-
PDF
- Split View
-
Views
-
Cite
Cite
Nobutaka Kawamoto, Yasuhiro Tsutani, Atsushi Kamigaichi, Manato Ohsawa, Takahiro Mimae, Yoshihiro Miyata, Morihito Okada, Tumour location predicts occult N1 nodal metastasis in clinical stage I non-small-cell lung cancer, European Journal of Cardio-Thoracic Surgery, Volume 63, Issue 2, February 2023, ezac575, https://doi.org/10.1093/ejcts/ezac575
Close - Share Icon Share
Abstract
Pathological lymph node metastases are often observed in patients with clinical N0 lung cancer. Identifying preoperative predictors of occult hilar nodal metastasis (OHNM) is important in determining the surgical procedure in patients with clinical stage I non-small-cell lung cancer. This study aimed to determine the frequency and predictors of OHNM by tumour location in these patients.
Between April 2007 and May 2019, data of patients who underwent lobectomy or segmentectomy for clinical stage I pure-solid non-small-cell lung cancer were retrospectively reviewed. The ratio of the distance from the pulmonary hilum to the proximal side of the tumour to the distance from the pulmonary hilum to the visceral pleural surface through the centre of the tumour, named ‘distance ratio (DR)’, was calculated. The relationship of the DR with clinicopathological findings and prognosis was discussed.
A total of 357 patients were enrolled. OHNM frequency was 14.6%. Patients were divided into 2 groups based on whether the DR was ≤0.67 (central type) or >0.67 (peripheral type). The frequency of OHNM was significantly higher in the DR ≤0.67 group (21.5% vs 7.4%; P < 0.001). Multivariable analysis revealed that DR was the only independent preoperative predictor of OHNM (odds ratio, 3.63; 95% confidence interval, 1.83–7.18; P < 0.001).
The frequency of OHNM was significantly lower in peripheral-type lung cancer; therefore, tumour location was the most important preoperative predictor of OHNM.
INTRODUCTION
The standard surgical procedure has been lobectomy for resectable non-small-cell lung cancer (NSCLC) for a long time [1]. Recent reports indicate that limited resection, such as segmentectomy or wedge resection, has been standardized for clinical stage I NSCLC with ground-glass opacity (GGO) [2, 3]. In addition, following the results of the JOCG0802/WJOG4607L trial, it is expected that for more patients with solid dominant, i.e. 0.5 < consolidation tumour ratio (CTR) ≤ 1.0, segmentectomy should also be performed for NSCLC [4]. Pathological lymph nodal involvement, known as occult nodal metastasis, is often observed in clinical N0 patients. Although mediastinal lymph nodes can be dissected regardless of the type of lung resection surgical procedure, hilar lymph nodes may be more difficult to dissect by segmentectomy than by lobectomy. Therefore, identification of preoperative predictors of occult hilar nodal metastasis (OHNM) is important in determining the surgical procedure in patients with clinical stage I NSCLC. Previous literature reported that tumour location [5, 6], tumour size [7, 8], maximum standardized uptake value (SUVmax) of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) [9–11] and serum carcinoembryonic antigen values [8] are preoperative predictors of OHNM. In the present study, we focused on tumour location, generally classified as central-type or peripheral-type. Although central-type lung cancer is reported to have more lymph node involvement than peripheral-type lung cancer [5, 6, 12], the definition of tumour location differs among investigators. As a specific example, peripheral-type tumours are defined in some studies as the centre of tumours in the outer one-third of the lung field [2–4], while in others, as the outer two-thirds [12]. However, these evaluations are subjective. In the present study, we measured the distance from the pulmonary hilum to the tumour using computed tomography (CT) images as an objective evaluation, and we calculated the ratio of the distance from the pulmonary hilum to visceral pleura. When considering the indications for segmentectomy, the distance from the pulmonary hilum to the proximal side of the tumour is important to ensure a sufficient resection margin from the tumour; hence, we selected this measurement method. Then, we retrospectively examined the association between the distance ratio (DR) and OHNM frequency. The purpose of this study was to evaluate the frequency and predictors of OHNM by tumour location in patients with clinical stage I NSCLC.
PATIENTS AND METHODS
Ethical statement
The institutional review board of Hiroshima University Hospital approved the study (E2018-1216–02; 30 November 2022). Written informed consent for patients to participate in the study was waived.
Patient enrolment
This is a database-based retrospective study. Patients with clinical stage I NSCLC who underwent lung resection at the Department of Thoracic Surgery, Hiroshima University Hospital between April 2007 and May 2019 were enrolled. Preoperative clinical staging and postoperative pathological staging were based on the TNM Classification of Lung Cancer, 8th Edition [13]. Preoperatively, high-resolution CT and FDG-PET/CT were performed in all cases. Clinical nodal metastasis was diagnosed as negative when the short diameter of the hilar or mediastinal lymph nodes was <10 mm on high-resolution CT and when FDG did not accumulate in these nodes on FDG-PET/CT scan. Generally, lung cancer with GGO has been reported to have a low frequency of lymph node metastasis [14, 15]. Cases of NSCLC with GGO were excluded from this study, and only pure-solid lesions, i.e. CTR = 1.0, were included. Since the aim of this study was to investigate the association between tumour location and the frequency of OHNM, lymph nodes had to be diagnosed histologically. Therefore, patients who underwent anatomical lung resection with hilar and mediastinal lymph nodal dissection (ND2a-1 or ND2a-2) and ≥6 lymph nodes dissected were enrolled.
Measurement method of tumour location

Measurement method of tumour location. The entrance of the lobar bronchus where the tumour was located was set as the pulmonary hilum, and tumour location was defined as follows. The ratio of the distance from the pulmonary hilum to the proximal side of the tumour to the distance from the pulmonary hilum to the visceral pleural surface through the centre of the tumour was calculated; the ratio of distance was named ‘distance ratio’.
The measurement of DR was performed by 1 investigator. The relationship of DR with clinicopathological findings and prognosis was examined.
