-
PDF
- Split View
-
Views
-
Cite
Cite
Stefan Fetcu, Takuya Osawa, Frank Klawonn, Thibault Schaeffer, Christoph Röhlig, Helena Staehler, Chiara Di Padua, Paul Philipp Heinisch, Nicole Piber, Alfred Hager, Peter Ewert, Jürgen Hörer, Masamichi Ono, Longitudinal analysis of systemic ventricular function and atrioventricular valve function after the Norwood procedure, European Journal of Cardio-Thoracic Surgery, Volume 65, Issue 3, March 2024, ezae058, https://doi.org/10.1093/ejcts/ezae058
Close - Share Icon Share
Abstract
To evaluate longitudinal systemic ventricular function and atrioventricular valve regurgitation in patients after the neonatal Norwood procedure.
Serial postoperative echocardiographic images before Fontan completion were assessed in neonates who underwent the Norwood procedure between 2001 and 2020. Ventricular function and atrioventricular valve regurgitation were compared between patients with modified Blalock–Taussig shunt and right ventricle to pulmonary artery conduit.
A total of 335 patients were identified including 273 hypoplastic left heart syndrome and 62 of its variants. Median age at Norwood was 8 (7–12) days. Modified Blalock–Taussig shunt was performed in 171 patients and the right ventricle to pulmonary artery conduit in 164 patients. Longitudinal ventricular function and atrioventricular valve regurgitation were evaluated using a total of 4352 echocardiograms. After the Norwood procedure, ventricular function was initially worse (1–30 days) but thereafter better (30 days to stage II) in the right ventricle to pulmonary artery conduit group (P < 0.001). After stage II, the ventricular function was inferior in the right ventricle to the pulmonary artery conduit group (P < 0.001). Atrioventricular valve regurgitation between the Norwood procedure and stage II was more frequent in the modified Blalock–Taussig shunt group (P < 0.001). After stage II, there was no significant difference in atrioventricular valve regurgitation between the groups (P = 0.171).
The effect of shunt type on haemodynamics after the Norwood procedure seems to vary according to the stage of palliation. After the Norwood, the modified Blalock–Taussig shunt is associated with poorer ventricular function and worse atrioventricular valve regurgitation compared to right ventricle to pulmonary artery conduit. Whereas, after stage II, modified Blalock–Taussig shunt is associated with better ventricular function and comparable atrioventricular valve regurgitation, compared to the right ventricle to pulmonary artery conduit.
INTRODUCTION
Despite the current era of advanced surgical procedures and modernized perioperative care, congenital heart diseases revolving around single-ventricle heart defects constitute a serious challenge for paediatric cardiologists and cardiac surgeons [1]. Affecting 13–19 newborns per 100 000 live births, hypoplastic left heart syndrome (HLHS) is the most frequently encountered anatomic deformity in children born with single-ventricle pathophysiology [2, 3]. They require a three-stage palliations to achieve long-term survival [4, 5]. The Norwood procedure, performed within the first 2 weeks after birth, remains one of the highest risk operations performed in congenital heart surgery. This can be achieved through a modified Blalock–Taussig shunt (MBTS) or a right ventricle to pulmonary artery conduit (RVPAC). The choice of shunt remains controversial [6–9]. MBTS is associated with a diastolic runoff that causes coronary ischaemia. However, its benefit lies in the potential for enhanced pulmonary artery (PA) growth. On the other hand, the downsides of RVPAC lie in the injury of the systemic ventricle and the reduced oxygen saturation. RVPAC provides pulmonary blood flow only during the systolic phase, resulting in a more stable haemodynamic during the postoperative period [8].
Even though over the last 30 years, the Norwood procedure has managed to improve the survival rates, it often leads to haemodynamic challenges, including poor systemic ventricular function (VF) and atrioventricular valve (AVV) regurgitation even after stage II palliation [10–12]. Understanding the evolution of systemic VF and AVV function after the Norwood procedure is crucial for optimizing patient care and identifying potential interventions [13–15]. Previous studies provided valuable insights into the long-term outcome [6, 7, 16, 17]. Nevertheless, longitudinal assessments that track the serial changes of systemic VF and AVV function after the Norwood procedure are limited.
Therefore, this study aims to conduct a longitudinal analysis of systemic VF and AVV function between the Norwood and the Fontan procedure concerning the techniques of MBTS or RVPAC.
METHODS
Ethical statement
This study was approved by the Institutional Review Board of the Technical University of Munich (305/20 S-KH on 2 June 2020). Because of the retrospective nature of the study, the need for individual patient consent was waived.
Patients and data collection
We reviewed the medical records of neonates with HLHS and its variants who underwent the neonatal Norwood procedure at our centre between 2001 and 2019. Patients who had a Norwood procedure later than the neonatal period (n = 5) and those who underwent bilateral PA banding (n = 7) were excluded. Early mortality is defined as death within 30 postoperative days, and late mortality as death on the 31st postoperative day or later. Patients’ data during the 1st hospital stay were collected. Post-discharge outcomes were determined from the outpatient clinic documentation in medical record of our institute or from correspondence with the referring paediatric cardiologists.
Operative techniques
The Norwood procedure was performed under cardiopulmonary bypass with hypothermic circulatory arrest [9, 14, 18]. Selective cerebral perfusion has been performed since 2009. The selection of MBTS or RVPAC depended on the surgeon's preference. MBTS was performed most frequently using a 3.5-mm heparin-coated Gore-Tex tube. The proximal anastomosis was placed in the brachiocephalic artery. The outflow end of the shunt was sewn to the right PA. RVPAC was performed usually using a 5-mm non-valved ring-reinforced Gore-Tex tube. The proximal end of the graft was bevelled and sewn to an incision in the systemic ventricle [18]. The proximal anastomosis was performed by conventional method before 2011, and a dunk technique was adopted since 2012. In most cases, the prostheses were placed to the right of the neo-aorta [18]. At the area of the pulmonary bifurcation, an oval-shaped pericardium or a Gore-Tex patch covered the main PA. The conduit was inserted either into the main PA at the cranial part of this patch or into the right PA. The stage II palliation consisted of a bidirectional cavopulmonary shunt (BCPS) [19, 20]. The Fontan procedure consisted of an extra-cardiac total cavopulmonary connection (TCPC). Indications for fenestration and AVV surgery have also been illustrated in our previous reports [21–23].
