-
PDF
- Split View
-
Views
-
Cite
Cite
Josephina Haunschild, Antonia van Kampen, Martin Misfeld, Konstantin Von Aspern, Jörg Ender, Waseem Zakhary, Michael A Borger, Christian D Etz, Is perioperative fast-track management the future of proximal aortic repair?, European Journal of Cardio-Thoracic Surgery, Volume 63, Issue 2, February 2023, ezac578, https://doi.org/10.1093/ejcts/ezac578
Close - Share Icon Share
Abstract
The Bentall procedure is the gold standard for patients with combined aortic root dilation and valve dysfunction. Over the past decade, fast-track (FT) perioperative anaesthetic management protocols have progressively evolved. We reviewed our results for selected patients undergoing Bentall surgery under an FT protocol.
We retrospectively analysed a consecutive cohort of patients who underwent elective Bentall procedures at our institution between 2000 and 2018. Complex aortic root repair (i.e. David and Ross procedure, redo surgery, major concomitant procedures, emergency repair for acute dissections) was excluded. Patients who underwent conventional perioperative treatment and those treated according to our institutional FT concept were compared following 1:1 propensity score matching.
Of 772 patients who fit the in- and exclusion criteria, 565 were treated conventionally post-surgery, while 207 were treated using the FT protocol. Propensity score matching resulted in 197 pairs, with no differences in baseline characteristics after matching. In-house mortality, 30-day mortality and overall all-cause long-term mortality were comparable between the FT and the conventionally treated cohort. Postoperative anaesthetic care unit/intensive care unit length-of-stay (6.2 vs 20.6 h, P = 0.03) and postoperative ventilation times (158.9 vs 465.5 min, P < 0.001) were significantly shorter in the FT cohort. There were no differences in rates of postoperative adverse events.
In centres with experienced anaesthesiologists, perioperative FT management is non-inferior to conventionally treated patients undergoing elective Bentall procedures without compromising patient safety.
- aortic valve
- coronary artery bypass surgery
- ross procedure
- anesthetics
- tissue dissection
- objective (goal)
- hospital mortality
- intensive care unit
- length of stay
- perioperative care
- surgical procedures, operative
- mortality
- surgery specialty
- aortic root dilatation
- intermediate care
- patient safety
- coronary inclusion technique
- aortic surgery
- gold standard
- anesthesiologists
- supraaortic valve area
- adverse event
- aortic root aneurysm
INTRODUCTION
Over the past 2 decades, fast-track (FT) concepts have been successfully implemented in cardiac surgery in up to 45% of cases, primarily in patients undergoing coronary artery bypass grafting (CABG), with no significant increases in morbidity and mortality [1–3]. The FT concept includes early tracheal extubation, administration of small amounts of short-acting opioids and propofol or volatile anaesthetics leading to a decrease in length of stay on the intensive care unit and postoperative ward [4]. Over time and with increasing experience, FT management has been applied in more complex cases with more comorbidities and higher surgical complexity. We aim to compare peri- and postoperative outcomes after Bentall operations over a 19-year period of time in a high-volume centre between 2 cohorts: those treated in the intensive care unit (ICU) postoperatively versus those treated following our institutional FT concept, bypassing the ICU investigating the safety and efficacy of this concept, including associations with changes regarding postoperative recovery and potential adverse events.
PATIENTS AND METHODS
Ethics statement
The study was approved by the ethics committee of the University of Leipzig, approval number: 177/15. Due to retrospective analysis of a large number of patients, anonymity was granted and no individual patient consent needed to be obtained.
Definitions
FT concept—fast awakening and extubation of the patient after surgery, different pain management and bypassing the intensive care unit going directly on the intermediate care unit.
Bentall procedure—combined replacement of the aortic valve and aortic root with reimplantation of the coronary arteries, mostly due to aortic valve dysfunction in combination with aortic root aneurysm.
Circulatory arrest—no perfusion of lower body while suturing of the distal aortic anastomosis of the aortic arch, performed in hypothermia (for organ protection), brain perfusion granted by selective antegrade cerebral perfusion.
Study design and patient cohort
This retrospective single-centre study was conducted and reported following the recommendations of the STROBE statement [5]. Our institutional database was retrospectively reviewed from 2000 to 2018. We identified all patients who had undergone the Bentall procedure. Patients with Stanford type A aortic dissection previous cardiac surgery, active endocarditis, major concomitant procedures (e.g. CABG, other/multiple valve surgery) and patients with intraoperative conversion from valve-sparing root replacement were excluded. Resection of hypertrophic septal tissue (Morrow’s resection) and pulmonary vein ablation were allowed as concomitant procedures. Patients were then divided into 2 groups: conventionally treated patients [CONV; i.e. spending the first night after the operation in the ICU before transfer to the intermediate care unit (IMCU)] versus FT patients (FT) who were treated solely in the postoperative anaesthetic care unit (PACU) and IMCU, bypassing the ICU.
We hypothesized that the application of the FT concept is equally safe as conventional treatment in patients undergoing Bentall surgery and possibly yields benefits regarding mechanical ventilation times and postoperative recovery. Primary outcomes were ventilation time, IMCU length of stay, and respiratory failure requiring reintubation; secondary outcomes were stroke, renal failure requiring dialysis, hospital length of stay and in-hospital mortality.
Surgical technique
The Bentall procedures were performed, as previously described [6], through a full median sternotomy (88.9%), or via a partial upper sternotomy (11.1%). Cardiopulmonary bypass (CPB) was established via distal ascending aorta/aortic arch and right atrial appendix cannulation in most instances. A left ventricular vent was used in all operations. Blood or crystalloid cardioplegia was delivered into the aortic root or, in case of relevant aortic regurgitation, directly into the coronary ostia using mushroom- or olive-tipped catheters.
Standard techniques were used to remove the aortic aneurysm and excise the native aortic valve and surrounding calcified tissue. The coronary ostia were preserved as buttons and reimplanted according to the modified Bentall technique. Standard valve-bearing conduits were implanted including commercially available prostheses (e.g. most mechanical valved conduits and porcine root conduits) or ‘tailor-made’ by the surgeon in the operating room by suturing standard aortic valve prostheses into a tubular Dacron grafts. Valve conduits were fixed to the aortic annulus using standard interrupted, pledgeted, mattress sutures. Mechanical or biological prostheses were chosen according to patient preference, age, and risk profile. Coronary reimplantation was conducted after creating buttonholes in the tubular graft, using continuous 5–0 polypropylene sutures. Distal anastomosis, de-airing, weaning from CPB and closure were performed in a standard fashion. Moderate hypothermia was the standard approach, but in cases including replacement of the proximal aortic arch, deep hypothermia with circulatory arrest and bilateral antegrade selective cerebral perfusion was applied.
Anaesthesiologic management
Our institutional FT protocol has been previously described in detail [7] and applied from 2009 onwards to an ever-increasing proportion of eligible elective cases (see Supplementary Material, Fig. S1). Both groups were treated identically regarding anaesthetic premedication (patients are admitted 1 day prior to surgery at our institution), induction and maintenance, prevention of body core temperature drifting post-CPB, intraoperative invasive pulmonary recruitment manoeuvres and analgetic therapy scheme (for details, see Table 1). After surgery, FT patients were admitted to the PACU for early recovery, weaning from the ventilator, and extubation prior to transfer to IMCU, without any stay in the ICU. Postoperative pain management on PACU included 1 g of metamizole i.v. and piritramide 0.1 mg/kg i.v. at arrival. We aimed for a maximum pain intensity below 4 on the pain numeric rating scale and used additional boluses of piritramide 0.02–0.03 mg/kg i.v. to achieve this. In contrast, patients of the CONV group received continuous infusion of sufentanyl while still sedated and a bolus of piritramide (0.1 mg/kg i.v.) when propofol infusion was stopped. After extubation pain management was identical to postoperative pain management on PACU. As previously described, our PACU is available until 10 pm every day and staffed with anaesthesiologists and specialized nursed. The IMCU is equipped with full telemetry including invasive blood pressure monitoring. Conventionally treated patients were transferred from the operating room directly to the ICU. Postoperative ventilation times were calculated as the time from the end of surgery until the time of extubation. Inclusion criteria for FT management and criteria for extubation and transfer from PACU to IMCU were standardized at our institution, as previously described [2], and are displayed in Table 2. Based on the available data, only patients without exclusion criteria for FT treatment were included and assignment to CONV versus FT perioperative treatment was mostly based on (i) when patients were operated (before or after introduction of the FT protocol and (ii) whether patients were operated on a day/at a time of day that the PACU was not available (holidays, weekends, late in the day).
