-
PDF
- Split View
-
Views
-
Cite
Cite
Conall T Morgan, Devin Chetan, Jaymie Varenbut, Christoph Haller, Mike Seed, Luc L Mertens, Osami Honjo, Mechanical atrioventricular valve replacement in patients with single ventricle palliation, European Journal of Cardio-Thoracic Surgery, Volume 64, Issue 3, September 2023, ezad317, https://doi.org/10.1093/ejcts/ezad317
Close - Share Icon Share
Abstract
Atrioventricular valve (AVV) replacements in patients with single-ventricle circulations pose significant surgical risks and are associated with high morbidity and mortality.
From 1997 to 2021, 16 consecutive patients with functionally single-ventricle physiology underwent mechanical AVV replacement. Primary outcome was transplant-free survival. Secondary outcomes included major postoperative morbidity.
The median age of AVV replacement was 2 years old (interquartile range 0.6–3.8 years). All AVV replacements were performed with a St. Jude Medical mechanical valve, median 24 mm (range, 19–31mm). Extracorporeal membrane oxygenation (ECMO) was required in 4 patients. Operative mortality was 38% (6/16). There were 2 late deaths and 3 transplants. Transplant-free survival was 50% at 1 year, 37.5% at 5 years, and 22% at 10 years. Transplant-free survival was higher for patients with preserved ventricular function (P = 0.01). Difference in transplant-free survival at 1 year was 75% vs 25%, at 5 years was 62.5% vs 12.5% and at 10 years was 57% vs 0%. Three (19%) patients had complete heart block requiring permanent pacemaker insertion. 6 of 13 patients (46%) patients reached Fontan completion (3 patients operated at/after Fontan). Significant bleeding events occurred in 8 patients (50%) with 3 patients suffering major cerebrovascular accidents. There were 6 events of valve thrombosis in 5 patients, resulting in 2 deaths and 2 heart transplants.
Mechanical valve replacement carries significant morbidity and mortality risk. While it successfully salvages about half of patients with preserved ventricular function, careful consideration of alternative options should be made before embarking upon mechanical valve replacement.
INTRODUCTION
Atrioventricular (AV) valve regurgitation is a known risk factor for morbidity and mortality in patients undergoing single-ventricle palliation strategies [1–11]. AV valve regurgitation and subsequent requirement for AV valve repair are common [1] in this population. Significant AV valve regurgitation does not preclude patients from reaching Fontan completion but long-term survival is suboptimal compared to patients with a competent native AV valve [8]. In particular, a common AV valve in single-ventricle physiology has a high propensity to become regurgitant and fail earlier than a mitral or systemic tricuspid valve [12]. Successful AV valve repair in the setting of preserved ventricular function does not negatively affect survival [1, 3, 13, 14]. Less is known about the short- and long-term outcomes of mechanical AV valve replacement in this patient population. Results from the limited data available are disappointing with regard to both short- and longer-term survival with a significant risk of postoperative heart block [15, 16]. Mechanical replacement of the AV valve in this population is a particular challenge due to patient size and challenges with anticoagulation in the single-ventricle population.
The purpose of this matched case–control study is to examine our experience with AV valve replacement in children with single-ventricle circulations and assess the relationship between early postoperative ventricular function and clinical outcome.
METHODS
Study design, patient population and ethics statement
We retrospectively reviewed patients with functionally single-ventricle physiology who underwent mechanical atrioventricular valve (AVV) replacement between January 1997 and October 2021. The Research Ethics Board at the Hospital for Sick Children approved the study (REB 1000051004; 12 August 2015) and waived the requirement for patient consent. Patient demographics are presented in Table 1. There was 1 patient who underwent bioprosthetic (Melody) AV valve replacement during the study period (reported previously) and was excluded from this analysis [17].
| . | Cohort (n = 16) . | Group 1 (n = 8) . | Group 2 (n = 8) . | P-Value . |
|---|---|---|---|---|
| Age (years), median (IQR) | 2.0 (0.6–3.8) | 1.7 (0.6–3.7) | 2.0 (0.6–3.8) | 0.161 |
| Weight (kg), median (IQR) | 11.4 (7.4–13.9) | 10.2 (7.0–15.2) | 11.4 (9.2–12.8) | 0.505 |
| Body surface area (m2), median (IQR) | 0.51 (0.42–0.56) | 0.48 (0.39–0.59) | 0.52 (0.46–0.54) | 0.950 |
