-
PDF
- Split View
-
Views
-
Cite
Cite
Elsa Armand, Alex Fourdrain, Chloé Lafouasse, Noémie Resseguier, Delphine Trousse, Xavier-Benoît D’Journo, Pascal-Alexandre Thomas, Is the Epithor conversion score reliable in robotic-assisted surgery anatomical lung resection?, European Journal of Cardio-Thoracic Surgery, Volume 64, Issue 3, September 2023, ezad283, https://doi.org/10.1093/ejcts/ezad283
Close - Share Icon Share
Abstract
Despite an improvement in surgical abilities, the need for an intraoperative switch from a minimally invasive procedure towards an open surgery (conversion) still remains. To anticipate this risk, the Epithor conversion score (ECS) has been described for video-assisted thoracoscopic surgery (VATS). Our objective was to determine if this score, developed for VATS, is applicable in robotic-assisted thoracoscopic surgery (RATS).
This was a retrospective monocentric study from January 2006 to June 2022, and data were obtained from the EPITHOR database. Patients included were those who underwent anatomic lung resection either by VATS or RATS. The ECS was calculated for all patients studied. Discrimination and calibration of the test were measured by the area under the curve and Hosmer–Lemeshow test.
A total of 1685 were included. There were 183/1299 conversions in the VATS group (14.1%) and 27/386 conversions in the RATS group (6.9%). Patients in the RATS group had fewer antiplatelet therapy and peripheral arterial disease. There were more segmentectomies in the VATS group. As for test discrimination, the area under the curve was 0.66 [0.56–0.78] in the RATS group and 0.64 [0.60–0.69] in the VATS group. Regarding the calibration, the Hosmer–Lemeshow test was not significant for both groups but more positive (better calibrated) for the VATS group (P = 0.12) compared to the RATS group (P = 0.08).
The ECS seems applicable for patients operated with RATS, with a correct discrimination but a lower calibration performance for patients operated with VATS. A new score could be developed to specifically anticipate conversion in patients operated on by RATS.
INTRODUCTION
Anatomical resection with systematic lymph node dissection is the treatment of choice in patient with operable early-stage non-small-cell lung cancer, favouring minimally invasive surgical approaches such as video-assisted thoracoscopic surgery (VATS) and robotic-assisted thoracoscopic surgery (RATS). Indeed, these techniques have been associated with decreased rates of postoperative complications, postoperative pain, shorter hospital stay and better short-term and long-term outcomes compared to conventional open thoracotomy [1, 2].
While these minimally invasive procedures lead to better outcomes in patients facing lung cancer, intraoperative conversion to open surgery may be necessary, mainly due to intraoperative difficulties, or less often emergently in case of serious intraoperative adverse event, especially haemorrhage. This change in the intraoperative course aims to preserve oncological goals [3] and patient safety, while altering the short-term outcomes compared to a full minimally invasive procedure [4]. Predictive factors for minimally invasive surgery conversions have been identified and permit a preoperative assessment to predict the risk of intraoperative conversion [5, 6], which may be useful to adapt the preoperative surgical and anaesthetic strategy. The Epithor conversion score (ECS) [7] has been developed to this end, allowing the preoperative estimation of three levels of risk for intraoperative conversion (low, intermediate and high risks). This score has been developed based on a cohort of 7943 patients treated with a VATS approach. However, even if VATS and RATS aim to the same curative intent, the surgical technique is different between the two procedures, and it is questionable if prognosticators for conversion may be similar in VATS and RATS anatomical resection.
The main objective of this study was to determine if the ECS is suitable to predict intraoperative conversion in RATS anatomical lung resection. Secondary objectives were to compare prognosticators of conversion between VATS and RATS anatomical resection.
MATERIALS AND METHODS
Ethical statement
The study was declared to our institution, in compliance with the General Data Protection Regulation (GRPD) and registered with the following reference: PADS HL82PT.
Patients
A single-centre retrospective study was conducted, using prospectively collected data regarding preoperative characteristics, perioperative variables and postoperative outcomes and follow-up in patients surgically treated in our institution between January 2006 and June 2022. Patient informed consent was obtained to gather the data that were extracted from our institutional database (CNIL 809833).
Data were extracted on all consecutive patients scheduled for VATS or RATS with anatomical lung resection, either lobar (lobectomy, bilobectomy or pneumonectomy) or sublobar (segmentectomy). We excluded patients operated through open surgery (thoracotomy), patients with non-anatomical lung resection (atypical/wedge resection, tumorectomy, surgical biopsy) and patients with missing data in whom the ECS was not calculable.