Surgical procedure and postoperative therapy
In our institute, anatomical lung resection was performed using hybrid video-assisted thoracic surgery as previously reported by Okada et al. [16]. In this study, the patients underwent anatomical lung resection, and systematic dissection or sampling of the hilar and mediastinal lymph nodes. Dissected lymph nodes were numbered according to their location. Based on the intraoperative findings, intraoperative frozen section diagnosis was performed in order to determine the extent of resection and assess lymph nodal status. The main indications of segmentectomy were as follows: tumour size of ≤20 mm, peripheral-type tumour and patients with marginal pulmonary function [17, 18]. However, if intraoperative frozen section diagnosis or postoperative histopathological diagnosis showed nodal metastasis, lobectomy was performed basically. Segmentectomy was performed according to a previously described method by Okada et al. [19]. Postoperative adjuvant chemotherapy was usually administered to patients with pathological stage IA with tumours of >20 mm size [20], stage IB or more advanced stages.
Follow-up evaluation
All patients were followed up from the day of surgery. Examinations were performed at intervals of 3–6 months for 5 years after surgery and at intervals of 1-year thereafter, until patients' death or the date of last follow-up. Evaluations included physical examination, blood tests including tumour markers, chest radiography, or CT scan. If recurrence was suspected, FDG-PET/CT scan, brain magnetic resonance imaging, or bone scintigraphy was performed. Recurrence was determined by imaging features or histopathological evidence and classified into 3 subgroups: (i) loco-regional; (ii) distant; and (iii) loco-regional and distant. Loco-regional recurrence was defined as ipsilateral hilar or mediastinal nodal metastasis, regional recurrence of resected margin and recurrence in the residual lobe after segmentectomy. The others were defined as distant recurrence.
Statistical analyses
Summarized data were presented as number (%) or median (interquartile range). Continuous variables between 2 groups were analysed using the Mann–Whitney U-test, while categorical tests were analysed using Fisher’s exact test or chi-squared test. Continuous variables other than DR were dichotomized and converted to categorical variables using the median values, and univariable and multivariable analyses were used to identify the predictors of OHNM. Overall survival (OS) was defined as the interval from the date of the surgery to the date of death from any cause or the last follow-up date. Recurrence-free survival (RFS) was defined as the interval from the date of surgery to the date of death or proven detection of recurrence or metastases. OS and RFS were calculated from the Kaplan–Meier curves, and the 2 groups were compared using univariable log-rank analysis. A difference of P-value <0.05 was deemed to indicate statistical significance. All data were statistically analysed using JMP, Version 13.0 (SAS Institute, Cary, NC).
RESULTS
Flow chart of patient enrolment
Overall, 1038 patients diagnosed with clinical stage I NSCLC underwent lung resection (Fig. 2). Patients with GGO (n = 481) were excluded from this study. Patients who underwent wedge resection (n = 118), hilar nodal dissection only (ND1) or <6 lymph nodes dissection (n = 77) or with incomplete information, such as cases in which the tumour location could not be identified on CT images (n = 5), were excluded. A total of 357 patients with clinical stage I pure-solid NSCLC who underwent anatomical lung resection with hilar and mediastinal nodal dissection (ND2a-1 or ND2a-2) and ≥6 lymph nodes dissection, were enrolled in this study.

Flow chart of patient enrolment. GGO: ground-glass opacity; NSCLC: non-small-cell lung cancer.
Relationship between distance ratio and occult hilar nodal metastasis
In this study, the frequency of OHNM was 14.6% (n = 52). Based on location, lung cancer is often classified into central and peripheral types, bordering the inner one-third, inner one-half and outer one-third of the lung field [2–4, 12, 21]; therefore, the DRs corresponding to these definitions, 0.33, 0.50 and 0.67, were used as cut-off values, respectively. The frequency of OHNM by these DRs was shown in Table 1. The frequency of OHNM was higher in the central-type, and lower in the peripheral-type. Additionally, the cohort was classified into 3 groups, DR ≤ 0.33, 0.33 < DR ≤ 0.67, and DR > 0.67, and the OHNM frequency was found to be progressively lower as the tumour was located peripherally (Table 1). Since lung cancers in the outer one-third of the lung field are often indicated for limited resection [2–4], we divided the cohort into 2 groups based on DR of ≤0.67 and >0.67 in the subsequent analysis. The frequency of OHNM was 21.5% for DR ≤ 0.67 and 7.4% for DR > 0.67 (P < 0.001).
Relationship between the frequency of occult hilar nodal metastasis and distance ratio
| . | DR ≤ 0.33 . | DR > 0.33 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 12/33 (36.4) | 40/324 (12.4) | <0.001 | |
| . | DR ≤ 0.33 . | DR > 0.33 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 12/33 (36.4) | 40/324 (12.4) | <0.001 | |
| . | DR ≤ 0.50 . | DR > 0.50 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 22/70 (31.4) | 30/287 (10.5) | <0.001 | |
| . | DR ≤ 0.50 . | DR > 0.50 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 22/70 (31.4) | 30/287 (10.5) | <0.001 | |
| . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 39/181 (21.5) | 13/176 (7.4) | <0.001 | |
| . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 39/181 (21.5) | 13/176 (7.4) | <0.001 | |
| . | DR ≤ 0.33 . | 0.33 < DR ≤ 0.67 . | DR > 0.67 . | P-Value . |
|---|---|---|---|---|
| OHNM frequency | 12/33 (36.4) | 27/148 (18.2) | 13/176 (7.4) | <0.001 |
| . | DR ≤ 0.33 . | 0.33 < DR ≤ 0.67 . | DR > 0.67 . | P-Value . |
|---|---|---|---|---|
| OHNM frequency | 12/33 (36.4) | 27/148 (18.2) | 13/176 (7.4) | <0.001 |
Values are represented as n (%).
DR: distance ratio; OHNM: occult hilar nodal metastasis.
