-
PDF
- Split View
-
Views
-
Cite
Cite
Juan José Menéndez, Amelia Caridad Sánchez-Galindo, Joan Balcells, María Ángeles Tejero-Hernández, Ángela Ferrer-Barba, Emilio Ibiza-Palacios, Constancio Medrano-López, Ferran Gran, Manuel Ángel Frías-Pérez, María García-Vieites, Ana Cano-Sánchez, Luz Polo, Juan-Miguel Gil-Jaurena, Raúl Felipe Abella, Carlos Merino-Cejas, Isaac Martínez-Bendayán, Félix Serrano, Luis García-Guereta, Short- and long-term survival of children treated with ventricular assist devices in Spain, based on 15 years’ experience, European Journal of Cardio-Thoracic Surgery, Volume 63, Issue 2, February 2023, ezad050, https://doi.org/10.1093/ejcts/ezad050
Close - Share Icon Share
Abstract
To describe the use of ventricular assist devices (VAD) in children in Spain and to identify variables related to survival.
This is an observational cohort study of all children younger than 18 years of age who underwent an initial implantation of a VAD at any of the 6 paediatric heart transplant centres from May 2006 to December 2020. Subjects were identified retrospectively from each hospital’s database.
Paracorporeal VADs were implanted in 118 children [pulsatile (63%), continuous (30.5%) or both types (5.9%)]. Small children (<0.7 m2 of body surface area) comprised the majority of this cohort (63.3%). Overall, 67% survived to VAD explantation, and 64.9% survived to hospital discharge. Non-central nervous system haemorrhage (39%) and stroke (38.1%) were the most common complications. Body weight <5 kg, congenital heart disease, pre-implantation bilirubin >34 μmol/l and bridge to decision strategy were associated with a higher mortality at hospital discharge and in the long-term. Interagency registry for mechanically assisted circulatory support (INTERMACS) status 1 and cardiac arrest prior to VAD implantation were related to long-term mortality, whereas pre-implantation renal replacement therapy and extracorporeal membrane oxygenation were not related to mortality.
In Spain, 67% of the VAD-supported children have been bridged to heart transplantation or to recovery. Body weight lower than 5 kg, congenital heart disease diagnosis, cholestatic liver dysfunction, bridge to decision as VAD strategy, INTERMACS-1 status and cardiac arrest were pre-implantation variables related to mortality, whereas pre-implantation renal replacement therapy and extracorporeal membrane oxygenation were not.
INTRODUCTION
Ventricular assist devices (VADs) have become the mainstay of management of end-stage heart failure in children waiting for heart transplant and their use has yielded a significant reduction in the waiting list mortality [1]. Currently, more than a third of paediatric patients are bridged to heart transplantation with VADs. Reported transplant rates after 1 year of support range between 45.1% [2] and 62.1% [3]. The use of VADs can be associated to serious complications [2, 3] but it is not associated with increased mortality after paediatric heart transplantation [4].
Spain has one of the highest organ donor rates in the world [median total heart donation rate: 278 heart donors/year—5.9 heart donors per million population; median paediatric (<16 years) heart donation rate: 21 heart donors/year—2.2 heart donors per million population—period: 2006–2020]. However, the number of paediatric heart donors is still insufficient and even those children listed in the highest priority status usually have to wait longer than 2 months to be transplanted. After an initial experience in 2002 in a single centre [5], the regular use of VADs in Spain began in 2006 and since then, over 40% of children receiving a heart transplant in Spain have reached transplant on VAD support [6].
The aim of this study is to describe the characteristics and outcomes of all children treated with VADs in Spain from 2006 to 2020, and to analyse factors related to short and long-term survival.
PATIENTS AND METHODS
Ethical statement
The ethics committee of Hospital Universitario La Paz (HULP: PI-4717), Hospital General y Universitario Gregorio Marañón (Acta 18/2021), Hospital Universitari Vall d’Hebron (Barcelona) [PR(AMI)409/2021], Hospital Universitario Reina Sofía (Córdoba) (5056), Complexo Hospitalario Universitario A Coruña (A Coruña) (2021/048) and Hospital Universitario y Politécnico La Fe (Valencia), Spain (Acta 503) approved this study. The need to obtain informed consent was waived due to the retrospective analysis of de-identified data.
Study population
This was an observational cohort study performed between May 2006 and December 2020 of all children (<18 years) who underwent an initial implantation of a VAD at any of the 6 Spanish paediatric heart transplant centres. The study population was followed until 31 December 2020. The indication of VAD support, VAD type and type of support was at the discretion of each hospital treating teams. Both paracorporeal pulsatile (PP) (Berlin Heart EXCOR®) and paracorporeal continuous (PC) (CentriMAG™/PediVAS™) flow VADs were implanted. Each centre followed its own antithrombotic protocol. All centres used at least 1 anticoagulant agent (non-fractionated heparin, low-molecular-weight heparin, bivalirudin or coumadin) combined with 1 or more anti-platelet agents (aspirin, dypiridamol, clopidogrel or prostacyclin).
Outcome assessment
Primary outcomes assessed were hospital mortality and long-term mortality. Secondary outcomes were competing outcomes leading to VAD explantation and VAD-related complications. Stroke was defined as any ischaemic or haemorrhagic intracranial event detected, clinically, by neuroimaging or by pathological study. Severe haemorrhage was defined as non-central nervous system bleeding that needed surgical revision or transfusion (>20 ml/kg/24 h of packed red cells).
Statistical analysis
Categorical variables were expressed as counts and/or percentage and compared using Fisher’s exact test. Equality of proportions was tested either by a Chi-squared goodness-of-fit test or a Chi-squared test for trend. Continuous variables were expressed as median and interquartile interval (IQI) unless stated otherwise and compared using Mann–Whitney test. Incidence of adverse events was described as rates of events per 100 patient-months and rate ratios were compared. Variables with more than 10% of missing values were not to be considered for analysis. All statistical comparisons were 2-sided. A P-value of <0.05 was considered statistically significant.
The 3 immediate outcomes of VAD implantation (transplant, death or recovery) were modelled as time-dependent competing events. A multivariable logistic regression model was used to identify variables related to hospital survival. Variables significantly associated with hospital mortality on descriptive analysis were forced into the model. The definitive model was constructed through a backward stepwise process were forced variables were invariably retained and the rest of the variables considered for inclusion were kept if they either had an adjusted P-value <0.1 or had a significant impact (>10% change) on any of the model coefficients (augmented backward elimination) [7]. The Kaplan–Meier method was applied to estimate long-term survival probability and log-rank analysis was performed to compare survival among groups. A Cox proportional model was used to assess the relationship between the variables included in the multivariate logistic regression model and long-term mortality. Listwise deletion was used to build regression models. Results are presented as odds ratios (ORs) or hazard ratios (HRs) and 95% confidence intervals (CIs).
All analyses were performed using R Statistical Software (v4.0.3; R Core Team 2020). Augmented backward elimination was carried out using the abe R package (Rok Blagus (2017). abe: Augmented Backward Elimination. R package version 3.0.1. https://CRAN.R-project.org/package=abe). The cmprsk R package (Bob Gray (2020). cmprsk: Subdistribution Analysis of Competing Risks. R package version 2.2–10. https://CRAN.R-project.org/package=cmprsk) was used to model competing risks. The finalfit package [Ewen Harrison, Tom Drake and Riinu Ots (2022). finalfit: Quickly Create Elegant Regression Results Tables and Plots when Modelling. R package version 1.0.5. https://CRAN.R-project.org/package=finalfit] was used to create OR plots.