Surgical procedure
After approval of a dedicated thoracic multidisciplinary tumour board, patients were scheduled for surgical resection with curative intent for proven or suspicion of primitive lung cancer. Anatomical lung resection, lobar or sublobar, consisted of an elective dissection of bronchial and vascular elements, with an intent to treat systematic complete hilar and mediastinal lymph node dissection. VATS approach was predominantly multiportal, using a 30° 10-mm endoscope and dedicated endoscopic instruments/staplers/ultrasonic energy and bipolar cautery. Rib retractors were not used. RATS approach was performed using the Da-Vinci Si from 2013 with a four-arm technique, switching towards Da-Vinci X with a five-arm technique in 2020. Surgical conversion consisted of a lateral (axillary) or posterolateral thoracotomy, using a rib retractor, based on the surgeon intraoperative preference.
Prediction of robotic-assisted thoracoscopic surgery conversion
The ECS is based on 13 prognosticators to estimate the risk of intraoperative conversion. This score was calculated for each included patient using the following formula: ECS = −2.9265 + body mass index × 0.0358 + forced expiratory volume in 1 s × (−0.00411) + the sum of the variables’ coefficients. The ECS identify 3 levels of risk: low risk (ECS <−2.44, risk of conversion <8%), intermediate risk (ECS between −2.43 and −1.99, risk of conversion 8–12%) and high risk (ECS >−1.99, risk of conversion >12%). Patient ECS based on preoperative characteristics was confronted to the occurrence of intraoperative conversion and analysed by measuring the area under the receiver operating characteristic curve, to determine the ECS’s ability to identify the patient level of risk for intraoperative conversion.
Statistical analysis
Continuous variables are displayed as the mean ± standard deviation, and categorical variables as the frequency (percentage). Categorical variables were compared between groups using the Chi-square test if expected numbers were equal or larger than 5, otherwise using Fisher’s exact test. Continuous variables were compared between groups using Student’s t-test if the Shapiro–Wilk test did not reject the normality assumption, otherwise using the Mann–Whitney test. The threshold for statistical significance was set to P ≤ 0.05. ECS’s discrimination for RATS was assessed by measuring the area under the receiver operating characteristic curve, and calibration was measured graphically in a Hosmer–Lemeshow test. All statistical analyses were performed using SPSS software for Windows version 22.0 (IBM Corp., Armonk, NY, USA) and R software.
RESULTS
Patients
Over a 16-year period (January 2006–June 2022), a total of 13 952 patients are registered in our institutional prospective database. Non-anatomic pulmonary resection (n = 1880) and other operations who were not anatomic pulmonary resection (n = 7689) were excluded. Among the 4383 patients with anatomic pulmonary resection, we focused on patients who were treated with minimally invasive surgery and excluded those who underwent open surgery (n = 2479). Lastly, 219 patients were excluded because of missing data. Hence, the study population consisted in 1685 patients with the intent to treat anatomical pulmonary resection through minimally invasive surgery, with 1299 VATS procedures and 386 RATS procedures (Fig. 1). Intraoperative conversion occurred in 183/1299 patients in the VATS group (14.1%) and in 27/386 patients in the RATS group (6.9%).
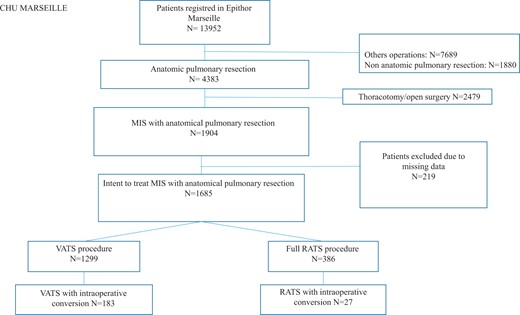
A total of 1469 patients/1685 (87.2%) had systematic lymph node dissection. The other patients received oriented lymph node dissection.
Comparison of video-assisted thoracoscopic surgery and robotic-assisted thoracoscopic surgery cohorts
A comparison between the VATS and RATS groups was performed regarding preoperative variables used in the ECS. Results are displayed in Table 1.