Relationship between the frequency of occult hilar nodal metastasis and distance ratio
| . | DR ≤ 0.33 . | DR > 0.33 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 12/33 (36.4) | 40/324 (12.4) | <0.001 | |
| . | DR ≤ 0.33 . | DR > 0.33 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 12/33 (36.4) | 40/324 (12.4) | <0.001 | |
| . | DR ≤ 0.50 . | DR > 0.50 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 22/70 (31.4) | 30/287 (10.5) | <0.001 | |
| . | DR ≤ 0.50 . | DR > 0.50 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 22/70 (31.4) | 30/287 (10.5) | <0.001 | |
| . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 39/181 (21.5) | 13/176 (7.4) | <0.001 | |
| . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . | |
|---|---|---|---|---|
| OHNM frequency | 39/181 (21.5) | 13/176 (7.4) | <0.001 | |
| . | DR ≤ 0.33 . | 0.33 < DR ≤ 0.67 . | DR > 0.67 . | P-Value . |
|---|---|---|---|---|
| OHNM frequency | 12/33 (36.4) | 27/148 (18.2) | 13/176 (7.4) | <0.001 |
| . | DR ≤ 0.33 . | 0.33 < DR ≤ 0.67 . | DR > 0.67 . | P-Value . |
|---|---|---|---|---|
| OHNM frequency | 12/33 (36.4) | 27/148 (18.2) | 13/176 (7.4) | <0.001 |
Values are represented as n (%).
DR: distance ratio; OHNM: occult hilar nodal metastasis.
Patient characteristics
The patient characteristics are summarized in Table 2. The 357 patients were divided into 2 groups according to whether DR was ≤0.67 (n = 181) or >0.67 (n = 176). More patients with DR >0.67 were males (76.7% vs 64.1%; P = 0.011), had greater pack-year of smoking (45 vs 36; P = 0.004), more cases that underwent segmentectomy (31.3% vs 16.6%; P = 0.001), smaller tumour size (20 mm vs 25 mm; P < 0.001) and lower SUVmax (3.8 vs 4.5; P = 0.011).
| . | Total . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . |
|---|---|---|---|---|
| No. cases | 357 | 181 | 176 | |
| Age (years) | 69 (63–75) | 70 (63–76) | 68 (63–75) | 0.39 |
| Sex, male/female | 251 (70.3)/106 (29.7) | 116 (64.1)/65 (35.9) | 135 (76.7)/41(23.3) | 0.011 |
| Pack-year smoking | 40 (0–60) | 36 (0–54) | 45 (5–69) | 0.004 |
| CCI ≥2 points | 94 (26.3) | 44 (24.3) | 50 (28.4) | 0.40 |
| CEA (ng/ml) | 3.1 (2.1–5.7) | 3.1 (2.1–6.1) | 3.0 (2.1–5.2) | 0.52 |
| Tumour size (mm) | 22 (16–29) | 25 (18–33) | 20 (15–26) | <0.001 |
| SUVmax | 4.3 (2.3–6.8) | 4.5 (2.7–7.7) | 3.8 (2.1–6.3) | 0.011 |
| Surgical procedure | 0.001 | |||
| Lobectomy | 272 (76.2) | 151 (83.4) | 121 (68.8) | |
| Segmentectomy | 85 (23.8) | 30 (16.6) | 55 (31.3) | |
| Number of dissected lymph nodes | 12 (9–16) | 12 (9–17) | 11 (9–16) | 0.38 |
| Histology | 0.50 | |||
| Adenocarcinoma | 227 (63.6) | 118 (65.2) | 109 (61.9) | |
| Squamous cell carcinoma | 82 (23.0) | 37 (20.4) | 45 (25.6) | |
| Others | 48 (13.4) | 26 (14.4) | 22 (12.5) | |
| Histological subtypea | 0.12 | |||
| Lepidic | 13 (5.7) | 9 (7.6) | 4 (3.7) | |
| Papillary | 146 (64.3) | 81 (68.6) | 65 (59.6) | |
| Acinar | 25 (11.0) | 12 (10.2) | 13 (11.9) | |
| Solid | 31 (13.7) | 12 (10.2) | 19 (17.4) | |
| Micropapillary | 5 (2.2) | 3 (2.5) | 2 (1.8) | |
| Others | 7 (3.1) | 1 (0.9) | 6 (5.5) | |
| Visceral pleural invasion | 100 (28.0) | 45 (24.9) | 55 (31.3) | 0.20 |
| Lymphatic invasion | 121 (33.9) | 65 (35.9) | 56 (31.8) | 0.44 |
| Vascular invasion | 154 (43.1) | 87 (48.1) | 67 (38.1) | 0.069 |
| Pulmonary metastasis | 10 (2.8) | 9 (5.0) | 1 (0.6) | 0.020 |
| Pathologic upstage | 88 (24.6) | 62 (34.3) | 26 (14.8) | <0.001 |
| Upstaging from T factor | 35 (9.8) | 24 (13.3) | 11 (6.3) | 0.032 |