Echocardiography
An experienced echocardiographer (Christoph Röhlig) reviewed the archived echocardiogram images from pre- and post-Norwood procedures and assessed the systemic VF and AVV regurgitation. We evaluated the VF according to the report from Margossian et al., which focusses on the measurement of systemic VF in patients with single ventricle and clearly describes the measurement of EF in patients with single ventricle [24]. The echocardiograms were interpreted to measure systemic ventricular end-systolic volume and systemic ventricular end-diastolic volume, and to determine the fractional area change and systemic ventricular ejection fraction (EF), and qualitatively graded by eyeballing as normal = 0, slightly (EF <50%) = 1, mildly (EF <40%) = 2, moderately (EF <30%) = 3 or severely reduced (EF <20%) = 4 [25]. AVV regurgitation was graded according to the width and length of the insufficient jet in the two-dimensional lateral and anteroposterior view [23, 25]. We evaluated the grade of AVVR. The following are our categories of grading AVVR: trivial AVVR was defined in width (vena contracta) within 2 mm and length within the middle of the atrium. Mild AVVR was defined in width (vena contracta) within 4 mm and length within the middle of the atrium. Moderate AVVR was defined in width (vena contracta) within 6 mm and length deeper than middle of the atrium. Severe AVVR was defined in width (vena contracta) more than 6 mm and regurgitate jet reaching the atrial wall. Finally, the grade of AVVR was determined by an experienced paediatric cardiologist by visualization of jet area.
Statistical analysis
Categorical variables are presented as absolute numbers and percentages. A chi-square test was used for categorical data. Continuous variables are expressed as medians with interquartile ranges. An independent sample t-test was used to compare normally distributed variables. The Mann–Whitney U-test was used for variables that were not normally distributed. Levene’s test was used to differentiate between normal and non-normal distributions. Comparison of longitudinal systemic VF and AVV regurgitation between MBTC and RVPAC was the primary end-point, and survival after the Norwood procedure was the 2nd end-point as the post-hoc analysis. For statistical analysis of longitudinal systemic VF and AVV regurgitation, the proportions of ventricular dysfunction and AVV regurgitation were examined in the period of pre Norwood, 0–30 days after the Norwood, 31–90 days after the Norwood, 91 days to BCPS, 0–30 days after BCPS, 31–90 days after BCPS and 91 days to TCPC. For patients who died before TCPC, the serial echocardiographic data before their death were used. Histograms illustrating the proportions of ventricular dysfunction and AVV regurgitation in each period are shown. Histograms based on the total number of patients in the vertical bar with percentages of ventricular dysfunction and AVV regurgitation in each period are also shown. For further analysis of longitudinal systemic VF and AVV regurgitation, scatter plots were used to visualize the potentially non-linear relation of the continuous predictors ‘logarithmic postoperative period’ and ‘modified Blalock–Taussig shunt’ to the outcome ‘VF and AVV regurgitation’. The scatter plots were endowed with 90% quantiles for longitudinal systemic VF and AVV regurgitation based on a sliding window of a logarithmic size of 0.19, i.e. the window size remains constant on the logarithmic scale but increases on the original scale measured in days. For each window, the 90% quantile was computed and the window was then shifted to stepwise to the right by an increment of 0.01 on the logarithmic scale. Survival after the Norwood procedure was calculated using the Kaplan–Meier method, and comparison between MBTS and RVPAC was performed using log-rank test. Data analysis was performed using SPSS version 28.0 for Windows (IBM, Ehningen, Germany) and R-statistical software (state package). Scatter plot analysis and corresponding graphs were generated with R, version 4.2.
RESULTS
Patient characteristics and perioperative data
During the study period, 335 consecutive patients who underwent neonatal Norwood procedure for HLHS and its variants were identified. Patient characteristics are presented in Table 1. Primary diagnosis included 273 HLHS (81.5%) and 62 (18.5%) variants. Pulmonary circulation was established by RVPAC in 164 (49.0%) patients and by MBTS in 171 (51.0%). RVPAC was more frequently used in patients with HLHS and aortic atresia. On the other hand, MBTS was more frequently used in patients with aortic stenosis and variants. Perioperative data are presented in Table 2. Age at Norwood procedure was significantly younger in patients with RVPAC. There are no significant differences in other variables between RVPAS and MBTS.
| Variables . | Total . | RVPAC . | MBTS . | P value . |
|---|---|---|---|---|
| . | 335 . | 164 (49.0) . | 171 (51.0) . | . |
| Characteristics | ||||
| Gestational age (week), median (IQR) | 39 (38–40) | 39 (38–40) | 39 (38–40) | 0.691 |
| Premature birth, n (%) | 39 (15.9) | 15 (15.6) | 24 (16.1) | 0.920 |
| Genetic anomalies, n (%) | 14 (4.4) | 7 (4.5) | 7 (4.3) | 0.933 |
| Extracardiac anomalies, n (%) | 44 (13.6) | 23 (14.6) | 21 (12.7) | 0.617 |
| Anatomic variables | ||||
| HLHS, n (%) | 273 (81.5) | 155 (94.5) | 118 (69.0) | <0.001 |
| AA/MA | 69 | 47 | 22 | 0.034 |
| AA/MS | 67 | 53 | 14 | <0.001 |
| AS/MA | 29 | 11 | 18 | 0.027 |
| AS/MS | 106 | 44 | 62 | <0.001 |
| Variant, n (%) | 62 (18.5) | 9 (5.5) | 53 (31.0) | <0.001 |
| DILV | 22 (6.6) | 2 (1.2) | 20 (11.7) | <0.001 |
| TA | 15 (4.5) | 1 (0.6) | 14 (8.2) | <0.001 |
| UAVSD | 15 (4.5) | 5 (3.0) | 10 (5.8) | 0.216 |
| Associated anomalies, n (%) | ||||
| TAPVC | 9 (2.7) | 4 (2.4) | 5 (2.9) | 0.784 |
| Echocardiography | ||||
| Reduced SV function | 25 | 6 | 19 | 0.007 |
| AVVR greater than or equal to moderate | 19 | 10 | 9 | 0.786 |
| Restrictive atrial septum | 108 | 60 | 48 | 0.064 |
| Variables . | Total . | RVPAC . | MBTS . | P value . |
|---|---|---|---|---|
| . | 335 . | 164 (49.0) . | 171 (51.0) . | . |
| Characteristics | ||||
| Gestational age (week), median (IQR) | 39 (38–40) | 39 (38–40) | 39 (38–40) | 0.691 |
| Premature birth, n (%) | 39 (15.9) | 15 (15.6) | 24 (16.1) | 0.920 |
| Genetic anomalies, n (%) | 14 (4.4) | 7 (4.5) | 7 (4.3) | 0.933 |
| Extracardiac anomalies, n (%) | 44 (13.6) | 23 (14.6) | 21 (12.7) | 0.617 |
| Anatomic variables | ||||
| HLHS, n (%) | 273 (81.5) | 155 (94.5) | 118 (69.0) | <0.001 |
| AA/MA | 69 | 47 | 22 | 0.034 |
| AA/MS | 67 | 53 | 14 | <0.001 |
| AS/MA | 29 | 11 | 18 | 0.027 |
| AS/MS | 106 | 44 | 62 | <0.001 |
| Variant, n (%) | 62 (18.5) | 9 (5.5) | 53 (31.0) | <0.001 |
| DILV | 22 (6.6) | 2 (1.2) | 20 (11.7) | <0.001 |
| TA | 15 (4.5) | 1 (0.6) | 14 (8.2) | <0.001 |
| UAVSD | 15 (4.5) | 5 (3.0) | 10 (5.8) | 0.216 |
| Associated anomalies, n (%) | ||||
| TAPVC | 9 (2.7) | 4 (2.4) | 5 (2.9) | 0.784 |
| Echocardiography | ||||
| Reduced SV function | 25 | 6 | 19 | 0.007 |
| AVVR greater than or equal to moderate | 19 | 10 | 9 | 0.786 |
| Restrictive atrial septum | 108 | 60 | 48 | 0.064 |
AA: aortic atresia; AS: aortic stenosis; AVVR: atrioventricular valve regurgitation; DILV: double inlet left ventricle; HLHS: hypoplastic left heart syndrome; IQR: interquartile range; MA: mitral atresia; MBTS: modified Blalock–Taussig shunt; MS: mitral stenosis; RVPAC: right ventricle to pulmonary artery conduit; SV: single ventricle; TA: tricuspid atresia; TAPVC: total anomalous pulmonary venous connection; UAVSD: unbalanced atrioventricular septal defect.