| . | VATS (n = 1299) . | RATS (n = 386) . | P-Value . |
|---|---|---|---|
| Age, mean (SD) | 65.2 (10.3) | 65.1 (9.5) | 0.447 |
| Sex, n (%) | 0.777 | ||
| Men | 743 (57.2) | 226 (58.5) | |
| Women | 555 (42.7) | 160 (41.5) | |
| BMI (kg/m2), mean (SD) | 25.2 (4.5) | 25.5 (4.2) | 0.076 |
| Smocking, n (%) | 0.288 | ||
| Yes | 930 (71.6) | 287 (74.4) | |
| No | 369 (28.4) | 99 (25.6) | |
| Preop. FEV1 (%), mean (SD) | 90.3 (19.7) | 91.6 (18.2) | 0.481 |
| Peripheral artery disease, n (%) | 130 (10) | 23 (6) | 0.015 |
| Antiplatelet therapy, n (%) | 206 (15.8) | 37 (9.5) | 0.002 |
| Chronic kidney failure, n (%) | 31 (2.4) | 7 (1.8) | 0.506 |
| Previous thoracic surgery, n (%) | 33 (2.5) | 11 (2.8) | 0.738 |
| Previous thoracic trauma, n (%) | 4 (0.3) | 0 | 0.353 |
| Drug addiction, n (%) | 12 (0.9) | 4 (1) | 0.517 |
| Cardiac arrhythmia, n (%) | 101 (7.8) | 19 (4.9) | 0.056 |
| Type II diabetes, n (%) | 130 (10) | 30 (7.8) | 0.188 |
| Histology, n (%) | 0.725 | ||
| Malignant (primary) | 1167 (89.8) | 345 (89.4) | |
| Metastasis | 57 (4.4) | 15 (3.9) | |
| Benignant | 75 (5.8) | 26 (6.7) | |
| Type of resection, n (%) | <0.001 | ||
| Lobectomy | 998 (76.8) | 338 (87.6) | |
| Segmentectomy | 289 (22.2) | 47 (12.2) | |
| Bilobectomy | 11 (0.8) | 1 (0.03) | |
| Pneumonectomy | 1 (0.1) | 0 | |
| cN stage, n (%) | 0.256 | ||
| 0 | 1250 (96.2) | 372 (96.4) | |
| 1 | 36 (2.8) | 7 (1.8) | |
| 2 | 13 (1) | 7 (41.8) |
| . | VATS (n = 1299) . | RATS (n = 386) . | P-Value . |
|---|---|---|---|
| Age, mean (SD) | 65.2 (10.3) | 65.1 (9.5) | 0.447 |
| Sex, n (%) | 0.777 | ||
| Men | 743 (57.2) | 226 (58.5) | |
| Women | 555 (42.7) | 160 (41.5) | |
| BMI (kg/m2), mean (SD) | 25.2 (4.5) | 25.5 (4.2) | 0.076 |
| Smocking, n (%) | 0.288 | ||
| Yes | 930 (71.6) | 287 (74.4) | |
| No | 369 (28.4) | 99 (25.6) | |
| Preop. FEV1 (%), mean (SD) | 90.3 (19.7) | 91.6 (18.2) | 0.481 |
| Peripheral artery disease, n (%) | 130 (10) | 23 (6) | 0.015 |
| Antiplatelet therapy, n (%) | 206 (15.8) | 37 (9.5) | 0.002 |
| Chronic kidney failure, n (%) | 31 (2.4) | 7 (1.8) | 0.506 |
| Previous thoracic surgery, n (%) | 33 (2.5) | 11 (2.8) | 0.738 |
| Previous thoracic trauma, n (%) | 4 (0.3) | 0 | 0.353 |
| Drug addiction, n (%) | 12 (0.9) | 4 (1) | 0.517 |
| Cardiac arrhythmia, n (%) | 101 (7.8) | 19 (4.9) | 0.056 |
| Type II diabetes, n (%) | 130 (10) | 30 (7.8) | 0.188 |
| Histology, n (%) | 0.725 | ||
| Malignant (primary) | 1167 (89.8) | 345 (89.4) | |
| Metastasis | 57 (4.4) | 15 (3.9) | |
| Benignant | 75 (5.8) | 26 (6.7) | |
| Type of resection, n (%) | <0.001 | ||
| Lobectomy | 998 (76.8) | 338 (87.6) | |
| Segmentectomy | 289 (22.2) | 47 (12.2) | |
| Bilobectomy | 11 (0.8) | 1 (0.03) | |
| Pneumonectomy | 1 (0.1) | 0 | |
| cN stage, n (%) | 0.256 | ||
| 0 | 1250 (96.2) | 372 (96.4) | |
| 1 | 36 (2.8) | 7 (1.8) | |
| 2 | 13 (1) | 7 (41.8) |
BMI: body mass index; FEV1: forced expiratory volume in 1 s; RATS: robot-assisted thoracoscopic surgery; SD: standard deviation; VATS: video-assisted thoracoscopic surgery.