Overview of the anaesthesiological management (modified from Ender et al. [7])
| Anaesthesiological management . | |
|---|---|
| Premedication | |
| One day before surgery | Dipotassium clorazepate |
| Day of surgery | Clonidine |
| Anaesthetic induction | Propofol (1–2 mg/kg) |
| Sufentanil (0.5–1 µg/kg) | |
| Rocuronium (0.6 mg/kg) | |
| Maintenance of anaesthesia (under CPB) | Remifentanil (0.2 µg/kg/min) |
| Sevoflurane (0.8–1.1% MAC) | |
| [Propofol (3 mg/kg/min)] | |
| Temperature control after weaning from CPB | Bairhugger© underbody blanket external convective warming system (goal core temperature >36°C) |
| Early analgetic control (intraoperative, before skin closure) | 1 g paracetamol systemically |
| Standardized postoperative analgetic therapy scheme | bolus of piritramide (0.1 mg/kg) if needed + paracetamol (1 g every 6 h) (goal APS 2–4) |
| immediately after extubation | 1 h of noninvasive ventilation |
| Anaesthesiological management . | |
|---|---|
| Premedication | |
| One day before surgery | Dipotassium clorazepate |
| Day of surgery | Clonidine |
| Anaesthetic induction | Propofol (1–2 mg/kg) |
| Sufentanil (0.5–1 µg/kg) | |
| Rocuronium (0.6 mg/kg) | |
| Maintenance of anaesthesia (under CPB) | Remifentanil (0.2 µg/kg/min) |
| Sevoflurane (0.8–1.1% MAC) | |
| [Propofol (3 mg/kg/min)] | |
| Temperature control after weaning from CPB | Bairhugger© underbody blanket external convective warming system (goal core temperature >36°C) |
| Early analgetic control (intraoperative, before skin closure) | 1 g paracetamol systemically |
| Standardized postoperative analgetic therapy scheme | bolus of piritramide (0.1 mg/kg) if needed + paracetamol (1 g every 6 h) (goal APS 2–4) |
| immediately after extubation | 1 h of noninvasive ventilation |
APS: analogue pain scale; CPB: cardiopulmonary bypass; MAC: minimal alveolar concentration.
Overview of the anaesthesiological management (modified from Ender et al. [7])
| Anaesthesiological management . | |
|---|---|
| Premedication | |
| One day before surgery | Dipotassium clorazepate |
| Day of surgery | Clonidine |
| Anaesthetic induction | Propofol (1–2 mg/kg) |
| Sufentanil (0.5–1 µg/kg) | |
| Rocuronium (0.6 mg/kg) | |
| Maintenance of anaesthesia (under CPB) | Remifentanil (0.2 µg/kg/min) |
| Sevoflurane (0.8–1.1% MAC) | |
| [Propofol (3 mg/kg/min)] | |
| Temperature control after weaning from CPB | Bairhugger© underbody blanket external convective warming system (goal core temperature >36°C) |
| Early analgetic control (intraoperative, before skin closure) | 1 g paracetamol systemically |
| Standardized postoperative analgetic therapy scheme | bolus of piritramide (0.1 mg/kg) if needed + paracetamol (1 g every 6 h) (goal APS 2–4) |
| immediately after extubation | 1 h of noninvasive ventilation |
| Anaesthesiological management . | |
|---|---|
| Premedication | |
| One day before surgery | Dipotassium clorazepate |
| Day of surgery | Clonidine |
| Anaesthetic induction | Propofol (1–2 mg/kg) |
| Sufentanil (0.5–1 µg/kg) | |
| Rocuronium (0.6 mg/kg) | |
| Maintenance of anaesthesia (under CPB) | Remifentanil (0.2 µg/kg/min) |
| Sevoflurane (0.8–1.1% MAC) | |
| [Propofol (3 mg/kg/min)] | |
| Temperature control after weaning from CPB | Bairhugger© underbody blanket external convective warming system (goal core temperature >36°C) |
| Early analgetic control (intraoperative, before skin closure) | 1 g paracetamol systemically |
| Standardized postoperative analgetic therapy scheme | bolus of piritramide (0.1 mg/kg) if needed + paracetamol (1 g every 6 h) (goal APS 2–4) |
| immediately after extubation | 1 h of noninvasive ventilation |
APS: analogue pain scale; CPB: cardiopulmonary bypass; MAC: minimal alveolar concentration.
Standardized postoperative handling according to the fast-track concept (modified from Zakhary et al. [2])
| Inclusion criteria for fast track | Haemodynamically stable ± low-dose inotropic support |
| No excessive bleeding | |
| Elective/urgent surgeries | |
| Clinical judgement and communication between anaesthesiologist and surgeon | |
| Criteria for extubation | Clinical criteria: fully awake and alert, hemodynamically stable, completely recovered motor function, no neurologic deficit |
| Objective criteria: bleeding <100 ml/h, temperature ≥36°C, acceptable blood gases on FiO2 < 0.5, sufficient tidal volume (pressure support 8 cmH2O and positive end-expiratory pressure 5 cmH2O), Normal lactate, mixed venous oxygen saturation, electrocardiogram and chest X-ray | |
| Criteria for transfer from PACU to IMCU | Fully awake and alert, no neurologic deficit |
| Haemodynamically stable ± low-dose inotropic support | |
| PaO2 > 90 mmHg and PaCO2 < 46 mmHg on 2–6 l/minO2 | |
| Urinary output >0.5 ml/kg/h | |
| Bleeding <50 ml/h | |
| Normal serum lactate, mixed venous oxygen saturation, cardiac enzymes and chest X-ray |
| Inclusion criteria for fast track | Haemodynamically stable ± low-dose inotropic support |
| No excessive bleeding | |
| Elective/urgent surgeries | |
| Clinical judgement and communication between anaesthesiologist and surgeon | |
| Criteria for extubation | Clinical criteria: fully awake and alert, hemodynamically stable, completely recovered motor function, no neurologic deficit |
| Objective criteria: bleeding <100 ml/h, temperature ≥36°C, acceptable blood gases on FiO2 < 0.5, sufficient tidal volume (pressure support 8 cmH2O and positive end-expiratory pressure 5 cmH2O), Normal lactate, mixed venous oxygen saturation, electrocardiogram and chest X-ray | |
| Criteria for transfer from PACU to IMCU | Fully awake and alert, no neurologic deficit |
| Haemodynamically stable ± low-dose inotropic support | |
| PaO2 > 90 mmHg and PaCO2 < 46 mmHg on 2–6 l/minO2 | |
| Urinary output >0.5 ml/kg/h | |
| Bleeding <50 ml/h | |
| Normal serum lactate, mixed venous oxygen saturation, cardiac enzymes and chest X-ray |
IMCU: intermediate care unit; PACU: postanaesthesia care unit; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen.