| Variables . | Total . | RVPAC . | MBTS . | P value . |
|---|---|---|---|---|
| . | 335 . | 164 (49.0) . | 171 (51.0) . | . |
| Characteristics | ||||
| Gestational age (week), median (IQR) | 39 (38–40) | 39 (38–40) | 39 (38–40) | 0.691 |
| Premature birth, n (%) | 39 (15.9) | 15 (15.6) | 24 (16.1) | 0.920 |
| Genetic anomalies, n (%) | 14 (4.4) | 7 (4.5) | 7 (4.3) | 0.933 |
| Extracardiac anomalies, n (%) | 44 (13.6) | 23 (14.6) | 21 (12.7) | 0.617 |
| Anatomic variables | ||||
| HLHS, n (%) | 273 (81.5) | 155 (94.5) | 118 (69.0) | <0.001 |
| AA/MA | 69 | 47 | 22 | 0.034 |
| AA/MS | 67 | 53 | 14 | <0.001 |
| AS/MA | 29 | 11 | 18 | 0.027 |
| AS/MS | 106 | 44 | 62 | <0.001 |
| Variant, n (%) | 62 (18.5) | 9 (5.5) | 53 (31.0) | <0.001 |
| DILV | 22 (6.6) | 2 (1.2) | 20 (11.7) | <0.001 |
| TA | 15 (4.5) | 1 (0.6) | 14 (8.2) | <0.001 |
| UAVSD | 15 (4.5) | 5 (3.0) | 10 (5.8) | 0.216 |
| Associated anomalies, n (%) | ||||
| TAPVC | 9 (2.7) | 4 (2.4) | 5 (2.9) | 0.784 |
| Echocardiography | ||||
| Reduced SV function | 25 | 6 | 19 | 0.007 |
| AVVR greater than or equal to moderate | 19 | 10 | 9 | 0.786 |
| Restrictive atrial septum | 108 | 60 | 48 | 0.064 |
| Variables . | Total . | RVPAC . | MBTS . | P value . |
|---|---|---|---|---|
| . | 335 . | 164 (49.0) . | 171 (51.0) . | . |
| Characteristics | ||||
| Gestational age (week), median (IQR) | 39 (38–40) | 39 (38–40) | 39 (38–40) | 0.691 |
| Premature birth, n (%) | 39 (15.9) | 15 (15.6) | 24 (16.1) | 0.920 |
| Genetic anomalies, n (%) | 14 (4.4) | 7 (4.5) | 7 (4.3) | 0.933 |
| Extracardiac anomalies, n (%) | 44 (13.6) | 23 (14.6) | 21 (12.7) | 0.617 |
| Anatomic variables | ||||
| HLHS, n (%) | 273 (81.5) | 155 (94.5) | 118 (69.0) | <0.001 |
| AA/MA | 69 | 47 | 22 | 0.034 |
| AA/MS | 67 | 53 | 14 | <0.001 |
| AS/MA | 29 | 11 | 18 | 0.027 |
| AS/MS | 106 | 44 | 62 | <0.001 |
| Variant, n (%) | 62 (18.5) | 9 (5.5) | 53 (31.0) | <0.001 |
| DILV | 22 (6.6) | 2 (1.2) | 20 (11.7) | <0.001 |
| TA | 15 (4.5) | 1 (0.6) | 14 (8.2) | <0.001 |
| UAVSD | 15 (4.5) | 5 (3.0) | 10 (5.8) | 0.216 |
| Associated anomalies, n (%) | ||||
| TAPVC | 9 (2.7) | 4 (2.4) | 5 (2.9) | 0.784 |
| Echocardiography | ||||
| Reduced SV function | 25 | 6 | 19 | 0.007 |
| AVVR greater than or equal to moderate | 19 | 10 | 9 | 0.786 |
| Restrictive atrial septum | 108 | 60 | 48 | 0.064 |
AA: aortic atresia; AS: aortic stenosis; AVVR: atrioventricular valve regurgitation; DILV: double inlet left ventricle; HLHS: hypoplastic left heart syndrome; IQR: interquartile range; MA: mitral atresia; MBTS: modified Blalock–Taussig shunt; MS: mitral stenosis; RVPAC: right ventricle to pulmonary artery conduit; SV: single ventricle; TA: tricuspid atresia; TAPVC: total anomalous pulmonary venous connection; UAVSD: unbalanced atrioventricular septal defect.