| Diagnosis, n (%) | ||||
| Hypoplastic left heart syndrome | 8 (50%) | 5 (63%) | 3 (28%) | 0.310 |
| Unbalanced atrioventricular septal defect | 4 (25%) | 2 (25%) | 2 (25%) | 0.715 |
| Tricuspid atresia | 2 (13%) | 0 (0%) | 2 (25%) | 0.233 |
| Pulmonary atresia/intact ventricular septum | 2 (13%) | 1 (13%) | 1 (13%) | 0.767 |
| Ventricular morphology, n (%) | ||||
| Dominant right ventricle | 12 (75%) | 7 (88%) | 5 (63%) | 0.285 |
| Dominant left ventricle | 4 (25%) | 1 (13%) | 3 (38%) | 0.285 |
| Timing of AV valve replacement, n (%) | ||||
| Between stage I and II | 2 (13%) | 1 (13%) | 1 (13%) | 0.767 |
| Stage II | 3 (19%) | 1 (13%) | 2 (25%) | 0.500 |
| Between stage II and III | 8 (50%) | 5 (63%) | 3 (28%) | 0.310 |
| Stage III | 1 (6%) | 0 (0%) | 1 (13%) | 0.500 |
| After stage III | 2 (25%) | 1 (13%) | 1 (13%) | 0.767 |
| Cardiopulmonary bypass time (min), median (IQR) | 182 (122–233) | 149 (97–197) | 218 (134–253) | 0.161 |
| Aortic cross-clamp time (min), median (IQR) | 97 (80–141) | 93 (77–114) | 116 (92–160) | 0.328 |
| Prosthesis size (mm), median (IQR) | 24 (23–25) | 24 (22–26) | 24 (23–25) | 0.878 |
| Intensive care unit length of stay (days), median (IQR) | 18 (6–33) | 21 (3–61) | 18 (11–27) | 0.999 |
| Hospital length of stay (days), median (IQR) | 36 (16–62) | 49 (11–88) | 30 (20–47) | 0.721 |
| Complications, n (%) | ||||
| Thrombosis | 6 (38%)a | 2 (25%) | 4 (50%)a | 0.304 |
| Major bleeding | 8 (50%) | 2 (25%) | 6 (75%) | 0.066 |
| Complete heart block requiring permanent pacemaker | 3 (19%) | 1 (13%) | 2 (25%) | 0.500 |
| Extracorporeal membrane oxygenation | 4 (25%) | 1 (13%) | 3 (28%) | 0.285 |
| Latest AV valve regurgitation (n = 5), n (%) | ||||
| None or mild | 5 (100%) | 4 (100%) | 1 (100%) | – |
| AV valve gradient (mmHg) | 7 (6–9) | 6 (6–8) | 9b | 0.667 |
| Latest ventricular dysfunction (n = 5), n (%) | ||||
| None or mild | 4 (80%) | 4 (100%) | 0 (0%) | 0.200 |
| Moderate or severe | 1 (20%) | 0 (0%) | 1 (100%) | 0.200 |
| Fontan completion, n (%) | 6/13 (46%)c | 4/7 (57%) | 2/6 (33%) | 0.592 |
| . | Cohort (n = 16) . | Group 1 (n = 8) . | Group 2 (n = 8) . | P-Value . |
|---|---|---|---|---|
| Age (years), median (IQR) | 2.0 (0.6–3.8) | 1.7 (0.6–3.7) | 2.0 (0.6–3.8) | 0.161 |
| Weight (kg), median (IQR) | 11.4 (7.4–13.9) | 10.2 (7.0–15.2) | 11.4 (9.2–12.8) | 0.505 |
| Body surface area (m2), median (IQR) | 0.51 (0.42–0.56) | 0.48 (0.39–0.59) | 0.52 (0.46–0.54) | 0.950 |
| Diagnosis, n (%) | ||||
| Hypoplastic left heart syndrome | 8 (50%) | 5 (63%) | 3 (28%) | 0.310 |
| Unbalanced atrioventricular septal defect | 4 (25%) | 2 (25%) | 2 (25%) | 0.715 |
| Tricuspid atresia | 2 (13%) | 0 (0%) | 2 (25%) | 0.233 |
| Pulmonary atresia/intact ventricular septum | 2 (13%) | 1 (13%) | 1 (13%) | 0.767 |
| Ventricular morphology, n (%) | ||||
| Dominant right ventricle | 12 (75%) | 7 (88%) | 5 (63%) | 0.285 |
| Dominant left ventricle | 4 (25%) | 1 (13%) | 3 (38%) | 0.285 |
| Timing of AV valve replacement, n (%) | ||||
| Between stage I and II | 2 (13%) | 1 (13%) | 1 (13%) | 0.767 |
| Stage II | 3 (19%) | 1 (13%) | 2 (25%) | 0.500 |
| Between stage II and III | 8 (50%) | 5 (63%) | 3 (28%) | 0.310 |
| Stage III | 1 (6%) | 0 (0%) | 1 (13%) | 0.500 |
| After stage III | 2 (25%) | 1 (13%) | 1 (13%) | 0.767 |
| Cardiopulmonary bypass time (min), median (IQR) | 182 (122–233) | 149 (97–197) | 218 (134–253) | 0.161 |
| Aortic cross-clamp time (min), median (IQR) | 97 (80–141) | 93 (77–114) | 116 (92–160) | 0.328 |
| Prosthesis size (mm), median (IQR) | 24 (23–25) | 24 (22–26) | 24 (23–25) | 0.878 |
| Intensive care unit length of stay (days), median (IQR) | 18 (6–33) | 21 (3–61) | 18 (11–27) | 0.999 |
| Hospital length of stay (days), median (IQR) | 36 (16–62) | 49 (11–88) | 30 (20–47) | 0.721 |
| Complications, n (%) | ||||
| Thrombosis | 6 (38%)a | 2 (25%) | 4 (50%)a | 0.304 |
| Major bleeding | 8 (50%) | 2 (25%) | 6 (75%) | 0.066 |
| Complete heart block requiring permanent pacemaker | 3 (19%) | 1 (13%) | 2 (25%) | 0.500 |
| Extracorporeal membrane oxygenation | 4 (25%) | 1 (13%) | 3 (28%) | 0.285 |
| Latest AV valve regurgitation (n = 5), n (%) | ||||
| None or mild | 5 (100%) | 4 (100%) | 1 (100%) | – |
| AV valve gradient (mmHg) | 7 (6–9) | 6 (6–8) | 9b | 0.667 |
| Latest ventricular dysfunction (n = 5), n (%) | ||||
| None or mild | 4 (80%) | 4 (100%) | 0 (0%) | 0.200 |
| Moderate or severe | 1 (20%) | 0 (0%) | 1 (100%) | 0.200 |
| Fontan completion, n (%) | 6/13 (46%)c | 4/7 (57%) | 2/6 (33%) | 0.592 |
One patient had 2 thrombotic events.
Data only available for 1 patient.
3 patients operated at/after Fontan.