Standardized postoperative handling according to the fast-track concept (modified from Zakhary et al. [2])
| Inclusion criteria for fast track | Haemodynamically stable ± low-dose inotropic support |
| No excessive bleeding | |
| Elective/urgent surgeries | |
| Clinical judgement and communication between anaesthesiologist and surgeon | |
| Criteria for extubation | Clinical criteria: fully awake and alert, hemodynamically stable, completely recovered motor function, no neurologic deficit |
| Objective criteria: bleeding <100 ml/h, temperature ≥36°C, acceptable blood gases on FiO2 < 0.5, sufficient tidal volume (pressure support 8 cmH2O and positive end-expiratory pressure 5 cmH2O), Normal lactate, mixed venous oxygen saturation, electrocardiogram and chest X-ray | |
| Criteria for transfer from PACU to IMCU | Fully awake and alert, no neurologic deficit |
| Haemodynamically stable ± low-dose inotropic support | |
| PaO2 > 90 mmHg and PaCO2 < 46 mmHg on 2–6 l/minO2 | |
| Urinary output >0.5 ml/kg/h | |
| Bleeding <50 ml/h | |
| Normal serum lactate, mixed venous oxygen saturation, cardiac enzymes and chest X-ray |
| Inclusion criteria for fast track | Haemodynamically stable ± low-dose inotropic support |
| No excessive bleeding | |
| Elective/urgent surgeries | |
| Clinical judgement and communication between anaesthesiologist and surgeon | |
| Criteria for extubation | Clinical criteria: fully awake and alert, hemodynamically stable, completely recovered motor function, no neurologic deficit |
| Objective criteria: bleeding <100 ml/h, temperature ≥36°C, acceptable blood gases on FiO2 < 0.5, sufficient tidal volume (pressure support 8 cmH2O and positive end-expiratory pressure 5 cmH2O), Normal lactate, mixed venous oxygen saturation, electrocardiogram and chest X-ray | |
| Criteria for transfer from PACU to IMCU | Fully awake and alert, no neurologic deficit |
| Haemodynamically stable ± low-dose inotropic support | |
| PaO2 > 90 mmHg and PaCO2 < 46 mmHg on 2–6 l/minO2 | |
| Urinary output >0.5 ml/kg/h | |
| Bleeding <50 ml/h | |
| Normal serum lactate, mixed venous oxygen saturation, cardiac enzymes and chest X-ray |
IMCU: intermediate care unit; PACU: postanaesthesia care unit; PaCO2: partial pressure of carbon dioxide; PaO2: partial pressure of oxygen.
Statistical analysis
Five-fold multiple imputation was applied to correct for missing data in baseline characteristics (proportion of missing data was 0.1% in the FT and 0.7% in the CONV group). Preoperative data were reported as mean with standard deviation or median with interquartile range (according to assessment for normal distribution using Kolmogorov–Smirnov test), or counts with percentages, and for the unmatched groups compared using Student’s t-test or Pearson’s chi-squared test with continuity correction, as appropriate. Preoperative variables of the 2 matched groups were compared using paired sample t-test or Wilcoxon signed rank test, as appropriate, and McNemar’s test. Using logistic regression, propensity scores were estimated for each individual and 1:1 propensity score matching was performed using a ‘nearest neighbor’ algorithm without replacement and a caliper setting of 0.3 standard deviations (as needed to accomplish balancing of all covariates). Outcome comparison of the matched groups was conducted using Wilcoxon sign-rank test, or, in case of missing data, Wilcoxon rank-sum test for continuous variables and exact McNemar’s chi-squared test with continuity correction for categorical variables. Continuous outcomes are reported as median with interquartile range, and categorical data as counts with percentages and odds ratios, when applicable. Survival was compared using Cox-proportional hazards model with adjusted standard errors using generalized estimating equations. Univariable and multivariable effect sizes are reported as hazard ratios with 95% confidence intervals.
Data analysis was performed using R version 3.6.1 [8]. Data preparation and descriptive statistics were supported by the ‘tidyverse’ [9] package. The ‘MatchIt’ [10] package was used for propensity score modelling and matching, ‘survival’ [11] for survival analysis and regression, and ‘ggplot2’ [12] for plotting.
RESULTS
Preoperative patient characteristics and propensity score matching
After application of in- and exclusion criteria, 772 consecutive patients who had received elective, isolated Bentall surgery at our institution between 2000 and 2018 were included in the analysis. Patient in- and exclusion are detailed in Fig. 1.
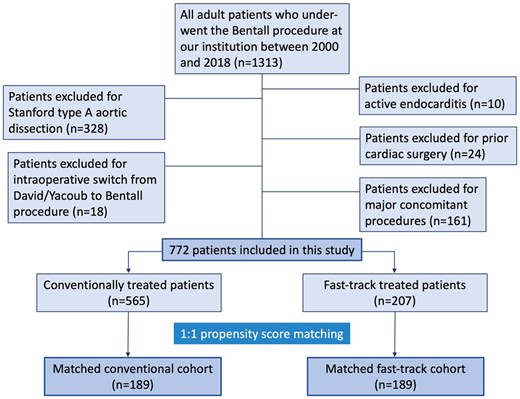
The unmatched CONV (n = 565) and FT (n = 207) cohorts were substantially different with regard to their baseline characteristics, reflecting changes in patient selection criteria and comorbidity profiles of the target population over time. The CONV cohort was older, had a lower proportion of women, higher peripheral artery disease and an overall higher EuroSCORE II (predicted rate of mortality). Furthermore, the rates of bicuspid aortic valve disease and arterial and pulmonary hypertension were higher in the FT group. Left ventricular ejection fraction ≥50%, the rate of chronic obstructive pulmonary disease, mid-ascending aortic diameter, and the distribution of severe aortic stenosis and regurgitation did not show significant differences between the unmatched groups.
A total of 189 patients from each cohort were matched 1:1. Variables included in the propensity score model were EuroSCORE II, arterial hypertension, pulmonary hypertension, sex, diabetes, chronic obstructive pulmonary disease, peripheral arterial disease, left ventricular ejection fraction ≥50%, body mass index, age, preoperative glomerular filtration rate, severe aortic regurgitation, bicuspid valve and time on cardiopulmonary bypass. Distribution of propensity scores in the unmatched cohorts is illustrated in Supplementary Material, Fig. S2.
The resulting cohorts had balanced preoperative characteristics with no statistically significant differences between groups. After matching, all standardized mean differences were <0.1. Baseline characteristics before and after matching are displayed in Table 3.
