-
PDF
- Split View
-
Views
-
Cite
Cite
Antonia Schulz, Edward Buratto, Shuta Ishigami, Igor E Konstantinov, Michael M H Cheung, Christian P Brizard, Bex-Nikaidoh operation and the impact of double root translocation on outcomes, European Journal of Cardio-Thoracic Surgery, Volume 65, Issue 3, March 2024, ezad407, https://doi.org/10.1093/ejcts/ezad407
Close - Share Icon Share
Abstract
The Bex-Nikaidoh operation can effectively relieve left ventricular outflow tract obstruction. However, if a conduit is used for right ventricular outflow tract reconstruction, a late reoperation can be anticipated. We examined the impact of double root translocation on outcomes.
We performed a retrospective single-centre study of patients who underwent aortic root translocation between 2006 and 2019.
Aortic root translocation was performed in 23 patients at a median age of 1.6 years [interquartile range (IQR) 0.9–2.5]. Concomitant repairs were done in 52.2% of patients (12/23) including the Senning atrial switch in 34.8% (8/23). The right ventricular outflow tract was reconstructed with valved conduits in 39.1% (9/23), direct anastomoses in 4.35% (1/23) and pulmonary autografts in 56.5% of patients (13/23). Aortic cross-clamp time was significantly longer in patients with double root translocation [308 min (IQR 270–259) vs 209 min (IQR 179–281), P = 0.02]; 2 patients in this group required temporary mechanical circulatory support. There were no early deaths. Median follow-up time was 7.5 years (IQR 3.3–10.5). The estimated 10-year survival was 90% [95% confidence interval (CI): 47.3%, 98.5%]. There was no recurrent left ventricular outflow tract obstruction. Freedom from any reoperation was 64.2% (95% CI: 40.8%, 80.3%) at 3 years and 44.5% (95% CI: 21.2%, 65.5%) at 6 years. The main indication for late reoperation was conduit degeneration. Freedom from a right ventricular outflow tract reoperation was significantly higher, and the number of reoperations per patient was lower when a double root translocation had been performed (P = 0.03).
The Bex-Nikaidoh operation effectively relieved left ventricular outflow tract obstruction. A double root translocation further increased procedural complexity but was associated with better mid-term freedom from a right ventricular outflow tract reoperation. It should be considered in suitable patients.
INTRODUCTION
Treatment of transposition of the great arteries (TGA) with ventricular septal defect (VSD) and left ventricular outflow tract obstruction (LVOTO) remains a surgical challenge with multiple management options. An arterial switch operation and VSD closure can be done if resection of the LVOTO is feasible. However, patients with complex LVOTO and hypoplastic or dysfunctional pulmonary valves may undergo either a Rastelli operation [1], a réparation à l’etage ventriculaire (REV) [2] or a Bex-Nikaidoh procedure [3, 4]. The choice of procedure is influenced by the anatomical substrate, in particular the location and size of the VSD, the position of the great vessels and the coronary anatomy [5, 6].
In the Bex-Nikaidoh operation the aortic root is harvested from the right ventricle (RV) and, after division of the outlet septum, is translocated posteriorly onto the left ventricle, creating a straight and short left ventricular outflow tract with little potential for recurrent LVOTO. The procedure can preserve RV volume by avoiding a tunnel from the left ventricle to the aorta and allows an orthotopic position of the RV to pulmonary artery (PA) connection. However, if a valved conduit is used for reconstruction of the right ventricular outflow tract (RVOT), an eventual reoperation needs to be anticipated in the growing child. In some patients, a double root translocation (DRT) or en bloc rotation of the outflow tracts may be considered to avoid the use of an RV-PA conduit by preserving the autologous pulmonary valve [7–9]. Even in patients with hypoplastic pulmonary valves that require an annulus enlargement, anastomosis of the posterior autologous pulmonary artery wall provides growth potential with reduced risk for development of right ventricular outflow tract obstruction.
We performed a retrospective study to determine the results after a Bex-Nikaidoh operation and examined the impact of DRT on outcomes.
PATIENTS AND METHODS
Ethics statement
This study was approved by the human research ethics committee of the Royal Children’s Hospital Melbourne (HREC number 42224) on 24 September 2020. The committee waived the need for informed written consent due to the retrospective study design.
Study design
This is a single-institution retrospective study. All patients who underwent aortic root translocation (ART) between 2006 and 2019 were included. Data were collected retrospectively from the hospital records. Follow-up data including echocardiographic assessment were collected via letters from the patients’ primary cardiologists where all patients had regular follow-up visits. Follow-up was closed in November 2021. Data were available for all patients unless stated otherwise in the Results section. Early mortality was defined as death within 30 days after surgery or before discharge from the hospital. Mortality was death from any cause. Perioperative complications assessed were need for mechanical circulatory support, re-exploration for bleeding, sepsis, mediastinitis, permanent and temporary neurological deficit and early cardiac reoperation including pacemaker insertion.
End points
Survival, reoperation, freedom from LVOTO and aortic valve function were evaluated. The impact of DRT on outcomes was explored. Patients who underwent DRT were compared to those in whom other RV-PA connections were used, and differences in survival, reoperation rates and perioperative complications were assessed.
Operative technique
Selection of the surgical procedure is displayed in Fig. 1. A detailed description of our operative technique for ART was previously published by Davies et al. [10]. In brief, patients were placed on cardiopulmonary bypass (CPB) and cooled to moderate hypothermia (28–32°C). Intermittent antegrade cold blood cardioplegia was administered. After transection of the aorta, the coronary arteries were detached and mobilized. The aortic and pulmonary roots were excised, and any LVOTO were resected. The VSD was closed and the aortic root reimplanted on the left ventricular outflow tract after a 180° rotation. The coronary artery buttons were reimplanted into the existing incision points. The RVOT was reconstructed with a valved conduit or the pulmonary autograft. The autologous pulmonary valve was used when the valve had at least 2 cusps with decent mobility and thinness. If necessary, the pulmonary annulus was augmented to normal size for the body surface area by using autologous aortic root tissue between the cusps. If the neocommissure was too wide, then a neocusp was added to create a tricuspid morphology. Additional procedures were performed when indicated.
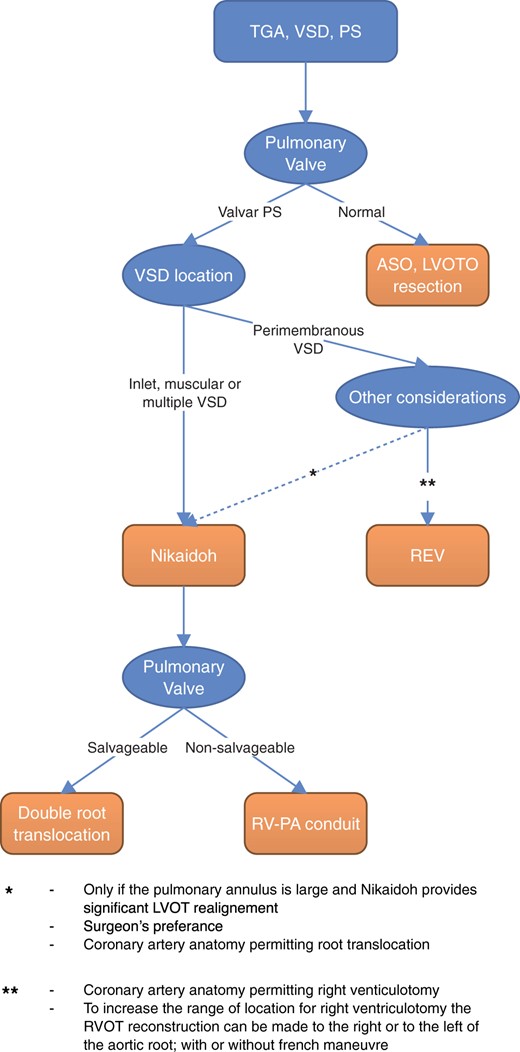
Procedure selection depending on anatomical considerations. ASO: arterial switch operation; LVOT: left ventricular outflow tract; LVOTO: LVOT obstruction; PA: pulmonary artery; PS: pulmonary stenosis; REV: réparation à l’etage ventriculaire; RV: right ventricle; TGA: transposition of the great arteries; VSD: ventricular septal defect.