AV: atrioventricular; IQR: interquartile range.
| . | Cohort (n = 16) . | Group 1 (n = 8) . | Group 2 (n = 8) . | P-Value . |
|---|---|---|---|---|
| Age (years), median (IQR) | 2.0 (0.6–3.8) | 1.7 (0.6–3.7) | 2.0 (0.6–3.8) | 0.161 |
| Weight (kg), median (IQR) | 11.4 (7.4–13.9) | 10.2 (7.0–15.2) | 11.4 (9.2–12.8) | 0.505 |
| Body surface area (m2), median (IQR) | 0.51 (0.42–0.56) | 0.48 (0.39–0.59) | 0.52 (0.46–0.54) | 0.950 |
| Diagnosis, n (%) | ||||
| Hypoplastic left heart syndrome | 8 (50%) | 5 (63%) | 3 (28%) | 0.310 |
| Unbalanced atrioventricular septal defect | 4 (25%) | 2 (25%) | 2 (25%) | 0.715 |
| Tricuspid atresia | 2 (13%) | 0 (0%) | 2 (25%) | 0.233 |
| Pulmonary atresia/intact ventricular septum | 2 (13%) | 1 (13%) | 1 (13%) | 0.767 |
| Ventricular morphology, n (%) | ||||
| Dominant right ventricle | 12 (75%) | 7 (88%) | 5 (63%) | 0.285 |
| Dominant left ventricle | 4 (25%) | 1 (13%) | 3 (38%) | 0.285 |
| Timing of AV valve replacement, n (%) | ||||
| Between stage I and II | 2 (13%) | 1 (13%) | 1 (13%) | 0.767 |
| Stage II | 3 (19%) | 1 (13%) | 2 (25%) | 0.500 |
| Between stage II and III | 8 (50%) | 5 (63%) | 3 (28%) | 0.310 |
| Stage III | 1 (6%) | 0 (0%) | 1 (13%) | 0.500 |
| After stage III | 2 (25%) | 1 (13%) | 1 (13%) | 0.767 |
| Cardiopulmonary bypass time (min), median (IQR) | 182 (122–233) | 149 (97–197) | 218 (134–253) | 0.161 |
| Aortic cross-clamp time (min), median (IQR) | 97 (80–141) | 93 (77–114) | 116 (92–160) | 0.328 |
| Prosthesis size (mm), median (IQR) | 24 (23–25) | 24 (22–26) | 24 (23–25) | 0.878 |
| Intensive care unit length of stay (days), median (IQR) | 18 (6–33) | 21 (3–61) | 18 (11–27) | 0.999 |
| Hospital length of stay (days), median (IQR) | 36 (16–62) | 49 (11–88) | 30 (20–47) | 0.721 |
| Complications, n (%) | ||||
| Thrombosis | 6 (38%)a | 2 (25%) | 4 (50%)a | 0.304 |
| Major bleeding | 8 (50%) | 2 (25%) | 6 (75%) | 0.066 |
| Complete heart block requiring permanent pacemaker | 3 (19%) | 1 (13%) | 2 (25%) | 0.500 |
| Extracorporeal membrane oxygenation | 4 (25%) | 1 (13%) | 3 (28%) | 0.285 |
| Latest AV valve regurgitation (n = 5), n (%) | ||||
| None or mild | 5 (100%) | 4 (100%) | 1 (100%) | – |
| AV valve gradient (mmHg) | 7 (6–9) | 6 (6–8) | 9b | 0.667 |
| Latest ventricular dysfunction (n = 5), n (%) | ||||
| None or mild | 4 (80%) | 4 (100%) | 0 (0%) | 0.200 |
| Moderate or severe | 1 (20%) | 0 (0%) | 1 (100%) | 0.200 |
| Fontan completion, n (%) | 6/13 (46%)c | 4/7 (57%) | 2/6 (33%) | 0.592 |
| . | Cohort (n = 16) . | Group 1 (n = 8) . | Group 2 (n = 8) . | P-Value . |
|---|---|---|---|---|
| Age (years), median (IQR) | 2.0 (0.6–3.8) | 1.7 (0.6–3.7) | 2.0 (0.6–3.8) | 0.161 |
| Weight (kg), median (IQR) | 11.4 (7.4–13.9) | 10.2 (7.0–15.2) | 11.4 (9.2–12.8) | 0.505 |
| Body surface area (m2), median (IQR) | 0.51 (0.42–0.56) | 0.48 (0.39–0.59) | 0.52 (0.46–0.54) | 0.950 |
| Diagnosis, n (%) | ||||
| Hypoplastic left heart syndrome | 8 (50%) | 5 (63%) | 3 (28%) | 0.310 |
| Unbalanced atrioventricular septal defect | 4 (25%) | 2 (25%) | 2 (25%) | 0.715 |
| Tricuspid atresia | 2 (13%) | 0 (0%) | 2 (25%) | 0.233 |
| Pulmonary atresia/intact ventricular septum | 2 (13%) | 1 (13%) | 1 (13%) | 0.767 |
| Ventricular morphology, n (%) | ||||
| Dominant right ventricle | 12 (75%) | 7 (88%) | 5 (63%) | 0.285 |
| Dominant left ventricle | 4 (25%) | 1 (13%) | 3 (38%) | 0.285 |
| Timing of AV valve replacement, n (%) | ||||
| Between stage I and II | 2 (13%) | 1 (13%) | 1 (13%) | 0.767 |
| Stage II | 3 (19%) | 1 (13%) | 2 (25%) | 0.500 |
| Between stage II and III | 8 (50%) | 5 (63%) | 3 (28%) | 0.310 |
| Stage III | 1 (6%) | 0 (0%) | 1 (13%) | 0.500 |
| After stage III | 2 (25%) | 1 (13%) | 1 (13%) | 0.767 |
| Cardiopulmonary bypass time (min), median (IQR) | 182 (122–233) | 149 (97–197) | 218 (134–253) | 0.161 |
| Aortic cross-clamp time (min), median (IQR) | 97 (80–141) | 93 (77–114) | 116 (92–160) | 0.328 |
| Prosthesis size (mm), median (IQR) | 24 (23–25) | 24 (22–26) | 24 (23–25) | 0.878 |
| Intensive care unit length of stay (days), median (IQR) | 18 (6–33) | 21 (3–61) | 18 (11–27) | 0.999 |
| Hospital length of stay (days), median (IQR) | 36 (16–62) | 49 (11–88) | 30 (20–47) | 0.721 |
| Complications, n (%) | ||||
| Thrombosis | 6 (38%)a | 2 (25%) | 4 (50%)a | 0.304 |
| Major bleeding | 8 (50%) | 2 (25%) | 6 (75%) | 0.066 |
| Complete heart block requiring permanent pacemaker | 3 (19%) | 1 (13%) | 2 (25%) | 0.500 |
| Extracorporeal membrane oxygenation | 4 (25%) | 1 (13%) | 3 (28%) | 0.285 |
| Latest AV valve regurgitation (n = 5), n (%) | ||||
| None or mild | 5 (100%) | 4 (100%) | 1 (100%) | – |
| AV valve gradient (mmHg) | 7 (6–9) | 6 (6–8) | 9b | 0.667 |
| Latest ventricular dysfunction (n = 5), n (%) | ||||
| None or mild | 4 (80%) | 4 (100%) | 0 (0%) | 0.200 |
| Moderate or severe | 1 (20%) | 0 (0%) | 1 (100%) | 0.200 |
| Fontan completion, n (%) | 6/13 (46%)c | 4/7 (57%) | 2/6 (33%) | 0.592 |
One patient had 2 thrombotic events.