Baseline characteristics and additional matching variables of the unmatched (n = 772) and matched (n = 378) cohorts
| . | Unmatched . | Matched . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Total, n = 772 . | CONV, n = 565 . | FT, n = 207 . | P-Value . | SMD . | Total, n = 378 . | CONV, n = 189 . | FT, n = 189 . | P-Value . | SMD . |
| Agea (years) | 59.91 (12.2) | 60.48 (11.8) | 58.34 (13) | 0.04 | 0.173 | 58.31 (12.9) | 57.5 (12.5) | 58.6 (12.8) | 0.4 | 0.093 |
| Male sexa | 602 (78.0) | 427 (75.6) | 175 (84.5) | 0.01 | 0.226 | 294 (82.6) | 156 (82.5) | 159 (84.1) | 0.8 | 0.043 |
| Art. hypertensiona | 588 (76.2) | 415 (73.5) | 173 (83.6) | 0.005 | 0.248 | 307 (81.2) | 152 (80.4) | 155 (82) | 0.9 | 0.041 |
| Pulm. hypertensiona | 86 (11.1) | 39 (6.9) | 47 (22.7) | <0.001 | 0.456 | 56 (14.8) | 25 (13.2) | 31 (16.4) | 0.5 | 0.089 |
| Bicuspid aortic valvea | 353 (45.7) | 223 (39.5) | 130 (62.8) | <0.001 | 0.48 | 228 (60.3) | 113 (59.8) | 115 (60.8) | 0.9 | 0.022 |
| Severe AS | 366 (47.4) | 274 (48.5) | 92 (44.4) | 0.4 | 0.081 | 174 (46) | 89 (47.1) | 85 (45) | 0.8 | 0.042 |
| Severe ARa | 452 (58.5) | 331 (59.1) | 120 (58.3) | 0.9 | 0.012 | 222 (58.7) | 114 (60.3) | 108 (57.1) | 0.8 | 0.065 |
| Diabetesa | 85 (11.0) | 70 (12.4) | 15 (7.2) | 0.058 | 0.173 | 24 (6.3) | 10 (5.3) | 14 (7.4) | 0.4 | 0.087 |
| COPDa | 28 (3.6) | 25 (4.4) | 3 (1.4) | 0.08 | 0.177 | 4 (1.1) | 1 (0.5) | 3 (1.6) | 0.6 | 0.096 |
| PAODa | 446 (57.8) | 348 (61.6) | 98 (47.3) | 0.001 | 0.289 | 189 (50) | 95 (50.3) | 94 (49.7) | 1 | 0.011 |
| LVEF ≥50a | 578 (74.9) | 408 (79.7) | 170 (83.3) | 0.3 | 0.238 | 308 (81.5) | 155 (82) | 153 (81) | 1 | 0.027 |
| BMI (kg/m2)a | 27.81 (4.6) | 27.82 (4.3) | 27.77 (4.8) | 0.9 | 0.01 | 27.72 (4.4) | 27.7 (4.7) | 27.8 (3.9) | 0.8 | 0.023 |
| EuroSCORE II (%)a | 4.91 (2) | 5.07 (2) | 4.47 (1.8) | 0.06 | 0.313 | 4.48 (1.8) | 4.40 (1.9) | 4.57 (1.8) | 0.4 | 0.089 |
| Time on CPB (min)b | 111 (93–133) | 114 (95–136) | 106 (88–126) | 0.9 | 0.352 | 107 (92–127.8) | 107 (92–127) | 107 (92–129) | 0.9 | 0.016 |
| . | Unmatched . | Matched . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Total, n = 772 . | CONV, n = 565 . | FT, n = 207 . | P-Value . | SMD . | Total, n = 378 . | CONV, n = 189 . | FT, n = 189 . | P-Value . | SMD . |
| Agea (years) | 59.91 (12.2) | 60.48 (11.8) | 58.34 (13) | 0.04 | 0.173 | 58.31 (12.9) | 57.5 (12.5) | 58.6 (12.8) | 0.4 | 0.093 |
| Male sexa | 602 (78.0) | 427 (75.6) | 175 (84.5) | 0.01 | 0.226 | 294 (82.6) | 156 (82.5) | 159 (84.1) | 0.8 | 0.043 |
| Art. hypertensiona | 588 (76.2) | 415 (73.5) | 173 (83.6) | 0.005 | 0.248 | 307 (81.2) | 152 (80.4) | 155 (82) | 0.9 | 0.041 |
| Pulm. hypertensiona | 86 (11.1) | 39 (6.9) | 47 (22.7) | <0.001 | 0.456 | 56 (14.8) | 25 (13.2) | 31 (16.4) | 0.5 | 0.089 |
| Bicuspid aortic valvea | 353 (45.7) | 223 (39.5) | 130 (62.8) | <0.001 | 0.48 | 228 (60.3) | 113 (59.8) | 115 (60.8) | 0.9 | 0.022 |
| Severe AS | 366 (47.4) | 274 (48.5) | 92 (44.4) | 0.4 | 0.081 | 174 (46) | 89 (47.1) | 85 (45) | 0.8 | 0.042 |
| Severe ARa | 452 (58.5) | 331 (59.1) | 120 (58.3) | 0.9 | 0.012 | 222 (58.7) | 114 (60.3) | 108 (57.1) | 0.8 | 0.065 |
| Diabetesa | 85 (11.0) | 70 (12.4) | 15 (7.2) | 0.058 | 0.173 | 24 (6.3) | 10 (5.3) | 14 (7.4) | 0.4 | 0.087 |
| COPDa | 28 (3.6) | 25 (4.4) | 3 (1.4) | 0.08 | 0.177 | 4 (1.1) | 1 (0.5) | 3 (1.6) | 0.6 | 0.096 |
| PAODa | 446 (57.8) | 348 (61.6) | 98 (47.3) | 0.001 | 0.289 | 189 (50) | 95 (50.3) | 94 (49.7) | 1 | 0.011 |
| LVEF ≥50a | 578 (74.9) | 408 (79.7) | 170 (83.3) | 0.3 | 0.238 | 308 (81.5) | 155 (82) | 153 (81) | 1 | 0.027 |
| BMI (kg/m2)a | 27.81 (4.6) | 27.82 (4.3) | 27.77 (4.8) | 0.9 | 0.01 | 27.72 (4.4) | 27.7 (4.7) | 27.8 (3.9) | 0.8 | 0.023 |
| EuroSCORE II (%)a | 4.91 (2) | 5.07 (2) | 4.47 (1.8) | 0.06 | 0.313 | 4.48 (1.8) | 4.40 (1.9) | 4.57 (1.8) | 0.4 | 0.089 |
| Time on CPB (min)b | 111 (93–133) | 114 (95–136) | 106 (88–126) | 0.9 | 0.352 | 107 (92–127.8) | 107 (92–127) | 107 (92–129) | 0.9 | 0.016 |
Unmatched data: continuous variables presented as mean (standard deviation) or median (interquartile range) according to the distribution, compared by Student’s t-test or Wilcoxon rank-sum test; categorical variables reported as numbers (percentages), compared by Pearson’s chi-squared test with continuity correction. Matched comparisons conducted using paired t-test for continuous and McNemar’s test for categorical variables. P < 0.05 indicates statistical significance.
Variables included in the propensity score model.
AR: aortic regurgitation; AS: aortic stenosis; BMI: body mass index; CONV: conventional treatment group; COPD: chronic obstructive pulmonary disease; FT: fast-track treatment group; LVEF: left ventricular ejection fraction; PAOD: peripheral arterial occlusive disease; SMD: standardized mean difference.
Baseline characteristics and additional matching variables of the unmatched (n = 772) and matched (n = 378) cohorts
| . | Unmatched . | Matched . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Total, n = 772 . | CONV, n = 565 . | FT, n = 207 . | P-Value . | SMD . | Total, n = 378 . | CONV, n = 189 . | FT, n = 189 . | P-Value . | SMD . |
| Agea (years) | 59.91 (12.2) | 60.48 (11.8) | 58.34 (13) | 0.04 | 0.173 | 58.31 (12.9) | 57.5 (12.5) | 58.6 (12.8) | 0.4 | 0.093 |
| Male sexa | 602 (78.0) | 427 (75.6) | 175 (84.5) | 0.01 | 0.226 | 294 (82.6) | 156 (82.5) | 159 (84.1) | 0.8 | 0.043 |
| Art. hypertensiona | 588 (76.2) | 415 (73.5) | 173 (83.6) | 0.005 | 0.248 | 307 (81.2) | 152 (80.4) | 155 (82) | 0.9 | 0.041 |
| Pulm. hypertensiona | 86 (11.1) | 39 (6.9) | 47 (22.7) | <0.001 | 0.456 | 56 (14.8) | 25 (13.2) | 31 (16.4) | 0.5 | 0.089 |
| Bicuspid aortic valvea | 353 (45.7) | 223 (39.5) | 130 (62.8) | <0.001 | 0.48 | 228 (60.3) | 113 (59.8) | 115 (60.8) | 0.9 | 0.022 |
| Severe AS | 366 (47.4) | 274 (48.5) | 92 (44.4) | 0.4 | 0.081 | 174 (46) | 89 (47.1) | 85 (45) | 0.8 | 0.042 |
| Severe ARa | 452 (58.5) | 331 (59.1) | 120 (58.3) | 0.9 | 0.012 | 222 (58.7) | 114 (60.3) | 108 (57.1) | 0.8 | 0.065 |
| Diabetesa | 85 (11.0) | 70 (12.4) | 15 (7.2) | 0.058 | 0.173 | 24 (6.3) | 10 (5.3) | 14 (7.4) | 0.4 | 0.087 |