Statistical analysis
Descriptive statistics include mean with standard deviation for normal continuous data, median with interquartile range (IQR) for non-normal continuous data and frequency with percentage for categorical data. Patients with and without DRT were compared. The Student t-test was used to compare normally distributed continuous variables, and the Mann–Whitney U test was used to compare not normally distributed continuous variables. The Fisher exact test was used to compare categorical data.
The distributions of survival and freedom from reoperation were estimated using Kaplan–Meier methodology. Distributions of time-to-event by selected patient groups were compared using the log-rank test. Data analysis was performed with Stata version 17 (StataCorp, College Station, TX, USA).
RESULTS
Baseline characteristics
Between 2006 and 2019, a total of 23 patients underwent the Bex-Nikaidoh procedure at a median age of 1.6 years (IQR 0.9–2.5 years), with the youngest patient being 2 months of age. Underlying diagnoses were dextro-TGA with VSD and pulmonary stenosis (PS) in 43.5% (10/23), congenitally corrected transposition of the great arteries (ccTGA) with VSD and PS in 34.8% (8/23), a double-outlet right ventricle with malposed great arteries and PS in 17.4% (4/23) and dextro-TGA with PS and complete atrioventricular septal defect in 4.3% (1/23). Two patients (8.7%) also had a straddling mitral valve. Further details on patient characteristics are provided in Table 1.
Baseline characteristics of all patients who underwent a Bex-Nikaidoh procedure (n = 23) and a comparison of patients with double root translocation (n = 13) versus other right ventricular-to-pulmonary artery connections (n = 10)
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connection (n = 10) . | P- value . |
|---|---|---|---|---|
| Age, years | 1.6 (0.9-2.5) | 1.8 (1.6-2.5) | 1.25 (0.7-1.8) | 0.17 |
| Weight, kg | 10.3 (8.7-12.3) | 10.9 (10-12.3) | 9.6 (6.6-11.3) | 0.28 |
| Diagnosis | 0.37 | |||
| dTGA/VSD/PS | 10 (43.5%) | 4 (30.8%) | 6 (60%) | |
| ccTGA/VSD/PS | 8 (34.8%) | 5 (38.5%) | 3 (30%) | |
| DORV/MGA/PS | 4 (17.4%) | 4 (30.8%) | - | |
| dTGA/AVSD/PS | 1 (4.3%) | - | 1 (10%) | |
| Straddling mitral valve | 2 (8.7%) | 2 (15.4%) | – | 0.49 |
| Situs | 0.16 | |||
| Solitus | 19 (82.6%) | 11 (84.6%) | 8 (80%) | |
| Inversus | 2 (8.7%) | 2 (15.4%) | - | |
| Ambiguus | 2 (8.7%) | - | 2 (20%) | |
| Cardiac position | 1 | |||
| Levocardia | 14 (60.9%) | 8 (61.5%) | 6 (60%) | |
| Dextrocardia | 2 (8.7%) | 1 (7.7%) | 1 (10%) | |
| Mesocardia | 7 (30.4%) | 4 (30.8%) | 3 (30%) | |
| Coronary anatomy | 0.62 | |||
| Usual arrangement | 18 (78.3%) | 11 (84.6%) | 7 (70%) | |
| Abnormal | 5 (21.7%) | 2 (15.4%) | 3 (30%) | |
| Yacoub D | 1 (4.3%) | - | 1 (10%) | |
| Yacoub E | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| VSD location | 0.28 | |||
| Paramembranous | 11 (47.8%) | 8 (61.5%) | 3 (30%) | |
| Inlet | 11 (47.8%) | 5 (38.5%) | 6 (60%) | |
| Remote | 1 (4.3%) | – | 1 (10%) | |
| Pulmonary stenosis | 0.03 | |||
| Valvar | 4 (17.4%) | - | 4 (40%) | |
| Subvalvular | 3 (13%) | 3 (23.1%) | - | |
| Multilevel | 16 (69.6%) | 10 (76.9%) | 6 (60%) | |
| LVOT Vmax, m/s | 4.1 (0.7) | 4.1 (0.6) | 4.1 (0.9) | 0.85 |
| Pulmonary-to-aortic valve diameter ratio | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.64 |
| Previous shunt insertion | 13 (56.5%) | 5 (38.5%) | 8 (80%) | 0.09 |
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connection (n = 10) . | P- value . |
|---|---|---|---|---|
| Age, years | 1.6 (0.9-2.5) | 1.8 (1.6-2.5) | 1.25 (0.7-1.8) | 0.17 |
| Weight, kg | 10.3 (8.7-12.3) | 10.9 (10-12.3) | 9.6 (6.6-11.3) | 0.28 |
| Diagnosis | 0.37 | |||
| dTGA/VSD/PS | 10 (43.5%) | 4 (30.8%) | 6 (60%) | |
| ccTGA/VSD/PS | 8 (34.8%) | 5 (38.5%) | 3 (30%) | |
| DORV/MGA/PS | 4 (17.4%) | 4 (30.8%) | - | |
| dTGA/AVSD/PS | 1 (4.3%) | - | 1 (10%) | |
| Straddling mitral valve | 2 (8.7%) | 2 (15.4%) | – | 0.49 |
| Situs | 0.16 | |||
| Solitus | 19 (82.6%) | 11 (84.6%) | 8 (80%) | |
| Inversus | 2 (8.7%) | 2 (15.4%) | - | |
| Ambiguus | 2 (8.7%) | - | 2 (20%) | |
| Cardiac position | 1 | |||
| Levocardia | 14 (60.9%) | 8 (61.5%) | 6 (60%) | |
| Dextrocardia | 2 (8.7%) | 1 (7.7%) | 1 (10%) | |
| Mesocardia | 7 (30.4%) | 4 (30.8%) | 3 (30%) | |
| Coronary anatomy | 0.62 | |||
| Usual arrangement | 18 (78.3%) | 11 (84.6%) | 7 (70%) | |
| Abnormal | 5 (21.7%) | 2 (15.4%) | 3 (30%) | |
| Yacoub D | 1 (4.3%) | - | 1 (10%) | |
| Yacoub E | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| VSD location | 0.28 | |||
| Paramembranous | 11 (47.8%) | 8 (61.5%) | 3 (30%) | |
| Inlet | 11 (47.8%) | 5 (38.5%) | 6 (60%) | |
| Remote | 1 (4.3%) | – | 1 (10%) | |
| Pulmonary stenosis | 0.03 | |||
| Valvar | 4 (17.4%) | - | 4 (40%) | |
| Subvalvular | 3 (13%) | 3 (23.1%) | - | |
| Multilevel | 16 (69.6%) | 10 (76.9%) | 6 (60%) | |
| LVOT Vmax, m/s | 4.1 (0.7) | 4.1 (0.6) | 4.1 (0.9) | 0.85 |
| Pulmonary-to-aortic valve diameter ratio | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.64 |
| Previous shunt insertion | 13 (56.5%) | 5 (38.5%) | 8 (80%) | 0.09 |
Values are median (interquartile range), mean (standard deviation) or number (%).
AVSD: atrioventricular septal defect, ccTGA: congenitally corrected transposition of the great arteries, DORV: double-outlet right ventricle, dTGA: dextro-transposition of the great arteries, LVOT: left ventricular outflow tract, MGA: malposition of the great arteries, PS: pulmonary stenosis; Vmax: maximum initial velocity; VSD: ventricular septal defect.