RESULTS
Patient population and functional status at implantation
Between May 2006 and December 2020, 118 children were implanted a VAD. Complete data were available for all variables except for 9 cases (7.6%) missing pre-implant bilirubin value. The number of patients treated per 5-year interval increased from 24 (20.3%; 2006–2010) to 55 (46.6%; 2016–2020) (P < 0.001) (Supplementary Material, Fig. S1). Patient demographic and pre-implantation characteristics are summarized in Table 1. Patients with dilated cardiomyopathy represented 51.7% of the study sample, while only 7.6% patients had congenital heart disease (CHD) with single ventricle physiology. Extracorporeal membrane oxygenation (ECMO) was used in 45 patients (38.1%) [duration of support 10 days (6–15)]. Patients supported with ECMO before implantation presented mostly with an interagency registry for mechanically assisted circulatory support (INTERMACS) patient profile 1 [INTERMACS 1, 38 (84.4%), INTERMACS 1-A, 4 (8.9%) and INTERMACS 2, 3 (6.7%)] at the moment of ECMO initiation, while at the moment of VAD implantation the predominant INTERMACS patient profile was 2 (Table 1).
| Characteristic . | Overall (%), n = 118 (100) . | PP (%), n = 75 (63.6) . | PC (%), n = 36 (30.5) . | Mixed (%), n = 7 (5.9) . | P-Valuea . |
|---|---|---|---|---|---|
| Age (years) | 2.3 (0.7–7.7) | 2.2 (0–15.8)b | 2.7 (0.2–15.0)b | 1.7 (0.2–5.8)b | 0.257 |
| Age group | |||||
| <1 year | 37 (31.4) | 24 (32.0) | 10 (27.8) | 3 (42.9) | 0.186 |
| 1–5 years | 46 (39.0) | 31 (41.3) | 11 (30.6) | 4 (57.1) | |
| 6–10 years | 19 (16.1) | 13 (17.3) | 6 (16.7) | 0 (0) | |
| 11–16 years | 16 (13.6) | 7 (9.3) | 9 (25.0) | 0 (0) | |
| Sex, male | 78 (66.1) | 49 (65.3) | 25 (69.4) | 4 (57.1) | 0.830 |
| Weight (kg) | 12 (6.5–20.8) | 12.0 (3.2–63.0)b | 12.0 (3.2–70.0)b | 10.0 (4.1–21.0)b | 0.195 |
| Weight, group | |||||
| <5 kg | 12 (10.2) | 10 (13.3) | 1 (2.8) | 1 (14.3) | 0.007 |
| 5–14 kg | 58 (49.2) | 35 (46.7) | 19 (52.8) | 4 (57.1) | |
| 15–29 kg | 30 (25.4) | 23 (30.7) | 5 (13.9) | 2 (28.6) | |
| 30–59 kg | 15 (12.7) | 5 (6.7) | 10 (27.8) | 0 (0) | |
| ≥60 kg | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| BSA (m2) | 0.5 (0.3–0.9) | 0.5 (0.2–1.8)b | 0.5 (0.2–1.8)b | 0.5 (0.3–1.1)b | 0.171 |
| BSA, group | |||||
| <0.7 m2 | 75 (63.6) | 49 (65.3) | 21 (58.3) | 5 (71.4) | 0.079 |
| 0.7–1.2 m2 | 32 (27.1) | 22 (29.3) | 8 (22.2) | 2 (28.6) | |
| >1.2 m2 | 11 (9.3) | 4 (5.3) | 7 (19.4) | 0 (0) | |
| Diagnosis | |||||
| Cardiomyopathy | 75 (63.6) | 54 (72) | 18 (50.0) | 3 (42.9) | 0.030c |
| Dilated | 61 (51.7) | 44 (58.7) | 14 (38.9) | 3 (42.9) | |
| Restrictive | 9 (7.6) | 6 (8.0) | 3 (8.3) | 0 (0) | |
| Hypertrophic | 2 (1.7) | 2 (2.7) | 0 (0) | 0 (0) | |
| Other | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| Myocarditis | 11 (9.3) | 4 (5.3) | 7(19.4) | 0 (0) | |
| Congenital heart disease | 29 (24.6) | 16 (21.3) | 9 (25.0) | 4 (57.1) | |
| Biventricular | 20 (16.9) | 11 (14.7) | 7 (19.4) | 2 (28.6) | |
| Single ventricle | 9 (7.6) | 5 (6.7) | 2 (5.6) | 2 (28.6) | |
| Other | 3 (2.5) | 1 (1.3) | 2 (5.6) | 0 (0) | |
| INTERMACS profile | |||||
| INTERMACS 1 | 19 (16.1) | 10 (13.3) | 9 (25.0) | 0 (0) | 0.383 |
| INTERMACS 2 | 61 (51.7) | 39 (52.0) | 18 (50.0) | 4 (57.1) | |
| INTERMACS 3 | 35 (29.7) | 24 (32.0) | 8 (22.2) | 3 (42.9) | |
| INTERMACS >3 | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| Admitted in PICU | 103 (87.3) | 67 (98.3) | 29 (80.6) | 7 (100) | 0.241 |
| Mechanical ventilation | 85 (72.0) | 53 (70.7) | 25 (69.4) | 7 (100) | 1 |
| Inotropes/vasopressors | 106 (89.8) | 69 (92.0) | 30 (83.3) | 7 (100) | 0.198 |
| RRT | 33 (28.0) | 21 (28.0) | 8 (22.2) | 4 (57.1) | 0.646 |
| Bilirubin >34 μmol/ld | 18 (16.5) | 14 (19.7) | 2 (6.5) | 2 (28.6) | 0.284 |
| ECMO | 45 (38.1) | 30 (40.0) | 10 (27.8) | 5 (71.4) | 0.291 |
| Previous cardiac arrest | 38 (32.2) | 22 (29.3) | 16 (44.4) | 0 (0) | 0.203 |
| Support type | |||||
| LVAD | 73 (61.9) | 42 (56.0) | 28 (77.8) | 3 (42.9) | 0.006 |
| BiVAD | 40 (33.9) | 30 (40.0) | 6 (16.7) | 4 (57.1) | |
| RVAD | 2 (1.7) | 0 (0) | 2 (5.6) | 0 (0) | |
| LVAD → BiVAD | 3 (2.5) | 3 (4.0) | 0 (0) | 0 (0) | |
| Device strategy | |||||
| Bridge to transplantation | 101 (85.6) | 70 (93.3) | 24 (66.7) | 7 (100) | <0.001 |
| Bridge to recovery | 6 (5.1) | 0 (0) | 6 (16.7) | 0 (0) | |
| Bridge to decision | 11 (9.3) | 5 (6.7) | 6 (16.7) | 0 (0) |
| Characteristic . | Overall (%), n = 118 (100) . | PP (%), n = 75 (63.6) . | PC (%), n = 36 (30.5) . | Mixed (%), n = 7 (5.9) . | P-Valuea . |
|---|---|---|---|---|---|
| Age (years) | 2.3 (0.7–7.7) | 2.2 (0–15.8)b | 2.7 (0.2–15.0)b | 1.7 (0.2–5.8)b | 0.257 |
| Age group | |||||
| <1 year | 37 (31.4) | 24 (32.0) | 10 (27.8) | 3 (42.9) | 0.186 |
| 1–5 years | 46 (39.0) | 31 (41.3) | 11 (30.6) | 4 (57.1) | |
| 6–10 years | 19 (16.1) | 13 (17.3) | 6 (16.7) | 0 (0) | |
| 11–16 years | 16 (13.6) | 7 (9.3) | 9 (25.0) | 0 (0) | |