| COPDa | 28 (3.6) | 25 (4.4) | 3 (1.4) | 0.08 | 0.177 | 4 (1.1) | 1 (0.5) | 3 (1.6) | 0.6 | 0.096 |
| PAODa | 446 (57.8) | 348 (61.6) | 98 (47.3) | 0.001 | 0.289 | 189 (50) | 95 (50.3) | 94 (49.7) | 1 | 0.011 |
| LVEF ≥50a | 578 (74.9) | 408 (79.7) | 170 (83.3) | 0.3 | 0.238 | 308 (81.5) | 155 (82) | 153 (81) | 1 | 0.027 |
| BMI (kg/m2)a | 27.81 (4.6) | 27.82 (4.3) | 27.77 (4.8) | 0.9 | 0.01 | 27.72 (4.4) | 27.7 (4.7) | 27.8 (3.9) | 0.8 | 0.023 |
| EuroSCORE II (%)a | 4.91 (2) | 5.07 (2) | 4.47 (1.8) | 0.06 | 0.313 | 4.48 (1.8) | 4.40 (1.9) | 4.57 (1.8) | 0.4 | 0.089 |
| Time on CPB (min)b | 111 (93–133) | 114 (95–136) | 106 (88–126) | 0.9 | 0.352 | 107 (92–127.8) | 107 (92–127) | 107 (92–129) | 0.9 | 0.016 |
| . | Unmatched . | Matched . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Total, n = 772 . | CONV, n = 565 . | FT, n = 207 . | P-Value . | SMD . | Total, n = 378 . | CONV, n = 189 . | FT, n = 189 . | P-Value . | SMD . |
| Agea (years) | 59.91 (12.2) | 60.48 (11.8) | 58.34 (13) | 0.04 | 0.173 | 58.31 (12.9) | 57.5 (12.5) | 58.6 (12.8) | 0.4 | 0.093 |
| Male sexa | 602 (78.0) | 427 (75.6) | 175 (84.5) | 0.01 | 0.226 | 294 (82.6) | 156 (82.5) | 159 (84.1) | 0.8 | 0.043 |
| Art. hypertensiona | 588 (76.2) | 415 (73.5) | 173 (83.6) | 0.005 | 0.248 | 307 (81.2) | 152 (80.4) | 155 (82) | 0.9 | 0.041 |
| Pulm. hypertensiona | 86 (11.1) | 39 (6.9) | 47 (22.7) | <0.001 | 0.456 | 56 (14.8) | 25 (13.2) | 31 (16.4) | 0.5 | 0.089 |
| Bicuspid aortic valvea | 353 (45.7) | 223 (39.5) | 130 (62.8) | <0.001 | 0.48 | 228 (60.3) | 113 (59.8) | 115 (60.8) | 0.9 | 0.022 |
| Severe AS | 366 (47.4) | 274 (48.5) | 92 (44.4) | 0.4 | 0.081 | 174 (46) | 89 (47.1) | 85 (45) | 0.8 | 0.042 |
| Severe ARa | 452 (58.5) | 331 (59.1) | 120 (58.3) | 0.9 | 0.012 | 222 (58.7) | 114 (60.3) | 108 (57.1) | 0.8 | 0.065 |
| Diabetesa | 85 (11.0) | 70 (12.4) | 15 (7.2) | 0.058 | 0.173 | 24 (6.3) | 10 (5.3) | 14 (7.4) | 0.4 | 0.087 |
| COPDa | 28 (3.6) | 25 (4.4) | 3 (1.4) | 0.08 | 0.177 | 4 (1.1) | 1 (0.5) | 3 (1.6) | 0.6 | 0.096 |
| PAODa | 446 (57.8) | 348 (61.6) | 98 (47.3) | 0.001 | 0.289 | 189 (50) | 95 (50.3) | 94 (49.7) | 1 | 0.011 |
| LVEF ≥50a | 578 (74.9) | 408 (79.7) | 170 (83.3) | 0.3 | 0.238 | 308 (81.5) | 155 (82) | 153 (81) | 1 | 0.027 |
| BMI (kg/m2)a | 27.81 (4.6) | 27.82 (4.3) | 27.77 (4.8) | 0.9 | 0.01 | 27.72 (4.4) | 27.7 (4.7) | 27.8 (3.9) | 0.8 | 0.023 |
| EuroSCORE II (%)a | 4.91 (2) | 5.07 (2) | 4.47 (1.8) | 0.06 | 0.313 | 4.48 (1.8) | 4.40 (1.9) | 4.57 (1.8) | 0.4 | 0.089 |
| Time on CPB (min)b | 111 (93–133) | 114 (95–136) | 106 (88–126) | 0.9 | 0.352 | 107 (92–127.8) | 107 (92–127) | 107 (92–129) | 0.9 | 0.016 |
Unmatched data: continuous variables presented as mean (standard deviation) or median (interquartile range) according to the distribution, compared by Student’s t-test or Wilcoxon rank-sum test; categorical variables reported as numbers (percentages), compared by Pearson’s chi-squared test with continuity correction. Matched comparisons conducted using paired t-test for continuous and McNemar’s test for categorical variables. P < 0.05 indicates statistical significance.
Variables included in the propensity score model.
AR: aortic regurgitation; AS: aortic stenosis; BMI: body mass index; CONV: conventional treatment group; COPD: chronic obstructive pulmonary disease; FT: fast-track treatment group; LVEF: left ventricular ejection fraction; PAOD: peripheral arterial occlusive disease; SMD: standardized mean difference.
Intraoperative data
No significant differences were detected in CONV versus FT patients regarding operation or aortic cross-clamp times. CPB time was used as a matching variable and was therefore comparable between the 2 groups. There was no significant difference in the use of biological and mechanical valve prostheses; detailed information on the implanted valve models is given in Table 4. Surgery via partial sternotomy, as well as concomitant septal myectomy and pulmonary vein ablation were performed in similar proportions of patients in each group. Intraoperative outcomes of the matched groups are summarized in Table 5.
| . | CONV, n = 189 . | FT, n = 189 . | P-Value . |
|---|---|---|---|
| Biological prostheses, n (%) | 106 (56.1) | 134 (70.9) | 0.08 |
| Biological valved grafts, n (%) | |||
| Medtronic Freestyle Aortic Root Model 995 | 44 (23.2) | 39 (20.6) | |
| Vascutek BioValsalva Conduit | 2 (1.1) | 9 (4.8) | |
| SJM Toronto Root | 26 (13.8) | 3 (1.6) | |
| Biological prostheses used for ‘tailor-made‘ grafts, n (%) | |||
| CE Perimount Aortic Model 2900 | 28 (14.8) | 72 (38.1) | |
| CE Perimount Magna Ease Aortic Model 3300TFX | 4 (2.1) | 9 (4.8) | |
| Medtronic Mosaic Aortic Model 305 | 2 (1.1) | 2 (1.1) | |
| Mechanical prostheses, n (%) | 83 (43.9) | 55 (29.1) | 0.08 |
| Mechanical valved grafts, n (%) | |||
| ATS Aortic Valved Graft Model 502AG | 64 (33.9) | 46 (24.3) | |
| SJM Aortic Valved Graft Model CAVGJ-514 | 18 (9.5) | 9 (4.8) | |
| ATS Aortic Model 500FA Open Pivot as ‘tailor made’ graft, n (%) | 1 (0.5) | 0 (0) | |
| . | CONV, n = 189 . | FT, n = 189 . | P-Value . |
|---|---|---|---|
| Biological prostheses, n (%) | 106 (56.1) | 134 (70.9) | 0.08 |
| Biological valved grafts, n (%) | |||
| Medtronic Freestyle Aortic Root Model 995 | 44 (23.2) | 39 (20.6) | |
| Vascutek BioValsalva Conduit | 2 (1.1) | 9 (4.8) | |
| SJM Toronto Root | 26 (13.8) | 3 (1.6) | |
| Biological prostheses used for ‘tailor-made‘ grafts, n (%) | |||
| CE Perimount Aortic Model 2900 | 28 (14.8) | 72 (38.1) | |
| CE Perimount Magna Ease Aortic Model 3300TFX | 4 (2.1) | 9 (4.8) | |
| Medtronic Mosaic Aortic Model 305 | 2 (1.1) | 2 (1.1) | |
| Mechanical prostheses, n (%) | 83 (43.9) | 55 (29.1) | 0.08 |
| Mechanical valved grafts, n (%) | |||
| ATS Aortic Valved Graft Model 502AG | 64 (33.9) | 46 (24.3) | |
| SJM Aortic Valved Graft Model CAVGJ-514 | 18 (9.5) | 9 (4.8) | |
| ATS Aortic Model 500FA Open Pivot as ‘tailor made’ graft, n (%) | 1 (0.5) | 0 (0) | |
Categorical data: compared by McNemar’s chi-squared test with continuity correction; reported as numbers (percentages). P < 0.05 indicates statistical significance.