Baseline characteristics of all patients who underwent a Bex-Nikaidoh procedure (n = 23) and a comparison of patients with double root translocation (n = 13) versus other right ventricular-to-pulmonary artery connections (n = 10)
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connection (n = 10) . | P- value . |
|---|---|---|---|---|
| Age, years | 1.6 (0.9-2.5) | 1.8 (1.6-2.5) | 1.25 (0.7-1.8) | 0.17 |
| Weight, kg | 10.3 (8.7-12.3) | 10.9 (10-12.3) | 9.6 (6.6-11.3) | 0.28 |
| Diagnosis | 0.37 | |||
| dTGA/VSD/PS | 10 (43.5%) | 4 (30.8%) | 6 (60%) | |
| ccTGA/VSD/PS | 8 (34.8%) | 5 (38.5%) | 3 (30%) | |
| DORV/MGA/PS | 4 (17.4%) | 4 (30.8%) | - | |
| dTGA/AVSD/PS | 1 (4.3%) | - | 1 (10%) | |
| Straddling mitral valve | 2 (8.7%) | 2 (15.4%) | – | 0.49 |
| Situs | 0.16 | |||
| Solitus | 19 (82.6%) | 11 (84.6%) | 8 (80%) | |
| Inversus | 2 (8.7%) | 2 (15.4%) | - | |
| Ambiguus | 2 (8.7%) | - | 2 (20%) | |
| Cardiac position | 1 | |||
| Levocardia | 14 (60.9%) | 8 (61.5%) | 6 (60%) | |
| Dextrocardia | 2 (8.7%) | 1 (7.7%) | 1 (10%) | |
| Mesocardia | 7 (30.4%) | 4 (30.8%) | 3 (30%) | |
| Coronary anatomy | 0.62 | |||
| Usual arrangement | 18 (78.3%) | 11 (84.6%) | 7 (70%) | |
| Abnormal | 5 (21.7%) | 2 (15.4%) | 3 (30%) | |
| Yacoub D | 1 (4.3%) | - | 1 (10%) | |
| Yacoub E | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| VSD location | 0.28 | |||
| Paramembranous | 11 (47.8%) | 8 (61.5%) | 3 (30%) | |
| Inlet | 11 (47.8%) | 5 (38.5%) | 6 (60%) | |
| Remote | 1 (4.3%) | – | 1 (10%) | |
| Pulmonary stenosis | 0.03 | |||
| Valvar | 4 (17.4%) | - | 4 (40%) | |
| Subvalvular | 3 (13%) | 3 (23.1%) | - | |
| Multilevel | 16 (69.6%) | 10 (76.9%) | 6 (60%) | |
| LVOT Vmax, m/s | 4.1 (0.7) | 4.1 (0.6) | 4.1 (0.9) | 0.85 |
| Pulmonary-to-aortic valve diameter ratio | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.64 |
| Previous shunt insertion | 13 (56.5%) | 5 (38.5%) | 8 (80%) | 0.09 |
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connection (n = 10) . | P- value . |
|---|---|---|---|---|
| Age, years | 1.6 (0.9-2.5) | 1.8 (1.6-2.5) | 1.25 (0.7-1.8) | 0.17 |
| Weight, kg | 10.3 (8.7-12.3) | 10.9 (10-12.3) | 9.6 (6.6-11.3) | 0.28 |
| Diagnosis | 0.37 | |||
| dTGA/VSD/PS | 10 (43.5%) | 4 (30.8%) | 6 (60%) | |
| ccTGA/VSD/PS | 8 (34.8%) | 5 (38.5%) | 3 (30%) | |
| DORV/MGA/PS | 4 (17.4%) | 4 (30.8%) | - | |
| dTGA/AVSD/PS | 1 (4.3%) | - | 1 (10%) | |
| Straddling mitral valve | 2 (8.7%) | 2 (15.4%) | – | 0.49 |
| Situs | 0.16 | |||
| Solitus | 19 (82.6%) | 11 (84.6%) | 8 (80%) | |
| Inversus | 2 (8.7%) | 2 (15.4%) | - | |
| Ambiguus | 2 (8.7%) | - | 2 (20%) | |
| Cardiac position | 1 | |||
| Levocardia | 14 (60.9%) | 8 (61.5%) | 6 (60%) | |
| Dextrocardia | 2 (8.7%) | 1 (7.7%) | 1 (10%) | |
| Mesocardia | 7 (30.4%) | 4 (30.8%) | 3 (30%) | |
| Coronary anatomy | 0.62 | |||
| Usual arrangement | 18 (78.3%) | 11 (84.6%) | 7 (70%) | |
| Abnormal | 5 (21.7%) | 2 (15.4%) | 3 (30%) | |
| Yacoub D | 1 (4.3%) | - | 1 (10%) | |
| Yacoub E | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| VSD location | 0.28 | |||
| Paramembranous | 11 (47.8%) | 8 (61.5%) | 3 (30%) | |
| Inlet | 11 (47.8%) | 5 (38.5%) | 6 (60%) | |
| Remote | 1 (4.3%) | – | 1 (10%) | |
| Pulmonary stenosis | 0.03 | |||
| Valvar | 4 (17.4%) | - | 4 (40%) | |
| Subvalvular | 3 (13%) | 3 (23.1%) | - | |
| Multilevel | 16 (69.6%) | 10 (76.9%) | 6 (60%) | |
| LVOT Vmax, m/s | 4.1 (0.7) | 4.1 (0.6) | 4.1 (0.9) | 0.85 |
| Pulmonary-to-aortic valve diameter ratio | 0.6 (0.1) | 0.6 (0.1) | 0.6 (0.1) | 0.64 |
| Previous shunt insertion | 13 (56.5%) | 5 (38.5%) | 8 (80%) | 0.09 |
Values are median (interquartile range), mean (standard deviation) or number (%).
AVSD: atrioventricular septal defect, ccTGA: congenitally corrected transposition of the great arteries, DORV: double-outlet right ventricle, dTGA: dextro-transposition of the great arteries, LVOT: left ventricular outflow tract, MGA: malposition of the great arteries, PS: pulmonary stenosis; Vmax: maximum initial velocity; VSD: ventricular septal defect.
The majority of patients (60.9%, 14/23) had undergone at least one previous operation, including four patients (17.4%, 4/23) with two previous operations. Those procedures included 16 aortopulmonary shunt placements or upgrades, one emergent postnatal central implantation of extracorporeal membrane oxygenation (ECMO) and one pacemaker implantation for congenital heart block.
In most patients, the PS was a combination of valvar and subvalvar stenosis (69.6%, 16/23). The mean Vmax over the pulmonary valve was 4.1 m/s (SD: 0.7). The mean pulmonary valve to aortic valve annulus diameter ratio was 0.6 (SD: 0.1).
Procedure
In addition to the ART, half of the patients (52.2%, 12/23) underwent further repairs at the time of the operation. Those included a Senning atrial switch in 34.8% (8/23), mitral valve repair in 17.4% (4/23) and tricuspid valve repair in 8.7% (2/23). Operative details are summarized in Table 2. The RVOT was reconstructed using a pulmonary autograft (a DRT) in 56.5% of patients (13/23) with en bloc rotation in 1 patient. The majority of patients with DRT (69.2%, 9/13) required pulmonary valve repair in order to achieve an unobstructed RVOT. Pulmonary valve intervention included addition of a transannular patch in 5 patients with additional cusp insertion in 3 patients (autologous pericardium n = 2, CardioCel patch n = 1). Other techniques used were leaflet mobilization with commissurotomy and valvotomy (n = 2) and enlargement of the annulus by incision of the interleaflet triangles (n = 2). The other patients received a Contegra valve in 26.1% (6/23), a pulmonary homograft in 8.7% (2/23), an aortic homograft in 4.35% (1/23) or a direct anastomosis in 4.35% (1/23). One patient with an additional atrioventricular septal defect repair initially underwent a DRT with pulmonary valve enlargement, but due to severe pulmonary regurgitation with unfavourable haemodynamics, the autograft was taken down and replaced with a Contegra valve.
