mechanical valve leaflets appear to trigger more protein adsorption and therefore more thrombin generation than bioprosthetic valve leaflets [18]. Our systematic review found similar rates of clinical valve thrombosis, stroke or systemic thromboembolism with DOACs compared to VKAs in patients with bioprosthetic valves. With regard to bleeding, the RE-ALIGN trial included both standard (150 mg twice daily) and high-dose (220 and 300 mg twice daily) dabigatran, which may at least partially explain the increased pericardial bleeding observed in the dabigatran arm. Although we did find a non-significant trend towards increased bleeding with DOACs compared to VKAs when initiated within 7 days of valve implantation, only 1 trial randomized patients within 7 days of surgical valve implantation. In 218 patients, there were 3 major bleeding events in the DOAC arm (1 of which was intrapericardial) and 1 major bleeding event in the VKA arm (not intrapericardial) [13].
More than 80% of patients in this systematic review underwent TAVR, and the interpretation of subgroup analyses in SAVR patients is limited by wide CIs. TAVR patients were older on average than SAVR patients (mean age 82 vs 64), and a greater proportion of TAVR patients had risk factors for bleeding such as hypertension (89% vs 59%) and previous stroke (16% vs 11%). Major bleeding was more common in TAVR patients (11.5%) than in SAVR patients (2%). In patients with similar baseline risk of bleeding, however, clinicians might expect bleeding rates to be higher after SAVR than TAVR given the extent of the surgical procedure and the need for intensive systemic anticoagulation during cardiopulmonary bypass. In major randomized trials of TAVR versus SAVR, 30-day bleeding rates in patients undergoing SAVR tended to be two- to five-fold higher than in patients undergoing TAVR (TAVR 11% vs SAVR 28% in intermediate-risk trials, RR 0.46, 95% CI 0.25–0.99 [19–22]; TAVR 2% vs SAVR 9% in low-risk trials [23, 24], RR 0.19, 95% CI 0.06–0.57; see Supplementary Material, Table S3). Patients in these trials were prescribed dual antiplatelet therapy, or single antiplatelet therapy plus oral anticoagulation if they had another indication for anticoagulation, for at least the first 30 days after valve implantation. The risk of bleeding after surgical bioprosthetic valve replacement in patients requiring anticoagulation is likely higher than this systematic review suggests. Thus, the lower-than-expected event rates and wide CIs of subgroup analyses in surgical (patients limit the application of this systematic review’s results to patients undergoing surgical bioprosthetic valve replacement.
Uncertainty also remains about the effect of DOACs versus VKAs on subclinical valve thrombosis. Subclinical valve thrombosis was first detected in 2015 on routine CT scans [25]. It is characterized by hypoattenuated lesions on valve leaflets, leaflet thickening and reduced leaflet motion with incidence around 5–15% at 30 days and 20–28% at 1 year [26–29]. The relationship between subclinical valve thrombosis and clinical outcomes such as valve failure, symptomatic valve thrombosis, stroke and systemic embolism remains uncertain. However, subclinical valve thrombosis is associated with increased transvalvular gradients on echocardiography, which raises concerns that it is a precursor to structural valve deterioration [30–32]. The ESC guidelines recommend consideration of anticoagulation in patients with subclinical valve thrombosis (class 2A, level of evidence B) without specifying DOACs or VKAs [15]. Given that only 2 of the included studies in this systematic review reported on subclinical valve thrombosis, any differential impact of DOACs versus VKAs on the incidence of subclinical valve thrombosis remains uncertain. Furthermore, given the short follow-up of included studies, clinical sequelae of subclinical valve thrombosis such as structural valve deterioration or valve failure were not captured. The ongoing Direct Oral Anticoagulation versus Warfarin after Cardiac Surgery (DANCE) trial (NCT04284839) will randomize 6215 patients with recent cardiac surgery (<14 days) and atrial fibrillation requiring oral anticoagulation to DOACs versus VKAs for the first 90 days after cardiac surgery. In a subset of 900 patients with bioprosthetic aortic valve replacement, the SUNDANCE substudy will also conduct CT scans in order to assess the impact of DOAC versus VKA on subclinical valve thrombosis and compare this to valve haemodynamics on echocardiography. Long-term follow-up to assess the impact of early DOAC versus VKA therapy on valve durability is planned.
Limitations
One limitation of this systematic review is that the majority of participants and events were from a single trial. ENVISAGE-TAVI-AF [11] comprised 67% of the included patients and an even larger proportion of the outcome events (78% of thrombotic events, 73% of bleeding events and 76% of deaths), which is not surprising given that it was also the only study that followed patients for longer than 1 year (median follow-up 18 months). The short duration of follow-up in most trials is a further limitation of this systematic review.
More than 80% of the patients included in this systematic review underwent TAVR. There were significant differences in baseline risk between TAVR and surgical valve recipients included in this systematic review, including age and comorbidities. We addressed this heterogeneity by using random effects modelling and conducting subgroup analyses for transcatheter and surgical valves, but ultimately, our results have wide CIs and our certainty in the evidence is at best moderate due to imprecision.
Included studies used 3 different factor Xa inhibitors, rivaroxaban, apixaban and edoxaban. While these drugs all work by the same mechanism and have comparable effectiveness with regard to prevention of thrombosis, there may be slight differences in the risk of bleeding (apixaban may be associated with lower bleeding risk than rivaroxaban) [33]. However, all factor Xa inhibitors have the same indications for use and in clinical practice can be used interchangeably. Given the small number of trials and events in this systematic review, we were not able to compare different types of DOACs.
Finally, patients in 3 of the 4 included studies had an indication for oral anticoagulation (most commonly atrial fibrillation) in addition to their bioprosthetic valve, while in the fourth study (ENAVLE [13]), conducted in surgical patients, patients were not required to have an additional indication for oral anticoagulation. As might be expected, the rate of thrombotic events in the ENAVLE was lower than in the other studies, and most likely was driven by the additional indication for oral anticoagulation rather than by valve-related thrombotic and thromboembolic events.
CONCLUSION
In the existing randomized literature on DOACs versus VKAs in the first 90 days after bioprosthetic valve implantation, there appears to be no difference with regard to thrombosis, bleeding or death. However, included studies are predominantly conducted in TAVR patients and have short (<18-month) follow-up. Like the DANCE trial and its SUNDANCE substudy, future randomized studies should focus on DOACs versus VKAs in the surgical valve population, and they should conduct CT scans and include long-term follow-up to assess the impact of therapy on subclinical valve thrombosis and valve durability.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENT
No new data were generated or analysed in support of this research.
Funding
This study was not funded.
Conflict of interest: none declared.
Author contributions
Rachel Eikelboom: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing. Richard P. Whitlock: Conceptualization; Investigation; Methodology; Supervision; Validation; Visualization; Writing—review & editing. Raveen Muzaffar: Data curation; Methodology; Software; Validation; Writing—review & editing. Renato D. Lopes: Conceptualization; Methodology; Supervision; Writing—review & editing. Deborah Siegal: Conceptualization; Formal analysis; Methodology; Supervision; Writing—review & editing. Sam Schulman: Conceptualization; Methodology; Supervision; Writing—review & editing. Emilie P. Belley-Côté: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Giuseppe Biondi-Zoccai, Marco Moscarelli and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the European Association of Cardio-Thoracic Surgery Annual Meeting, Milan, 5–8 October 2022.
REFERENCES
ABBREVIATIONS
- CI
Confidence interval
- CT
Computed tomography
- DOACs
Direct oral anticoagulants
- ESC
European Society of Cardiology
- RR
Relative risk
- VKAs
Vitamin K antagonists