| Sex, male | 78 (66.1) | 49 (65.3) | 25 (69.4) | 4 (57.1) | 0.830 |
| Weight (kg) | 12 (6.5–20.8) | 12.0 (3.2–63.0)b | 12.0 (3.2–70.0)b | 10.0 (4.1–21.0)b | 0.195 |
| Weight, group | |||||
| <5 kg | 12 (10.2) | 10 (13.3) | 1 (2.8) | 1 (14.3) | 0.007 |
| 5–14 kg | 58 (49.2) | 35 (46.7) | 19 (52.8) | 4 (57.1) | |
| 15–29 kg | 30 (25.4) | 23 (30.7) | 5 (13.9) | 2 (28.6) | |
| 30–59 kg | 15 (12.7) | 5 (6.7) | 10 (27.8) | 0 (0) | |
| ≥60 kg | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| BSA (m2) | 0.5 (0.3–0.9) | 0.5 (0.2–1.8)b | 0.5 (0.2–1.8)b | 0.5 (0.3–1.1)b | 0.171 |
| BSA, group | |||||
| <0.7 m2 | 75 (63.6) | 49 (65.3) | 21 (58.3) | 5 (71.4) | 0.079 |
| 0.7–1.2 m2 | 32 (27.1) | 22 (29.3) | 8 (22.2) | 2 (28.6) | |
| >1.2 m2 | 11 (9.3) | 4 (5.3) | 7 (19.4) | 0 (0) | |
| Diagnosis | |||||
| Cardiomyopathy | 75 (63.6) | 54 (72) | 18 (50.0) | 3 (42.9) | 0.030c |
| Dilated | 61 (51.7) | 44 (58.7) | 14 (38.9) | 3 (42.9) | |
| Restrictive | 9 (7.6) | 6 (8.0) | 3 (8.3) | 0 (0) | |
| Hypertrophic | 2 (1.7) | 2 (2.7) | 0 (0) | 0 (0) | |
| Other | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| Myocarditis | 11 (9.3) | 4 (5.3) | 7(19.4) | 0 (0) | |
| Congenital heart disease | 29 (24.6) | 16 (21.3) | 9 (25.0) | 4 (57.1) | |
| Biventricular | 20 (16.9) | 11 (14.7) | 7 (19.4) | 2 (28.6) | |
| Single ventricle | 9 (7.6) | 5 (6.7) | 2 (5.6) | 2 (28.6) | |
| Other | 3 (2.5) | 1 (1.3) | 2 (5.6) | 0 (0) | |
| INTERMACS profile | |||||
| INTERMACS 1 | 19 (16.1) | 10 (13.3) | 9 (25.0) | 0 (0) | 0.383 |
| INTERMACS 2 | 61 (51.7) | 39 (52.0) | 18 (50.0) | 4 (57.1) | |
| INTERMACS 3 | 35 (29.7) | 24 (32.0) | 8 (22.2) | 3 (42.9) | |
| INTERMACS >3 | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| Admitted in PICU | 103 (87.3) | 67 (98.3) | 29 (80.6) | 7 (100) | 0.241 |
| Mechanical ventilation | 85 (72.0) | 53 (70.7) | 25 (69.4) | 7 (100) | 1 |
| Inotropes/vasopressors | 106 (89.8) | 69 (92.0) | 30 (83.3) | 7 (100) | 0.198 |
| RRT | 33 (28.0) | 21 (28.0) | 8 (22.2) | 4 (57.1) | 0.646 |
| Bilirubin >34 μmol/ld | 18 (16.5) | 14 (19.7) | 2 (6.5) | 2 (28.6) | 0.284 |
| ECMO | 45 (38.1) | 30 (40.0) | 10 (27.8) | 5 (71.4) | 0.291 |
| Previous cardiac arrest | 38 (32.2) | 22 (29.3) | 16 (44.4) | 0 (0) | 0.203 |
| Support type | |||||
| LVAD | 73 (61.9) | 42 (56.0) | 28 (77.8) | 3 (42.9) | 0.006 |
| BiVAD | 40 (33.9) | 30 (40.0) | 6 (16.7) | 4 (57.1) | |
| RVAD | 2 (1.7) | 0 (0) | 2 (5.6) | 0 (0) | |
| LVAD → BiVAD | 3 (2.5) | 3 (4.0) | 0 (0) | 0 (0) | |
| Device strategy | |||||
| Bridge to transplantation | 101 (85.6) | 70 (93.3) | 24 (66.7) | 7 (100) | <0.001 |
| Bridge to recovery | 6 (5.1) | 0 (0) | 6 (16.7) | 0 (0) | |
| Bridge to decision | 11 (9.3) | 5 (6.7) | 6 (16.7) | 0 (0) |
Data are n (%) or median (interquartile interval).
P-value for comparison between pulsatile and continuous devices groups.
Median (range).
Comparison between main diagnostic groups (cardiomyopathy, myocarditis, congenital heart disease and other).
Data were missing for 9 patients (n = 109); bilirubin >2 mg/dl.
BiVAD: biventricular assist device; BSA: body surface area; ECMO: extracorporeal membrane oxygenation; INTERMACS: Interagency Registry for Mechanical Circulatory Support; LVAD: left ventricular assist device; PC: paracorporeal continuous; PICU: Paediatric Intensive Care Unit; PP: paracorporeal pulsatile; RRT: renal replacement therapy; RVAD: right ventricular assist device.
| Characteristic . | Overall (%), n = 118 (100) . | PP (%), n = 75 (63.6) . | PC (%), n = 36 (30.5) . | Mixed (%), n = 7 (5.9) . | P-Valuea . |
|---|---|---|---|---|---|
| Age (years) | 2.3 (0.7–7.7) | 2.2 (0–15.8)b | 2.7 (0.2–15.0)b | 1.7 (0.2–5.8)b | 0.257 |
| Age group | |||||
| <1 year | 37 (31.4) | 24 (32.0) | 10 (27.8) | 3 (42.9) | 0.186 |
| 1–5 years | 46 (39.0) | 31 (41.3) | 11 (30.6) | 4 (57.1) | |
| 6–10 years | 19 (16.1) | 13 (17.3) | 6 (16.7) | 0 (0) | |
| 11–16 years | 16 (13.6) | 7 (9.3) | 9 (25.0) | 0 (0) | |
| Sex, male | 78 (66.1) | 49 (65.3) | 25 (69.4) | 4 (57.1) | 0.830 |
| Weight (kg) | 12 (6.5–20.8) | 12.0 (3.2–63.0)b | 12.0 (3.2–70.0)b | 10.0 (4.1–21.0)b | 0.195 |
| Weight, group | |||||
| <5 kg | 12 (10.2) | 10 (13.3) | 1 (2.8) | 1 (14.3) | 0.007 |
| 5–14 kg | 58 (49.2) | 35 (46.7) | 19 (52.8) | 4 (57.1) | |
| 15–29 kg | 30 (25.4) | 23 (30.7) | 5 (13.9) | 2 (28.6) | |
| 30–59 kg | 15 (12.7) | 5 (6.7) | 10 (27.8) | 0 (0) | |
| ≥60 kg | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| BSA (m2) | 0.5 (0.3–0.9) | 0.5 (0.2–1.8)b | 0.5 (0.2–1.8)b | 0.5 (0.3–1.1)b | 0.171 |
| BSA, group | |||||
| <0.7 m2 | 75 (63.6) | 49 (65.3) | 21 (58.3) | 5 (71.4) | 0.079 |
| 0.7–1.2 m2 | 32 (27.1) | 22 (29.3) | 8 (22.2) | 2 (28.6) | |
| >1.2 m2 | 11 (9.3) | 4 (5.3) | 7 (19.4) | 0 (0) | |
| Diagnosis | |||||
| Cardiomyopathy | 75 (63.6) | 54 (72) | 18 (50.0) | 3 (42.9) | 0.030c |
| Dilated | 61 (51.7) | 44 (58.7) | 14 (38.9) | 3 (42.9) | |
| Restrictive | 9 (7.6) | 6 (8.0) | 3 (8.3) | 0 (0) | |
| Hypertrophic | 2 (1.7) | 2 (2.7) | 0 (0) | 0 (0) | |