| Upstaging from N factor | 63 (17.6) | 45 (24.9) | 18 (10.2) | <0.001 |
| Hilar nodal metastasis | 52 (14.6) | 39 (21.5) | 13 (7.4) | <0.001 |
| Mediastinal nodal metastasis | 22 (6.2) | 12 (6.6) | 10 (5.7) | 0.83 |
| EGFR mutationa, positive/negative/unknown | 67 (29.5)/113 (49.8)/47 (20.7) | 47 (39.8)/50 (42.4)/21 (17.8) | 20 (18.3)/63 (57.8)/26 (23.9) | 0.002 |
| Adjuvant chemotherapy | 123 (34.5) | 69 (38.1) | 54 (30.7) | 0.15 |
| Recurrence | 81 (22.7) | 47 (26.0) | 34 (19.3) | 0.16 |
| Recurrence pattern | 0.59 | |||
| Loco-regional | 9 (11.1) | 5 (10.6) | 4 (11.8) | |
| Distant | 48 (59.3) | 30 (63.8) | 18 (52.9) | |
| Loco-regional and distant | 24 (29.6) | 12 (25.5) | 12 (35.3) |
| . | Total . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . |
|---|---|---|---|---|
| No. cases | 357 | 181 | 176 | |
| Age (years) | 69 (63–75) | 70 (63–76) | 68 (63–75) | 0.39 |
| Sex, male/female | 251 (70.3)/106 (29.7) | 116 (64.1)/65 (35.9) | 135 (76.7)/41(23.3) | 0.011 |
| Pack-year smoking | 40 (0–60) | 36 (0–54) | 45 (5–69) | 0.004 |
| CCI ≥2 points | 94 (26.3) | 44 (24.3) | 50 (28.4) | 0.40 |
| CEA (ng/ml) | 3.1 (2.1–5.7) | 3.1 (2.1–6.1) | 3.0 (2.1–5.2) | 0.52 |
| Tumour size (mm) | 22 (16–29) | 25 (18–33) | 20 (15–26) | <0.001 |
| SUVmax | 4.3 (2.3–6.8) | 4.5 (2.7–7.7) | 3.8 (2.1–6.3) | 0.011 |
| Surgical procedure | 0.001 | |||
| Lobectomy | 272 (76.2) | 151 (83.4) | 121 (68.8) | |
| Segmentectomy | 85 (23.8) | 30 (16.6) | 55 (31.3) | |
| Number of dissected lymph nodes | 12 (9–16) | 12 (9–17) | 11 (9–16) | 0.38 |
| Histology | 0.50 | |||
| Adenocarcinoma | 227 (63.6) | 118 (65.2) | 109 (61.9) | |
| Squamous cell carcinoma | 82 (23.0) | 37 (20.4) | 45 (25.6) | |
| Others | 48 (13.4) | 26 (14.4) | 22 (12.5) | |
| Histological subtypea | 0.12 | |||
| Lepidic | 13 (5.7) | 9 (7.6) | 4 (3.7) | |
| Papillary | 146 (64.3) | 81 (68.6) | 65 (59.6) | |
| Acinar | 25 (11.0) | 12 (10.2) | 13 (11.9) | |
| Solid | 31 (13.7) | 12 (10.2) | 19 (17.4) | |
| Micropapillary | 5 (2.2) | 3 (2.5) | 2 (1.8) | |
| Others | 7 (3.1) | 1 (0.9) | 6 (5.5) | |
| Visceral pleural invasion | 100 (28.0) | 45 (24.9) | 55 (31.3) | 0.20 |
| Lymphatic invasion | 121 (33.9) | 65 (35.9) | 56 (31.8) | 0.44 |
| Vascular invasion | 154 (43.1) | 87 (48.1) | 67 (38.1) | 0.069 |
| Pulmonary metastasis | 10 (2.8) | 9 (5.0) | 1 (0.6) | 0.020 |
| Pathologic upstage | 88 (24.6) | 62 (34.3) | 26 (14.8) | <0.001 |
| Upstaging from T factor | 35 (9.8) | 24 (13.3) | 11 (6.3) | 0.032 |
| Upstaging from N factor | 63 (17.6) | 45 (24.9) | 18 (10.2) | <0.001 |
| Hilar nodal metastasis | 52 (14.6) | 39 (21.5) | 13 (7.4) | <0.001 |
| Mediastinal nodal metastasis | 22 (6.2) | 12 (6.6) | 10 (5.7) | 0.83 |
| EGFR mutationa, positive/negative/unknown | 67 (29.5)/113 (49.8)/47 (20.7) | 47 (39.8)/50 (42.4)/21 (17.8) | 20 (18.3)/63 (57.8)/26 (23.9) | 0.002 |
| Adjuvant chemotherapy | 123 (34.5) | 69 (38.1) | 54 (30.7) | 0.15 |
| Recurrence | 81 (22.7) | 47 (26.0) | 34 (19.3) | 0.16 |
| Recurrence pattern | 0.59 | |||
| Loco-regional | 9 (11.1) | 5 (10.6) | 4 (11.8) | |
| Distant | 48 (59.3) | 30 (63.8) | 18 (52.9) | |
| Loco-regional and distant | 24 (29.6) | 12 (25.5) | 12 (35.3) |
Values are represented as median (interquartile range) or n (%).
Only adenocarcinoma cases are eligible.
CCI: Charlson Comorbidity Index; CEA: carcinoembryonic antigen; DR: distance ratio; EGFR: epidermal growth factor receptor; SUVmax: maximum standardized uptake value.