| . | VATS (n = 1299) . | RATS (n = 386) . | P-Value . |
|---|---|---|---|
| Age, mean (SD) | 65.2 (10.3) | 65.1 (9.5) | 0.447 |
| Sex, n (%) | 0.777 | ||
| Men | 743 (57.2) | 226 (58.5) | |
| Women | 555 (42.7) | 160 (41.5) | |
| BMI (kg/m2), mean (SD) | 25.2 (4.5) | 25.5 (4.2) | 0.076 |
| Smocking, n (%) | 0.288 | ||
| Yes | 930 (71.6) | 287 (74.4) | |
| No | 369 (28.4) | 99 (25.6) | |
| Preop. FEV1 (%), mean (SD) | 90.3 (19.7) | 91.6 (18.2) | 0.481 |
| Peripheral artery disease, n (%) | 130 (10) | 23 (6) | 0.015 |
| Antiplatelet therapy, n (%) | 206 (15.8) | 37 (9.5) | 0.002 |
| Chronic kidney failure, n (%) | 31 (2.4) | 7 (1.8) | 0.506 |
| Previous thoracic surgery, n (%) | 33 (2.5) | 11 (2.8) | 0.738 |
| Previous thoracic trauma, n (%) | 4 (0.3) | 0 | 0.353 |
| Drug addiction, n (%) | 12 (0.9) | 4 (1) | 0.517 |
| Cardiac arrhythmia, n (%) | 101 (7.8) | 19 (4.9) | 0.056 |
| Type II diabetes, n (%) | 130 (10) | 30 (7.8) | 0.188 |
| Histology, n (%) | 0.725 | ||
| Malignant (primary) | 1167 (89.8) | 345 (89.4) | |
| Metastasis | 57 (4.4) | 15 (3.9) | |
| Benignant | 75 (5.8) | 26 (6.7) | |
| Type of resection, n (%) | <0.001 | ||
| Lobectomy | 998 (76.8) | 338 (87.6) | |
| Segmentectomy | 289 (22.2) | 47 (12.2) | |
| Bilobectomy | 11 (0.8) | 1 (0.03) | |
| Pneumonectomy | 1 (0.1) | 0 | |
| cN stage, n (%) | 0.256 | ||
| 0 | 1250 (96.2) | 372 (96.4) | |
| 1 | 36 (2.8) | 7 (1.8) | |
| 2 | 13 (1) | 7 (41.8) |
| . | VATS (n = 1299) . | RATS (n = 386) . | P-Value . |
|---|---|---|---|
| Age, mean (SD) | 65.2 (10.3) | 65.1 (9.5) | 0.447 |
| Sex, n (%) | 0.777 | ||
| Men | 743 (57.2) | 226 (58.5) | |
| Women | 555 (42.7) | 160 (41.5) | |
| BMI (kg/m2), mean (SD) | 25.2 (4.5) | 25.5 (4.2) | 0.076 |
| Smocking, n (%) | 0.288 | ||
| Yes | 930 (71.6) | 287 (74.4) | |
| No | 369 (28.4) | 99 (25.6) | |
| Preop. FEV1 (%), mean (SD) | 90.3 (19.7) | 91.6 (18.2) | 0.481 |
| Peripheral artery disease, n (%) | 130 (10) | 23 (6) | 0.015 |
| Antiplatelet therapy, n (%) | 206 (15.8) | 37 (9.5) | 0.002 |
| Chronic kidney failure, n (%) | 31 (2.4) | 7 (1.8) | 0.506 |
| Previous thoracic surgery, n (%) | 33 (2.5) | 11 (2.8) | 0.738 |
| Previous thoracic trauma, n (%) | 4 (0.3) | 0 | 0.353 |
| Drug addiction, n (%) | 12 (0.9) | 4 (1) | 0.517 |
| Cardiac arrhythmia, n (%) | 101 (7.8) | 19 (4.9) | 0.056 |
| Type II diabetes, n (%) | 130 (10) | 30 (7.8) | 0.188 |
| Histology, n (%) | 0.725 | ||
| Malignant (primary) | 1167 (89.8) | 345 (89.4) | |
| Metastasis | 57 (4.4) | 15 (3.9) | |
| Benignant | 75 (5.8) | 26 (6.7) | |
| Type of resection, n (%) | <0.001 | ||
| Lobectomy | 998 (76.8) | 338 (87.6) | |
| Segmentectomy | 289 (22.2) | 47 (12.2) | |
| Bilobectomy | 11 (0.8) | 1 (0.03) | |
| Pneumonectomy | 1 (0.1) | 0 | |
| cN stage, n (%) | 0.256 | ||
| 0 | 1250 (96.2) | 372 (96.4) | |
| 1 | 36 (2.8) | 7 (1.8) | |
| 2 | 13 (1) | 7 (41.8) |
BMI: body mass index; FEV1: forced expiratory volume in 1 s; RATS: robot-assisted thoracoscopic surgery; SD: standard deviation; VATS: video-assisted thoracoscopic surgery.