| Variables . | Total . | RVPAC . | MBTS . | P value . |
|---|---|---|---|---|
| . | 335 . | 164 (49.0) . | 171 (51.0) . | . |
| Norwood procedure, median (IQR) | ||||
| Age at Norwood (days) | 8 (7–12) | 8 (7–11) | 9 (7–12) | 0.026 |
| Weight at Norwood (kg) | 3.2 (2.9–3.5) | 3.2 (2.9–3.5) | 3.2 (2.8–3.5) | 0.130 |
| CPB time (min) | 138 (109–165) | 135 (105–163) | 141 (110–167) | 0.455 |
| AXC time (min) | 49 (41–59) | 47 (41–57) | 49 (40–60) | 0.406 |
| Postoperative variables | ||||
| Intubation (days), median (IQR) | 5 (4–9) | 6 (4–13) | 5 (3–8) | 0.351 |
| ICU stay (days), median (IQR) | 13 (8–21) | 14 (10–22) | 12 (8–20) | 0.663 |
| HSP stay (days), median (IQR) | 24 (15–37) | 25 (18–37) | 23 (14–40) | 0.234 |
| ECMO, n (%) | 46 (13.8) | 23 (14.0) | 23 (13.5) | 0.896 |
| Re-intubation, n (%) | 56 (16.8) | 25 (15.3) | 31 (18.2) | 0.480 |
| Shunt intervention, n (%) | 50 (15.0) | 29 (17.7) | 21 (12.4) | 0.179 |
| Re CoA intervention, n (%) | 19 (5.7) | 10 (6.1) | 9 (5.3) | 0.761 |
| Peritoneal dialysis, n (%) | 40 (11.9) | 25 (15.2) | 15 (8.8) | 0.068 |
| Necrotizing enterocolitis, n (%) | 22 (6.6) | 7 (4.3) | 15 (8.8) | 0.099 |
| Variables . | Total . | RVPAC . | MBTS . | P value . |
|---|---|---|---|---|
| . | 335 . | 164 (49.0) . | 171 (51.0) . | . |
| Norwood procedure, median (IQR) | ||||
| Age at Norwood (days) | 8 (7–12) | 8 (7–11) | 9 (7–12) | 0.026 |
| Weight at Norwood (kg) | 3.2 (2.9–3.5) | 3.2 (2.9–3.5) | 3.2 (2.8–3.5) | 0.130 |
| CPB time (min) | 138 (109–165) | 135 (105–163) | 141 (110–167) | 0.455 |
| AXC time (min) | 49 (41–59) | 47 (41–57) | 49 (40–60) | 0.406 |
| Postoperative variables | ||||
| Intubation (days), median (IQR) | 5 (4–9) | 6 (4–13) | 5 (3–8) | 0.351 |
| ICU stay (days), median (IQR) | 13 (8–21) | 14 (10–22) | 12 (8–20) | 0.663 |
| HSP stay (days), median (IQR) | 24 (15–37) | 25 (18–37) | 23 (14–40) | 0.234 |
| ECMO, n (%) | 46 (13.8) | 23 (14.0) | 23 (13.5) | 0.896 |
| Re-intubation, n (%) | 56 (16.8) | 25 (15.3) | 31 (18.2) | 0.480 |
| Shunt intervention, n (%) | 50 (15.0) | 29 (17.7) | 21 (12.4) | 0.179 |
| Re CoA intervention, n (%) | 19 (5.7) | 10 (6.1) | 9 (5.3) | 0.761 |
| Peritoneal dialysis, n (%) | 40 (11.9) | 25 (15.2) | 15 (8.8) | 0.068 |
| Necrotizing enterocolitis, n (%) | 22 (6.6) | 7 (4.3) | 15 (8.8) | 0.099 |
AA: aortic atresia; AS: aortic stenosis; AXC: aortic cross clamp; CoA: coarctation of the aorta; CPB: cardiopulmonary bypass; DILV: double inlet left ventricle; ECMO: extracorporeal membrane oxygenation; HSP: hospital; ICU: intensive care unit; IQR: interquartile range; MA: mitral atresia; MBTS: modified Blalock–Taussig shunt; MS: mitral stenosis; RVPAC: right ventricle to pulmonary artery conduit; SV: single ventricle; TA: tricuspid atresia; TAPVC: total anomalous pulmonary venous connection; UAVSD: unbalanced atrioventricular septal defect.
| Variables . | Total . | RVPAC . | MBTS . | P value . |
|---|---|---|---|---|
| . | 335 . | 164 (49.0) . | 171 (51.0) . | . |
| Norwood procedure, median (IQR) | ||||
| Age at Norwood (days) | 8 (7–12) | 8 (7–11) | 9 (7–12) | 0.026 |
| Weight at Norwood (kg) | 3.2 (2.9–3.5) | 3.2 (2.9–3.5) | 3.2 (2.8–3.5) | 0.130 |
| CPB time (min) | 138 (109–165) | 135 (105–163) | 141 (110–167) | 0.455 |
| AXC time (min) | 49 (41–59) | 47 (41–57) | 49 (40–60) | 0.406 |
| Postoperative variables | ||||
| Intubation (days), median (IQR) | 5 (4–9) | 6 (4–13) | 5 (3–8) | 0.351 |
| ICU stay (days), median (IQR) | 13 (8–21) | 14 (10–22) | 12 (8–20) | 0.663 |
| HSP stay (days), median (IQR) | 24 (15–37) | 25 (18–37) | 23 (14–40) | 0.234 |
| ECMO, n (%) | 46 (13.8) | 23 (14.0) | 23 (13.5) | 0.896 |
| Re-intubation, n (%) | 56 (16.8) | 25 (15.3) | 31 (18.2) | 0.480 |
| Shunt intervention, n (%) | 50 (15.0) | 29 (17.7) | 21 (12.4) | 0.179 |
| Re CoA intervention, n (%) | 19 (5.7) | 10 (6.1) | 9 (5.3) | 0.761 |
| Peritoneal dialysis, n (%) | 40 (11.9) | 25 (15.2) | 15 (8.8) | 0.068 |
| Necrotizing enterocolitis, n (%) | 22 (6.6) | 7 (4.3) | 15 (8.8) | 0.099 |
| Variables . | Total . | RVPAC . | MBTS . | P value . |
|---|---|---|---|---|
| . | 335 . | 164 (49.0) . | 171 (51.0) . | . |
| Norwood procedure, median (IQR) | ||||
| Age at Norwood (days) | 8 (7–12) | 8 (7–11) | 9 (7–12) | 0.026 |
| Weight at Norwood (kg) | 3.2 (2.9–3.5) | 3.2 (2.9–3.5) | 3.2 (2.8–3.5) | 0.130 |
| CPB time (min) | 138 (109–165) | 135 (105–163) | 141 (110–167) | 0.455 |
| AXC time (min) | 49 (41–59) | 47 (41–57) | 49 (40–60) | 0.406 |
| Postoperative variables | ||||
| Intubation (days), median (IQR) | 5 (4–9) | 6 (4–13) | 5 (3–8) | 0.351 |
| ICU stay (days), median (IQR) | 13 (8–21) | 14 (10–22) | 12 (8–20) | 0.663 |
| HSP stay (days), median (IQR) | 24 (15–37) | 25 (18–37) | 23 (14–40) | 0.234 |
| ECMO, n (%) | 46 (13.8) | 23 (14.0) | 23 (13.5) | 0.896 |
| Re-intubation, n (%) | 56 (16.8) | 25 (15.3) | 31 (18.2) | 0.480 |
| Shunt intervention, n (%) | 50 (15.0) | 29 (17.7) | 21 (12.4) | 0.179 |
| Re CoA intervention, n (%) | 19 (5.7) | 10 (6.1) | 9 (5.3) | 0.761 |
| Peritoneal dialysis, n (%) | 40 (11.9) | 25 (15.2) | 15 (8.8) | 0.068 |
| Necrotizing enterocolitis, n (%) | 22 (6.6) | 7 (4.3) | 15 (8.8) | 0.099 |
AA: aortic atresia; AS: aortic stenosis; AXC: aortic cross clamp; CoA: coarctation of the aorta; CPB: cardiopulmonary bypass; DILV: double inlet left ventricle; ECMO: extracorporeal membrane oxygenation; HSP: hospital; ICU: intensive care unit; IQR: interquartile range; MA: mitral atresia; MBTS: modified Blalock–Taussig shunt; MS: mitral stenosis; RVPAC: right ventricle to pulmonary artery conduit; SV: single ventricle; TA: tricuspid atresia; TAPVC: total anomalous pulmonary venous connection; UAVSD: unbalanced atrioventricular septal defect.