| Other | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| Myocarditis | 11 (9.3) | 4 (5.3) | 7(19.4) | 0 (0) | |
| Congenital heart disease | 29 (24.6) | 16 (21.3) | 9 (25.0) | 4 (57.1) | |
| Biventricular | 20 (16.9) | 11 (14.7) | 7 (19.4) | 2 (28.6) | |
| Single ventricle | 9 (7.6) | 5 (6.7) | 2 (5.6) | 2 (28.6) | |
| Other | 3 (2.5) | 1 (1.3) | 2 (5.6) | 0 (0) | |
| INTERMACS profile | |||||
| INTERMACS 1 | 19 (16.1) | 10 (13.3) | 9 (25.0) | 0 (0) | 0.383 |
| INTERMACS 2 | 61 (51.7) | 39 (52.0) | 18 (50.0) | 4 (57.1) | |
| INTERMACS 3 | 35 (29.7) | 24 (32.0) | 8 (22.2) | 3 (42.9) | |
| INTERMACS >3 | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| Admitted in PICU | 103 (87.3) | 67 (98.3) | 29 (80.6) | 7 (100) | 0.241 |
| Mechanical ventilation | 85 (72.0) | 53 (70.7) | 25 (69.4) | 7 (100) | 1 |
| Inotropes/vasopressors | 106 (89.8) | 69 (92.0) | 30 (83.3) | 7 (100) | 0.198 |
| RRT | 33 (28.0) | 21 (28.0) | 8 (22.2) | 4 (57.1) | 0.646 |
| Bilirubin >34 μmol/ld | 18 (16.5) | 14 (19.7) | 2 (6.5) | 2 (28.6) | 0.284 |
| ECMO | 45 (38.1) | 30 (40.0) | 10 (27.8) | 5 (71.4) | 0.291 |
| Previous cardiac arrest | 38 (32.2) | 22 (29.3) | 16 (44.4) | 0 (0) | 0.203 |
| Support type | |||||
| LVAD | 73 (61.9) | 42 (56.0) | 28 (77.8) | 3 (42.9) | 0.006 |
| BiVAD | 40 (33.9) | 30 (40.0) | 6 (16.7) | 4 (57.1) | |
| RVAD | 2 (1.7) | 0 (0) | 2 (5.6) | 0 (0) | |
| LVAD → BiVAD | 3 (2.5) | 3 (4.0) | 0 (0) | 0 (0) | |
| Device strategy | |||||
| Bridge to transplantation | 101 (85.6) | 70 (93.3) | 24 (66.7) | 7 (100) | <0.001 |
| Bridge to recovery | 6 (5.1) | 0 (0) | 6 (16.7) | 0 (0) | |
| Bridge to decision | 11 (9.3) | 5 (6.7) | 6 (16.7) | 0 (0) |
| Characteristic . | Overall (%), n = 118 (100) . | PP (%), n = 75 (63.6) . | PC (%), n = 36 (30.5) . | Mixed (%), n = 7 (5.9) . | P-Valuea . |
|---|---|---|---|---|---|
| Age (years) | 2.3 (0.7–7.7) | 2.2 (0–15.8)b | 2.7 (0.2–15.0)b | 1.7 (0.2–5.8)b | 0.257 |
| Age group | |||||
| <1 year | 37 (31.4) | 24 (32.0) | 10 (27.8) | 3 (42.9) | 0.186 |
| 1–5 years | 46 (39.0) | 31 (41.3) | 11 (30.6) | 4 (57.1) | |
| 6–10 years | 19 (16.1) | 13 (17.3) | 6 (16.7) | 0 (0) | |
| 11–16 years | 16 (13.6) | 7 (9.3) | 9 (25.0) | 0 (0) | |
| Sex, male | 78 (66.1) | 49 (65.3) | 25 (69.4) | 4 (57.1) | 0.830 |
| Weight (kg) | 12 (6.5–20.8) | 12.0 (3.2–63.0)b | 12.0 (3.2–70.0)b | 10.0 (4.1–21.0)b | 0.195 |
| Weight, group | |||||
| <5 kg | 12 (10.2) | 10 (13.3) | 1 (2.8) | 1 (14.3) | 0.007 |
| 5–14 kg | 58 (49.2) | 35 (46.7) | 19 (52.8) | 4 (57.1) | |
| 15–29 kg | 30 (25.4) | 23 (30.7) | 5 (13.9) | 2 (28.6) | |
| 30–59 kg | 15 (12.7) | 5 (6.7) | 10 (27.8) | 0 (0) | |
| ≥60 kg | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| BSA (m2) | 0.5 (0.3–0.9) | 0.5 (0.2–1.8)b | 0.5 (0.2–1.8)b | 0.5 (0.3–1.1)b | 0.171 |
| BSA, group | |||||
| <0.7 m2 | 75 (63.6) | 49 (65.3) | 21 (58.3) | 5 (71.4) | 0.079 |
| 0.7–1.2 m2 | 32 (27.1) | 22 (29.3) | 8 (22.2) | 2 (28.6) | |
| >1.2 m2 | 11 (9.3) | 4 (5.3) | 7 (19.4) | 0 (0) | |
| Diagnosis | |||||
| Cardiomyopathy | 75 (63.6) | 54 (72) | 18 (50.0) | 3 (42.9) | 0.030c |
| Dilated | 61 (51.7) | 44 (58.7) | 14 (38.9) | 3 (42.9) | |
| Restrictive | 9 (7.6) | 6 (8.0) | 3 (8.3) | 0 (0) | |
| Hypertrophic | 2 (1.7) | 2 (2.7) | 0 (0) | 0 (0) | |
| Other | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| Myocarditis | 11 (9.3) | 4 (5.3) | 7(19.4) | 0 (0) | |
| Congenital heart disease | 29 (24.6) | 16 (21.3) | 9 (25.0) | 4 (57.1) | |
| Biventricular | 20 (16.9) | 11 (14.7) | 7 (19.4) | 2 (28.6) | |
| Single ventricle | 9 (7.6) | 5 (6.7) | 2 (5.6) | 2 (28.6) | |
| Other | 3 (2.5) | 1 (1.3) | 2 (5.6) | 0 (0) | |
| INTERMACS profile | |||||
| INTERMACS 1 | 19 (16.1) | 10 (13.3) | 9 (25.0) | 0 (0) | 0.383 |
| INTERMACS 2 | 61 (51.7) | 39 (52.0) | 18 (50.0) | 4 (57.1) | |
| INTERMACS 3 | 35 (29.7) | 24 (32.0) | 8 (22.2) | 3 (42.9) | |
| INTERMACS >3 | 3 (2.5) | 2 (2.7) | 1 (2.8) | 0 (0) | |
| Admitted in PICU | 103 (87.3) | 67 (98.3) | 29 (80.6) | 7 (100) | 0.241 |
| Mechanical ventilation | 85 (72.0) | 53 (70.7) | 25 (69.4) | 7 (100) | 1 |
| Inotropes/vasopressors | 106 (89.8) | 69 (92.0) | 30 (83.3) | 7 (100) | 0.198 |
| RRT | 33 (28.0) | 21 (28.0) | 8 (22.2) | 4 (57.1) | 0.646 |
| Bilirubin >34 μmol/ld | 18 (16.5) | 14 (19.7) | 2 (6.5) | 2 (28.6) | 0.284 |
| ECMO | 45 (38.1) | 30 (40.0) | 10 (27.8) | 5 (71.4) | 0.291 |
| Previous cardiac arrest | 38 (32.2) | 22 (29.3) | 16 (44.4) | 0 (0) | 0.203 |
| Support type | |||||
| LVAD | 73 (61.9) | 42 (56.0) | 28 (77.8) | 3 (42.9) | 0.006 |
| BiVAD | 40 (33.9) | 30 (40.0) | 6 (16.7) | 4 (57.1) | |
| RVAD | 2 (1.7) | 0 (0) | 2 (5.6) | 0 (0) | |
| LVAD → BiVAD | 3 (2.5) | 3 (4.0) | 0 (0) | 0 (0) | |
| Device strategy | |||||
| Bridge to transplantation | 101 (85.6) | 70 (93.3) | 24 (66.7) | 7 (100) | <0.001 |
| Bridge to recovery | 6 (5.1) | 0 (0) | 6 (16.7) | 0 (0) | |
| Bridge to decision | 11 (9.3) | 5 (6.7) | 6 (16.7) | 0 (0) |
Data are n (%) or median (interquartile interval).