Patients treated with the RATS approach had significantly less antiplatelet therapy (P = 0.002) and peripheral artery disease (P = 0.015) than patients treated with the VATS approach. The type of resection performed was significantly different for each group, with a higher rate of segmental resection in the VATS group (22.2% vs 12.2%, P < 0.001).
Conversion occurred in 183/1.299 patients in the VATS group (14.1%) and was non-emergent in 128 cases (70%) (technical difficulties or oncological reasons) and emergent in 55 cases (30%) (bleeding or severe intraoperative complication). A conversion was significantly less common in the RATS group (P < 0.001), occurred in 27/386 patients (6.9%) and was non-emergent in 20 cases (74%) (technical difficulties or oncological reasons) and emergent in 7 cases (26%) (bleeding or severe intraoperative complication).
No differences were observed between preoperative variables of interest from the ECS in patient undergoing intraoperative conversion between the the VATS and RATS groups (Table 2).
| . | VATS conversion (n = 183) . | RATS conversion (n = 27) . | P-Value . |
|---|---|---|---|
| Age, mean (SD) | 68.01 (8.9) | 65.30 (8.3) | 0.100 |
| Sex, n (%) | 0.521 | ||
| Men | 120 (65.5) | 16 (59.3) | |
| Women | 63 (34.4) | 11 (40.7) | |
| BMI (kg/m2), mean (SD) | 26.1 (5.2) | 28.01 (4.9) | 0.039 |
| Smocking, n (%) | 0.615 | ||
| Yes | 134 (73.2) | 27 (77.8) | |
| No | 49 (26.8) | 6 (22.2) | |
| Preop. FEV1 (%), mean (SD) | 84.6 (18.8) | 83.88 (15.9) | 0.873 |
| Peripheral artery disease, n (%) | 33 (18) | 2 (7.4) | 0.131 |
| Antiplatelet therapy, n (%) | 39 (21.3) | 5 (18.5) | 0.739 |
| Chronic kidney failure, n (%) | 11 (6) | 1 (3.7) | 0.999 |
| Previous thoracic surgery, n (%) | 5 (2.7) | 0 | 0.999 |
| Previous thoracic trauma, n (%) | 1 (0.5) | 0 | 0.999 |
| Drug addiction, n (%) | 3 (1.6) | 0 | 0.999 |
| Cardiac arrhythmia, n (%) | 23 (12.6) | 2 (7.4) | 0.749 |
| Type II diabetes, n (%) | 25 (13.7) | 6 (22.2) | 0.249 |
| Histology, n (%) | 0.805 | ||
| Malignant (primary) | 171 (93.4) | 25 (92.6) | |
| Metastasis | 8 (4.4) | 1 (3.7) | |
| Benignant | 4 (2.2) | 1 (3.7) | |
| Type of resection, n (%) | 0.999 | ||
| Lobectomy | 148 (80.9) | 23 (85.2) | |
| Segmentectomy | 30 (16.4) | 4 (14.8) | |
| Bilobectomy | 4 (2.2) | 0 | |
| Pneumonectomy | 1 (0.5) | 0 | |
| cN stage, n (%) | 0.999 | ||
| 0 | 174 (72.1) | 27 (100) | |
| 1 | 5 (2.7) | 0 | |
| 2 | 4 (2.2) | 0 |
| . | VATS conversion (n = 183) . | RATS conversion (n = 27) . | P-Value . |
|---|---|---|---|
| Age, mean (SD) | 68.01 (8.9) | 65.30 (8.3) | 0.100 |
| Sex, n (%) | 0.521 | ||
| Men | 120 (65.5) | 16 (59.3) | |
| Women | 63 (34.4) | 11 (40.7) | |
| BMI (kg/m2), mean (SD) | 26.1 (5.2) | 28.01 (4.9) | 0.039 |
| Smocking, n (%) | 0.615 | ||
| Yes | 134 (73.2) | 27 (77.8) | |
| No | 49 (26.8) | 6 (22.2) | |