Data only available for 1 patient.
3 patients operated at/after Fontan.
AV: atrioventricular; IQR: interquartile range.
Surgical decision-making and techniques
Preoperative decisions were made in multidisciplinary conferences including cardiovascular surgeons and cardiologists. Indication for primary AV valve replacement was persistent severe AV valve regurgitation after at least 1 attempted AV valve repair. If a patient needed a second AV valve intervention after a failed AV valve repair, the second AV valve repair is attempted with consideration of AV valve replacement in the same operation if residual AV valve regurgitation is moderate or greater and/or AV valve stenosis becomes unacceptable (mean inflow gradient >6–8 mmHg).
Procedures were performed with mild hypothermic cardiopulmonary bypass and antegrade cardioplegia. Standard blood cardioplegia was used until 2015 and Del Nido cardioplegia solution has been used thereafter. The leaflets and subvalvular apparatus were preserved as long as they did not obstruct the artificial valve inflow or ventricular outflow tract. 2–0 or 3–0 braided polyester sutures (TiCron™, Medtronic, MN) with small pledgets were used to suture the mechanical valves in place. The sutures were anchored to the valve leaflet only along the area of the conduction system to avoid heart block.
The size of mechanical valve is chosen based on the preoperative echocardiographic measurement of the AV valve annulus size and confirmed by direct measurement with a Hegar dilator intraoperatively. We try not to oversize the valve too much to avoid compression on the conduction system and the posterior AV groove. We also avoid very small mechanical valves (15 or 17 mm) so that the patients do not need frequent upsizing procedures. Regarding common AV valve replacement in the setting of unbalanced AVSD, we tend to close off the hypoplastic annulus and place the mechanical valve in the dominant AV valve annulus.
Anticoagulation strategy
We routinely use an unfractionated heparin infusion for anticoagulation in the acute recovery phase. Once a patient is stabilized, patients under 2 years of age are transitioned to low molecular weight heparin and patients over 2 years of age are transitioned to warfarin.
Data collection and outcome assessment
Data collected included age and weight at time of surgery, underlying anatomic diagnosis, morphology of the replaced valve (tricuspid, mitral or common AV valve), timing of AV valve replacement, and baseline perioperative data. Patients were stratified into 2 groups based on early postoperative ventricular function. Those with preserved ventricular function (group 1) were compared to those with moderate or severe ventricular dysfunction (group 2).
The primary outcome was transplant-free survival. Secondary outcomes included major postoperative morbidity: complete heart block requiring permanent pacemaker insertion, need for ECMO, and major thrombotic or bleeding events. Operative mortality was defined as death within thirty days of surgery. A secondary analysis in which patients were case-matched to a group of patients that underwent valve repair based on weight and valve morphology was performed. Only 4 of 16 (25%) match patients had undergone a previous attempt at valve repair.
Echocardiographic variables
Preoperative, early postoperative (within 7 days of repair) and 1-year follow-up echocardiograms were reviewed by a single echocardiographer (Conall T. Morgan) to assess valve morphology, ventricular function qualitatively (normal, mildly, moderately or severely decreased), degree of AVV regurgitation (none, mild, moderate or severe) and degree of AVV stenosis (none, mild, moderate or severe). In cases where echo data could be not be retrieved (no digitized echoes available for review), data were obtained from the report (n = 2).
Statistical analysis
Continuous data are presented as median (interquartile range). Discrete data are presented as frequency (percentage). Patients were stratified by ventricular function on their first postoperative echocardiogram following valve replacement. Those with preserved ventricular function (group 1) were compared to those with moderate or severe ventricular dysfunction (group 2). Continuous variables were compared with a Mann–Whitney U-test. Event frequencies were compared using the chi-square test or Fisher’s exact test. Transplant-free survival was compared between patients with preserved ventricular function and those with ventricular dysfunction as well as for patients with AV valve replacement versus matched controls with valve repair using a log-rank test and displayed using the Kaplan–Meier method.
RESULTS
Preoperative characteristics
AV valve replacement was performed in 16 patients. Two (13%) patients had right atrial isomerism. Dysfunctional systemic AV valve morphology was tricuspid in 8 patients (50%), mitral in 4 patients (25%) and common AV valve in 4 patients (25%). In our cohort of 16 patients, there were a total of 25 attempts at AVV repair. In an operation that occurred prior to AV valve replacement, 14 patients had 16 attempts at AV valve repair. Additional attempts were made to repair the AV valve in 9 patients in the same operation before replacing it with a mechanical prosthesis. In 2 patients, no prior AV valve repair occurred before valve replacement; this was an intraoperative decision by the surgeon based on their assessment of the AV valve appearance. One patient who did not have previous valve repair suffered intraoperative damage to the valve apparatus which was deemed unrepairable and another patient had an unsuccessful attempt at valve repair and the surgeon felt that further attempts were futile so the valve was replaced in the same operation. All patients had undergone at least their first stage of single-ventricle palliation prior to AV valve replacement. Prior to AV valve replacement, 15 patients (94%) had moderate-to-severe regurgitation. The most common timing of AV valve replacement was between stage II and III palliations in 8 patients (50%). There was no difference in anatomic diagnosis, age or weight at time of surgery or timing of AV valve replacement between group 1 and group 2 (Table 1).
