-
PDF
- Split View
-
Views
-
Cite
Cite
Roberto Lorusso, Justine M Ravaux, Francesco Pollari, Thierry A Folliguet, Utz Kappert, Bart Meuris, Malakh L Shrestha, Eric E Roselli, Nikolaos Bonaros, Olivier Fabre, Pierre Corbi, Giovanni Troise, Martin Andreas, Frederic Pinaud, Steffen Pfeiffer, Sami Kueri, Erwin Tan, Pierre Voisine, Evaldas Girdauskas, Filip Rega, Julio Garcia-Puente, Theodor Fischlein, on behalf the PERSIST-AVR Investigators , Pacemaker implantation after sutureless or stented valve: results from a controlled randomized trial, European Journal of Cardio-Thoracic Surgery, Volume 62, Issue 4, October 2022, ezac164, https://doi.org/10.1093/ejcts/ezac164
Close - Share Icon Share
Abstract
Sutureless aortic valves demonstrated non-inferiority to standard stented valves for major cardiovascular and cerebral events at 1 year after aortic valve replacement. We aim to assess the factors correlating with permanent pacemaker implantation (PPI) in both cohorts.
PERSIST-AVR is a prospective, randomized, open-label trial. Patients undergoing aortic valve replacement were randomized to receive a sutureless aortic valve replacement (Su-AVR) or stented sutured bioprosthesis (SAVR). Multivariable analysis was performed to identify possible independent risk factors associated with PPI. A logistic regression analysis was performed to estimate the risk of PPI associated to different valve size.
The 2 groups (Su-AVR; n = 450, SAVR n = 446) were well balanced in terms of preoperative risk factors. Early PPI rates were 10.4% in the Su-AVR group and 3.1% in the SAVR. PPI prevalence correlated with valve size XL (P = 0.0119) and preoperative conduction disturbances (P = 0.0079) in the Su-AVR group. No predictors were found in the SAVR cohort. Logistic regression analysis showed a significantly higher risk for PPI with size XL compared to each individual sutureless valve sizes [odds ratio (OR) 0.272 vs size S (95%confidence interval 0.07–0.95), 0.334 vs size M (95% CI 0,16–0; 68), 0.408 vs size L (95% CI 0,21–0.81)] but equivalent risk of PPI rates for all other combination of valve sizes.
Su-AVR is associated with higher PPI rate as compared to SAVR. However, the increased PPI rate appears to be size-dependent with significant higher rate only for size XL. The combination of preoperative conduction disorder and a size XL can lead to a higher probability of early PPI in Su-AVR.
NCT02673697.
INTRODUCTION
The comparison between sutureless valves and standard stented valves has been investigated in previous studies, demonstrating decreased cross-clamp time using the Perceval prosthesis and similar results for major cardiovascular and cerebral events over the short- to mid-term follow-up [1–3]. The Perceval sutureless aortic valve (CORCYM, Saluggia, Italy) is a bovine pericardial valve nitinol-stent mounted offering an alternative to traditional flexible prostheses [4]. Higher permanent pacemaker implantation (PPI) rate after sutureless valve has been already highlighted although with a wide range of occurrence of such perioperative event [5–7] Indeed, recent studies report a PPI rate after sutureless aortic valve replacement (Su-AVR) from 3% to 13.3% [5–7], while the incidence of conduction disorders leading to PPI after aortic valve replacement with a stented valves (SAVR) varies between 3% and 7% [8–10]. However, the identification of predictive factors associated with PPI remains still controversial [11]. A recent meta-analysis demonstrated a two-fold greater risk of PPI after rapid deployment prosthesis (including Su-AVR) than in a SAVR cohort [12], independently of the type of the valve used. The impact of postoperative PPI on late morality after Su-AVR is still under investigation [13] and the matter of PPI after Su-ARV might represent a limitation for an extended use of sutureless valves despite shorter operative times, and enhancement of minimally invasive procedures [14]. Nevertheless, data from international registry as ‘Sutureless and Rapid Deployment International Registry’ show a temporal decreasing trend in PPI after Su-AVR [15]. However, dedicated, objective and in-depth analysis of such an issue has been lacking. The aim of the present study was, therefore, to assess the incidence and related factors correlated with PPI after either Su-AVR or SAVR in a prospective, randomized study.