Operative details of all patients who underwent the Bex-Nikaidoh procedure (n = 23) and a comparison of patients with double root translocation (n = 13) versus other right ventricular-to- pulmonary artery connections (n = 10)
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connection (n = 10) . | P- value . |
|---|---|---|---|---|
| CPB time, min | 372 (296-463) | 403 (361-463) | 302 (291-438) | 0.13 |
| Cross-clamp time, min | 271 (205-321) | 308 (270-259) | 209 (179-281) | 0.02 |
| Resection of infundibular septum | 13 (56.5%) | 6 (46.2%) | 7 (70%) | 0.4 |
| VSD closure type | 1 | |||
| Direct closure | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| Patch closure | 19 (82.6%) | 11 (84.6%) | 8 (80%) | |
| LeCompte manoeuvre | 16 (69.6%) | 8 (61.5%) | 8 (80%) | 0.4 |
| RVOT reconstruction | <0.001 | |||
| Direct anastomosis | 1 (4.3%) | – | 1 (10%) | |
| Pulmonary homograft | 2 (8.7%) | – | 2 (20%) | |
| Aortic homograft | 1 (4.3%) | – | 1 (10%) | |
| Contegra conduit | 6 (26.1%) | – | 6 (60%) | |
| Pulmonary autograft | 13 (56.5%) | 13 (100%) | – | |
| Additional procedures | 12 (52.2%) | 8 (61.5%) | 4 (40%) | 0.41 |
| Senning atrial switch | 8 (34.8%) | 5 (38.5%) | 3 (30%) | 1 |
| Mitral valve repair | 4 (17.4%) | 3 (23.1%) | 1 (10%) | 0.6 |
| Tricuspid valve repair | 2 (8.7%) | 1 (7.7%) | 1 (10%) | 1 |
| Warden procedure | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Coronary artery augmentation | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Open chest | 6 (26.1%) | 5 (38.5%) | 1 (10%) | 0.18 |
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connection (n = 10) . | P- value . |
|---|---|---|---|---|
| CPB time, min | 372 (296-463) | 403 (361-463) | 302 (291-438) | 0.13 |
| Cross-clamp time, min | 271 (205-321) | 308 (270-259) | 209 (179-281) | 0.02 |
| Resection of infundibular septum | 13 (56.5%) | 6 (46.2%) | 7 (70%) | 0.4 |
| VSD closure type | 1 | |||
| Direct closure | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| Patch closure | 19 (82.6%) | 11 (84.6%) | 8 (80%) | |
| LeCompte manoeuvre | 16 (69.6%) | 8 (61.5%) | 8 (80%) | 0.4 |
| RVOT reconstruction | <0.001 | |||
| Direct anastomosis | 1 (4.3%) | – | 1 (10%) | |
| Pulmonary homograft | 2 (8.7%) | – | 2 (20%) | |
| Aortic homograft | 1 (4.3%) | – | 1 (10%) | |
| Contegra conduit | 6 (26.1%) | – | 6 (60%) | |
| Pulmonary autograft | 13 (56.5%) | 13 (100%) | – | |
| Additional procedures | 12 (52.2%) | 8 (61.5%) | 4 (40%) | 0.41 |
| Senning atrial switch | 8 (34.8%) | 5 (38.5%) | 3 (30%) | 1 |
| Mitral valve repair | 4 (17.4%) | 3 (23.1%) | 1 (10%) | 0.6 |
| Tricuspid valve repair | 2 (8.7%) | 1 (7.7%) | 1 (10%) | 1 |
| Warden procedure | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Coronary artery augmentation | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Open chest | 6 (26.1%) | 5 (38.5%) | 1 (10%) | 0.18 |
Values are median (interquartile range) or number (%).
CPB: cardiopulmonary bypass; PA: pulmonary artery; RV: right ventricle; RVOT: right ventricular outflow tract; VSD: ventricular septal defect.
Operative details of all patients who underwent the Bex-Nikaidoh procedure (n = 23) and a comparison of patients with double root translocation (n = 13) versus other right ventricular-to- pulmonary artery connections (n = 10)
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connection (n = 10) . | P- value . |
|---|---|---|---|---|
| CPB time, min | 372 (296-463) | 403 (361-463) | 302 (291-438) | 0.13 |
| Cross-clamp time, min | 271 (205-321) | 308 (270-259) | 209 (179-281) | 0.02 |
| Resection of infundibular septum | 13 (56.5%) | 6 (46.2%) | 7 (70%) | 0.4 |
| VSD closure type | 1 | |||
| Direct closure | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| Patch closure | 19 (82.6%) | 11 (84.6%) | 8 (80%) | |
| LeCompte manoeuvre | 16 (69.6%) | 8 (61.5%) | 8 (80%) | 0.4 |
| RVOT reconstruction | <0.001 | |||
| Direct anastomosis | 1 (4.3%) | – | 1 (10%) | |
| Pulmonary homograft | 2 (8.7%) | – | 2 (20%) | |
| Aortic homograft | 1 (4.3%) | – | 1 (10%) | |
| Contegra conduit | 6 (26.1%) | – | 6 (60%) | |
| Pulmonary autograft | 13 (56.5%) | 13 (100%) | – | |
| Additional procedures | 12 (52.2%) | 8 (61.5%) | 4 (40%) | 0.41 |
| Senning atrial switch | 8 (34.8%) | 5 (38.5%) | 3 (30%) | 1 |
| Mitral valve repair | 4 (17.4%) | 3 (23.1%) | 1 (10%) | 0.6 |
| Tricuspid valve repair | 2 (8.7%) | 1 (7.7%) | 1 (10%) | 1 |
| Warden procedure | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Coronary artery augmentation | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Open chest | 6 (26.1%) | 5 (38.5%) | 1 (10%) | 0.18 |
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connection (n = 10) . | P- value . |
|---|---|---|---|---|
| CPB time, min | 372 (296-463) | 403 (361-463) | 302 (291-438) | 0.13 |
| Cross-clamp time, min | 271 (205-321) | 308 (270-259) | 209 (179-281) | 0.02 |
| Resection of infundibular septum | 13 (56.5%) | 6 (46.2%) | 7 (70%) | 0.4 |
| VSD closure type | 1 | |||
| Direct closure | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| Patch closure | 19 (82.6%) | 11 (84.6%) | 8 (80%) | |
| LeCompte manoeuvre | 16 (69.6%) | 8 (61.5%) | 8 (80%) | 0.4 |
| RVOT reconstruction | <0.001 | |||
| Direct anastomosis | 1 (4.3%) | – | 1 (10%) | |
| Pulmonary homograft | 2 (8.7%) | – | 2 (20%) | |
| Aortic homograft | 1 (4.3%) | – | 1 (10%) | |
| Contegra conduit | 6 (26.1%) | – | 6 (60%) | |
| Pulmonary autograft | 13 (56.5%) | 13 (100%) | – | |
| Additional procedures | 12 (52.2%) | 8 (61.5%) | 4 (40%) | 0.41 |
| Senning atrial switch | 8 (34.8%) | 5 (38.5%) | 3 (30%) | 1 |
| Mitral valve repair | 4 (17.4%) | 3 (23.1%) | 1 (10%) | 0.6 |
| Tricuspid valve repair | 2 (8.7%) | 1 (7.7%) | 1 (10%) | 1 |
| Warden procedure | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Coronary artery augmentation | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Open chest | 6 (26.1%) | 5 (38.5%) | 1 (10%) | 0.18 |
Values are median (interquartile range) or number (%).