Operative details
All patients received a St. Jude’s Medical mechanical valve ranging in size from 19 to 31 mm (median 24 mm). There was no significant difference in cardiopulmonary bypass time or aortic cross-clamp time between group 1 and group 2. At the time of replacement, the median age was 2 years (0.6–3.8 years) and weight was 11.4 kg (7.4–13.9 kg) with no significant differences between group 1 and group 2. Four patients required ECMO postoperatively.
Mortality
Overall, transplant-free survival was 5 of 16 (31%). Figure 1 shows freedom from death or transplant stratified according to early postoperative ventricular function. Transplant-free survival rates at 1, 5, and 10 years were 75%, 62.5%, and 57%, respectively, for patients with preserved ventricular function. Transplant-free survival was significantly lower (P = 0.01) in patients with ventricular dysfunction with estimates of 1-, 5-, and 10-year survival of 25%, 12.5%, and 0%, respectively. Figure 2 demonstrates the outcome of the cohort stratified by palliative stage at time of AV valve replacement surgery. There were 6 operative deaths (38%). The cause of the operative deaths were valve thrombosis in 2 patients, catastrophic haemorrhage in 2 patients, and irrecoverable myocardial dysfunction in 2 patients. Of 10 survivors to hospital discharge, 3 underwent heart transplantation between 260 and 600 days post-valve replacement and 2 late deaths occurred at 4 months post-valve replacement (acute valve thrombosis) and 7.5 years post-valve replacement (variceal bleeding in the context of a failing Fontan circulation). The median follow-up for the 5 event-free survivors was 8.2 years (interquartile range, 3.7–9.6 years). At latest follow-up, 5 patients were alive with their mechanical AVV, 4 had normal ventricular function, and 1 patient had moderately reduced function.
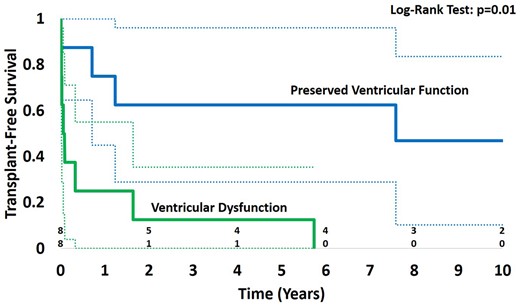
Survival for the whole cohort stratified by ventricular function early after atrioventricular valve replacement.

Cohort outcomes stratified by palliative stage at time of AV valve replacement surgery.
Morbidity
Three patients (19%) had postoperative complete heart block that did not recover requiring permanent pacemaker insertion. Three patients suffered early haemorrhagic strokes, 2 of whom required partial craniectomy and insertion of a ventriculoperitoneal shunt. There were 6 valve thrombosis events in 5 patients. Ultimately, 2 of those patients died and 2 were transplanted (1 of which was medically managed with systemic thrombolysis on 2 occasions). There were no reports of prosthetic valve endocarditis, late cerebrovascular accidents, or major late bleeding events.
Ventricular function
Figure 3 shows changes in ventricular function from the preoperative period to 1-year post-valve replacement. Preoperative ventricular function was normal or mildly reduced in 14 patients (88%) and severely reduced in 2 patients (12%). Both patients with severe preoperative ventricular dysfunction died. Early postoperative ventricular function was normal or mildly reduced in 8 patients (50%), moderately reduced in 3 patients (19%) and severely reduced in 5 patients (31%). Ventricular function improved over time in the majority of the cases. At 1-year post-valve replacement, ventricular function was normal in 6 patients, mildly reduced in 1 patient, and severely reduced in 1 patient. No patient had more than mild mechanical AVV regurgitation or stenosis.
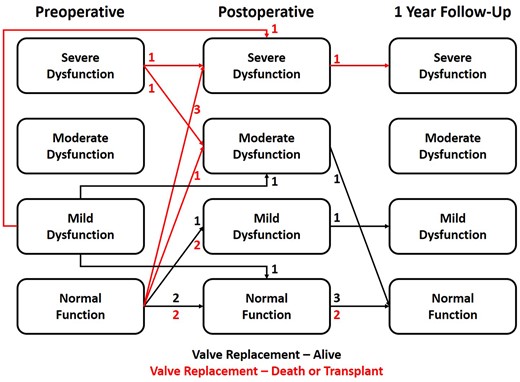
Change in ventricular dysfunction from the preoperative period to 1-year post-valve replacement.
Case–control outcomes
There were no significant differences in the age, weight, BSA, diagnosis, ventricular morphology, valve morphology, timing of AV valve replacement, and preoperative or early postoperative ventricular function between the cohort and the matched control group that underwent AV valve repair (Table 2). Figure 4 shows transplant-free survival of the AV valve replacement cohort compared with a matched cohort of single-ventricle patients who underwent AV valve repair. Transplant-free survival was significantly decreased in the replacement cohort vs the repair cohort (P = 0.016) with estimates at 1 year of 50% vs 80%, 5 years of 37.5% vs 73%, and 10 years of 22% vs 73%.