METHODS
Ethical statement
Ethical approval was provided by the local ethics committee before patient recruitment (Medical Ethics Research Committee, 151138). The study was registered at clinical-trials.gov (NCT02673697) and performed in accordance with the Declaration of Helsinki. All subjects gave written informed consent.
Patients and methods
PERSIST-AVR is a multicentre, prospective, randomized, open-label, interventional post-market trial, with a parallel assignment schema. The design of the study has been previously published [16]. For the record, 910 patients underwent randomization (1:1 blocked randomization). The choice of the surgical bioprosthesis in the stented valve arm was left to the discretion of the surgeon. Patients were enrolled 47 sites in in Europe, Canada, USA, Chile and Israel from March 2016 to September 2018. Clinical, echocardiographic and blood test outcomes were collected preoperatively, at discharge and at each follow-up (1-year follow-up completed).
Statistical analyses
Categorical variables are presented as absolute number and percentages. Continuous variables are described by the mean (± standard deviation). The actual treatment population was the analysed population. Cumulative freedom from events has been evaluated using the method of Kaplan–Meier. Comparison of curves among arms has been performed with the log-rank test. Multivariable analysis on Perceval and stented cohorts was run to identify possible independent risk factors associated with occurrence of PPI. Selection of analysed variables was based on previous literature reporting on potentials factors influencing PPI rate [12, 13]. The following variables were considered potential predictors of PPI: valve size (M, L, XL), age, female sex, surgical approach by full sternotomy, concomitant procedure and preoperative conduction disorder. Multiple logistic regression models with simultaneous consideration of all clinically relevant variables (covariates) that influence the PPI rates were used. After 4 steps, the backward selection reached a model fit with P = 0.994 (higher better) leading to the inclusion of only 2 covariates of interest into the final model (valve size and preoperative conduction). Every covariate with a cut off P-values of >0.1 was excluded from the final model by the backward selection. Valve size S was used as a reference and corresponded to the intercept.
RESULTS
A total of 914 patients were enrolled, and 910 underwent randomization, at 47 international centres. The actual treatment population consists of 450 patients with Perceval valve implanted and 446 with a traditional stented valve implanted. The population in the primary outcome analysis (per protocol) involved 819 patients, 407 in the sutureless group and 412 in the stented group [17]. The actual treatment population consists of 450 patients with a Perceval valve implanted and 446 with a traditional stented valve implanted.
Preoperative patient profiles are reported in Table 1, demonstrating no significant differences in preoperative risk (EuroSCORE II/STS score) and baseline characteristics between Perceval and stented valve cohorts. Operative data are summarized in Table 2. A mini-sternotomy approach was used in almost 50% of the patients in both groups. The number of concomitant procedures was also well balanced between the 2 cohorts. Most patients were successfully implanted at the first attempt in both groups. In the stented valve group, there were 10 cases where the valve was not successfully implanted, due to valve deficiency discovered after implant, sizing, positioning difficulties, anatomical patient features. In the Perceval group, there were 5 cases of valves not successfully implanted due to valve deficiency observed at the first attempt in 4 patients and 1 sizing issue.