Outcomes after the Norwood procedure
There were 20 early deaths (within 30 days) in patients with RVPAC and 21 in those with MBTS (P = 0.981). There were 15 late death before BCPS in RVPAC and 12 in MBTS (P = 0.806). In patients with RVPAC, 126 (76.8%) underwent BCPS at a median age of 4.0 (3.2–4.9) months. In patients with MBTS, 125 (73.1%) underwent BCPS at a median age of 3.8 (3.1–4.8) months. Median arterial oxygen saturation at BCPS was higher in MBTS than in RVPAC [77 (interquartile range 73–80) % vs 73 (67–78)%, P < 0.001], and median Qp/Qs at BCPS was higher in MBTS than in RVPAC [1.22 (1.00–1.69) vs 0.91 (0.67–1.22), P < 0.001]. There was no significant difference between the groups in the rate of patients who reached BCPS (P = 0.431). After BCPS, 19 patients (11.6%) in the RVPAC group died, 24 were waiting for TCPC and the remaining 83 (50.6%) underwent TCPC at a median age of 1.9 (1.6–2.4) years, while 13 patients (7.6%) in the MBTS group died, 24 were waiting for TCPC and the remaining 88 (51.5%) underwent TCPC at a median age of 2.1 (1.7–2.5) years. There was no difference in post-BCPS mortality (P = 0.215) or TCPC completion rate (P = 0.876) between the groups.
The median follow-up after the Norwood procedure was 4.7 (0.4–10.2) years. The Kaplan–Meier survival curve is shown in Supplementary Material, Fig. S1. Because no patient underwent heart transplantation, survival is the same as transplant-free survival. When survival is compared between RVPAC and MBTS, survival at 0.5, 1, 3 and 6 years was 79.7%, 73.1%, 67.8% and 66.2% in patients with RVPAC and 74.3%, 70.5%, 65.7% and 64.9% in patients with MBTS (Fig. 1). There was no significant difference in survival between the 2 groups (P = 0.800).
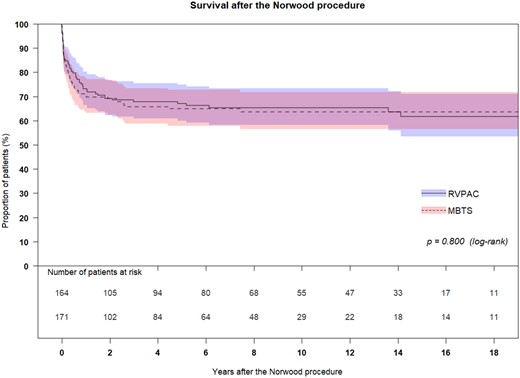
The Kaplan–Meier survival curve compared between RVPAC and MBTS. Because no patient underwent heart transplantation, survival is the same as transplant-free survival. Survival at 0.5, 1, 3 and 6 years was 79.7%, 73.1%, 67.8% and 66.2% in patients with RVPAC and 74.3%, 70.5%, 65.7% and 64.9% in patients with MBTS. There was no significant difference in survival between the 2 groups (P = 0.800). MBTS: modifies Blalock–Taussig shunt; RVPAC: right ventricle to pulmonary artery conduit.
Longitudinal analysis of systemic ventricular function and atrioventricular valve regurgitation
We collected a total of 4352 echocardiograms (2065 with RVPAC and 2287 with MBTS). The median interval between the last recorded postoperative echocardiographic assessment and the time of the Norwood procedure was 142 (interquartile range 38–718) days. Fig. 2 showed the proportions of VF and AVV regurgitation over time. VF was reduced over the first 30 days after the Norwood procedure but recovered thereafter. Post-BCPS, systemic VF decreased again over the first 90 postoperative days. The recovery of VF was observed thereafter. AVV regurgitation was significantly increased immediately after the Norwood procedure, and the proportion remained after that. Fig. 3 shows the comparison of VF between the groups. Initially, after the Norwood procedure (0–30 days), VF was better in the MBTS group (P < 0.001). However, thereafter (31–90 and 91 days to BCPS), VF was better in the RVPAC group (P < 0.001 and P = 0.011, respectively). After BCPS, during the period of 31–90 days and 91 days to TCPC, VF was inferior in the RVPAC group (P < 0.001 and P < 0.001, respectively). Histograms based on the total number of patients in the vertical bar with percentages of ventricular dysfunction in each period are shown in Supplementary Material, Fig. S2. Figure 4 shows the comparison of AVV regurgitation between the 2 shunts. AVV regurgitation after the Norwood procedure before BCPS (31–90 and 91 days to BCPS) was more frequent in the MBTS group (P < 0.001 and P < 0.001, respectively). However, there was no significant difference between both groups in AVVR after BCPS (P = 0.171). Histograms based on the total number of patients in the vertical bar with percentages of AVV regurgitation in each period are shown in Supplementary Material, Fig. S3.
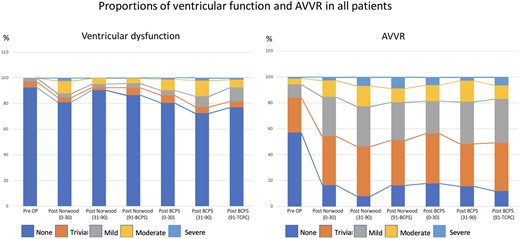
Histograms illustrating the proportions of ventricular dysfunction and atrioventricular valve regurgitation after the Norwood procedure over time by serial echocardiogram. AVVR: atrioventricular valve regurgitation; BCPS: bidirectional cavopulmonary shunt; Pre OP: preoperative; TCPC: total cavopulmonary connection.
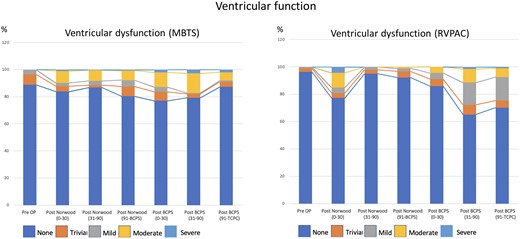
Histograms illustrating the progression of ventricular dysfunction after the Norwood procedure over time by serial echocardiogram: comparison between MBTS and RVPAC. BCPS: bidirectional cavopulmonary shunt; MBTS: modified Blalock–Taussig shunt; Pre OP: preoperative; RVPAC: right ventricle to pulmonary artery conduit; TCPC: total cavopulmonary connection.
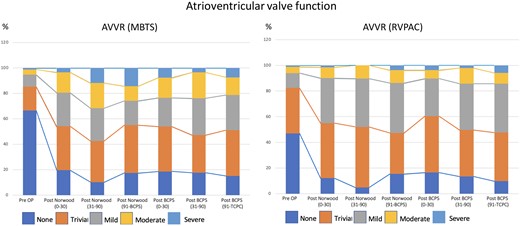
Histograms illustrating the progression of AVVR after the Norwood procedure over time by serial echocardiogram: comparison between MBTS and RVPAC. AVVR: atrioventricular valve regurgitation; BCPS: bidirectional cavopulmonary shunt; MBTS: modified Blalock–Taussig shunt; Pre OP: preoperative; RVPAC: right ventricle to pulmonary artery conduit; TCPC: total cavopulmonary connection.