| . | Total . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . |
|---|---|---|---|---|
| No. cases | 357 | 181 | 176 | |
| Age (years) | 69 (63–75) | 70 (63–76) | 68 (63–75) | 0.39 |
| Sex, male/female | 251 (70.3)/106 (29.7) | 116 (64.1)/65 (35.9) | 135 (76.7)/41(23.3) | 0.011 |
| Pack-year smoking | 40 (0–60) | 36 (0–54) | 45 (5–69) | 0.004 |
| CCI ≥2 points | 94 (26.3) | 44 (24.3) | 50 (28.4) | 0.40 |
| CEA (ng/ml) | 3.1 (2.1–5.7) | 3.1 (2.1–6.1) | 3.0 (2.1–5.2) | 0.52 |
| Tumour size (mm) | 22 (16–29) | 25 (18–33) | 20 (15–26) | <0.001 |
| SUVmax | 4.3 (2.3–6.8) | 4.5 (2.7–7.7) | 3.8 (2.1–6.3) | 0.011 |
| Surgical procedure | 0.001 | |||
| Lobectomy | 272 (76.2) | 151 (83.4) | 121 (68.8) | |
| Segmentectomy | 85 (23.8) | 30 (16.6) | 55 (31.3) | |
| Number of dissected lymph nodes | 12 (9–16) | 12 (9–17) | 11 (9–16) | 0.38 |
| Histology | 0.50 | |||
| Adenocarcinoma | 227 (63.6) | 118 (65.2) | 109 (61.9) | |
| Squamous cell carcinoma | 82 (23.0) | 37 (20.4) | 45 (25.6) | |
| Others | 48 (13.4) | 26 (14.4) | 22 (12.5) | |
| Histological subtypea | 0.12 | |||
| Lepidic | 13 (5.7) | 9 (7.6) | 4 (3.7) | |
| Papillary | 146 (64.3) | 81 (68.6) | 65 (59.6) | |
| Acinar | 25 (11.0) | 12 (10.2) | 13 (11.9) | |
| Solid | 31 (13.7) | 12 (10.2) | 19 (17.4) | |
| Micropapillary | 5 (2.2) | 3 (2.5) | 2 (1.8) | |
| Others | 7 (3.1) | 1 (0.9) | 6 (5.5) | |
| Visceral pleural invasion | 100 (28.0) | 45 (24.9) | 55 (31.3) | 0.20 |
| Lymphatic invasion | 121 (33.9) | 65 (35.9) | 56 (31.8) | 0.44 |
| Vascular invasion | 154 (43.1) | 87 (48.1) | 67 (38.1) | 0.069 |
| Pulmonary metastasis | 10 (2.8) | 9 (5.0) | 1 (0.6) | 0.020 |
| Pathologic upstage | 88 (24.6) | 62 (34.3) | 26 (14.8) | <0.001 |
| Upstaging from T factor | 35 (9.8) | 24 (13.3) | 11 (6.3) | 0.032 |
| Upstaging from N factor | 63 (17.6) | 45 (24.9) | 18 (10.2) | <0.001 |
| Hilar nodal metastasis | 52 (14.6) | 39 (21.5) | 13 (7.4) | <0.001 |
| Mediastinal nodal metastasis | 22 (6.2) | 12 (6.6) | 10 (5.7) | 0.83 |
| EGFR mutationa, positive/negative/unknown | 67 (29.5)/113 (49.8)/47 (20.7) | 47 (39.8)/50 (42.4)/21 (17.8) | 20 (18.3)/63 (57.8)/26 (23.9) | 0.002 |
| Adjuvant chemotherapy | 123 (34.5) | 69 (38.1) | 54 (30.7) | 0.15 |
| Recurrence | 81 (22.7) | 47 (26.0) | 34 (19.3) | 0.16 |
| Recurrence pattern | 0.59 | |||
| Loco-regional | 9 (11.1) | 5 (10.6) | 4 (11.8) | |
| Distant | 48 (59.3) | 30 (63.8) | 18 (52.9) | |
| Loco-regional and distant | 24 (29.6) | 12 (25.5) | 12 (35.3) |
| . | Total . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . |
|---|---|---|---|---|
| No. cases | 357 | 181 | 176 | |
| Age (years) | 69 (63–75) | 70 (63–76) | 68 (63–75) | 0.39 |
| Sex, male/female | 251 (70.3)/106 (29.7) | 116 (64.1)/65 (35.9) | 135 (76.7)/41(23.3) | 0.011 |
| Pack-year smoking | 40 (0–60) | 36 (0–54) | 45 (5–69) | 0.004 |
| CCI ≥2 points | 94 (26.3) | 44 (24.3) | 50 (28.4) | 0.40 |
| CEA (ng/ml) | 3.1 (2.1–5.7) | 3.1 (2.1–6.1) | 3.0 (2.1–5.2) | 0.52 |
| Tumour size (mm) | 22 (16–29) | 25 (18–33) | 20 (15–26) | <0.001 |
| SUVmax | 4.3 (2.3–6.8) | 4.5 (2.7–7.7) | 3.8 (2.1–6.3) | 0.011 |
| Surgical procedure | 0.001 | |||
| Lobectomy | 272 (76.2) | 151 (83.4) | 121 (68.8) | |
| Segmentectomy | 85 (23.8) | 30 (16.6) | 55 (31.3) | |
| Number of dissected lymph nodes | 12 (9–16) | 12 (9–17) | 11 (9–16) | 0.38 |
| Histology | 0.50 | |||
| Adenocarcinoma | 227 (63.6) | 118 (65.2) | 109 (61.9) | |
| Squamous cell carcinoma | 82 (23.0) | 37 (20.4) | 45 (25.6) | |
| Others | 48 (13.4) | 26 (14.4) | 22 (12.5) | |
| Histological subtypea | 0.12 | |||
| Lepidic | 13 (5.7) | 9 (7.6) | 4 (3.7) | |
| Papillary | 146 (64.3) | 81 (68.6) | 65 (59.6) | |
| Acinar | 25 (11.0) | 12 (10.2) | 13 (11.9) | |
| Solid | 31 (13.7) | 12 (10.2) | 19 (17.4) | |
| Micropapillary | 5 (2.2) | 3 (2.5) | 2 (1.8) | |
| Others | 7 (3.1) | 1 (0.9) | 6 (5.5) | |
| Visceral pleural invasion | 100 (28.0) | 45 (24.9) | 55 (31.3) | 0.20 |
| Lymphatic invasion | 121 (33.9) | 65 (35.9) | 56 (31.8) | 0.44 |
| Vascular invasion | 154 (43.1) | 87 (48.1) | 67 (38.1) | 0.069 |
| Pulmonary metastasis | 10 (2.8) | 9 (5.0) | 1 (0.6) | 0.020 |
| Pathologic upstage | 88 (24.6) | 62 (34.3) | 26 (14.8) | <0.001 |
| Upstaging from T factor | 35 (9.8) | 24 (13.3) | 11 (6.3) | 0.032 |
| Upstaging from N factor | 63 (17.6) | 45 (24.9) | 18 (10.2) | <0.001 |
| Hilar nodal metastasis | 52 (14.6) | 39 (21.5) | 13 (7.4) | <0.001 |
| Mediastinal nodal metastasis | 22 (6.2) | 12 (6.6) | 10 (5.7) | 0.83 |
| EGFR mutationa, positive/negative/unknown | 67 (29.5)/113 (49.8)/47 (20.7) | 47 (39.8)/50 (42.4)/21 (17.8) | 20 (18.3)/63 (57.8)/26 (23.9) | 0.002 |
| Adjuvant chemotherapy | 123 (34.5) | 69 (38.1) | 54 (30.7) | 0.15 |
| Recurrence | 81 (22.7) | 47 (26.0) | 34 (19.3) | 0.16 |
| Recurrence pattern | 0.59 | |||
| Loco-regional | 9 (11.1) | 5 (10.6) | 4 (11.8) | |
| Distant | 48 (59.3) | 30 (63.8) | 18 (52.9) | |
| Loco-regional and distant | 24 (29.6) | 12 (25.5) | 12 (35.3) |
Values are represented as median (interquartile range) or n (%).