P-value for comparison between pulsatile and continuous devices groups.
Median (range).
Comparison between main diagnostic groups (cardiomyopathy, myocarditis, congenital heart disease and other).
Data were missing for 9 patients (n = 109); bilirubin >2 mg/dl.
BiVAD: biventricular assist device; BSA: body surface area; ECMO: extracorporeal membrane oxygenation; INTERMACS: Interagency Registry for Mechanical Circulatory Support; LVAD: left ventricular assist device; PC: paracorporeal continuous; PICU: Paediatric Intensive Care Unit; PP: paracorporeal pulsatile; RRT: renal replacement therapy; RVAD: right ventricular assist device.
Device type and strategy
The most frequently used devices were PP devices (63.3%), followed by PC devices (30.5%) and finally, a combination of both (PP–PC) (5.9%). The initial type of support provided was left VAD (LVAD) in the majority of cases (n = 76, 64.4%) [biventricular VAD (BiVAD) (n = 40, 33.9%); right VAD (RVAD) [n = 2, 1.7%)]. The type of support was changed in 3 patients (2.5%) from LVAD to BiVAD. The primary indication for VAD implantation was bridge to transplantation (BtT) (n = 101; 85.6%).
Device selection differed significantly based on body size, diagnosis, type of support and device strategy (Table 1). PC devices were scarcely used as BiVAD compared to PP devices (16.7% vs 40%), and only PC devices were used as isolated RVADs.
Outcomes
Median support time on a device was 30.5 days (IQI 13–81.8; range 0–352). Patient status at the end of mechanical circulatory support is depicted in Table 2. Overall, 83 children (70.3%) had a favourable VAD outcome (transplant, recovery or ongoing). The estimated probability of being transplanted while on VAD support was 26% (95% CI 18–34), 46% (95% CI 37–55), 58% (95% CI 18–66) and 62% (95% CI 52–70) at 1, 3, 6 and 9 months, respectively. The probability of dying while on VAD support at the same time points was estimated at 16% (95% CI 10–24), 27% (95% CI 19–35), 30% (95% CI 22–38) and 30% (95% CI 22–38), respectively (Fig. 1).
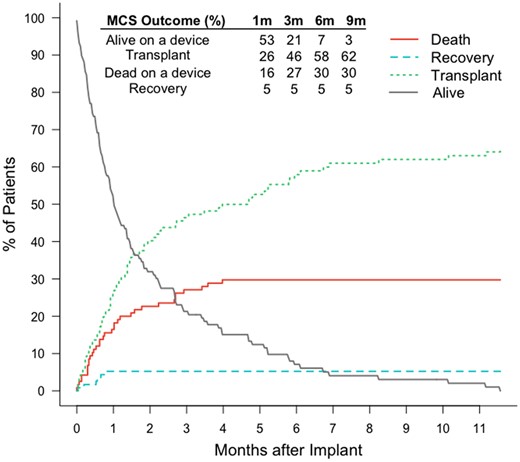
Competing outcomes description of the overall cohort. Estimated cumulative incidence of each outcome. m: months; MCS: mechanical circulatory support.
| Outcome . | Overall (%), n = 118 (100) . | PP (%), n = 75 (63.6) . | PC (%), n = 36 (30.5) . | Mixed (%), n = 7 (5.9) . | P-Valuea . |
|---|---|---|---|---|---|
| Transplanted | 73 (61.9) | 54 (72.0) | 15 (41.7) | 4 (57.1) | <0.001 |
| Weaned (recovery) | 6 (5.1) | 0 (0) | 6 (16.7) | 0 (0) | |
| Weaned (haemorrhage) | 1 (0.8) | 0 (0) | 1 (2.8) | 0 (0) | |
| Died | 34 (28.8) | 19 (25.3) | 12 (33.3) | 3 (42.9) | |
| On going | 4 (3.4) | 2 (2.7) | 2 (5.6) | 0 (0) |
| Outcome . | Overall (%), n = 118 (100) . | PP (%), n = 75 (63.6) . | PC (%), n = 36 (30.5) . | Mixed (%), n = 7 (5.9) . | P-Valuea . |
|---|---|---|---|---|---|
| Transplanted | 73 (61.9) | 54 (72.0) | 15 (41.7) | 4 (57.1) | <0.001 |
| Weaned (recovery) | 6 (5.1) | 0 (0) | 6 (16.7) | 0 (0) | |
| Weaned (haemorrhage) | 1 (0.8) | 0 (0) | 1 (2.8) | 0 (0) | |
| Died | 34 (28.8) | 19 (25.3) | 12 (33.3) | 3 (42.9) | |
| On going | 4 (3.4) | 2 (2.7) | 2 (5.6) | 0 (0) |
Data are n (%).
P-value for comparison between pulsatile and continuous devices groups.
PC: paracorporeal continuous; PP: paracorporeal pulsatile.
| Outcome . | Overall (%), n = 118 (100) . | PP (%), n = 75 (63.6) . | PC (%), n = 36 (30.5) . | Mixed (%), n = 7 (5.9) . | P-Valuea . |
|---|---|---|---|---|---|
| Transplanted | 73 (61.9) | 54 (72.0) | 15 (41.7) | 4 (57.1) | <0.001 |
| Weaned (recovery) | 6 (5.1) | 0 (0) | 6 (16.7) | 0 (0) | |
| Weaned (haemorrhage) | 1 (0.8) | 0 (0) | 1 (2.8) | 0 (0) | |
| Died | 34 (28.8) | 19 (25.3) | 12 (33.3) | 3 (42.9) | |
| On going | 4 (3.4) | 2 (2.7) | 2 (5.6) | 0 (0) |
| Outcome . | Overall (%), n = 118 (100) . | PP (%), n = 75 (63.6) . | PC (%), n = 36 (30.5) . | Mixed (%), n = 7 (5.9) . | P-Valuea . |
|---|---|---|---|---|---|
| Transplanted | 73 (61.9) | 54 (72.0) | 15 (41.7) | 4 (57.1) | <0.001 |
| Weaned (recovery) | 6 (5.1) | 0 (0) | 6 (16.7) | 0 (0) | |
| Weaned (haemorrhage) | 1 (0.8) | 0 (0) | 1 (2.8) | 0 (0) | |
| Died | 34 (28.8) | 19 (25.3) | 12 (33.3) | 3 (42.9) | |
| On going | 4 (3.4) | 2 (2.7) | 2 (5.6) | 0 (0) |
Data are n (%).