Supplementary Material, Fig. S4 shows the scatter plot of VF and AVV regurgitation in patients with MBTS and RVPAC. When we compared the VF after the Norwood between the groups, initial VF between 0 and 30 days after the Norwood procedure was inferior in RVPAC patients, but thereafter (30–150 days), VF was inferior in MBTS patients (Supplementary Material, Fig. S4A). AVV regurgitation after the Norwood procedure was initially similar between the groups, but AVV regurgitation was worse in MBTS group between 30 and 150 days after the Norwood procedure (Supplementary Material, Fig. S4B). VF after the BCPS was initially worse in MBTS between 10 and 50 days after BCPS. However, it was worse in RVPAC between 300 and 800 days after BCPS (Supplementary Material, Fig. S4C). AVV regurgitation after BCPS was similar between the groups (Supplementary Material, Fig. S4D).
Correlation of ventricular dysfunction and atrioventricular valve regurgitation
We found a significant correlation between VF and AVV regurgitation in both patients with MBTS and RVPAC (Table 3). In patients with AVV regurgitation greater than or equal to moderate, 21.4% of the patients had associated significant ventricular dysfunction (greater than or equal to moderate) in the entire period (P < 0.001). On the other hand, only 6.4% of patients with AVV regurgitation less than moderate developed significant ventricular dysfunction (P < 0.001). Importantly, the correlation between AVV regurgitation greater than or equal to moderate and ventricular dysfunction was observed during the first 90 days after the Norwood and after BCPS. In the period between 91 days after Norwood and BCPS, there was no correlation between AVV regurgitation and ventricular dysfunction. When we performed the same analysis using the cut-off value of mild ventricular dysfunction and mild AVV regurgitation, we obtained a significant correlation between VF and AVV regurgitation in MBTS between 31 and 90 days after Norwood and 0–30 days after BCPS, but not in RVPAC (Supplementary Material, Table S1).
| Variables . | AVVR < moderate . | AVVR ≥ moderate . | ||||
|---|---|---|---|---|---|---|
| . | Total . | MBTS . | RVPAC . | Total . | MBTS . | RVPAC . |
| Total period | ||||||
| Dysfunction < moderate | 3333 | 1620 | 1713 | 607 | 421 | 186 |
| Dysfunction ≥ moderate | 227 | 127 | 100 | 165 | 103 | 62 |
| % incidence of dysfunction ≥ moderate | 6.4 | 7.3 | 5.5 | 21.4** | 19.7** | 25.0** |
| Post-Norwood (0–30) | ||||||
| Dysfunction < moderate | 1005 | 545 | 460 | 150 | 120 | 30 |
| Dysfunction ≥ moderate | 109 | 49 | 60 | 50 | 23 | 27 |
| %incidence of dysfunction ≥ moderate | 9.8 | 8.2 | 11.5 | 25.0** | 16.1** | 47.4** |
| Post-Norwood (31–90) | ||||||
| Dysfunction < moderate | 402 | 200 | 202 | 110 | 87 | 23 |
| Dysfunction ≥ moderate | 14 | 14 | 0 | 12 | 12 | 0 |
| %incidence of dysfunction ≥ moderate | 3.4 | 6.5 | 0.0 | 9.8** | 12.1 | 0.0 |
| Post-Norwood (91-BCPS) | ||||||
| Dysfunction < moderate | 269 | 114 | 155 | 66 | 40 | 26 |
| Dysfunction ≥ moderate | 12 | 10 | 2 | 3 | 3 | 0 |
| %incidence of dysfunction ≥ moderate | 4.3 | 8.1 | 1.3 | 4.3 | 7.0 | 0.0 |
| Post-BCPS (0–30) | ||||||
| Dysfunction < moderate | 602 | 349 | 253 | 108 | 82 | 26 |
| Dysfunction ≥ moderate | 39 | 30 | 9 | 38 | 34 | 4 |
| % incidence of dysfunction ≥ moderate | 6.1 | 7.9 | 3.4 | 26.0** | 29.3** | 13.3* |
| Post-BCPS (31–90) | ||||||
| Dysfunction < moderate | 210 | 98 | 112 | 34 | 21 | 13 |
| Dysfunction ≥ moderate | 19 | 10 | 9 | 20 | 13 | 7 |
| %incidence of dysfunction ≥ moderate | 8.3 | 9.3 | 7.4 | 37.0** | 38.2** | 35.0** |
| Post-BCPS (91-TCPC) | ||||||
| Dysfunction < moderate | 844 | 313 | 531 | 139 | 71 | 68 |
| Dysfunction ≥ moderate | 33 | 14 | 19 | 42 | 18 | 24 |
| %incidence of dysfunction ≥ moderate | 3.8 | 4.3 | 3.5 | 23.2** | 20.2** | 26.1** |
| Variables . | AVVR < moderate . | AVVR ≥ moderate . | ||||
|---|---|---|---|---|---|---|
| . | Total . | MBTS . | RVPAC . | Total . | MBTS . | RVPAC . |
| Total period | ||||||
| Dysfunction < moderate | 3333 | 1620 | 1713 | 607 | 421 | 186 |
| Dysfunction ≥ moderate | 227 | 127 | 100 | 165 | 103 | 62 |
| % incidence of dysfunction ≥ moderate | 6.4 | 7.3 | 5.5 | 21.4** | 19.7** | 25.0** |
| Post-Norwood (0–30) | ||||||
| Dysfunction < moderate | 1005 | 545 | 460 | 150 | 120 | 30 |
| Dysfunction ≥ moderate | 109 | 49 | 60 | 50 | 23 | 27 |
| %incidence of dysfunction ≥ moderate | 9.8 | 8.2 | 11.5 | 25.0** | 16.1** | 47.4** |
| Post-Norwood (31–90) | ||||||
| Dysfunction < moderate | 402 | 200 | 202 | 110 | 87 | 23 |
| Dysfunction ≥ moderate | 14 | 14 | 0 | 12 | 12 | 0 |
| %incidence of dysfunction ≥ moderate | 3.4 | 6.5 | 0.0 | 9.8** | 12.1 | 0.0 |
| Post-Norwood (91-BCPS) | ||||||
| Dysfunction < moderate | 269 | 114 | 155 | 66 | 40 | 26 |
| Dysfunction ≥ moderate | 12 | 10 | 2 | 3 | 3 | 0 |
| %incidence of dysfunction ≥ moderate | 4.3 | 8.1 | 1.3 | 4.3 | 7.0 | 0.0 |
| Post-BCPS (0–30) | ||||||
| Dysfunction < moderate | 602 | 349 | 253 | 108 | 82 | 26 |
| Dysfunction ≥ moderate | 39 | 30 | 9 | 38 | 34 | 4 |
| % incidence of dysfunction ≥ moderate | 6.1 | 7.9 | 3.4 | 26.0** | 29.3** | 13.3* |
| Post-BCPS (31–90) | ||||||
| Dysfunction < moderate | 210 | 98 | 112 | 34 | 21 | 13 |
| Dysfunction ≥ moderate | 19 | 10 | 9 | 20 | 13 | 7 |
| %incidence of dysfunction ≥ moderate | 8.3 | 9.3 | 7.4 | 37.0** | 38.2** | 35.0** |
| Post-BCPS (91-TCPC) | ||||||
| Dysfunction < moderate | 844 | 313 | 531 | 139 | 71 | 68 |
| Dysfunction ≥ moderate | 33 | 14 | 19 | 42 | 18 | 24 |
| %incidence of dysfunction ≥ moderate | 3.8 | 4.3 | 3.5 | 23.2** | 20.2** | 26.1** |
P < 0.05.