CPB: cardiopulmonary bypass; PA: pulmonary artery; RV: right ventricle; RVOT: right ventricular outflow tract; VSD: ventricular septal defect.
Early outcomes
An ART successfully eliminated any LVOTO in all patients, and no patient had more than mild AR after the procedure. Early outcomes are presented in Table 3.
Early outcomes of all patients who underwent the Bex-Nikaidoh procedure (n = 23) and a comparison of patients with a double root translocation (n = 13) versus other right ventricular-to- pulmonary artery connections (n = 10)
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connections (n = 10) . | P-value . |
|---|---|---|---|---|
| Ventilation time, h | 53.5 (16-96.5) | 68.5 (17-111) | 23 (15-82) | 0.54 |
| ICU stay, h | 114 (43.5-158.5) | 128 (95-164) | 57 (36-133) | 0.18 |
| Hospital stay, days | 15 (7-24) | 16 (9-26) | 8.5 (6-21) | 0.25 |
| Complications | ||||
| Primary ECMO implant | 2 (8.7%) | 2 (15.4%) | - | 0.49 |
| Re-exploration for bleeding | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Sepsis/mediastinitis | 3 (13%) | 2 (15.4%) | 1 (10%) | 1 |
| Renal insufficiency | 2 (8.7%) | 2 (15.4%) | - | 0.49 |
| Temporary neurological deficit | 1 (4.3%) | - | 1 (10%) | 0.43 |
| Pacemaker insertion | 3 (13%) | 2 (15.4%) | 1 (10%) | 1 |
| Early cardiac reoperation* | 3 (13%) | 3 (23.1%) | - | 0.23 |
| Early death | 0 (0%) | - | - | |
| Echocardiographic data | ||||
| LVOTO | 0 (0%) | – | – | |
| LVOT Vmax, m/s | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) | 0.45 |
| Aortic regurgitation | 0.66 | |||
| None | 11 (47.8%) | 6 (46.2%) | 5 (50%) | |
| Trivial | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Mild | 5 (21.7%) | 2 (15.4%) | 3 (30%) | |
| Pulmonary regurgitation | 0.5 | |||
| None | 5 (21.7%) | 4 (30.8%) | 1 (10%) | |
| Trivial | 2 (8.7%) | 2 (15.4%) | – | |
| Mild | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Moderate | 1 (4.3%) | – | 1 (10%) | |
| Severe | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| Not evaluated | 4 (17.4%) | – | 4 (40%) | |
| Pulmonary stenosis | 0.59 | |||
| None | 15 (65.2%) | 10 (76.9%) | 5 (50%) | |
| Mild | 3 (13%) | 3 (23.1%) | – | |
| Moderate | 1 (4.3%) | – | 1 (10%) | |
| Not evaluated | 4 (17.4%) | – | 4 (40%) | |
| Mitral regurgitation | 0.04 | |||
| None | 9 (39.1%) | 2 (15.4%) | 7 (70%) | |
| Trivial | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Mild | 4 (17.4%) | 4 (30.8%) | – | |
| Moderate | 2 (8.7%) | 1 (7.7%) | 1 (10%) | |
| Valve replaced | 1 (4.3%) | 1 (7.7%) | – | |
| Residual VSD | 0.48 | |||
| None | 18 (78.3%) | 10 (76.9%) | 9 (90%) | |
| Trivial | 2 (8.7%) | 2 (15.4%) | – | |
| Small | 3 (13%) | 1 (7.7%) | 2 (20%) | |
| LV function | 0.56 | |||
| Normal | 20 (87%) | 12 (92.3%) | 8 (80%) | |
| Mildly impaired | 3 (13%) | 1 (7.7%) | 2 (20%) |
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connections (n = 10) . | P-value . |
|---|---|---|---|---|
| Ventilation time, h | 53.5 (16-96.5) | 68.5 (17-111) | 23 (15-82) | 0.54 |
| ICU stay, h | 114 (43.5-158.5) | 128 (95-164) | 57 (36-133) | 0.18 |
| Hospital stay, days | 15 (7-24) | 16 (9-26) | 8.5 (6-21) | 0.25 |
| Complications | ||||
| Primary ECMO implant | 2 (8.7%) | 2 (15.4%) | - | 0.49 |
| Re-exploration for bleeding | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Sepsis/mediastinitis | 3 (13%) | 2 (15.4%) | 1 (10%) | 1 |
| Renal insufficiency | 2 (8.7%) | 2 (15.4%) | - | 0.49 |
| Temporary neurological deficit | 1 (4.3%) | - | 1 (10%) | 0.43 |
| Pacemaker insertion | 3 (13%) | 2 (15.4%) | 1 (10%) | 1 |
| Early cardiac reoperation* | 3 (13%) | 3 (23.1%) | - | 0.23 |
| Early death | 0 (0%) | - | - | |
| Echocardiographic data | ||||
| LVOTO | 0 (0%) | – | – | |
| LVOT Vmax, m/s | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) | 0.45 |
| Aortic regurgitation | 0.66 | |||
| None | 11 (47.8%) | 6 (46.2%) | 5 (50%) | |
| Trivial | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Mild | 5 (21.7%) | 2 (15.4%) | 3 (30%) | |
| Pulmonary regurgitation | 0.5 | |||
| None | 5 (21.7%) | 4 (30.8%) | 1 (10%) | |
| Trivial | 2 (8.7%) | 2 (15.4%) | – | |
| Mild | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Moderate | 1 (4.3%) | – | 1 (10%) | |
| Severe | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| Not evaluated | 4 (17.4%) | – | 4 (40%) | |
| Pulmonary stenosis | 0.59 | |||
| None | 15 (65.2%) | 10 (76.9%) | 5 (50%) | |
| Mild | 3 (13%) | 3 (23.1%) | – | |
| Moderate | 1 (4.3%) | – | 1 (10%) | |
| Not evaluated | 4 (17.4%) | – | 4 (40%) | |
| Mitral regurgitation | 0.04 | |||
| None | 9 (39.1%) | 2 (15.4%) | 7 (70%) | |
| Trivial | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Mild | 4 (17.4%) | 4 (30.8%) | – | |
| Moderate | 2 (8.7%) | 1 (7.7%) | 1 (10%) | |
| Valve replaced | 1 (4.3%) | 1 (7.7%) | – | |
| Residual VSD | 0.48 | |||
| None | 18 (78.3%) | 10 (76.9%) | 9 (90%) | |
| Trivial | 2 (8.7%) | 2 (15.4%) | – | |
| Small | 3 (13%) | 1 (7.7%) | 2 (20%) | |
| LV function | 0.56 | |||
| Normal | 20 (87%) | 12 (92.3%) | 8 (80%) | |
| Mildly impaired | 3 (13%) | 1 (7.7%) | 2 (20%) |
Values are median (interquartile range), mean (standard deviation) or number (%).
Unplanned cardiac reoperations excluding pacemaker procedures.
ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; LV: left ventricle; LVOT: left ventricular outflow tract; LVOTO: left ventricular outflow tract obstruction; PA: pulmonary artery; RV: right ventricle; VSD: ventricular septal defect.