| Preop. FEV1 (%), mean (SD) | 84.6 (18.8) | 83.88 (15.9) | 0.873 |
| Peripheral artery disease, n (%) | 33 (18) | 2 (7.4) | 0.131 |
| Antiplatelet therapy, n (%) | 39 (21.3) | 5 (18.5) | 0.739 |
| Chronic kidney failure, n (%) | 11 (6) | 1 (3.7) | 0.999 |
| Previous thoracic surgery, n (%) | 5 (2.7) | 0 | 0.999 |
| Previous thoracic trauma, n (%) | 1 (0.5) | 0 | 0.999 |
| Drug addiction, n (%) | 3 (1.6) | 0 | 0.999 |
| Cardiac arrhythmia, n (%) | 23 (12.6) | 2 (7.4) | 0.749 |
| Type II diabetes, n (%) | 25 (13.7) | 6 (22.2) | 0.249 |
| Histology, n (%) | 0.805 | ||
| Malignant (primary) | 171 (93.4) | 25 (92.6) | |
| Metastasis | 8 (4.4) | 1 (3.7) | |
| Benignant | 4 (2.2) | 1 (3.7) | |
| Type of resection, n (%) | 0.999 | ||
| Lobectomy | 148 (80.9) | 23 (85.2) | |
| Segmentectomy | 30 (16.4) | 4 (14.8) | |
| Bilobectomy | 4 (2.2) | 0 | |
| Pneumonectomy | 1 (0.5) | 0 | |
| cN stage, n (%) | 0.999 | ||
| 0 | 174 (72.1) | 27 (100) | |
| 1 | 5 (2.7) | 0 | |
| 2 | 4 (2.2) | 0 |
BMI: body mass index; FEV1: forced expiratory volume in 1 s; RATS: robot-assisted thoracoscopic surgery; SD: standard deviation; VATS: video-assisted thoracoscopic surgery.
| . | VATS conversion (n = 183) . | RATS conversion (n = 27) . | P-Value . |
|---|---|---|---|
| Age, mean (SD) | 68.01 (8.9) | 65.30 (8.3) | 0.100 |
| Sex, n (%) | 0.521 | ||
| Men | 120 (65.5) | 16 (59.3) | |
| Women | 63 (34.4) | 11 (40.7) | |
| BMI (kg/m2), mean (SD) | 26.1 (5.2) | 28.01 (4.9) | 0.039 |
| Smocking, n (%) | 0.615 | ||
| Yes | 134 (73.2) | 27 (77.8) | |
| No | 49 (26.8) | 6 (22.2) | |
| Preop. FEV1 (%), mean (SD) | 84.6 (18.8) | 83.88 (15.9) | 0.873 |
| Peripheral artery disease, n (%) | 33 (18) | 2 (7.4) | 0.131 |
| Antiplatelet therapy, n (%) | 39 (21.3) | 5 (18.5) | 0.739 |
| Chronic kidney failure, n (%) | 11 (6) | 1 (3.7) | 0.999 |
| Previous thoracic surgery, n (%) | 5 (2.7) | 0 | 0.999 |
| Previous thoracic trauma, n (%) | 1 (0.5) | 0 | 0.999 |
| Drug addiction, n (%) | 3 (1.6) | 0 | 0.999 |
| Cardiac arrhythmia, n (%) | 23 (12.6) | 2 (7.4) | 0.749 |
| Type II diabetes, n (%) | 25 (13.7) | 6 (22.2) | 0.249 |
| Histology, n (%) | 0.805 | ||
| Malignant (primary) | 171 (93.4) | 25 (92.6) | |
| Metastasis | 8 (4.4) | 1 (3.7) | |
| Benignant | 4 (2.2) | 1 (3.7) | |
| Type of resection, n (%) | 0.999 | ||
| Lobectomy | 148 (80.9) | 23 (85.2) | |
| Segmentectomy | 30 (16.4) | 4 (14.8) | |
| Bilobectomy | 4 (2.2) | 0 | |
| Pneumonectomy | 1 (0.5) | 0 | |
| cN stage, n (%) | 0.999 | ||
| 0 | 174 (72.1) | 27 (100) | |
| 1 | 5 (2.7) | 0 | |
| 2 | 4 (2.2) | 0 |
| . | VATS conversion (n = 183) . | RATS conversion (n = 27) . | P-Value . |
|---|---|---|---|
| Age, mean (SD) | 68.01 (8.9) | 65.30 (8.3) | 0.100 |
| Sex, n (%) | 0.521 | ||
| Men | 120 (65.5) | 16 (59.3) | |
| Women | 63 (34.4) | 11 (40.7) | |
| BMI (kg/m2), mean (SD) | 26.1 (5.2) | 28.01 (4.9) | 0.039 |