P-value for comparison between pulsatile and continuous devices groups.
PC: paracorporeal continuous; PP: paracorporeal pulsatile.
Survival
Median follow-up time was 2.1 years (range <1 day to 14.2 years) with a total follow-up time of 417.4 patient-years. Overall 74 (64.9%) children survived to hospital discharge. Among non-survivors, 34 (85%) died while on VAD support and 6 (15%) died after VAD explantation and before hospital discharge. Primary causes of death were multisystem organ failure (n = 17, 42.5%), stroke (n = 11, 27.5%), shock (n = 6, 15%), brain death (n = 2, 5%), primary graft failure (n = 2, 5%), hypoxic-ischaemic encephalopathy (n = 1, 2.5%) and sepsis (n = 1, 2.5%).
Survival at hospital discharge
Patient characteristics of survivors and non-survivors at hospital discharge are depicted in Supplementary Material, Table S1. Mortality was higher in patients bridged to decision (63.6%) as compared to patients bridged to transplant (34%) or to recovery (0%) (P = 0.034). Hospital survival differed among diagnostic categories. Survival was higher in patients with cardiomyopathy (74%) and with myocarditis (72.7%) compared to patients with CHD (40.7%) or other diagnosis (33.3%) (P = 0.007). Patients on renal replacement therapy (RRT) or ECMO had a higher mortality than patients without either type of extracorporeal support [58.1% vs 26.5% (P = 0.004) and 48.8% vs 26.8% (P = 0.025), respectively]. Survival was 44.4% in patients with elevated bilirubin (>34 μmol/l) vs 70.1% in patients with normal bilirubin (P = 0.055). Patients who had suffered a previous cardiac arrest had a survival of 52.6% compared to a 71.1% survival of patients without previous cardiac arrest (P = 0.063).
A multivariable logistic regression model was built to identify factors independently associated with a higher risk of death and to identify other factors that might either be confounders or might be clinically relevant due to an effect on the estimated risk of death. Variables forced in the model were diagnostic category (CHD/other versus cardiomyopathy/myocarditis), pre-VAD use of RRT, pre-VAD use of ECMO and device strategy [bridge to decision (BtD) versus BtT/bridge to recovery]. Variables considered for inclusion in the model were: treating hospital, era (2006–2010, 2011–2015, 2016–2020), device type (PP, PC or both), support type (LVAD, BiVAD or RVAD), sex, weight (<5 vs ≥5 kg), INTERMACS patient profile 1 vs >1, bilirubin level pre-VAD (>34 vs ≤34 μmol/l) and previous cardiac arrest. Eight variables were kept in the model (Table 3). Four variables were identified as significantly and independently associated with a higher mortality risk: weight <5 kg (P = 0.021), diagnosis of CHD/other (P = 0.025), elevated bilirubin level (>34 μmol/l) (P = 0.013) and BtD strategy (P = 0.004). The use of RRT (P = 0.315) or ECMO (P = 0.369) pre-VAD implant did not have a significant independent effect on mortality. Previous cardiac arrest (P = 0.063) and INTERMACS patient profile 1 (P = 0.19) are variables that either are close to be statistically significant or modify the mortality risk estimate. Neither a statistically significant association nor an influence on effect estimates could be demonstrated in relation to the other variables considered (treating hospital, era, sex, device type or support type); thus, this variables were excluded from the final model. Unadjusted OR for the variables included in the multivariable model are depicted in Supplementary Material, Table S2.
| Characteristic . | Adjusted OR . | 95% CI . | P-Valuea . |
|---|---|---|---|
| Bridge to decisionb (reference: bridge to transplantation/bridge to recovery) | 13.5 | 2.5–93.1 | 0.004 |
| Weight <5 kg | 6.8 | 1.4–37.2 | 0.021 |
| Bilirubin >34 μmol/l (>2 mg/dl) | 5.0 | 1.4–18.5 | 0.013 |
| Diagnosis CHD/otherb (reference: cardiomyopathy/myocarditis) | 3.4 | 1.2–10.2 | 0.025 |
| Previous cardiac arrest | 2.7 | 1.0–8.0 | 0.063 |
| INTERMACS patient profile 1 (reference: INTERMACS patient profile >1) | 2.5 | 0.6–10.6 | 0.190 |
| ECMOb | 1.8 | 0.5–6.5 | 0.369 |
| Renal replacement therapyb | 1.8 | 0.6–6.1 | 0.315 |
| Characteristic . | Adjusted OR . | 95% CI . | P-Valuea . |
|---|---|---|---|
| Bridge to decisionb (reference: bridge to transplantation/bridge to recovery) | 13.5 | 2.5–93.1 | 0.004 |
| Weight <5 kg | 6.8 | 1.4–37.2 | 0.021 |
| Bilirubin >34 μmol/l (>2 mg/dl) | 5.0 | 1.4–18.5 | 0.013 |
| Diagnosis CHD/otherb (reference: cardiomyopathy/myocarditis) | 3.4 | 1.2–10.2 | 0.025 |
| Previous cardiac arrest | 2.7 | 1.0–8.0 | 0.063 |
| INTERMACS patient profile 1 (reference: INTERMACS patient profile >1) | 2.5 | 0.6–10.6 | 0.190 |
| ECMOb | 1.8 | 0.5–6.5 | 0.369 |
| Renal replacement therapyb | 1.8 | 0.6–6.1 | 0.315 |
Risk factors present at VAD implantation. Patients with ongoing support at the end of the study period (n = 6) and patients with missing bilirubin values (n = 9) were excluded (n = 105).
Multivariable logistic regression with augmented backward elimination. Variables considered for inclusion: treating hospital, era (2006–2010, 2011–2015, 2016–2020), device type (paracorporeal pulsatile, paracorporeal continuous or both), support type (left ventricular assist device, biventricular assist device or right ventricular assist device), sex, weight (<5 vs ≥5 kg), INTERMACS patient profile 1 vs >1, bilirubin level previous to VAD implantation (>34 vs ≤34 μmol/l) and previous cardiac arrest.
Variables forced in the model.
CHD: congenital heart disease; CI: confidence interval; ECMO: extracorporeal membrane oxygenation; INTERMACS: Interagency Registry for Mechanical Circulatory Support; OR: odds ratio.
| Characteristic . | Adjusted OR . | 95% CI . | P-Valuea . |
|---|---|---|---|
| Bridge to decisionb (reference: bridge to transplantation/bridge to recovery) | 13.5 | 2.5–93.1 | 0.004 |
| Weight <5 kg | 6.8 | 1.4–37.2 | 0.021 |
| Bilirubin >34 μmol/l (>2 mg/dl) | 5.0 | 1.4–18.5 | 0.013 |
| Diagnosis CHD/otherb (reference: cardiomyopathy/myocarditis) | 3.4 | 1.2–10.2 | 0.025 |
| Previous cardiac arrest | 2.7 | 1.0–8.0 | 0.063 |
| INTERMACS patient profile 1 (reference: INTERMACS patient profile >1) | 2.5 | 0.6–10.6 | 0.190 |
| ECMOb | 1.8 | 0.5–6.5 | 0.369 |
| Renal replacement therapyb | 1.8 | 0.6–6.1 | 0.315 |
| Characteristic . | Adjusted OR . | 95% CI . | P-Valuea . |
|---|---|---|---|
| Bridge to decisionb (reference: bridge to transplantation/bridge to recovery) | 13.5 | 2.5–93.1 | 0.004 |
| Weight <5 kg | 6.8 | 1.4–37.2 | 0.021 |
| Bilirubin >34 μmol/l (>2 mg/dl) | 5.0 | 1.4–18.5 | 0.013 |
| Diagnosis CHD/otherb (reference: cardiomyopathy/myocarditis) | 3.4 | 1.2–10.2 | 0.025 |
| Previous cardiac arrest | 2.7 | 1.0–8.0 | 0.063 |
| INTERMACS patient profile 1 (reference: INTERMACS patient profile >1) | 2.5 | 0.6–10.6 | 0.190 |
| ECMOb | 1.8 | 0.5–6.5 | 0.369 |
| Renal replacement therapyb | 1.8 | 0.6–6.1 | 0.315 |
Risk factors present at VAD implantation. Patients with ongoing support at the end of the study period (n = 6) and patients with missing bilirubin values (n = 9) were excluded (n = 105).