CONV: conventional treatment group; FT: fast-track treatment group.
| . | CONV, n = 189 . | FT, n = 189 . | P-Value . |
|---|---|---|---|
| Biological prostheses, n (%) | 106 (56.1) | 134 (70.9) | 0.08 |
| Biological valved grafts, n (%) | |||
| Medtronic Freestyle Aortic Root Model 995 | 44 (23.2) | 39 (20.6) | |
| Vascutek BioValsalva Conduit | 2 (1.1) | 9 (4.8) | |
| SJM Toronto Root | 26 (13.8) | 3 (1.6) | |
| Biological prostheses used for ‘tailor-made‘ grafts, n (%) | |||
| CE Perimount Aortic Model 2900 | 28 (14.8) | 72 (38.1) | |
| CE Perimount Magna Ease Aortic Model 3300TFX | 4 (2.1) | 9 (4.8) | |
| Medtronic Mosaic Aortic Model 305 | 2 (1.1) | 2 (1.1) | |
| Mechanical prostheses, n (%) | 83 (43.9) | 55 (29.1) | 0.08 |
| Mechanical valved grafts, n (%) | |||
| ATS Aortic Valved Graft Model 502AG | 64 (33.9) | 46 (24.3) | |
| SJM Aortic Valved Graft Model CAVGJ-514 | 18 (9.5) | 9 (4.8) | |
| ATS Aortic Model 500FA Open Pivot as ‘tailor made’ graft, n (%) | 1 (0.5) | 0 (0) | |
| . | CONV, n = 189 . | FT, n = 189 . | P-Value . |
|---|---|---|---|
| Biological prostheses, n (%) | 106 (56.1) | 134 (70.9) | 0.08 |
| Biological valved grafts, n (%) | |||
| Medtronic Freestyle Aortic Root Model 995 | 44 (23.2) | 39 (20.6) | |
| Vascutek BioValsalva Conduit | 2 (1.1) | 9 (4.8) | |
| SJM Toronto Root | 26 (13.8) | 3 (1.6) | |
| Biological prostheses used for ‘tailor-made‘ grafts, n (%) | |||
| CE Perimount Aortic Model 2900 | 28 (14.8) | 72 (38.1) | |
| CE Perimount Magna Ease Aortic Model 3300TFX | 4 (2.1) | 9 (4.8) | |
| Medtronic Mosaic Aortic Model 305 | 2 (1.1) | 2 (1.1) | |
| Mechanical prostheses, n (%) | 83 (43.9) | 55 (29.1) | 0.08 |
| Mechanical valved grafts, n (%) | |||
| ATS Aortic Valved Graft Model 502AG | 64 (33.9) | 46 (24.3) | |
| SJM Aortic Valved Graft Model CAVGJ-514 | 18 (9.5) | 9 (4.8) | |
| ATS Aortic Model 500FA Open Pivot as ‘tailor made’ graft, n (%) | 1 (0.5) | 0 (0) | |
Categorical data: compared by McNemar’s chi-squared test with continuity correction; reported as numbers (percentages). P < 0.05 indicates statistical significance.
CONV: conventional treatment group; FT: fast-track treatment group.
| . | CONV, n = 189 . | FT, n = 189 . | P-Value . |
|---|---|---|---|
| Operation time (min), median (IQR) | 188 (160–210) | 185 (160–221) | 0.8 |
| Bypass time (min), median (IQR) | 107 (92–127) | 107 (91–129) | 0.9 |
| X-clamp time (min), median (IQR) | 80 (69–94) | 86 (72–100) | 0.06 |
| Biological valve/conduit, n (%) | 106 (56.1) | 134 (70.9) | 0.08 |
| Morrow resection, n (%) | 9 (4.8) | 8 (9) | 1 |
| Ablation, n (%) | 9 (4.8) | 18 (9.6) | 0.1 |
| Partial sternotomy, n (%) | 20 (10.6) | 19 (10.1) | 1 |
| . | CONV, n = 189 . | FT, n = 189 . | P-Value . |
|---|---|---|---|
| Operation time (min), median (IQR) | 188 (160–210) | 185 (160–221) | 0.8 |
| Bypass time (min), median (IQR) | 107 (92–127) | 107 (91–129) | 0.9 |
| X-clamp time (min), median (IQR) | 80 (69–94) | 86 (72–100) | 0.06 |
| Biological valve/conduit, n (%) | 106 (56.1) | 134 (70.9) | 0.08 |
| Morrow resection, n (%) | 9 (4.8) | 8 (9) | 1 |
| Ablation, n (%) | 9 (4.8) | 18 (9.6) | 0.1 |
| Partial sternotomy, n (%) | 20 (10.6) | 19 (10.1) | 1 |
Continuous data: compared by Wilcoxon signed rank test with continuity correction; presented as median (interquartile range). Categorical data: compared by McNemar’s chi-squared test with continuity correction; reported as numbers (percentages). P < 0.05 indicates statistical significance.
CONV: conventional treatment group; FT: fast-track treatment group; IQR: interquartile range; X-clamp: aortic cross-clamp.
| . | CONV, n = 189 . | FT, n = 189 . | P-Value . |
|---|---|---|---|
| Operation time (min), median (IQR) | 188 (160–210) | 185 (160–221) | 0.8 |
| Bypass time (min), median (IQR) | 107 (92–127) | 107 (91–129) | 0.9 |
| X-clamp time (min), median (IQR) | 80 (69–94) | 86 (72–100) | 0.06 |
| Biological valve/conduit, n (%) | 106 (56.1) | 134 (70.9) | 0.08 |
| Morrow resection, n (%) | 9 (4.8) | 8 (9) | 1 |
| Ablation, n (%) | 9 (4.8) | 18 (9.6) | 0.1 |
| Partial sternotomy, n (%) | 20 (10.6) | 19 (10.1) | 1 |
| . | CONV, n = 189 . | FT, n = 189 . | P-Value . |
|---|---|---|---|
| Operation time (min), median (IQR) | 188 (160–210) | 185 (160–221) | 0.8 |
| Bypass time (min), median (IQR) | 107 (92–127) | 107 (91–129) | 0.9 |
| X-clamp time (min), median (IQR) | 80 (69–94) | 86 (72–100) | 0.06 |
| Biological valve/conduit, n (%) | 106 (56.1) | 134 (70.9) | 0.08 |
| Morrow resection, n (%) | 9 (4.8) | 8 (9) | 1 |
| Ablation, n (%) | 9 (4.8) | 18 (9.6) | 0.1 |
| Partial sternotomy, n (%) | 20 (10.6) | 19 (10.1) | 1 |
Continuous data: compared by Wilcoxon signed rank test with continuity correction; presented as median (interquartile range). Categorical data: compared by McNemar’s chi-squared test with continuity correction; reported as numbers (percentages). P < 0.05 indicates statistical significance.
CONV: conventional treatment group; FT: fast-track treatment group; IQR: interquartile range; X-clamp: aortic cross-clamp.
Postoperative outcomes
Univariate analysis revealed substantial differences between the 2 groups with regard to postoperative outcomes. PACU/ICU length of stay was significantly shorter in the FT than in the CONV group (6.7 vs 21.7 h, P < 0.001), with no significant difference in the IMCU length of stay (26.5 vs 33.1 h, P = 0.2). Postoperative ventilation time was reduced significantly in the fast-tracked patients (CONV: 517.1 versus FT: 165.5 min, P < 0.001). No significant differences were detected regarding respiratory failures requiring reintubation, stroke, renal failure requiring dialysis, revisions for bleeding or other postoperative complications. Hospital length of stay showed no significant difference between the 2 cohorts. Detailed information on postoperative outcomes is displayed in Table 6.