Only adenocarcinoma cases are eligible.
CCI: Charlson Comorbidity Index; CEA: carcinoembryonic antigen; DR: distance ratio; EGFR: epidermal growth factor receptor; SUVmax: maximum standardized uptake value.
Histopathological findings and prognosis
The patient histopathological findings and recurrence pattern are summarized in Table 2. Despite the no significant difference in positive rate of lymphatic invasion between the 2 groups (35.9% vs 31.8%; P = 0.44), hilar nodal metastasis was significantly higher in the DR ≤0.67 group (21.5% vs 7.4%; P < 0.001). On the other hand, mediastinal nodal metastasis was not significantly different between the 2 groups (6.6% vs 5.7%; P = 0.83). The frequency of OHNM for cases with clinical tumour size ≤20, >20 and ≤30 mm, or >30 and ≤40 mm was 24.6%, 17.4% and 23.6%, respectively, in the DR ≤0.67 group and 6.7%, 8.3% and 7.7%, respectively, in the DR >0.67 group (Table 3 and Supplementary Material, Tables S1–S3). The frequency of OHNM was similar regardless of the tumour size. Preoperative predictors of OHNM were shown in Table 4. The univariable analysis results showed that only DR was a preoperative predictor of OHNM (odds ratio, 3.44; 95% confidence interval, 1.77–6.71; P < 0.001). Therefore, multivariable analysis was performed with DR and previously reported preoperative predictors of OHNM (carcinoembryonic antigen value, tumour size and SUVmax) [7–11]. DR was the only independent preoperative predictor of OHNM (odds ratio, 3.63; 95% confidence interval, 1.83–7.18; P < 0.001). Multivariable analysis using histopathological findings and DR as OHNM predictors showed that lymphovascular invasion and DR were independent predictors of OHNM (Supplementary Material, Tables S4 and S5). All the patients in this study were followed up for a median of 60 (interquartile range, 36–96) months. There was no difference in recurrence pattern between the 2 groups (P = 0.59) (Table 2). Kaplan–Meier curves for OS and RFS are shown in Fig. 3. The 5-year OS rate was 76.3% for DR ≤0.67 and 74.8% for DR >0.67 (P = 0.92), while the 5-year RFS rate was 62.1% for DR ≤0.67 and 69.3% for DR >0.67 (P = 0.19).
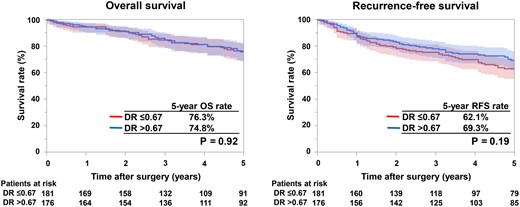
Kaplan–Meier curves for overall survival (OS) and recurrence-free survival (RFS). OS and RFS curves for patients with tumour location with DR ≤0.67 (red lines) and DR >0.67 (blue lines). DR: distance ratio.
| Tumour size . | Frequency of OHNM . | |||
|---|---|---|---|---|
| Total . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . | |
| ≤20 mm | 20/147 (13.6) | 14/57 (24.6) | 6/90 (6.7) | 0.003 |
| >20 and ≤30 mm | 17/129 (13.2) | 12/69 (17.4) | 5/60 (8.3) | 0.19 |
| >30 and ≤40 mm | 15/81 (18.5) | 13/55 (23.6) | 2/26 (7.7) | 0.13 |
| Tumour size . | Frequency of OHNM . | |||
|---|---|---|---|---|
| Total . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . | |
| ≤20 mm | 20/147 (13.6) | 14/57 (24.6) | 6/90 (6.7) | 0.003 |
| >20 and ≤30 mm | 17/129 (13.2) | 12/69 (17.4) | 5/60 (8.3) | 0.19 |
| >30 and ≤40 mm | 15/81 (18.5) | 13/55 (23.6) | 2/26 (7.7) | 0.13 |
Values are represented as n (%).
DR: distance ratio; OHNM: occult hilar nodal metastasis.
| Tumour size . | Frequency of OHNM . | |||
|---|---|---|---|---|
| Total . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . | |
| ≤20 mm | 20/147 (13.6) | 14/57 (24.6) | 6/90 (6.7) | 0.003 |
| >20 and ≤30 mm | 17/129 (13.2) | 12/69 (17.4) | 5/60 (8.3) | 0.19 |
| >30 and ≤40 mm | 15/81 (18.5) | 13/55 (23.6) | 2/26 (7.7) | 0.13 |
| Tumour size . | Frequency of OHNM . | |||
|---|---|---|---|---|
| Total . | DR ≤ 0.67 . | DR > 0.67 . | P-Value . | |
| ≤20 mm | 20/147 (13.6) | 14/57 (24.6) | 6/90 (6.7) | 0.003 |
| >20 and ≤30 mm | 17/129 (13.2) | 12/69 (17.4) | 5/60 (8.3) | 0.19 |
| >30 and ≤40 mm | 15/81 (18.5) | 13/55 (23.6) | 2/26 (7.7) | 0.13 |
Values are represented as n (%).