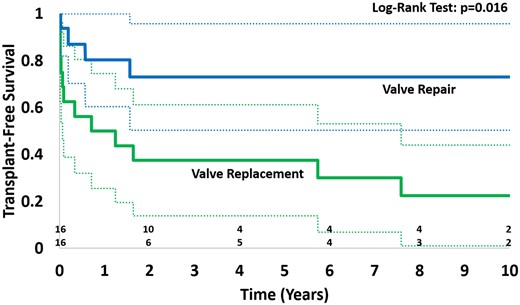
Transplant-free survival of patients with atrioventricular valve replacement compared to matched controls with atrioventricular valve repair.
| . | Cohort (n = 16) . | Match (n = 16) . | P-Value . |
|---|---|---|---|
| Age (years), median (IQR) | 2.0 (0.6–3.8) | 1.7 (0.5–3.3) | 0.59 |
| Weight (kg), median (IQR) | 11.4 (7.4–13.9) | 10.9 (7.2–11.8) | 0.64 |
| Body surface area, median (IQR) | 0.51 (0.42–0.56) | 0.50 (0.42–0.56) | 0.87 |
| Diagnosis, n (%) | |||
| Hypoplastic left heart syndrome | 8 (50%) | 8 (50%) | 0.99 |
| Unbalanced atrioventricular septal defect | 4 (25%) | 5 (31%) | 0.99 |
| Tricuspid atresia | 2 (13%) | 1 (6%) | 0.99 |
| Pulmonary atresia/intact ventricular septum | 2 (13%) | – | 0.48 |
| Double inlet left ventricle | – | 2 (13%) | 0.48 |
| Ventricular morphology, n (%) | |||
| Dominant right ventricle | 12 (75%) | 9 (56%) | 0.46 |
| Dominant left ventricle | 4 (25%) | 7 (44%) | 0.46 |
| Valve morphology, n (%) | |||
| Tricuspid valve | 8 (50%) | 8 (50%) | 0.99 |
| Mitral valve | 4 (25%) | 3 (19%) | 0.99 |
| Common AV valve | 4 (25%) | 5 (31%) | 0.99 |
| Timing of AV valve replacement, n (%) | |||
| Stage I | – | 1 (6%) | 0.99 |
| Between stage I and II | 2 (13%) | 1 (6%) | 0.99 |
| Stage II | 3 (19%) | 8 (50%) | 0.14 |
| Between stage II and III | 8 (50%) | 3 (19%) | 0.14 |
| Stage III | 1 (6%) | 3 (19%) | 0.60 |
| After stage III | 2 (25%) | – | 0.48 |
| Preoperative ventricular function, n (%) | |||
| Normal function | 11 (69%) | 13 (81%) | 0.69 |
| Mild dysfunction | 3 (19%) | 1 (6%) | 0.60 |
| Moderate dysfunction | 0 (0%) | 1 (6%) | 0.99 |
| Severe dysfunction | 2 (13%) | 1 (6%) | 0.99 |
| Postoperative ventricular function, n (%) | |||
| Normal function | 5 (31%) | 11 (69%) | 0.076 |
| Mild dysfunction | 3 (19%) | 3 (19%) | 0.99 |
| Moderate dysfunction | 3 (19%) | 1 (6%) | 0.60 |
| Severe dysfunction | 5 (31%) | 1 (6%) | 0.17 |
| . | Cohort (n = 16) . | Match (n = 16) . | P-Value . |
|---|---|---|---|
| Age (years), median (IQR) | 2.0 (0.6–3.8) | 1.7 (0.5–3.3) | 0.59 |
| Weight (kg), median (IQR) | 11.4 (7.4–13.9) | 10.9 (7.2–11.8) | 0.64 |
| Body surface area, median (IQR) | 0.51 (0.42–0.56) | 0.50 (0.42–0.56) | 0.87 |
| Diagnosis, n (%) | |||
| Hypoplastic left heart syndrome | 8 (50%) | 8 (50%) | 0.99 |
| Unbalanced atrioventricular septal defect | 4 (25%) | 5 (31%) | 0.99 |
| Tricuspid atresia | 2 (13%) | 1 (6%) | 0.99 |
| Pulmonary atresia/intact ventricular septum | 2 (13%) | – | 0.48 |
| Double inlet left ventricle | – | 2 (13%) | 0.48 |
| Ventricular morphology, n (%) | |||
| Dominant right ventricle | 12 (75%) | 9 (56%) | 0.46 |
| Dominant left ventricle | 4 (25%) | 7 (44%) | 0.46 |
| Valve morphology, n (%) | |||
| Tricuspid valve | 8 (50%) | 8 (50%) | 0.99 |
| Mitral valve | 4 (25%) | 3 (19%) | 0.99 |
| Common AV valve | 4 (25%) | 5 (31%) | 0.99 |
| Timing of AV valve replacement, n (%) | |||
| Stage I | – | 1 (6%) | 0.99 |
| Between stage I and II | 2 (13%) | 1 (6%) | 0.99 |
| Stage II | 3 (19%) | 8 (50%) | 0.14 |
| Between stage II and III | 8 (50%) | 3 (19%) | 0.14 |
| Stage III | 1 (6%) | 3 (19%) | 0.60 |
| After stage III | 2 (25%) | – | 0.48 |
| Preoperative ventricular function, n (%) | |||
| Normal function | 11 (69%) | 13 (81%) | 0.69 |
| Mild dysfunction | 3 (19%) | 1 (6%) | 0.60 |
| Moderate dysfunction | 0 (0%) | 1 (6%) | 0.99 |
| Severe dysfunction | 2 (13%) | 1 (6%) | 0.99 |
| Postoperative ventricular function, n (%) | |||
| Normal function | 5 (31%) | 11 (69%) | 0.076 |
| Mild dysfunction | 3 (19%) | 3 (19%) | 0.99 |
| Moderate dysfunction | 3 (19%) | 1 (6%) | 0.60 |
| Severe dysfunction | 5 (31%) | 1 (6%) | 0.17 |
AV: atrioventricular; IQR: interquartile range.