P < 0.01.
AVVR: atrioventricular valve regurgitation; BCPS: bidirectional cavopulmonary shunt; MBTS: modified Blalock–Taussig shunt; RVPAC: right ventricle to pulmonary artery conduit; TCPC: total cavopulmonary connection.
| Variables . | AVVR < moderate . | AVVR ≥ moderate . | ||||
|---|---|---|---|---|---|---|
| . | Total . | MBTS . | RVPAC . | Total . | MBTS . | RVPAC . |
| Total period | ||||||
| Dysfunction < moderate | 3333 | 1620 | 1713 | 607 | 421 | 186 |
| Dysfunction ≥ moderate | 227 | 127 | 100 | 165 | 103 | 62 |
| % incidence of dysfunction ≥ moderate | 6.4 | 7.3 | 5.5 | 21.4** | 19.7** | 25.0** |
| Post-Norwood (0–30) | ||||||
| Dysfunction < moderate | 1005 | 545 | 460 | 150 | 120 | 30 |
| Dysfunction ≥ moderate | 109 | 49 | 60 | 50 | 23 | 27 |
| %incidence of dysfunction ≥ moderate | 9.8 | 8.2 | 11.5 | 25.0** | 16.1** | 47.4** |
| Post-Norwood (31–90) | ||||||
| Dysfunction < moderate | 402 | 200 | 202 | 110 | 87 | 23 |
| Dysfunction ≥ moderate | 14 | 14 | 0 | 12 | 12 | 0 |
| %incidence of dysfunction ≥ moderate | 3.4 | 6.5 | 0.0 | 9.8** | 12.1 | 0.0 |
| Post-Norwood (91-BCPS) | ||||||
| Dysfunction < moderate | 269 | 114 | 155 | 66 | 40 | 26 |
| Dysfunction ≥ moderate | 12 | 10 | 2 | 3 | 3 | 0 |
| %incidence of dysfunction ≥ moderate | 4.3 | 8.1 | 1.3 | 4.3 | 7.0 | 0.0 |
| Post-BCPS (0–30) | ||||||
| Dysfunction < moderate | 602 | 349 | 253 | 108 | 82 | 26 |
| Dysfunction ≥ moderate | 39 | 30 | 9 | 38 | 34 | 4 |
| % incidence of dysfunction ≥ moderate | 6.1 | 7.9 | 3.4 | 26.0** | 29.3** | 13.3* |
| Post-BCPS (31–90) | ||||||
| Dysfunction < moderate | 210 | 98 | 112 | 34 | 21 | 13 |
| Dysfunction ≥ moderate | 19 | 10 | 9 | 20 | 13 | 7 |
| %incidence of dysfunction ≥ moderate | 8.3 | 9.3 | 7.4 | 37.0** | 38.2** | 35.0** |
| Post-BCPS (91-TCPC) | ||||||
| Dysfunction < moderate | 844 | 313 | 531 | 139 | 71 | 68 |
| Dysfunction ≥ moderate | 33 | 14 | 19 | 42 | 18 | 24 |
| %incidence of dysfunction ≥ moderate | 3.8 | 4.3 | 3.5 | 23.2** | 20.2** | 26.1** |
| Variables . | AVVR < moderate . | AVVR ≥ moderate . | ||||
|---|---|---|---|---|---|---|
| . | Total . | MBTS . | RVPAC . | Total . | MBTS . | RVPAC . |
| Total period | ||||||
| Dysfunction < moderate | 3333 | 1620 | 1713 | 607 | 421 | 186 |
| Dysfunction ≥ moderate | 227 | 127 | 100 | 165 | 103 | 62 |
| % incidence of dysfunction ≥ moderate | 6.4 | 7.3 | 5.5 | 21.4** | 19.7** | 25.0** |
| Post-Norwood (0–30) | ||||||
| Dysfunction < moderate | 1005 | 545 | 460 | 150 | 120 | 30 |
| Dysfunction ≥ moderate | 109 | 49 | 60 | 50 | 23 | 27 |
| %incidence of dysfunction ≥ moderate | 9.8 | 8.2 | 11.5 | 25.0** | 16.1** | 47.4** |
| Post-Norwood (31–90) | ||||||
| Dysfunction < moderate | 402 | 200 | 202 | 110 | 87 | 23 |
| Dysfunction ≥ moderate | 14 | 14 | 0 | 12 | 12 | 0 |
| %incidence of dysfunction ≥ moderate | 3.4 | 6.5 | 0.0 | 9.8** | 12.1 | 0.0 |
| Post-Norwood (91-BCPS) | ||||||
| Dysfunction < moderate | 269 | 114 | 155 | 66 | 40 | 26 |
| Dysfunction ≥ moderate | 12 | 10 | 2 | 3 | 3 | 0 |
| %incidence of dysfunction ≥ moderate | 4.3 | 8.1 | 1.3 | 4.3 | 7.0 | 0.0 |
| Post-BCPS (0–30) | ||||||
| Dysfunction < moderate | 602 | 349 | 253 | 108 | 82 | 26 |
| Dysfunction ≥ moderate | 39 | 30 | 9 | 38 | 34 | 4 |
| % incidence of dysfunction ≥ moderate | 6.1 | 7.9 | 3.4 | 26.0** | 29.3** | 13.3* |
| Post-BCPS (31–90) | ||||||
| Dysfunction < moderate | 210 | 98 | 112 | 34 | 21 | 13 |
| Dysfunction ≥ moderate | 19 | 10 | 9 | 20 | 13 | 7 |
| %incidence of dysfunction ≥ moderate | 8.3 | 9.3 | 7.4 | 37.0** | 38.2** | 35.0** |
| Post-BCPS (91-TCPC) | ||||||
| Dysfunction < moderate | 844 | 313 | 531 | 139 | 71 | 68 |
| Dysfunction ≥ moderate | 33 | 14 | 19 | 42 | 18 | 24 |
| %incidence of dysfunction ≥ moderate | 3.8 | 4.3 | 3.5 | 23.2** | 20.2** | 26.1** |
P < 0.05.
P < 0.01.
AVVR: atrioventricular valve regurgitation; BCPS: bidirectional cavopulmonary shunt; MBTS: modified Blalock–Taussig shunt; RVPAC: right ventricle to pulmonary artery conduit; TCPC: total cavopulmonary connection.