| Smocking, n (%) | 0.615 | ||
| Yes | 134 (73.2) | 27 (77.8) | |
| No | 49 (26.8) | 6 (22.2) | |
| Preop. FEV1 (%), mean (SD) | 84.6 (18.8) | 83.88 (15.9) | 0.873 |
| Peripheral artery disease, n (%) | 33 (18) | 2 (7.4) | 0.131 |
| Antiplatelet therapy, n (%) | 39 (21.3) | 5 (18.5) | 0.739 |
| Chronic kidney failure, n (%) | 11 (6) | 1 (3.7) | 0.999 |
| Previous thoracic surgery, n (%) | 5 (2.7) | 0 | 0.999 |
| Previous thoracic trauma, n (%) | 1 (0.5) | 0 | 0.999 |
| Drug addiction, n (%) | 3 (1.6) | 0 | 0.999 |
| Cardiac arrhythmia, n (%) | 23 (12.6) | 2 (7.4) | 0.749 |
| Type II diabetes, n (%) | 25 (13.7) | 6 (22.2) | 0.249 |
| Histology, n (%) | 0.805 | ||
| Malignant (primary) | 171 (93.4) | 25 (92.6) | |
| Metastasis | 8 (4.4) | 1 (3.7) | |
| Benignant | 4 (2.2) | 1 (3.7) | |
| Type of resection, n (%) | 0.999 | ||
| Lobectomy | 148 (80.9) | 23 (85.2) | |
| Segmentectomy | 30 (16.4) | 4 (14.8) | |
| Bilobectomy | 4 (2.2) | 0 | |
| Pneumonectomy | 1 (0.5) | 0 | |
| cN stage, n (%) | 0.999 | ||
| 0 | 174 (72.1) | 27 (100) | |
| 1 | 5 (2.7) | 0 | |
| 2 | 4 (2.2) | 0 |
BMI: body mass index; FEV1: forced expiratory volume in 1 s; RATS: robot-assisted thoracoscopic surgery; SD: standard deviation; VATS: video-assisted thoracoscopic surgery.
Epithor conversion score assessment in video-assisted thoracoscopic surgery and robotic-assisted thoracoscopic surgery cohort
Regarding discriminant power, the ECS had acceptable levels of sensitivity and specificity in our cohort for both groups: the area under the curve [95% confidence interval] was 0.66 [0.56–0.78] in the RATS group and 0.64 [0.60–0.69] in the VATS group (Fig. 2).
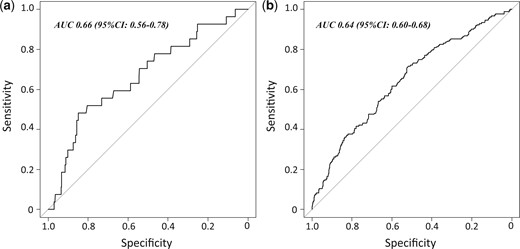
Discrimination of the Epithor conversion score assessed through area under curve in (a) the RATS group and (b) the VATS group. RATS: robot-assisted thoracoscopic surgery; VATS: video-assisted thoracoscopic surgery.
Regarding the calibration of the ECS, Hosmer–Lemeshow test showed similar calibration, trending to be slightly inferior in the RATS group (P = 0.08) compared to the VATS group (P = 0.12) (Fig. 3).
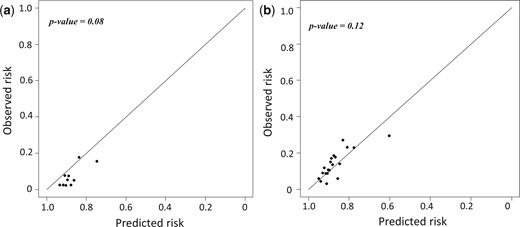
Calibration plot of the Epithor conversion score assessed through Hosmer–Lemeshow test in (a) the RATS group and (b) the VATS group. RATS: robot-assisted thoracoscopic surgery; VATS: video-assisted thoracoscopic surgery.
DISCUSSION
The ECS seems applicable to predict intraoperative conversion in patients treated with anatomical resection through RATS approach, with a similar measured discrimination but slightly lower calibration compared to VATS approach.
Our main hypothesis to explain the lower calibration in the RATS group is the difference in baseline characteristics between the 2 groups, especially in variables involved in the calculation of the ECS (antiplatelet therapy, peripheral artery disease and type of resection).