| . | Cohort (n = 16) . | Match (n = 16) . | P-Value . |
|---|---|---|---|
| Age (years), median (IQR) | 2.0 (0.6–3.8) | 1.7 (0.5–3.3) | 0.59 |
| Weight (kg), median (IQR) | 11.4 (7.4–13.9) | 10.9 (7.2–11.8) | 0.64 |
| Body surface area, median (IQR) | 0.51 (0.42–0.56) | 0.50 (0.42–0.56) | 0.87 |
| Diagnosis, n (%) | |||
| Hypoplastic left heart syndrome | 8 (50%) | 8 (50%) | 0.99 |
| Unbalanced atrioventricular septal defect | 4 (25%) | 5 (31%) | 0.99 |
| Tricuspid atresia | 2 (13%) | 1 (6%) | 0.99 |
| Pulmonary atresia/intact ventricular septum | 2 (13%) | – | 0.48 |
| Double inlet left ventricle | – | 2 (13%) | 0.48 |
| Ventricular morphology, n (%) | |||
| Dominant right ventricle | 12 (75%) | 9 (56%) | 0.46 |
| Dominant left ventricle | 4 (25%) | 7 (44%) | 0.46 |
| Valve morphology, n (%) | |||
| Tricuspid valve | 8 (50%) | 8 (50%) | 0.99 |
| Mitral valve | 4 (25%) | 3 (19%) | 0.99 |
| Common AV valve | 4 (25%) | 5 (31%) | 0.99 |
| Timing of AV valve replacement, n (%) | |||
| Stage I | – | 1 (6%) | 0.99 |
| Between stage I and II | 2 (13%) | 1 (6%) | 0.99 |
| Stage II | 3 (19%) | 8 (50%) | 0.14 |
| Between stage II and III | 8 (50%) | 3 (19%) | 0.14 |
| Stage III | 1 (6%) | 3 (19%) | 0.60 |
| After stage III | 2 (25%) | – | 0.48 |
| Preoperative ventricular function, n (%) | |||
| Normal function | 11 (69%) | 13 (81%) | 0.69 |
| Mild dysfunction | 3 (19%) | 1 (6%) | 0.60 |
| Moderate dysfunction | 0 (0%) | 1 (6%) | 0.99 |
| Severe dysfunction | 2 (13%) | 1 (6%) | 0.99 |
| Postoperative ventricular function, n (%) | |||
| Normal function | 5 (31%) | 11 (69%) | 0.076 |
| Mild dysfunction | 3 (19%) | 3 (19%) | 0.99 |
| Moderate dysfunction | 3 (19%) | 1 (6%) | 0.60 |
| Severe dysfunction | 5 (31%) | 1 (6%) | 0.17 |
| . | Cohort (n = 16) . | Match (n = 16) . | P-Value . |
|---|---|---|---|
| Age (years), median (IQR) | 2.0 (0.6–3.8) | 1.7 (0.5–3.3) | 0.59 |
| Weight (kg), median (IQR) | 11.4 (7.4–13.9) | 10.9 (7.2–11.8) | 0.64 |
| Body surface area, median (IQR) | 0.51 (0.42–0.56) | 0.50 (0.42–0.56) | 0.87 |
| Diagnosis, n (%) | |||
| Hypoplastic left heart syndrome | 8 (50%) | 8 (50%) | 0.99 |
| Unbalanced atrioventricular septal defect | 4 (25%) | 5 (31%) | 0.99 |
| Tricuspid atresia | 2 (13%) | 1 (6%) | 0.99 |
| Pulmonary atresia/intact ventricular septum | 2 (13%) | – | 0.48 |
| Double inlet left ventricle | – | 2 (13%) | 0.48 |
| Ventricular morphology, n (%) | |||
| Dominant right ventricle | 12 (75%) | 9 (56%) | 0.46 |
| Dominant left ventricle | 4 (25%) | 7 (44%) | 0.46 |
| Valve morphology, n (%) | |||
| Tricuspid valve | 8 (50%) | 8 (50%) | 0.99 |
| Mitral valve | 4 (25%) | 3 (19%) | 0.99 |
| Common AV valve | 4 (25%) | 5 (31%) | 0.99 |
| Timing of AV valve replacement, n (%) | |||
| Stage I | – | 1 (6%) | 0.99 |
| Between stage I and II | 2 (13%) | 1 (6%) | 0.99 |
| Stage II | 3 (19%) | 8 (50%) | 0.14 |
| Between stage II and III | 8 (50%) | 3 (19%) | 0.14 |
| Stage III | 1 (6%) | 3 (19%) | 0.60 |
| After stage III | 2 (25%) | – | 0.48 |
| Preoperative ventricular function, n (%) | |||
| Normal function | 11 (69%) | 13 (81%) | 0.69 |
| Mild dysfunction | 3 (19%) | 1 (6%) | 0.60 |
| Moderate dysfunction | 0 (0%) | 1 (6%) | 0.99 |
| Severe dysfunction | 2 (13%) | 1 (6%) | 0.99 |
| Postoperative ventricular function, n (%) | |||
| Normal function | 5 (31%) | 11 (69%) | 0.076 |
| Mild dysfunction | 3 (19%) | 3 (19%) | 0.99 |
| Moderate dysfunction | 3 (19%) | 1 (6%) | 0.60 |
| Severe dysfunction | 5 (31%) | 1 (6%) | 0.17 |
AV: atrioventricular; IQR: interquartile range.
DISCUSSION
While the survival of patients undergoing single-ventricle palliation has improved substantially, the challenge of managing the single-ventricle patient with significant AVV dysfunction has yet to be overcome. When possible, repair of a dysfunctional AV valve is preferred to replacement as it carries a significantly lower risk of mortality. However, that is not always possible. Our study found that in those circumstances, mechanical AV valve replacement salvages about half of patients at 1 year with substantially better mortality outcomes in patients with preserved ventricular function early after replacement. This study also confirmed that survivors still experience a significant risk of morbidity with almost 20% of patients having complete heart block requiring permanent pacemaker insertion, 50% having significant bleeding events with a subset suffering major cerebrovascular accidents, and over 25% having a valve thrombosis resulting in mortality or need for re-replacement.