Multivariable logistic regression with augmented backward elimination. Variables considered for inclusion: treating hospital, era (2006–2010, 2011–2015, 2016–2020), device type (paracorporeal pulsatile, paracorporeal continuous or both), support type (left ventricular assist device, biventricular assist device or right ventricular assist device), sex, weight (<5 vs ≥5 kg), INTERMACS patient profile 1 vs >1, bilirubin level previous to VAD implantation (>34 vs ≤34 μmol/l) and previous cardiac arrest.
Variables forced in the model.
CHD: congenital heart disease; CI: confidence interval; ECMO: extracorporeal membrane oxygenation; INTERMACS: Interagency Registry for Mechanical Circulatory Support; OR: odds ratio.
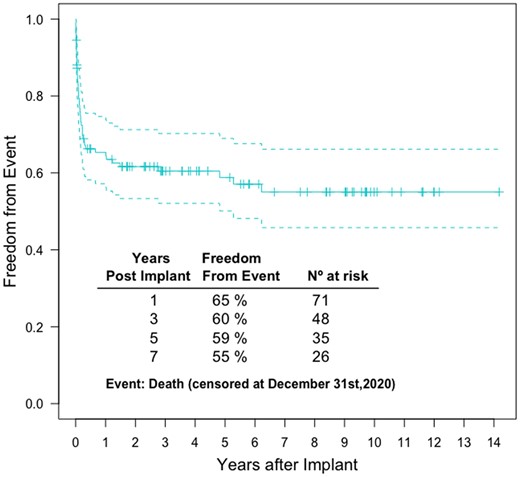
Long-term survival
Kaplan–Meier actuarial survival curve for the overall cohort [median follow-up time: 2.1 years (range: 0–14.2 years); no patient lost to follow-up] shows that most of the mortality occurred during the 1st year after implant (n = 40), which corresponds to in-hospital mortality (Fig. 2). Eight patients died during the following 6 years of follow-up (after hospital discharge). No additional deaths occurred among those patients (n = 26) who survived beyond 7 years [median follow-up time 9.7 years (range 7.5–14.2)]. Additional Kaplan–Meier actuarial survival curves stratified by the variables included in the hospital mortality model were constructed and unadjusted comparisons were made among subgroups (Supplementary Material, Figs S1–S9).
A multivariable Cox regression model including the same variables considered for in-hospital mortality was used to assess if these variables were also related to long-term survival after adjustment (Fig. 3). All variables identified as independent predictors of mortality at hospital discharge were identified as independent predictors of long-term survival: weight <5 kg, diagnosis CHD/other, bilirubin >34 μmol/l and device strategy BtD. Similarly, RRT or ECMO were not independently associated with long-term survival. Interestingly, both INTERMACS patient profile 1 and previous cardiac arrest were independently related to long-term survival after adjustment. Unadjusted HR for the variables included in the multivariable model are depicted in Supplementary Material, Table S2.

Forest plot of adjusted hazard ratios for death obtained with a Cox proportional hazards model. AIC: Akaike’s information criterion (bilirubin ≤34 vs >34 μmol/l); BtD: bridge to decision; BtR: bridge to recovery; BtT: bridge to transplant; CHD: congenital heart disease; ECMO: extracorporeal membrane oxygenation; INTERMACS: Interagency Registry for Mechanical Circulatory Support patient profile; MCP/MCD: cardiomyopathy/myocarditis; PCA: previous cardiac arrest; RRT: renal replacement therapy; STRATEGY: device strategy. *P-value <0.05; ***P-value <0.001.
Adverse events
Severe non-central nervous system haemorrhage was the most frequent major adverse event (Supplementary Material, Table S3). The incidence of severe bleeding was higher in the PC group compared to the PP group [rate ratio 2.5; 95% CI 1.3–4.8 (P = 0.007)]. Overall 45 children (38.1%) experienced at least 1 episode of stroke. The majority of children with stroke suffered only 1 stroke episode (n = 33, 73.3%), while 10 (22.2%) suffered 2 episodes and 2 (4.4%) suffered 3 episodes.
There were no differences either in the proportion of patients suffering a stroke or in the stroke type distribution between both groups (PP versus PC, P = 0.682 and P = 0.665, respectively). The stroke event rate was higher in the PC group compared to the PP [rate ratio 2.7; 95% CI 1.6–4.9 (P < 0.001)]. The proportion of patients with stroke did not change significantly over time [2006–2010 50%; 2011–2015 30.8%; 2016–2020 38.2% (P = 0.312)], but the incidence of stroke in the PP group decreased significantly from 2006–2010 (38.3 events/100 patient-months) to 2016–2020 (14.0 events/100 patient-months) [rate ratio 0.37; 95% CI 0.15–0.89 (P = 0.02)].
The event rate for device exchange was 42.6 exchanges per 100 patient-months. The exchange rate was significantly higher in the PC group [rate ratio 8.4; 95% CI 5.4–13.1 (P < 0.001)]. The main reason for pump exchange was fibrin or thrombus formation (n = 70, 72.2%). Only 3 (3.1%) pumps were exchanged due to device malfunction (device malfunction rate: 11.2 events/100 patient-months).
DISCUSSION
This is the first study to describe the characteristics and outcomes of all children treated with VADs from 2006 to 2020 in any of the 6 Spanish paediatric heart transplant centres.
Patient characteristics
Young children comprised the largest proportion of patients (70.4% below 5 and 31.4% below 1 years of age), a proportion that is greater than in the Pedimacs (47.2% below 5 and 25.5% below 1 year of age) [3] and in the Paedi-EUROMACS (43.4% below 5 and 19.5% below 1 years of age) [2] latest reports. Only 13.6% of the patients were older than 10 years of age (38% in the American and 41.9% in the European series). Children with CHD constituted almost a quarter of the patient population, comparable to that in the Pedimacs (25%), but greater to that in the Paedi-EUROMACS (15%) registries. This should be taken into account when interpreting the results because younger patients and those with CHD have worse outcomes than other groups, such as dilated cardiomyopathy or older patients [3].
Interestingly, no child was treated with an intracorporeal VAD, while in the European and North American latest reports, ∼41–47% of the patients received this type of support. Children treated with intracorporeal VADs appear to have better outcomes and lower complications, probably because they are usually older, less ill and rarely have CHD, compared to those who received paracorporeal VADs [3].