Early outcomes of all patients who underwent the Bex-Nikaidoh procedure (n = 23) and a comparison of patients with a double root translocation (n = 13) versus other right ventricular-to- pulmonary artery connections (n = 10)
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connections (n = 10) . | P-value . |
|---|---|---|---|---|
| Ventilation time, h | 53.5 (16-96.5) | 68.5 (17-111) | 23 (15-82) | 0.54 |
| ICU stay, h | 114 (43.5-158.5) | 128 (95-164) | 57 (36-133) | 0.18 |
| Hospital stay, days | 15 (7-24) | 16 (9-26) | 8.5 (6-21) | 0.25 |
| Complications | ||||
| Primary ECMO implant | 2 (8.7%) | 2 (15.4%) | - | 0.49 |
| Re-exploration for bleeding | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Sepsis/mediastinitis | 3 (13%) | 2 (15.4%) | 1 (10%) | 1 |
| Renal insufficiency | 2 (8.7%) | 2 (15.4%) | - | 0.49 |
| Temporary neurological deficit | 1 (4.3%) | - | 1 (10%) | 0.43 |
| Pacemaker insertion | 3 (13%) | 2 (15.4%) | 1 (10%) | 1 |
| Early cardiac reoperation* | 3 (13%) | 3 (23.1%) | - | 0.23 |
| Early death | 0 (0%) | - | - | |
| Echocardiographic data | ||||
| LVOTO | 0 (0%) | – | – | |
| LVOT Vmax, m/s | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) | 0.45 |
| Aortic regurgitation | 0.66 | |||
| None | 11 (47.8%) | 6 (46.2%) | 5 (50%) | |
| Trivial | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Mild | 5 (21.7%) | 2 (15.4%) | 3 (30%) | |
| Pulmonary regurgitation | 0.5 | |||
| None | 5 (21.7%) | 4 (30.8%) | 1 (10%) | |
| Trivial | 2 (8.7%) | 2 (15.4%) | – | |
| Mild | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Moderate | 1 (4.3%) | – | 1 (10%) | |
| Severe | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| Not evaluated | 4 (17.4%) | – | 4 (40%) | |
| Pulmonary stenosis | 0.59 | |||
| None | 15 (65.2%) | 10 (76.9%) | 5 (50%) | |
| Mild | 3 (13%) | 3 (23.1%) | – | |
| Moderate | 1 (4.3%) | – | 1 (10%) | |
| Not evaluated | 4 (17.4%) | – | 4 (40%) | |
| Mitral regurgitation | 0.04 | |||
| None | 9 (39.1%) | 2 (15.4%) | 7 (70%) | |
| Trivial | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Mild | 4 (17.4%) | 4 (30.8%) | – | |
| Moderate | 2 (8.7%) | 1 (7.7%) | 1 (10%) | |
| Valve replaced | 1 (4.3%) | 1 (7.7%) | – | |
| Residual VSD | 0.48 | |||
| None | 18 (78.3%) | 10 (76.9%) | 9 (90%) | |
| Trivial | 2 (8.7%) | 2 (15.4%) | – | |
| Small | 3 (13%) | 1 (7.7%) | 2 (20%) | |
| LV function | 0.56 | |||
| Normal | 20 (87%) | 12 (92.3%) | 8 (80%) | |
| Mildly impaired | 3 (13%) | 1 (7.7%) | 2 (20%) |
| . | All patients (n = 23) . | Double root translocation (n = 13) . | Other RV-PA connections (n = 10) . | P-value . |
|---|---|---|---|---|
| Ventilation time, h | 53.5 (16-96.5) | 68.5 (17-111) | 23 (15-82) | 0.54 |
| ICU stay, h | 114 (43.5-158.5) | 128 (95-164) | 57 (36-133) | 0.18 |
| Hospital stay, days | 15 (7-24) | 16 (9-26) | 8.5 (6-21) | 0.25 |
| Complications | ||||
| Primary ECMO implant | 2 (8.7%) | 2 (15.4%) | - | 0.49 |
| Re-exploration for bleeding | 1 (4.3%) | 1 (7.7%) | - | 1 |
| Sepsis/mediastinitis | 3 (13%) | 2 (15.4%) | 1 (10%) | 1 |
| Renal insufficiency | 2 (8.7%) | 2 (15.4%) | - | 0.49 |
| Temporary neurological deficit | 1 (4.3%) | - | 1 (10%) | 0.43 |
| Pacemaker insertion | 3 (13%) | 2 (15.4%) | 1 (10%) | 1 |
| Early cardiac reoperation* | 3 (13%) | 3 (23.1%) | - | 0.23 |
| Early death | 0 (0%) | - | - | |
| Echocardiographic data | ||||
| LVOTO | 0 (0%) | – | – | |
| LVOT Vmax, m/s | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) | 0.45 |
| Aortic regurgitation | 0.66 | |||
| None | 11 (47.8%) | 6 (46.2%) | 5 (50%) | |
| Trivial | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Mild | 5 (21.7%) | 2 (15.4%) | 3 (30%) | |
| Pulmonary regurgitation | 0.5 | |||
| None | 5 (21.7%) | 4 (30.8%) | 1 (10%) | |
| Trivial | 2 (8.7%) | 2 (15.4%) | – | |
| Mild | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Moderate | 1 (4.3%) | – | 1 (10%) | |
| Severe | 4 (17.4%) | 2 (15.4%) | 2 (20%) | |
| Not evaluated | 4 (17.4%) | – | 4 (40%) | |
| Pulmonary stenosis | 0.59 | |||
| None | 15 (65.2%) | 10 (76.9%) | 5 (50%) | |
| Mild | 3 (13%) | 3 (23.1%) | – | |
| Moderate | 1 (4.3%) | – | 1 (10%) | |
| Not evaluated | 4 (17.4%) | – | 4 (40%) | |
| Mitral regurgitation | 0.04 | |||
| None | 9 (39.1%) | 2 (15.4%) | 7 (70%) | |
| Trivial | 7 (30.4%) | 5 (38.5%) | 2 (20%) | |
| Mild | 4 (17.4%) | 4 (30.8%) | – | |
| Moderate | 2 (8.7%) | 1 (7.7%) | 1 (10%) | |
| Valve replaced | 1 (4.3%) | 1 (7.7%) | – | |
| Residual VSD | 0.48 | |||
| None | 18 (78.3%) | 10 (76.9%) | 9 (90%) | |
| Trivial | 2 (8.7%) | 2 (15.4%) | – | |
| Small | 3 (13%) | 1 (7.7%) | 2 (20%) | |
| LV function | 0.56 | |||
| Normal | 20 (87%) | 12 (92.3%) | 8 (80%) | |
| Mildly impaired | 3 (13%) | 1 (7.7%) | 2 (20%) |
Values are median (interquartile range), mean (standard deviation) or number (%).
Unplanned cardiac reoperations excluding pacemaker procedures.
ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; LV: left ventricle; LVOT: left ventricular outflow tract; LVOTO: left ventricular outflow tract obstruction; PA: pulmonary artery; RV: right ventricle; VSD: ventricular septal defect.
There were no early deaths. However, perioperative complications occurred in 47.8% (11/23) patients. Two patients (8.7%, 2/23) required temporary ECMO support due to prolonged cross-clamp times (CCT) with unstable haemodynamics. One patient who had ccTGA/VSD/PS underwent a DRT with a Senning atrial switch with a CCT of 6 h. The second patient had a double-outlet right ventricle/malposition of the great arteries/PS and a straddling mitral valve. In addition to DRT, the patient required translocation of the anterior mitral papillary muscle and mitral valve re-repair with a CCT of 6.2 h. In both patients the cardiac function recovered, and they were successfully weaned from ECMO. No patient had coronary ischaemia.
An unplanned early cardiac reoperation within 30 days of surgery was necessary in 6 patients (26.1%, 6/23). Both patients with preoperative straddling mitral valves developed severe mitral regurgitation after the initial repair, with a need for mitral valve re-repair in one and mitral valve replacement in the other patient. One patient needed a reoperation for pulmonary artery bifurcation stenosis with subsequent patch augmentation. Three patients required early pacemaker implants (13%, 3/23). Among the patients with ccTGA (n = 8), 2 patients needed pacemakers implanted for iatrogenic atrioventricular block (28.6%, 2/7). One patient had a previously implanted pacemaker due to congenital heart block.