Risk of mortality compared with valve repair
Mechanical AV valve replacement carries a substantial risk of mortality. We found an operative mortality rate of 38%, similar to other recently reported series. Analysis of the Paediatric Cardiac Care Consortium data reported on 38 SV patients with mechanical AV valve replacement showed in-hospital mortality of 42% and of 15 patients that had follow-up data, death or transplantation occurred in 10 patients [18]. Similarly, a multicentre report from Japan noted that of 56 SV patients with AV valve replacement, 36% died during follow-up (3.7 ± 2.6 years) with younger age and smaller prosthesis emerging as risk factors for mortality [16]. In 2001, Mahle et al. [15] reported hospital mortality of 29%; however, the mortality rate decreased in those in the more recent era (1994–2000). Due to the small sample size of our cohort, we were not able to identify risk factors for mortality. Nevertheless, patients with early postoperative ventricular dysfunction (moderate or greater) had increased mortality compared to those with normal function or mild dysfunction, underscoring the importance of preserved ventricular function for long-term survival.
Replacement of a regurgitant AV valve can unmask occult ventricular dysfunction. In 2011, our institution [1] reported a series of 219 patients undergoing a Norwood operation for a single systemic right ventricle, of which 25% had tricuspid valve repair at various stages of their single-ventricle palliation. Right ventricular function was preserved by tricuspid valve repair and intervention on the valve did not compromise survival, which was 82% at 5 years. Similarly, our study showed that a matched cohort of patients having valve repair had significantly higher survival than patients undergoing valve replacement. All but 1 patient in our series had prior attempts at valve repair. Unfortunately, the patient, who did not, suffered intraoperative damage to the valve apparatus which was deemed unrepairable.
Complete heart block, bleeding and thrombotic events
In our population, 19% of patients developed postoperative complete heart block requiring permanent pacemaker insertion. This is comparable to the other small studies in this population with reported rates ranging from 9% to 44% [15, 16, 19]. Complete heart block was seen following replacement of 1 mitral, 1 tricuspid, and 1 common AV valve in our study. Those series with lower incidences had less or no patients with heterotaxy or a common AV valve, theoretically reducing the risk of complete heart block.
We reported a high incidence of valve thrombosis as well as significant bleeding and stroke events. Our institutional practice is to anticoagulate with unfractionated heparin until patients are stable enough to tolerate either oral warfarin (if patients are over 2 years old) or low molecular weight heparin (under 2 years old) targeted to accepted international guidelines [20]. Anticoagulation levels are managed by a dedicated Thrombosis team who regularly monitor anti-Xa levels and INR. Given the modest size of the cohort, it is difficult to know whether these differences are based on different institutional anticoagulation practices. Anticoagulation in young children is a significant challenge with age-related changes in developmental hemostasis, differential distribution and binding of antithrombotic drugs, and lack of evidence for treatment guidelines [21]. Furthermore, patients with single-ventricle circulations from stage I up to and including the Fontan are known to have derangement in their coagulation factors and reported incidences of thromboembolic events may be as high as 20–30% in this population [22–24]. Deficiencies in protein C and S and antithrombin III have been found in patients prior to the Fontan and even the cavopulmonary connection suggesting that these patients are at a higher risk of thromboembolism throughout their course of surgical palliation [25, 26]. In addition to challenges with anticoagulation, there is a significant mismatch between a relatively generous-sized mechanical valve and marginal or low cardiac output, which may lead to stagnation of blood inflow through the mechanical valve and subsequent thrombus formation. While this highlights the challenges of long-term effective anticoagulation in single-ventricle patients requiring mechanical AVVs, it needs to be balanced against the risk of significant bleeding events, also a significant risk with 3 patients in our series having early haemorrhagic strokes.
Alternative options
The option of using a biological valve in place of a mechanical valve may be a more promising alternative. Recent reports have shown very promising results of surgical Melody mitral valve insertion with a small number of single-ventricle patients in 2 of these series [27–29] These studies showed low mortality and morbidity in the cohort overall however outcomes were not separated for patients with single-ventricle circulations. We have also recently published our experience using a Melody valve in a patient with unbalanced AVSD and severe AV valve regurgitation despite prior attempts at repair. This approach provided an improvement in the haemodynamics while awaiting heart transplantation, without mortality or morbidity [17]. While we need to accrue more experience with this technique, the early experience has been encouraging. Surgical modification of melody AV valve may become a useful therapeutic tool, even if used as a means of temporizing patients to a more stable circulation while awaiting transplantation.
Limitations
The major limitation of this study is that it represents a single-centre experience with a small sample size, and its retrospective nature, which limits risk factor analysis including the identification of preoperative variables that may contribute to postoperative ventricular dysfunction. This study may be underpowered to detect differences in demographic profiles between the groups and statistical analysis of these groups should be interpreted cautiously. Mechanical AVV replacements are rare in the single-ventricle population. Multicentre collaboration should be considered to improve our understanding of this disease.
CONCLUSION
Mechanical valve replacement carries significant morbidity and mortality risk. While it successfully salvages about half of patients with preserved ventricular function early after replacement, careful consideration of alternative options including transplantation and bioprosthetic valves (as a bridge to transplantation) should be made before embarking upon mechanical valve replacement. Earlier consideration of assessment and listing for transplant should be pursued if a patient has a dysfunctional valve that is challenging to repair or has significant regurgitation following repair. Further research is warranted to determine better long-term solutions to this vexing clinical challenge.
Funding
There was no external funding for this study. The authors have no financial disclosures.
Conflict of interest: none declared.
DATA AVAILABILITY
The data underlying this article cannot be shared publicly for the privacy of individuals that participated in the study. The data will be shared on reasonable request to the corresponding author.
Author contributions
Conall T. Morgan: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing—original draft; Writing—review & editing. Devin Chetan: Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing—original draft; Writing—review & editing. Jaymie Varenbut: Data curation; Investigation. Christoph Haller: Conceptualization; Investigation; Supervision; Writing—review & editing. Mike Seed: Conceptualization; Methodology; Supervision; Writing—review & editing. Luc L. Mertens: Conceptualization; Investigation; Methodology; Supervision; Writing—review & editing. Osami Honjo: Conceptualization; Formal analysis; Investigation; Methodology; Resources; Supervision; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Erle H. Austin, Katarzyna Januszewska and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.