| . | CONV, n = 189 . | FT, n = 189 . | OR . | 95% CI . | P-Value . |
|---|---|---|---|---|---|
| Postoperative ventilation time (min), median (IQR) | 517.1 (320.2–905) | 165.5 (124.8–290.7) | – | – | <0.001 |
| ICU/PACU stay (h), median (IQR) | 21.7 (16.5–26.3) | 6.7 (33.7–21.6) | – | – | <0.001 |
| IMCU stay (h), median (IQR) | 33.1 (21.7–66.2) | 26.5 (19.9–51.2) | – | – | 0.2 |
| Hospital stay (days), median (IQR) | 11 (8–14) | 10 (8–13) | – | – | 0.3 |
| ICU re-admission, n (%) | 9 (4.8) | 11 (5.8) | 1.2 | 0.5–3.3 | 0.8 |
| IMCU re-admission, n (%) | 31 (16.4) | 17 (9) | 0.55 | 0.3–1.02 | 0.06 |
| Re-intubation, n (%) | 7 (3.7) | 1 (0.4) | 0.14 | 0.003–1.1 | 0.07 |
| Revision for bleeding, n (%) | 10 (5.3) | 10 (5.3) | 1 | 0.4–2.7 | 1 |
| Number of RBC units transfused, median (IQR) | 0 (0–2) | 0 (0–2) | – | – | 0.3 |
| Stroke, n (%) | 4 (2.1) | 2 (1.1) | 0.5 | 0.05–3.5 | 1 |
| Dialysis, n (%) | 7 (3.7) | 5 (2.6) | 0.7 | 0.2–2.6 | 0.8 |
| Postoperative PCI, n (%) | 0 (0) | 1 (0.5) | – | – | 1 |
| In-house mortality, n (%) | 3 (1.6) | 1 (0.5) | 3 | 0.01–4.2 | 0.6 |
| 30-Day mortality, n (%) | 3 (1.6) | 1 (0.5) | 3 | 0.01–4.2 | 0.6 |
| Follow-up (days), median (IQR) | 1952 (394–3057) | 394 (37–1447) | – | – | <0.001 |
| . | CONV, n = 189 . | FT, n = 189 . | OR . | 95% CI . | P-Value . |
|---|---|---|---|---|---|
| Postoperative ventilation time (min), median (IQR) | 517.1 (320.2–905) | 165.5 (124.8–290.7) | – | – | <0.001 |
| ICU/PACU stay (h), median (IQR) | 21.7 (16.5–26.3) | 6.7 (33.7–21.6) | – | – | <0.001 |
| IMCU stay (h), median (IQR) | 33.1 (21.7–66.2) | 26.5 (19.9–51.2) | – | – | 0.2 |
| Hospital stay (days), median (IQR) | 11 (8–14) | 10 (8–13) | – | – | 0.3 |
| ICU re-admission, n (%) | 9 (4.8) | 11 (5.8) | 1.2 | 0.5–3.3 | 0.8 |
| IMCU re-admission, n (%) | 31 (16.4) | 17 (9) | 0.55 | 0.3–1.02 | 0.06 |
| Re-intubation, n (%) | 7 (3.7) | 1 (0.4) | 0.14 | 0.003–1.1 | 0.07 |
| Revision for bleeding, n (%) | 10 (5.3) | 10 (5.3) | 1 | 0.4–2.7 | 1 |
| Number of RBC units transfused, median (IQR) | 0 (0–2) | 0 (0–2) | – | – | 0.3 |
| Stroke, n (%) | 4 (2.1) | 2 (1.1) | 0.5 | 0.05–3.5 | 1 |
| Dialysis, n (%) | 7 (3.7) | 5 (2.6) | 0.7 | 0.2–2.6 | 0.8 |
| Postoperative PCI, n (%) | 0 (0) | 1 (0.5) | – | – | 1 |
| In-house mortality, n (%) | 3 (1.6) | 1 (0.5) | 3 | 0.01–4.2 | 0.6 |
| 30-Day mortality, n (%) | 3 (1.6) | 1 (0.5) | 3 | 0.01–4.2 | 0.6 |
| Follow-up (days), median (IQR) | 1952 (394–3057) | 394 (37–1447) | – | – | <0.001 |
Continuous data: compared by Wilcoxon signed rank test; presented as median (interquartile range). Categorical data: compared by exact McNemar’s chi-squared test with continuity correction; reported as numbers (percentages). P < 0.05 indicates statistical significance.
CI: confidence interval; CONV: conventional treatment group; FT: fast-track treatment group; ICU: intensive care unit; IMCU: intermediate care unit; IQR: interquartile range; OR: odds ratio; PACU: postanaesthesia care unit; PCI: percutaneous coronary intervention; RBC: red blood cells.
| . | CONV, n = 189 . | FT, n = 189 . | OR . | 95% CI . | P-Value . |
|---|---|---|---|---|---|
| Postoperative ventilation time (min), median (IQR) | 517.1 (320.2–905) | 165.5 (124.8–290.7) | – | – | <0.001 |
| ICU/PACU stay (h), median (IQR) | 21.7 (16.5–26.3) | 6.7 (33.7–21.6) | – | – | <0.001 |
| IMCU stay (h), median (IQR) | 33.1 (21.7–66.2) | 26.5 (19.9–51.2) | – | – | 0.2 |
| Hospital stay (days), median (IQR) | 11 (8–14) | 10 (8–13) | – | – | 0.3 |
| ICU re-admission, n (%) | 9 (4.8) | 11 (5.8) | 1.2 | 0.5–3.3 | 0.8 |
| IMCU re-admission, n (%) | 31 (16.4) | 17 (9) | 0.55 | 0.3–1.02 | 0.06 |
| Re-intubation, n (%) | 7 (3.7) | 1 (0.4) | 0.14 | 0.003–1.1 | 0.07 |
| Revision for bleeding, n (%) | 10 (5.3) | 10 (5.3) | 1 | 0.4–2.7 | 1 |
| Number of RBC units transfused, median (IQR) | 0 (0–2) | 0 (0–2) | – | – | 0.3 |
| Stroke, n (%) | 4 (2.1) | 2 (1.1) | 0.5 | 0.05–3.5 | 1 |
| Dialysis, n (%) | 7 (3.7) | 5 (2.6) | 0.7 | 0.2–2.6 | 0.8 |
| Postoperative PCI, n (%) | 0 (0) | 1 (0.5) | – | – | 1 |
| In-house mortality, n (%) | 3 (1.6) | 1 (0.5) | 3 | 0.01–4.2 | 0.6 |
| 30-Day mortality, n (%) | 3 (1.6) | 1 (0.5) | 3 | 0.01–4.2 | 0.6 |
| Follow-up (days), median (IQR) | 1952 (394–3057) | 394 (37–1447) | – | – | <0.001 |
| . | CONV, n = 189 . | FT, n = 189 . | OR . | 95% CI . | P-Value . |
|---|---|---|---|---|---|
| Postoperative ventilation time (min), median (IQR) | 517.1 (320.2–905) | 165.5 (124.8–290.7) | – | – | <0.001 |
| ICU/PACU stay (h), median (IQR) | 21.7 (16.5–26.3) | 6.7 (33.7–21.6) | – | – | <0.001 |
| IMCU stay (h), median (IQR) | 33.1 (21.7–66.2) | 26.5 (19.9–51.2) | – | – | 0.2 |
| Hospital stay (days), median (IQR) | 11 (8–14) | 10 (8–13) | – | – | 0.3 |
| ICU re-admission, n (%) | 9 (4.8) | 11 (5.8) | 1.2 | 0.5–3.3 | 0.8 |
| IMCU re-admission, n (%) | 31 (16.4) | 17 (9) | 0.55 | 0.3–1.02 | 0.06 |
| Re-intubation, n (%) | 7 (3.7) | 1 (0.4) | 0.14 | 0.003–1.1 | 0.07 |
| Revision for bleeding, n (%) | 10 (5.3) | 10 (5.3) | 1 | 0.4–2.7 | 1 |
| Number of RBC units transfused, median (IQR) | 0 (0–2) | 0 (0–2) | – | – | 0.3 |
| Stroke, n (%) | 4 (2.1) | 2 (1.1) | 0.5 | 0.05–3.5 | 1 |
| Dialysis, n (%) | 7 (3.7) | 5 (2.6) | 0.7 | 0.2–2.6 | 0.8 |
| Postoperative PCI, n (%) | 0 (0) | 1 (0.5) | – | – | 1 |
| In-house mortality, n (%) | 3 (1.6) | 1 (0.5) | 3 | 0.01–4.2 | 0.6 |
| 30-Day mortality, n (%) | 3 (1.6) | 1 (0.5) | 3 | 0.01–4.2 | 0.6 |
| Follow-up (days), median (IQR) | 1952 (394–3057) | 394 (37–1447) | – | – | <0.001 |
Continuous data: compared by Wilcoxon signed rank test; presented as median (interquartile range). Categorical data: compared by exact McNemar’s chi-squared test with continuity correction; reported as numbers (percentages). P < 0.05 indicates statistical significance.
CI: confidence interval; CONV: conventional treatment group; FT: fast-track treatment group; ICU: intensive care unit; IMCU: intermediate care unit; IQR: interquartile range; OR: odds ratio; PACU: postanaesthesia care unit; PCI: percutaneous coronary intervention; RBC: red blood cells.