DR: distance ratio; OHNM: occult hilar nodal metastasis.
Univariable and multivariable analyses for occult hilar nodal metastasis with patient background
| Variables . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| DR, ≤0.67 vs >0.67 | 3.44 | 1.77–6.71 | <0.001 | 3.63 | 1.83–7.18 | <0.001 |
| Age, ≥69a vs <69 years | 0.62 | 0.34–1.12 | 0.12 | |||
| Sex, male vs female | 1.17 | 0.61–2.27 | 0.64 | |||
| Pack-year smoking, ≥40a vs <40 | 0.75 | 0.41–1.35 | 0.33 | |||
| CCI, ≥2 vs ≤1 point | 0.72 | 0.35–1.46 | 0.36 | |||
| CEA, >5.0 vs ≤5.0 ng/ml | 1.23 | 0.66–2.28 | 0.52 | 1.08 | 0.56–2.07 | 0.83 |
| Tumour size, ≥22a vs <22 mm | 0.99 | 0.55–1.79 | 0.97 | 0.66 | 0.34–1.28 | 0.22 |
| SUVmax, ≥4.3a vs <4.3 | 1.41 | 0.78–2.55 | 0.26 | 1.48 | 0.77–2.86 | 0.24 |
| Variables . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| DR, ≤0.67 vs >0.67 | 3.44 | 1.77–6.71 | <0.001 | 3.63 | 1.83–7.18 | <0.001 |
| Age, ≥69a vs <69 years | 0.62 | 0.34–1.12 | 0.12 | |||
| Sex, male vs female | 1.17 | 0.61–2.27 | 0.64 | |||
| Pack-year smoking, ≥40a vs <40 | 0.75 | 0.41–1.35 | 0.33 | |||
| CCI, ≥2 vs ≤1 point | 0.72 | 0.35–1.46 | 0.36 | |||
| CEA, >5.0 vs ≤5.0 ng/ml | 1.23 | 0.66–2.28 | 0.52 | 1.08 | 0.56–2.07 | 0.83 |
| Tumour size, ≥22a vs <22 mm | 0.99 | 0.55–1.79 | 0.97 | 0.66 | 0.34–1.28 | 0.22 |
| SUVmax, ≥4.3a vs <4.3 | 1.41 | 0.78–2.55 | 0.26 | 1.48 | 0.77–2.86 | 0.24 |
Continuous variables were dichotomized and converted to categorical variables using the median values.
CCI: Charlson Comorbidity Index; CEA: carcinoembryonic antigen; CI: confidence interval; DR: distance ratio; OR: odds ratio; SUVmax: maximum standardized uptake value.
Univariable and multivariable analyses for occult hilar nodal metastasis with patient background
| Variables . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| DR, ≤0.67 vs >0.67 | 3.44 | 1.77–6.71 | <0.001 | 3.63 | 1.83–7.18 | <0.001 |
| Age, ≥69a vs <69 years | 0.62 | 0.34–1.12 | 0.12 | |||
| Sex, male vs female | 1.17 | 0.61–2.27 | 0.64 | |||
| Pack-year smoking, ≥40a vs <40 | 0.75 | 0.41–1.35 | 0.33 | |||
| CCI, ≥2 vs ≤1 point | 0.72 | 0.35–1.46 | 0.36 | |||
| CEA, >5.0 vs ≤5.0 ng/ml | 1.23 | 0.66–2.28 | 0.52 | 1.08 | 0.56–2.07 | 0.83 |
| Tumour size, ≥22a vs <22 mm | 0.99 | 0.55–1.79 | 0.97 | 0.66 | 0.34–1.28 | 0.22 |
| SUVmax, ≥4.3a vs <4.3 | 1.41 | 0.78–2.55 | 0.26 | 1.48 | 0.77–2.86 | 0.24 |
| Variables . | Univariable . | Multivariable . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P-Value . | OR . | 95% CI . | P-Value . | |
| DR, ≤0.67 vs >0.67 | 3.44 | 1.77–6.71 | <0.001 | 3.63 | 1.83–7.18 | <0.001 |
| Age, ≥69a vs <69 years | 0.62 | 0.34–1.12 | 0.12 | |||
| Sex, male vs female | 1.17 | 0.61–2.27 | 0.64 | |||
| Pack-year smoking, ≥40a vs <40 | 0.75 | 0.41–1.35 | 0.33 | |||
| CCI, ≥2 vs ≤1 point | 0.72 | 0.35–1.46 | 0.36 | |||
| CEA, >5.0 vs ≤5.0 ng/ml | 1.23 | 0.66–2.28 | 0.52 | 1.08 | 0.56–2.07 | 0.83 |
| Tumour size, ≥22a vs <22 mm | 0.99 | 0.55–1.79 | 0.97 | 0.66 | 0.34–1.28 | 0.22 |
| SUVmax, ≥4.3a vs <4.3 | 1.41 | 0.78–2.55 | 0.26 | 1.48 | 0.77–2.86 | 0.24 |
Continuous variables were dichotomized and converted to categorical variables using the median values.
CCI: Charlson Comorbidity Index; CEA: carcinoembryonic antigen; CI: confidence interval; DR: distance ratio; OR: odds ratio; SUVmax: maximum standardized uptake value.