DISCUSSION
Our data showed the different profiles in VF and AVV regurgitation in patients with RVPAC and MBTS. Serial and longitudinal analysis found greater ventricular dysfunction in MBTS patients from 30 days after the Norwood procedure to the time of BCPS than in RVPAC patients. However, after BCPS, the degree of ventricular dysfunction was higher in RVPAC than in MBTS. AVV regurgitation was more prominent in MBTS patients from 30 days after the Norwood procedure to the time of BCPS, but the degree of AVV regurgitation became similar between the groups after BCPS.
Different haemodynamics of right ventricle to pulmonary artery conduit and modified Blalock–Taussig shunt after the Norwood procedure
In 1981, Norwood et al. 1st introduced the concept of RVPAC in stage I palliation for HLHS [26]. The shunt materials they used were 8-mm non-valved Gore-Tex tubes in 2 patients and 12-mm Dacron valved conduits in 2 patients. Their survival was not satisfactory. In 1998, Sano et al. popularized this concept using a small non-valved polytetrafluoroethylene tube with a diameter of 4 or 5 mm and demonstrated an early survival of 89% in 19 consecutive patients [27]. Subsequent studies showed improved hospital survival after the Norwood procedure using RVPAC [8, 10]. RVPAC results in higher diastolic pressure, and thus higher coronary artery driving pressure, leading to a more stable haemodynamic state after the procedure. However, this modification needs a small incision in the systemic ventricle. Therefore, late ventricular dysfunction due to the scar in the systemic ventricle is an issue of concern.
Systemic ventricular function
The systemic VF in patients with RVPAC was initially better (30 days postoperatively to BCPS) than in those with MBTS, especially before BCPS. However, after BCPS, progression of systemic ventricular dysfunction was observed in patients after RVPAC, whereas progression was not seen in patients after MBTS. Frommelt et al. demonstrated increased end-systolic volume of the systemic ventricle and decreased EF in patients with RVPAC [28]. Ruotsalainen et al. found that the systemic ventricular fractional area change was higher in RVPAC patients before BCPS but better in MBTS patients after BCPS [29]. Other echocardiographic and post-mortem studies also indicated that fibrotic-formation scar after BCPS caused reduced growth in the scar region relative to the remote myocardial wall [30]. The results of these studies and our current study could be interpreted that the volume overload of MBTS has a negative impact on the systemic VF before BCPS, and the scar formation of the RV after the closure of RVPAC negatively affects the systemic VF after BCPS. According to these findings, we think that the negative impact of right ventriculotomy is negligible after stage II palliation and that MBTS might have better VF in the long term.
Atrioventricular valve function
Our results demonstrated a significant difference in AVV function before BCPS between the MBTS and RVPAC. Between the Norwood procedure and BCPS, patients with MBTS had greater AVV regurgitation than those with RVPAC. This may be due to greater volume loading of the systemic ventricle in patients with MBTS compared to those with RVPAC. However, after volume unloading of the systemic ventricle by BCPS, AVV regurgitation in patients with RVPAC tended to be progressive. The mechanisms of progression of AVV regurgitation in patients after RVPAC remains unclear. Our previous study demonstrated that the site of regurgitation varied from antero-septal, antero-posterior or septal-posterior commissure in RVPAC patients, compared to MBTS (mainly from antero-septal commissure) [14]. Our results suggested that a scar of the systemic ventricle might have a negative effect on AVV regurgitation after BCPS. However, the clinical significance of these results should be evaluated in further studies.
Future perspective
The negative influence of systemic ventriculotomy on the systemic VF in patients with RVPAC might be relevant after BCPS. Long-term VF was more stable in patients with MBTS than those with RVPAC. On the other hand, the haemodynamic advantage of RVPAC over MBTS is also relevant between the Norwood procedure and BCPS. Long-term results might be improved by refining the surgical and medical management after the Norwood procedure with MBTS, and the indication for RVPAC might be limited to specific anatomical subtypes. Our current strategy is to prefer MBTS rather than RVPAC.
Limitations
This study was limited by its retrospective, non-randomized and single-centre design. The choice of shunt type depended on the discretion of the surgeon. The nature of this study may affect the validity of the analysis, and our ability to control patient selection bias was limited. Surgical and medical management may have changed during the study period, probably influencing the long-term outcomes. Although experienced paediatric cardiologist performed the evaluation of the systemic VF, there still remain valid questions raised about the methodology of portraying systemic ventricular dysfunction and its impact on the analysis presented. As many tests were conducted without adjustment for multiple testing, analyses were exploratory in nature, 95% confidence intervals were not adjusted for multiple comparisons, and inferences drawn from them may not be reproducible. Echocardiographic data of those who suffered from operative, inter-stage and post-BCPS mortality were included, and might be biased.
CONCLUSIONS
After the Norwood procedure, the RVPAC group displayed better values of recovery and surpassed the MBTS group until BCPS in the prevalence of ventricular dysfunction. Thereafter, the VF of the group receiving MBTS was superior. Regarding all of the phases before TCPC, the degree of AVV regurgitation was similar between the patients after MBTS and RVPAC.
Presented at the 8th World Congress of Pediatric Cardiology and Cardiac Surgery, Washington DC, USA, 27 August to 1 September 2023.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
FUNDING STATEMENT
This study had no financial support.
Conflict of interest: none declared.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author contributions
Stefan Fetcu: Data curation; Investigation; Writing—original draft. Takuya Osawa: Supervision; Writing—review and editing. Frank Klawonn: Software; Writing—review and editing. Thibault Schaeffer: Writing—review and editing. Christoph Röhlig: Supervision. Helena Staehler: Software; Writing—review and editing. Chiara Di Padua: Writing—review and editing. Paul Philipp Heinisch: Writing—review and editing. Nicole Piber: Writing—review and editing. Alfred Hager: Project administration; Supervision; Validation; Writing—review and editing. Peter Ewert: Project administration; Supervision; Validation; Writing—review and editing. Jürgen Hörer: Project administration; Supervision; Validation; Writing—review and editing. Masamichi Ono: Conceptualization; Data curation; Formal analysis; Project administration; Supervision; Validation; Visualization; Writing—original draft.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Ali Dodge-Khatami, Christian Pierre Brizard and the other anonymous reviewers for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- AVV
Atrioventricular valve
- BCPS
Bidirectional cavopulmonary shunt
- EF
Ejection fraction
- HLHS
Hypoplastic left heart syndrome
- MBTS
Modified Blalock–Taussig shunt
- PA
Pulmonary artery
- RVPAC
Right ventricle to pulmonary artery conduit
- TCPC
Total cavopulmonary connection
- VF
Ventricular function
Author notes
Stefan Fetcu and Takuya Osawa contributed equally to this study.
- hypoplastic left heart syndrome
- echocardiography
- pulmonary artery
- ventricular function
- modified blalock-taussig shunt
- conduit implant
- repair of single ventricle with aortic outflow obstruction and aortic arch hypoplasia (hypoplastic left heart syndrome) (eg, norwood procedure)
- right ventricle
- vomiting
- atrioventricular valve
- shunt