The median durations of the stays in the intensive care unit and the hospital were 4.75 days (IQR 1.8–6.6) and 15 days (IQR 7–24), respectively.
Late outcomes
Survival
The median follow-up time was 7.5 years (IQR 3.3–10.5). Follow-up was complete for all patients. One patient died 7.7 years after ART in the postoperative period after a conduit change. Estimated survival after 10 years was 90% [95% confidence interval (CI): 47.3%, 98.5%] (Fig. 2A).
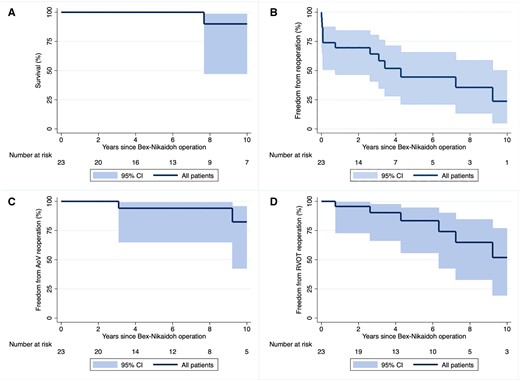
Kaplan–Meier estimates showing overall survival (A); freedom from any cardiac reoperation including a pacemaker procedure (B); freedom from an aortic valve reoperation (C); and freedom from a right ventricular outflow tract reoperation (D) following a Bex-Nikaidoh operation. Shaded areas represent 95% confidence bands. AoV: aortic valve; CI: confidence interval; RVOT: right ventricular outflow tract.
Reoperation
Overall, 56.5% (13/23) of the patients underwent a total of 22 cardiac reoperations, 6 of which occurred early and 16 late. The main indication for late reoperation was conduit exchange (n = 10). Other procedures included isolated pulmonary artery patch plasty (n = 2), a Senning revision (n = 2) and a pacemaker/ implantable cardioverter defibrillator procedure (n = 2).
Two patients underwent aortic valve repair (8.7%, 2/23) 3.1 and 9.2 years after ART. Both patients had mild AR initially. In 1 patient, AR progressed to severe in the context of aortic root dilatation (z-score +3.3) and an elongated cusp. The elongation was corrected with a small triangular resection. A circular annuloplasty was done, and a Gore-tex band was placed around the aorta. The other patient had only mild AR at the level of the anterior commissure. The indication for surgery was severe RV-PA conduit stenosis, but the aortic root did not sufficiently hold cardioplegia pressure. A commissural debridement and commissuroplasty were done. No patient required aortic valve replacement.
Freedom from any reoperation was 64.2% (95% CI: 40.8%, 80.3%) at 3 years and 44.5% (95% CI: 21.2%, 65.5%) at 6 years (Fig. 2B). Freedom from aortic valve reoperation was 94.1% (95% CI: 65.0%, 99.1%) at 5 years and 82.3% (95% CI: 42.6%, 95.7%) at 10 years (Fig. 2C). Freedom from RVOT reoperation was 83.4% (95% CI: 55.9%, 94.5%) at 5 years and 51.9% (95% CI: 19.5%, 76.8%) at 10 years (Fig. 2D).
Echocardiographic follow-up data
Echocardiographic follow-up data were available for all patients at a median time of 7.5 years (IQR 2.8–10.5). No patient had recurrent LVOTO. There was no aortic regurgitation (AR) in 39.1% (9/23), trivial AR in 21.7% (5/23), mild AR in 30.4% (7/23) and mild–moderate AR in 4.35% (1/23). One patient had moderate-severe AR (1/23, 4.35%) 2.9 years after DRT. Cardiac function was normal in 91.3% (21/23) of the patients. One patient had mildly reduced left ventricular function, and one patient mild-moderately reduced right ventricular function. No patient had more than moderate PS, and only one patient had more than moderate insufficiency.
Double root translocation
We compared 13 patients who underwent ART using their pulmonary autograft for RVOT reconstruction (DRT group) with 10 patients in whom other RV-PA connections, mainly valved conduits, were used (non-DRT group). Baseline patient characteristics were largely similar in both groups (Table 1). However, whereas PS was mostly multilevel in both groups, the DRT group also had 3 patients with subvalvar PS. In these patients, the indication for DRT rather than LVOTO resection and the arterial switch procedure had been that the pulmonary roots were found to be very thin and were judged not able to withstand systemic blood pressure without significant risk of root dilation. Also, both patients with straddling mitral valves were in the DRT group.
Additional procedures were performed in 61.5% (8/13) of patients in the DRT group and in 40% (4/10) in the non-DRT group. This included the Senning atrial switch procedure in 38.5% (5/13) and 30% (3/10) of patients, respectively. A complete comparison of procedural data is listed in Table 2. The median CPB time and CCT tended to be longer in the DRT group.
The incidence of perioperative complications was not different between the groups, but both patients who required ECMO support had undergone DRT.
Freedom from any reoperation was 61.5% (95% CI: 30.8%, 81.8%) at 3 and 6 years in the DRT group versus 68.6% (95% CI: 30.5%, 88.7%) and 34.3% (95% CI: 8.2%, 63.3%) at 3 and 6 years in the other patients, respectively (Fig. 3A). The number of reoperations per patient was lower for those with DRT [median 0 (IQR 0–1) vs 1 (IQR 1–3), P = 0.03]. There was no RVOT reoperation in the DRT group. Freedom from RVOT reoperation was 67.5% (95% CI: 29.1%, 88.25%) after 5 years and 33.75% (95% CI: 8%, 62.7%) after 10 years in patients with other RVOT reconstruction types (Fig. 3B, P = 0.03). No patient with DRT had an aortic valve reoperation, and there was no statistical difference in freedom from aortic valve reoperation between both groups (P = 0.32).

Kaplan–Meier estimates showing freedom from any cardiac reoperation (A) and a right ventricular outflow tract reoperation (B) comparing patients with and without double root translocation. Shaded areas represent 95% confidence bands. CI: confidence interval; PA: pulmonary artery; RV: right ventricle; RVOT: right ventricular outflow tract.
Pulmonary valve function was assessed at the last follow-up. In the DRT group, 11 patients had no, trivial or mild pulmonary regurgitation (PR); 1 patient had mild to moderate PR; 1 patient had severe PR and no patient had more than mild PS. In the non-DRT group, 8 patients had no, trivial or mild PR; 1 patient had mild to moderate PR; and 1 patient had moderate PR. Eight patients had no or mild PS and 2 patients had moderate PS.
DISCUSSION
The management of patients with TGA, VSD and PS is challenging. Several treatment strategies have evolved and been refined over time. The Rastelli operation has been the standard procedure for a long time with good early outcomes [11]. Suboptimal long-term results with LVOTO recurrence at the level of the VSD or intraventricular tunnel and frequent RV-PA conduit obstruction due to a non-anatomical location have led to exploration of alternative surgical strategies, although the Rastelli operation remains a good option for selected patients [11–14]. The goal of the Bex-Nikaidoh operation is to align both outflow tracts and to create a more normal anatomy that could result in a better long-term functional outcome [15, 16]. However, an optimal treatment strategy has to be chosen on an individual basis and is dependent on many anatomical considerations. At our institution, ART is performed when the arterial switch operation with LVOTO resection and intraventricular rerouting by REV is not preferable as in patients with restrictive, remote or inlet VSD, atrioventricular valve anomalies, small RV or an unfavourable location for the right ventriculotomy. We summarized our institutional approach in Fig. 1.