DISCUSSION
This study reveals an association between tumour location and frequency of OHNM in patients with clinical stage I pure-solid NSCLC. To the best of our knowledge, this is the first report to objectively evaluate tumour location from the pulmonary hilum to the tumour using CT images, and analyse the association between tumour location and the frequency of OHNM in patients with clinical stage I pure-solid NSCLC. In this study, the ratio of the distance of the tumour from pulmonary hilum was calculated. Our study showed that the frequency of OHNM was higher in the central type than in the peripheral type. Since lung cancers in the outer one-third of the lung field are often indicated for limited resection [2–4], DR = 0.67 was used as the cut-off value for analysis in this study. DR was calculated based on the distance from pulmonary hilum to the proximal side of the tumour. Thus, DR tended to be smaller with larger tumour size (P < 0.001). Generally, SUVmax tended to be proportional to tumour size [22]; SUVmax was also significantly higher in the group with DR ≤0.67 (P = 0.011). Unlike past reports, tumour size and SUVmax were not significant preoperative predictors of OHNM in univariable and multivariable analyses, and DR was the only independent preoperative predictor. One possible reason for the results is that this study included only patients with pure-solid NSCLC. In addition, since there was no significant difference in the positive rate of lymphatic invasion between the 2 groups, the frequency of OHNM was found to be simply dependent on DR. Although tumours in the outer one-third of the lung field have been empirically determined as peripheral-type lung cancer, by determining the frequency of OHNM, the present study validated the findings objectively.
In this study, OHNM was present in 14.6% of cases. The frequency of OHNM in patients with clinical stage I pure-solid NSCLC was reported to be 11.3–16.2% [11, 23, 24], which is similar to that of our data. The frequency of OHNM was significantly higher in the DR ≤0.67 group (central type) than in the DR >0.67 group (peripheral type). To avoid missed opportunities for preoperative treatment or postoperative adjuvant therapy, more aggressive preoperative nodal staging using endobronchial ultrasound-guided transbronchial needle aspiration and more aggressive hilar nodal dissection may be desirable in central-type lung cancer. Central-type lung cancer tended to have a higher recurrence rate and shorter 5-year RFS rate than peripheral-type lung cancers (26.0% vs 19.3%; P = 0.16, 62.1% vs 69.3%; P = 0.19, respectively). However, 5-year OS rate was similar between the 2 groups (76.3% vs 74.8%; P = 0.92). This may be due to the significantly higher rate of epidermal growth factor receptor mutation positivity in central-type lung cancers compared to that in peripheral-type lung cancers in adenocarcinoma cases in this study (39.8% vs 18.3%; P = 0.002).
The JCOG0802/WJOG4607L trial reported that OS was significantly better in the segmentectomy group than in the lobectomy group in patients with CTR >0.5 and tumour size ≤20-mm peripheral-type clinical stage I NSCLC [4]. In the JCOG0802/WJOG4607L trial, the peripheral-type lung cancer was defined as when the centre of the tumour was located in the outer one-third of the lung field, which is generally similar to the definition of the peripheral-type lung cancer in this study. The JCOG0802/WJOG4607L trial included 51.4% of pure-solid tumours, and the frequency of OHNM was 3.0%. Most of the cases with OHNM were pure-solid NSCLC, and if limited to pure-solid cases only, the frequency of OHNM would be about 6%. This study included ≤40-mm-sized pure-solid tumours, and the frequency of OHNM was 7.4% in the DR >0.67 group (peripheral-type): The frequency of OHNM was similar to that of the JCOG0802/WJOG4607L trial. In addition, the frequency of OHNM in peripheral-type lung cancer was similar regardless of tumour size (6.7% in ≤20 mm, 8.3% in >20 and ≤30 mm and 7.7% in >30 and ≤40 mm), which demonstrates the reproducibility of this study. Therefore, the frequency of OHNM is low in peripheral-type clinical stage I NSCLC, even with pure-solid tumours, and the risk of leaving behind hilar nodal metastasis when performing segmentectomy is considered low. However, when performing segmentectomy, the hilar lymph nodes should be well dissected to prevent loco-regional recurrence. In peripheral-type pure-solid early NSCLC, segmentectomy with systematic hilar nodal dissection may not increase the risk of leaving behind hilar nodal metastasis. Based on the results of this study, indications for segmentectomy may be expanded in the future, and further validation with multicentre studies is needed.
Limitations
The present study has several limitations. One is that it was a retrospective analysis in a single centre and with a small cohort. In addition, CTR and DR were not evaluated by an independent data monitoring committee. Thus, the determination of CTR and DR depended on individual decisions. Greater data accumulation through multicentre studies may solve these problems. Another is that we limited our analysis to patients in whom >6 lymph nodes were dissected. The guidelines of the European Society of Thoracic Surgeons recommend that at least 6 lymph nodes be dissected to assure proper pathologic classification [25]. Thus, we followed this guideline to examine lymph node metastasis in more detail.
CONCLUSION
In this study, the distance from the pulmonary hilum to the tumour was measured to objectively evaluate the tumour location. The frequency of OHNM was significantly lower when the tumour was located more peripherally in patients with clinical stage I pure-solid NSCLC. Therefore, tumour location from the pulmonary hilum is the most important preoperative predictor of OHNM.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
ACKNOWLEDGEMENTS
We thank Editage (http://www.editage.jp) for English language editing.
Conflict of interest: none declared.
Data Availability
The data underlying this article cannot be shared publicly due to the privacy of the individuals who participated in the study. Data will be shared if the corresponding author has a reasonable request.
Author contributions
Nobutaka Kawamoto: Conceptualization; Data curation; Formal analysis; Investigation; Writing—original draft. Yasuhiro Tsutani: Conceptualization; Data curation; Supervision; Writing—review & editing. Atsushi Kamigaichi: Writing—review & editing. Manato Ohsawa: Formal analysis; Writing—review & editing. Takahiro Mimae: Data curation; Writing—review & editing. Yoshihiro Miyata: Writing—review & editing. Morihito Okada: Supervision.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Angelo Carretta, Jeffrey A. Hagen, Larry R Kaiser and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
REFERENCES
ABBREVIATIONS
- CT
Computed tomography
- CTR
Consolidation tumour ratio
- DR
Distance ratio
- FDG-PET/CT
18F-fluorodeoxyglucose positron emission tomography/computed tomography
- GGO
Ground-glass opacity
- NSCLC
Non-small-cell lung cancer
- OHNM
Occult hilar nodal metastasis
- OS
Overall survival
- RFS
Recurrence-free survival
- SUVmax
Maximum standardized uptake value