The conversion rate in this cohort is 12.5% (n = 210/1685), with a higher proportion of conversion in VATS compared to RATS (14.1% vs 6.9%). Those results are in line with data from the literature, with a VATS conversion rate which is mildly higher than the French mean conversion rate, reported at 10.6% in a multicentric national study on conversion in VATS [7], and other reports: from 9.3% for Bongiolatti et al. [8], 6.5% for Augustin et al. [9], 8.8% for Li et al. [10] and 7% for Puri et al. [11]. These conversion rates are hardly comparable with Tong et al. [12] study, with a 1% conversion rate (in a study including 25% of wedge resection).
The decreased conversion rate we observed in the RATS cohort compared to VATS is consistent with already published reports. Chen et al. described a 2.4% conversion rate for the RATS procedure vs 25.1% for the VATS procedure [13], Herrera et al. described a 3.6% conversion rate for the RATS procedure vs 12.9% for the VATS procedure [14] and Servais et al. observed a similar trend with a 6% conversion rate for the RATS procedure vs 11% for the VATS procedure [5].
Without being fully prevented, the risk of conversion can be predicted and consequently permit peroperative prevention through the score.
This prevention enables anticipation and adaptation of per operative strategy such as the patient's care pathway (surgical unit or Intensive Care Unit), the use of a more advanced loco-regional anaesthesia (epidural), the surgical procedure, being perfectly oncologic and the information of patient concerning the higher risk of conversion to a thoracotomy if needed.
The ECS can be adapted to RATS resections with additional variables possibly having a specific impact in robot-assisted surgery. These variables may be related to technical difficulties (body mass index), dissection difficulties (complex cases, tumour size, emphysema, lymph node invasion, etc.) requiring an additional specific evaluation.
Furthermore, there exists an inverse relationship between the conversion rate and the experience of the operating surgeon, but we must detail several situations where this was not evidenced.
If a young operating surgeon is supervised by a trained senior surgeon (the example of academic centres), then there should not be any more conversions. Also, each surgeon is different and their experience will be varied, even depending on the number of lobectomies they perform, because the access to training is variable (simulator access, etc.).
We have not considered this association also because we do not have all training parameters for each individual surgeon in our database and we wanted to analyse patients-based variables. A study focused on the relationship between intraoperative conversion and the surgeon's experience would be interesting.
Limitations
This study displays several limitations. First, the design of the study is monocentric with a small number of patients in converted groups, which limits the strength of the study. The study is also retrospective, which may lead to biases in the data report. However, a retrospective design is almost unavoidable when studying intraoperative conversion, and biases are reduced by the use of an indexed prospective database. This study is the first to assess the ECS following its development and also the first to evaluate this score in an RATS cohort.
This study enables us to consider the use of the ECS in patients undergoing anatomical resection with RATS, in a comparable way as it was designed in patients treated with VATS. Also, as the rate of the RATS procedure is increasing, another perspective is the development of a dedicated RATS conversion score, which would probably be better calibrated than the ECS. In this study, we only considered the 13 variables used in the ECS (out of 35 variables initially analysed in the cohort used for establishing the ECS).
Among the other relevant variables, it would be interesting to determine the impact of the type of resection, particularly the type of segmentectomy (simple/complex segments), and the laterality/location of the resection. Institutional variables would also be interesting (number of resections by operator, number of resections in the centre, type of centre), but these variables are not based on the patient.
CONCLUSION
In conclusion, the ECS seems applicable to predict intraoperative conversion in patients treated with anatomical resection through the RATS approach, with a similar discrimination but slightly lower calibration compared to the VATS approach. As the RATS procedure is broadening worldwide, more predictive studies regarding intraoperative conversion focused on RATS resection should be performed to enhance patients’ perioperative surgical and anaesthetic strategies.
Conflict of interest: none declared.
DATA AVAILABILITY
The data underlying this article cannot be shared publicly due to the APHM and EPITHOR policies. The data will be shared on reasonable request to the corresponding author.
Author contributions
Elsa Armand: Conceptualization; Data curation; Funding acquisition; Investigation; Writing—original draft. Alex Fourdrain: Conceptualization; Formal analysis; Funding acquisition; Methodology; Project administration; Supervision; Validation; Writing—original draft. Chloé Lafouasse: Data curation; Investigation; Writing—original draft. Noémie Resseguier: Data curation; Formal analysis; Methodology. Delphine Trousse: Supervision; Validation; Visualization. Xavier-Benoît D’Journo: Supervision; Validation; Visualization. Pascal-Alexandre Thomas: Conceptualization; Project administration; Resources; Supervision; Validation; Visualization; Writing—original draft.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Alper Toker, Filippo Tommaso Gallina and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- ECS
Epithor conversion score
- RATS
Robotic-assisted thoracoscopic surgery
- VATS
Video-assisted thoracoscopic surgery