The Bex-Nikaidoh operation is a complex procedure, but early outcome reports from multiple centres with small patient cohorts have been encouraging with low mortality, and no recurrence of LVOTO has been reported so far [13, 14, 16–18]. A recent analysis of 119 patients from The Society of Thoracic Surgeons Congenital Heart Surgery Database who underwent the Bex-Nikaidoh operation revealed an operative mortality of 4.4% [19]. However, major postoperative morbidities occurred in 25% of patients, unplanned early reoperations in 19.8% and a more extensive use of ECMO (9.5%) compared to patients who underwent a Rastelli or REV procedure. In our cohort, we observed no recurrence of LVOTO and had no early deaths. However, nearly half of the patients also experienced postoperative morbidities, reflecting the complexity of the disease and treatment. Indeed, in our cohort, many patients had additional procedures for other complex cardiac lesions like the Senning atrial switch for ccTGA or atrioventricular valve repairs. The early reoperation rate was 26.1% and was related to pacemaker placement in 13%, but also to mitral valve reoperation in patients with straddling mitral valves. Because some patients required multiple complex procedures during a single operation, the median CPB and CCT were rather long with 6.2 h and 4.5 h, respectively. This outcome compares well with the report from Tam et al., who analysed outcomes of the Bex-Nikaidoh procedure in combination with the Senning atrial switch in patients with ccTGA [20]. The CCT was 5.1 h in their series, with 1 patient (1/8, 12.5%) needing ECMO support. We used perioperative mechanical circulatory support in 2 patients (8.7%), and both recovered with normal cardiac function. We did not observe any coronary ischaemia, which is of particular concern when the aortic root is translocated without detaching the coronary arteries as described in the original technique [4, 21]. We choose full mobilization of the aortic root with excision and reimplantation of both coronary artery buttons in order to avoid any distortion. However, we did not assess long-term coronary artery patency in this study.
Translocation of the aortic root also carries the risk of causing aortic valve regurgitation, which remains a concern because long-term data after Bex-Nikaidoh operations are still scarce. Moderate AR has been reported in 27% (3/11 patients) after the Bex-Nikaidoh procedure by Morell et al. during a median follow-up time of 33 months [16]. Raju et al. also observed moderate AR in 6.25% (2/32 patients), with aortic valvuloplasty done in 1 patient during a median follow-up time of 21 months [17]. Hongu et al. found moderate AR in 14% (2/14 patients) after a half-turned truncal switch with the need for aortic valvuloplasty in 1 patient during a median follow-up time of 5.2 years [22]. In our cohort, no patient had more than mild AR at hospital discharge, but 2 patients (8.7%) eventually underwent aortic valve repair 3 and 9 years after the operation. Both patients had mild AR initially that progressed in 1 patient to severe AR in the context of aortic root dilatation and cusp prolapse. Freedom from aortic valve reoperation was 94.1% and 82.3% at 5 and 10 years, respectively. One more patient had at least moderate AR at the last follow-up.
Overall freedom from any reoperation in our study was 44.5% after 5 years. The main indication for late reoperation was RV-PA conduit exchange. Even though the conduit is located in an orthotopic position after aortic root translocation, with less risk for compression by the sternum compared to the Rastelli operation, a valved conduit must eventually be exchanged in the growing child. Therefore, in patients with suitable pulmonary valves, a DRT or en bloc rotation of the outflow tracts might mitigate the risk for late reoperation by maintaining growth potential. We have used this technique increasingly over the past decade. We compared the reoperation rates between patients who underwent DRT and those who had other RVOT reconstruction types, mainly valved conduits. No patient with DRT required a pulmonary valve reoperation, and the freedom from RVOT reoperation was only 67.5% after 5 years with other reconstruction strategies. Because RV-PA conduits have to be changed several times over the years in the growing child, it is not surprising that the number of reoperations per patient was significantly higher when a valved conduit had been implanted. This result is comparable to the reports from the Boston group, who described an RVOT reintervention rate of 42% when a valved conduit had been used during ART with a follow-up time of 4.3 years [13] and a trend for fewer late reoperations when a valved conduit was avoided [17]. Therefore, in patients with a suitable pulmonary valve, a DRT is certainly worth considering to avoid a late reoperation. In our series, a DRT was feasible even when further complex repairs like a Senning atrial switch were done. However, it further increased procedural difficulty and was associated with longer cross-clamp times. Both patients who needed ECMO support had a DRT in addition to other complex cardiac procedures. The potential benefits of lower late reoperation rates need to be weighed against the increasing procedural complexity when additional lesions have to be addressed during the same operation. Afterall, the overall freedom of reoperation was not different due to higher early reoperation rates in the DRT group; rather, it was related mainly to mitral valve pathologies.
In a recently published retrospective multicentre study, Stoica and colleagues compared the outcomes of 43 patients who underwent ART with those of 27 patients who had en bloc rotation of the outflow tracts [23]. They reported an in-hospital mortality rate of 5.7%, cardiac complications in 43.5%, non-cardiac complications in 15.9% and an early reoperation rate of 15.9% for the entire cohort. They used mechanical circulatory support in 10.1% of patients. The en bloc rotation group had longer cross-clamp times, but also lower RVOT reoperation rates, which resulted in significantly better freedom from any cardiac reintervention. Freedom from RVOT reintervention was also comparable to that in our study, with 69.2% in the ART group and 100% in the en bloc rotation group at 5 years. Freedom from any reintervention was 60.6% and 45.1% after 5 and 10 years, respectively. Of note, Stoica et al. found that en bloc rotation was associated with moderate or worse AR during the follow-up period (16% vs 2.6%), with the need for aortic valvuloplasty in 1 patient. The authors hypothesized that this was related to oversizing of the VSD patch in this cohort. Although we used the en bloc rotation technique in only 1 patient, we did not find any difference between ART and DRT with respect to late aortic valve dysfunction. We did not use a VSD patch in the patient who later developed aortic root dilatation, but implanted the aortic root directly onto the crest of the VSD. This patient was also the only one in whom the left coronary artery was kept attached to the aortic root. Therefore, direct insertion of the autograft without a VSD patch appears not stable enough in the long run. Overall, we did not observe any difference in freedom from aortic valve reoperation or in the incidence of moderate or worse AR between the ART and DRT group in our study. It seems important with both techniques to size the VSD patch appropriately to avoid alterations of the aortic root.
Limitations
The limitations of this study include its retrospective design and the variable follow-up between the treatment groups. A DRT was performed with increasing frequency in recent years. Because of the rarity of the procedure, this study cohort is small, which limited the significance of the statistical analysis and the possibility to adjust for potential confounders. Results from this heterogeneous group of patients cannot be generalized to all patients. Long-term follow-up is still needed to confirm the lower RVOT intervention rate in the DRT group and late aortic valve function.
CONCLUSION
Aortic root translocation effectively relieved LVOTO and had excellent immediate and long-term survival, even when performed in conjunction with other complex cardiac procedures. Reoperation was not uncommon but was mainly related to RV-PA conduit failure or associated procedures. There was no recurrence of LVOTO, and late aortic valve reoperations were rare. A DRT significantly improved mid-term freedom from RVOT reoperation and led to fewer reoperations per patient. It should therefore be considered for suitable patients.
FUNDING
This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest: The authors declare no conflicts of interest.
DATA AVAILABILITY
The data underlying this article are available in the article and in its online supplementary material.
REFERENCES
Abbreviations
- AR
aortic regurgitation
- ART
aortic root translocation
- CCT
cross-clamp time
- ccTGA
congenitally corrected transposition of the great arteries
- CI
confidence interval
- CPB
cardiopulmonary bypass
- DRT
double root translocation
- ICU
intensive care unit
- IQR
interquartile range
- LVOTO
left ventricular outflow tract obstruction
- PA
pulmonary artery
- PR
pulmonary regurgitation
- PS
pulmonary stenosis
- REV
réparation à l’etage ventriculaire
- RV
right ventricle
- RVOT
right ventricular outflow tract
- RVOTO
right ventricular outflow tract obstruction
- SD
standard deviation
- TGA
transposition of the great arteries
- VSD
ventricular septal defect