Patient outcomes
Studies examining the BtT strategy in paediatric patients have reported success rates between 48% and 89%, and 1-year post-transplant survival rates from 62% to 100% [2, 3]. In this study, mortality rate at the end of VAD support (28.8%) was higher than mortality at 12 months in the Pedimacs (18.8%) and in the Paedi-EUROMACS (20.8%) reports [2, 3]. However, 61.9% of the patients were successfully bridged to heart transplantation, while 5.9% were weaned from the VAD due to myocardial recovery. The probability of being transplanted by 3, 6 and 9 months after VAD implantation was 46%, 58% and 62%, respectively. In contrast, the transplantation rates reported at 3, 6, 9 and 12 months in PEDIMACS [3] were 35.3%, 52%, 58.7% and 62.1%, while in Paedi-EUROMACS [2] were 33.2%, 45.1% and 54.5% at 6, 12 and 24 months post VAD implantation. These results highlight the differences in transplantation rates among different countries making the results of the Paedi-EUROMACS registry not generalizable to Spain. The faster and higher transplantation rate in Spanish centres compared to the European experience and the higher proportion of younger patients and patients with CHD (thus higher mortality) may explain the shorter time on VAD support (median Spain: 30.5 days versus Paedi-EUROMACS: 5.6 months) and the lack of use of long-term IC devices for older patients.
VAD associated complications
In agreement with previous reports [2, 3], severe non-central nervous system haemorrhage was the most frequent complication (39%), closely followed by stroke (38.1%), and the need for device exchange (33.9%). In the group of PC VAD, the rate of severe bleeding (RR 2.5; 95% CI 1.3–4.8) and stroke (RR 2.7; 95% CI 1.6–4.9) were more than two-fold higher, and the rate of pump exchange (RR 8.4; 95% CI 5.4–13.1) was more than eight-fold higher than those in the PP VAD group. Notably, there has been a significant decrease of stroke incidence in the PP group, probably related to the progressive refinement of anticoagulation protocols. Actual stroke incidence rates for this group of patients (14.0 events/100 patient-months) are similar to those reported in the 5th PEDIMACS report [3].
Survival-related conditions
As in many other paediatric VAD series, CHD (OR 3.4; HR 3.3) [8–10] diagnosis and BtD (OR 13.5; HR 8.2) as VAD strategy [11] were related with higher risk of mortality at hospital discharge and also in the long-term. In addition, increased preimplantation bilirubin serum levels (OR 5; HR 4.6) [8, 12] and body weight <5 kg (OR 6.8; HR 6.4) [9, 11] were identified as risk factors for mortality at hospital discharge and in the long term. Preimplantation RRT and ECMO were associated with higher mortality in the non-adjusted comparative analysis, but they were not found to be associated with short-term (hospital) or long-term mortality in multivariable analysis. INTERMACS-1 status [3, 12] and cardiac arrest prior to VAD implantation [13] have proved to be variables of interest as they relate to mortality both in the short term and long term, and should also be taken into consideration when it comes to the decision of initiating mechanical circulatory support.
The timing of VAD implantation is of critical importance. The decision dilemma the clinician is usually faced with is to avoid premature exposure to mechanical support and device-related complications, but at the same time, to avoid the onset of any end-organ dysfunction that may compromise the final outcome. Several paediatric studies have argued that ECMO should not be used as a BtT therapy because the complication and mortality rates are higher compared to those achieved with VADs [4, 9]. However, since INTERMACS-1 status or cardiac arrest prior to VAD implantation are associated with poorer long-term survival, the timely use of extracorporeal life support therapies such as RRT or ECMO, should remain as strategic allies to achieve patient stabilization and preimplantation end-organ recovery, as their use is not associated with worse outcomes [14, 15]. In these patients, delaying VAD implantation and waiting list inclusion might be valuable in achieving higher odds of long-term survival.
Limitations
The main limitations of this study are its observational retrospective design and its sample size. The study design is open to bias that may not be fully controlled by statistical methods. Sample size, though relatively large from the clinical standpoint, does not allow for meaningful comparisons between centres’ performance (including the possible role of surgeons’, teams’, centres’ volumes, experiences and protocols).
Sample size is also a limitation for precise estimates of effect in the multivariable models. Although we could not detect any effect of treating era on mortality, we cannot exclude that risk factors for mortality may change over time. The strengths of our study are that it describes in detail the experience of all the centres with an active paediatric VAD program in Spain, and that it comprehensively analyses outcomes and risk factors for short and long-term mortality.
CONCLUSION
The use of VADs for children in Spain has progressively increased since 2006. More than two-thirds of the patients have been successfully bridged to heart transplantation or to recovery. Body weight lower than 5 kg, CHD diagnosis, preimplantation cholestatic liver dysfunction and BtD as VAD strategy were related with higher short- and long-term mortality. INTERMACS-1 status and cardiac arrest prior to VAD implantation were related only with long-term mortality, whereas preimplantation RRT and ECMO were not independently related to mortality.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENTS
We thank Santiago Pérez-Hoyos from the Statistics & Bioinformatics Unit of Vall d'Hebron Institut de Recerca for providing thoughtful insights on statistical methods.
Funding
This research did not receive any funding.
Conflict of interest: Joan Balcells reports on being on an advisory board and receiving consultancy fees from Air Liquide. The other authors report no conflict of interest.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request. Sharing of the data is conditioned to approval from the Institutional Review Boards of all the participating centres.
Author contributions
Juan José Menéndez: Conceptualization; Data curation; Investigation; Writing—original draft; Writing—review & editing. Amelia Caridad Sánchez-Galindo: Conceptualization; Data curation; Investigation; Writing—original draft; Writing—review & editing. Joan Balcells: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Writing—original draft; Writing—review & editing. María Ángeles Tejero-Hernández: Conceptualization; Data curation; Investigation; Writing—original draft; Writing—review & editing. Ángela Ferrer-Barba: Conceptualization; Data curation; Investigation; Writing—original draft; Writing—review & editing. Emilio Ibiza-Palacios: Conceptualization; Data curation; Investigation; Writing—original draft; Writing—review & editing. Constancio Medrano-López: Data curation; Investigation; Writing—review & editing. Ferran Gran: Data curation; Investigation; Writing—review & editing. Manuel Ángel Frías-Pérez: Data curation; Investigation; Writing—review & editing. María García-Vieites: Data curation; Investigation; Writing—review & editing. Ana Cano-Sánchez: Conceptualization; Investigation; Writing—review & editing. Luz Polo: Data curation; Investigation; Writing—review & editing. Juan-Miguel Gil-Jaurena: Data curation; Investigation; Writing—review & editing. Raúl Felipe Abella: Data curation; Investigation; Writing—review & editing. Carlos Merino-Cejas: Data curation; Investigation; Writing—review & editing. Isaac Martínez-Bendayán: Data curation; Investigation; Writing—review & editing. Félix Serrano: Data curation; Investigation; Writing—review & editing. Luis García-Guereta: Data curation; Investigation; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Kevin Ruixuan An, Dominique Vervoort, Dominik Wiedemann and the other anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
Organización Nacional de Trasplantes. Actividad de donación y trasplante cardiaco. España
ABBREVIATIONS
- BiVAD
Biventricular assist device
- BtD
Bridge to decision
- BtT
Bridge to transplantation
- CHD
Congenital heart disease
- CIs
Confidence intervals
- ECMO
Extracorporeal membrane oxygenation
- HRs
Hazard ratios
- INTERMACS
Interagency registry for mechanically assisted circulatory support
- LVAD
Left ventricular assist device
- ORs
Odds ratios
- PC
Paracorporeal continuous
- PP
Paracorporeal pulsatile
- PP–PC
Paracorporeal pulsatile and paracorporeal continuous
- RRT
Renal replacement therapy
- RVAD
Right ventricular assist device
- VADs
Ventricular assist devices
- cardiac arrest
- heart transplantation
- extracorporeal membrane oxygenation
- hemorrhage
- cardiac support procedures
- cerebrovascular accident
- ischemic stroke
- congenital heart disease
- bilirubin
- ventricular assist device
- child
- patient discharge
- renal replacement therapy
- spain
- diagnosis
- mortality
- ventricular assist device implantation
- intermacs registry