Survival
There were no differences in in-house mortality (CONV: 1.6% vs FT: 0.5%, P = 0.6) or 30-day mortality (CONV: 1.6% vs FT: 0.5%, P = 0.6) between groups. Cox-proportional hazards analysis revealed no association between perioperative FT management and overall long-term survival. The only variable showing predictive effect for long-term survival was age (Table 7).
| . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P-Value . | HR . | 95% CI . | P-Value . |
| Fast-track management | 0.59 | 0.23–1.5 | 0.3 | 0.57 | 0.22–1.51 | 0.3 |
| Age | 1.09 | 1.04–1.14 | <0.001 | 1.09 | 1.04–1.14 | <0.001 |
| Female sex | 1.29 | 0.52–3.2 | 0.6 | 1.04 | 0.43–2.5 | 0.9 |
| . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P-Value . | HR . | 95% CI . | P-Value . |
| Fast-track management | 0.59 | 0.23–1.5 | 0.3 | 0.57 | 0.22–1.51 | 0.3 |
| Age | 1.09 | 1.04–1.14 | <0.001 | 1.09 | 1.04–1.14 | <0.001 |
| Female sex | 1.29 | 0.52–3.2 | 0.6 | 1.04 | 0.43–2.5 | 0.9 |
Cox-proportional hazards model with adjusted standard errors using generalized estimating equations. Univariable and multivariable effect sizes are reported as hazard ratios. Bold P-values are <0.05, indicating statistical significance.
CI: confidence interval; HR: hazards ratio.
| . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P-Value . | HR . | 95% CI . | P-Value . |
| Fast-track management | 0.59 | 0.23–1.5 | 0.3 | 0.57 | 0.22–1.51 | 0.3 |
| Age | 1.09 | 1.04–1.14 | <0.001 | 1.09 | 1.04–1.14 | <0.001 |
| Female sex | 1.29 | 0.52–3.2 | 0.6 | 1.04 | 0.43–2.5 | 0.9 |
| . | Univariable analysis . | Multivariable analysis . | ||||
|---|---|---|---|---|---|---|
| . | HR . | 95% CI . | P-Value . | HR . | 95% CI . | P-Value . |
| Fast-track management | 0.59 | 0.23–1.5 | 0.3 | 0.57 | 0.22–1.51 | 0.3 |
| Age | 1.09 | 1.04–1.14 | <0.001 | 1.09 | 1.04–1.14 | <0.001 |
| Female sex | 1.29 | 0.52–3.2 | 0.6 | 1.04 | 0.43–2.5 | 0.9 |
Cox-proportional hazards model with adjusted standard errors using generalized estimating equations. Univariable and multivariable effect sizes are reported as hazard ratios. Bold P-values are <0.05, indicating statistical significance.
CI: confidence interval; HR: hazards ratio.
DISCUSSION
This retrospective single-centre study included 772 patients with complex aortic root surgery and demonstrated shorter ventilation times and ICU stay in patients with perioperative FT management compared to conventional anaesthesiologic management. Operative and in-house mortality were the same and rate of reintubation was not different between the groups, supporting FT management as non-inferiority can be concluded from our data.
Improved postoperative outcomes, particularly a reduction of early mortality, have been found to be associated with growing institutional experience [13]. The Bentall operation is the gold standard for patients with proximal aortic root disease and non-pliable aortic valve cusps and may serve as a standard benchmark when comparing outcomes between studies and institutions [14]. A large contemporary multicentre study on 954 patients revealed a 30-day mortality rate of 3.6% for elective Bentall operations [15]. Etz et al. [14] published one of the largest single-centre analysis in 2010, presenting outcomes of 597 consecutive patients with an overall in-hospital mortality rate of 3.9% and elective 30-day mortality rates of 3.7% vs 1.4% following biological versus mechanical Bentall, respectively. Comparing these more recent findings to earlier published data underlines the role of evolving surgical techniques and perioperative care, as former studies had found early mortality rates of 6.9% [16] to 11.8% [17].
At our institution, we have a large experience with Bentall surgery, reflected by the size of our current study cohort. A total of 772 elective patients were included in this analysis, while emergencies and those involving major concomitant procedures were excluded. We thereby attempted to minimize possible confounding factors when comparing CONV and FT-managed patients [7]. Propensity score matching was thereafter applied to further reduce selection bias between patient groups. The FT concept was initially applied at our institution in very selected, purely elective patients (as displayed in Supplementary Material, Fig. S1) undergoing rather low-risk operations such as CABG or isolated valve surgery. However, promising outcomes in our initial experience led to a more widespread utilization of the FT concept in order to potentially accelerate recovery in patients undergoing more complex procedures. Nowadays, the selection process has been reverted: only patients expected to have prolonged recovery times (e.g. those with high-risk profile or those undergoing emergency surgery) are not planned for FT management.
In the comparison of non-matched cohorts, FT was younger and had significantly higher rates of arterial and pulmonary hypertension than the CONV group. After propensity matching, we confirmed statistically significant reductions in mechanical ventilation times and PACU/ICU length of stay in FT patients. There were no significant differences between the 2 matched cohorts regarding stroke, or renal failure requiring dialysis. In addition, we observed very low in-house mortality rates in each group, lower than in any of the above-mentioned previous studies.
Previous analyses of FT concepts in cardiac surgery included patients undergoing CABG or valve surgery [18–21], and the current study is the first to examine the FT management of complex aortic surgery patients. Risk of FT failure in patients undergoing complex procedures [22] might have discouraged others from implementing FT management in patients undergoing aortic root surgery. Zakhary et al. have examined risk factors for failure to implement the FT protocol (i.e. unplanned transfer of FT patients from PACU/IMCU to ICU) in another study from our centre, and identified female sex as independent risk factor [2]. Interestingly, in the unmatched cohort of this study, there were significantly less women in the FT group than in the CONV group. This might in addition be related to the fact that women present for surgery at a more advanced age, and age-associated comorbidities might have favoured a decision for CONV treatment.
Limitations
The current study is a retrospective analysis with all of the inherent limitations thereof. We compared the outcomes between propensity-matched groups to minimize patient selection bias and therefore the detected differences might not apply to patients falling outside the characteristics of this study cohort. Moreover, unmeasured factors, especially undocumented communication between surgeon and anaesthesiologist (Table 2), may have contributed to bias with regard to whether a patient underwent FT or CONV management. As mentioned above, there might be differences between men and women undergoing Bentall surgery with FT versus CONV treatment. Yet, the number of women in the presented cohort was too small to provide a comprehensive subgroup analysis with regard to FT management.
CONCLUSION
In summary, this study demonstrated non-inferiority of a perioperative FT approach in patients undergoing complex aortic root surgery, with no associated increase of perioperative complications and a reduction in ventilation and ICU/PACU times. Besides the protective value of reduced ventilation times, the herein-presented FT protocol might yield economic benefit by bypassing the ICU and keeping patients in the PACU for a shorter length of time.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENT
Antonia van Kampen is supported by the American Heart Association Postdoctoral Fellowship.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Author contributions
Josephina Haunschild: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing—original draft. Antonia van Kampen: Data curation; Formal analysis; Investigation; Methodology; Visualization; Writing—original draft. Martin Misfeld: Methodology; Supervision; Validation; Writing—review & editing. Konstantin von Aspern: Formal analysis; Investigation; Methodology; Writing—review & editing. Jörg Ender: Investigation; Project administration; Supervision; Validation; Writing—review & editing. Waseem Zakhary: Investigation; Supervision; Validation; Writing—review & editing. Michael A. Borger: Resources; Software; Supervision; Writing—review & editing. Christian D. Etz: Conceptualization; Investigation; Supervision; Validation; Writing—review & editing.
Reviewer information:
European Journal of Cardio-Thoracic Surgery thanks Torsten Loop, Giacomo Murana, Gabriele Piffaretti and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- CABG
Coronary artery bypass grafting
- CPB
Cardiopulmonary bypass
- FT
Fast track
- ICU
Intensive care unit
- IMCU
Intermediate care unit
- PACU
Postoperative anaesthetic care unit
Author notes
Josephina Haunschild and Antonia van Kampen contributed equally to this work.