| . | Perceval (n = 450) . | Stented (n = 446) . |
|---|---|---|
| Age | 75.5 ± 5.7 | 75.0 ± 6.2 |
| Female sex | 234 (52.0) | 189 (42.4) |
| STS score | 2.4 ± 1.8 | 2.1 ± 1.3 |
| STS score high (>8) | 12 (2.7) | 1 (0.2) |
| STS intermediate (4–8) | 33 (7.3) | 30 (6.7) |
| STS low (<4) | 395 (87.8) | 407 (91.3) |
| EuroSCORE II | 2.2 ± 1.9 | 2.0 ± 1.4 |
| NYHA class | ||
| NYHA I | 0 | 0 |
| NYHA II | 290 (64.4) | 284 (63.7) |
| NYHA III | 152 (33.8) | 158 (35.4) |
| NYHA IV | 7 (1.6) | 2 (0.4) |
| Comorbid conditions | ||
| Systemic hypertension | 370 (82.2) | 360 (80.7) |
| Dyslipidaemia | 251 (55.8) | 283 (63.5) |
| Diabetes | 125 (27.8) | 123 (27.6) |
| Tobacco user | 98 (21.8) | 130 (29.1) |
| Coronary artery disease | 181 (40.2) | 162 (36.3) |
| Chronic lung disease | 54 (12.0) | 45 (10.1) |
| Neoplasia | 37 (8.2) | 38 (8.5) |
| Pulmonary hypertension | 33 (7.3) | 41 (9.2) |
| Peripheral vascular disease | 34 (7.6) | 34 (7.6) |
| Angina | 68 (15.1) | 54 (12.1) |
| Carotid artery disease | 50 (11.1) | 55 (12.3) |
| Heart failure | 23 (5.1) | 26 (5.8) |
| Transient ischaemic attack | 21 (4.7) | 6 (1.3) |
| Stroke | 22 (4.9) | 13 (2.9) |
| Myocardial infarction | 19 (4.2) | 17 (3.8) |
| Endocarditis | 1 (0.2) | 1 (0.2) |
| Previous cardiovascular procedures | 50 (11.1) | 61 (13.7) |
| CABG | 1 (0.2) | 2 (0.4) |
| PCI | 40 (8.9) | 52 (11.7) |
| Pulse generator implant | 9 (2.0) | 10 (2.2) |
| Arrhythmia treatment | 1 (0.2) | 3 (0.7) |
| Site-reported preoperative hemodynamic data | ||
| Mean pressure gradient (mmHg) | 52.1 ± 15.2 | 46.6 ± 11.3 |
| Peak pressure gradient (mmHg) | 82.7 ± 24.9 | 75.8 ± 17.5 |
| Effective orifice area (cm2) | 0.7 ± 0.2 | 0.7 ± 0.2 |
| . | Perceval (n = 450) . | Stented (n = 446) . |
|---|---|---|
| Age | 75.5 ± 5.7 | 75.0 ± 6.2 |
| Female sex | 234 (52.0) | 189 (42.4) |
| STS score | 2.4 ± 1.8 | 2.1 ± 1.3 |
| STS score high (>8) | 12 (2.7) | 1 (0.2) |
| STS intermediate (4–8) | 33 (7.3) | 30 (6.7) |
| STS low (<4) | 395 (87.8) | 407 (91.3) |
| EuroSCORE II | 2.2 ± 1.9 | 2.0 ± 1.4 |
| NYHA class | ||
| NYHA I | 0 | 0 |
| NYHA II | 290 (64.4) | 284 (63.7) |
| NYHA III | 152 (33.8) | 158 (35.4) |
| NYHA IV | 7 (1.6) | 2 (0.4) |
| Comorbid conditions | ||
| Systemic hypertension | 370 (82.2) | 360 (80.7) |
| Dyslipidaemia | 251 (55.8) | 283 (63.5) |
| Diabetes | 125 (27.8) | 123 (27.6) |
| Tobacco user | 98 (21.8) | 130 (29.1) |
| Coronary artery disease | 181 (40.2) | 162 (36.3) |
| Chronic lung disease | 54 (12.0) | 45 (10.1) |
| Neoplasia | 37 (8.2) | 38 (8.5) |
| Pulmonary hypertension | 33 (7.3) | 41 (9.2) |
| Peripheral vascular disease | 34 (7.6) | 34 (7.6) |
| Angina | 68 (15.1) | 54 (12.1) |
| Carotid artery disease | 50 (11.1) | 55 (12.3) |
| Heart failure | 23 (5.1) | 26 (5.8) |
| Transient ischaemic attack | 21 (4.7) | 6 (1.3) |
| Stroke | 22 (4.9) | 13 (2.9) |
| Myocardial infarction | 19 (4.2) | 17 (3.8) |
| Endocarditis | 1 (0.2) | 1 (0.2) |
| Previous cardiovascular procedures | 50 (11.1) | 61 (13.7) |
| CABG | 1 (0.2) | 2 (0.4) |
| PCI | 40 (8.9) | 52 (11.7) |
| Pulse generator implant | 9 (2.0) | 10 (2.2) |
| Arrhythmia treatment | 1 (0.2) | 3 (0.7) |
| Site-reported preoperative hemodynamic data | ||
| Mean pressure gradient (mmHg) | 52.1 ± 15.2 | 46.6 ± 11.3 |
| Peak pressure gradient (mmHg) | 82.7 ± 24.9 | 75.8 ± 17.5 |
| Effective orifice area (cm2) | 0.7 ± 0.2 | 0.7 ± 0.2 |
Values are mean ± standard deviation or n (%).
CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; NYHA: New-York heart association class; STS score: society of thorax surgery score.
| . | Perceval (n = 450) . | Stented (n = 446) . |
|---|---|---|
| Age | 75.5 ± 5.7 | 75.0 ± 6.2 |
| Female sex | 234 (52.0) | 189 (42.4) |
| STS score | 2.4 ± 1.8 | 2.1 ± 1.3 |
| STS score high (>8) | 12 (2.7) | 1 (0.2) |
| STS intermediate (4–8) | 33 (7.3) | 30 (6.7) |
| STS low (<4) | 395 (87.8) | 407 (91.3) |
| EuroSCORE II | 2.2 ± 1.9 | 2.0 ± 1.4 |
| NYHA class | ||
| NYHA I | 0 | 0 |
| NYHA II | 290 (64.4) | 284 (63.7) |
| NYHA III | 152 (33.8) | 158 (35.4) |
| NYHA IV | 7 (1.6) | 2 (0.4) |
| Comorbid conditions | ||
| Systemic hypertension | 370 (82.2) | 360 (80.7) |
| Dyslipidaemia | 251 (55.8) | 283 (63.5) |
| Diabetes | 125 (27.8) | 123 (27.6) |
| Tobacco user | 98 (21.8) | 130 (29.1) |
| Coronary artery disease | 181 (40.2) | 162 (36.3) |
| Chronic lung disease | 54 (12.0) | 45 (10.1) |
| Neoplasia | 37 (8.2) | 38 (8.5) |
| Pulmonary hypertension | 33 (7.3) | 41 (9.2) |
| Peripheral vascular disease | 34 (7.6) | 34 (7.6) |
| Angina | 68 (15.1) | 54 (12.1) |
| Carotid artery disease | 50 (11.1) | 55 (12.3) |
| Heart failure | 23 (5.1) | 26 (5.8) |
| Transient ischaemic attack | 21 (4.7) | 6 (1.3) |
| Stroke | 22 (4.9) | 13 (2.9) |
| Myocardial infarction | 19 (4.2) | 17 (3.8) |
| Endocarditis | 1 (0.2) | 1 (0.2) |
| Previous cardiovascular procedures | 50 (11.1) | 61 (13.7) |
| CABG | 1 (0.2) | 2 (0.4) |
| PCI | 40 (8.9) | 52 (11.7) |
| Pulse generator implant | 9 (2.0) | 10 (2.2) |
| Arrhythmia treatment | 1 (0.2) | 3 (0.7) |
| Site-reported preoperative hemodynamic data | ||
| Mean pressure gradient (mmHg) | 52.1 ± 15.2 | 46.6 ± 11.3 |
| Peak pressure gradient (mmHg) | 82.7 ± 24.9 | 75.8 ± 17.5 |
| Effective orifice area (cm2) | 0.7 ± 0.2 | 0.7 ± 0.2 |
| . | Perceval (n = 450) . | Stented (n = 446) . |
|---|---|---|
| Age | 75.5 ± 5.7 | 75.0 ± 6.2 |
| Female sex | 234 (52.0) | 189 (42.4) |
| STS score | 2.4 ± 1.8 | 2.1 ± 1.3 |
| STS score high (>8) | 12 (2.7) | 1 (0.2) |
| STS intermediate (4–8) | 33 (7.3) | 30 (6.7) |
| STS low (<4) | 395 (87.8) | 407 (91.3) |
| EuroSCORE II | 2.2 ± 1.9 | 2.0 ± 1.4 |
| NYHA class | ||
| NYHA I | 0 | 0 |
| NYHA II | 290 (64.4) | 284 (63.7) |
| NYHA III | 152 (33.8) | 158 (35.4) |
| NYHA IV | 7 (1.6) | 2 (0.4) |
| Comorbid conditions | ||
| Systemic hypertension | 370 (82.2) | 360 (80.7) |
| Dyslipidaemia | 251 (55.8) | 283 (63.5) |
| Diabetes | 125 (27.8) | 123 (27.6) |
| Tobacco user | 98 (21.8) | 130 (29.1) |
| Coronary artery disease | 181 (40.2) | 162 (36.3) |
| Chronic lung disease | 54 (12.0) | 45 (10.1) |
| Neoplasia | 37 (8.2) | 38 (8.5) |
| Pulmonary hypertension | 33 (7.3) | 41 (9.2) |
| Peripheral vascular disease | 34 (7.6) | 34 (7.6) |
| Angina | 68 (15.1) | 54 (12.1) |
| Carotid artery disease | 50 (11.1) | 55 (12.3) |
| Heart failure | 23 (5.1) | 26 (5.8) |
| Transient ischaemic attack | 21 (4.7) | 6 (1.3) |
| Stroke | 22 (4.9) | 13 (2.9) |
| Myocardial infarction | 19 (4.2) | 17 (3.8) |
| Endocarditis | 1 (0.2) | 1 (0.2) |
| Previous cardiovascular procedures | 50 (11.1) | 61 (13.7) |
| CABG | 1 (0.2) | 2 (0.4) |
| PCI | 40 (8.9) | 52 (11.7) |
| Pulse generator implant | 9 (2.0) | 10 (2.2) |
| Arrhythmia treatment | 1 (0.2) | 3 (0.7) |
| Site-reported preoperative hemodynamic data | ||
| Mean pressure gradient (mmHg) | 52.1 ± 15.2 | 46.6 ± 11.3 |
| Peak pressure gradient (mmHg) | 82.7 ± 24.9 | 75.8 ± 17.5 |
| Effective orifice area (cm2) | 0.7 ± 0.2 | 0.7 ± 0.2 |
Values are mean ± standard deviation or n (%).
CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; NYHA: New-York heart association class; STS score: society of thorax surgery score.
| . | Perceval (n = 450) . | Stented (n = 446) . |
|---|---|---|
| Operative characteristics, n (%) | ||
| Surgical approach | ||
| Full sternotomy | 222 (49.3) | 236 (52.9) |
| Mini-sternotomy | 228 (50.7) | 210 (47.1) |
| Bicuspid aortic valvea | 47 (10.4) | 54 (12.1) |
| Valve size | ||
| S (21 mm) | 41 (9.1) | NA |
| M (23 mm) | 147 (32.7) | NA |
| L (25 mm) | 151 (33.6) | NA |
| XL (27 mm) | 111 (24.7) | NA |
| 19 mm | NA | 22 (4.9) |
| 21 mm | NA | 125 (28.0) |
| 23 mm | NA | 183 (41.0) |
| 25 mm | NA | 104 (23.3) |
| 27 mm | NA | 11 (2.5) |
| 29 mm | NA | 1 (0.2) |
| Concomitant procedures | 136 (30.2) | 127 (28.5) |
| CABG | 108 (24.0) | 98 (22.0) |
| Septal myectomy | 17 (3.8) | 14 (3.1) |
| Aortic annulus enlargement | 0 (0.0) | 4 (0.9) |
| Others | 18 (4.0) | 24 (5.4) |
| . | Perceval (n = 450) . | Stented (n = 446) . |
|---|---|---|
| Operative characteristics, n (%) | ||
| Surgical approach | ||
| Full sternotomy | 222 (49.3) | 236 (52.9) |
| Mini-sternotomy | 228 (50.7) | 210 (47.1) |
| Bicuspid aortic valvea | 47 (10.4) | 54 (12.1) |
| Valve size | ||
| S (21 mm) | 41 (9.1) | NA |
| M (23 mm) | 147 (32.7) | NA |
| L (25 mm) | 151 (33.6) | NA |
| XL (27 mm) | 111 (24.7) | NA |
| 19 mm | NA | 22 (4.9) |
| 21 mm | NA | 125 (28.0) |
| 23 mm | NA | 183 (41.0) |
| 25 mm | NA | 104 (23.3) |
| 27 mm | NA | 11 (2.5) |
| 29 mm | NA | 1 (0.2) |
| Concomitant procedures | 136 (30.2) | 127 (28.5) |
| CABG | 108 (24.0) | 98 (22.0) |
| Septal myectomy | 17 (3.8) | 14 (3.1) |
| Aortic annulus enlargement | 0 (0.0) | 4 (0.9) |
| Others | 18 (4.0) | 24 (5.4) |
Sievers type 1 only allowed per protocol.
CABG: coronary artery bypass graft; NA: not applicable.
| . | Perceval (n = 450) . | Stented (n = 446) . |
|---|---|---|
| Operative characteristics, n (%) | ||
| Surgical approach | ||
| Full sternotomy | 222 (49.3) | 236 (52.9) |
| Mini-sternotomy | 228 (50.7) | 210 (47.1) |
| Bicuspid aortic valvea | 47 (10.4) | 54 (12.1) |
| Valve size | ||
| S (21 mm) | 41 (9.1) | NA |
| M (23 mm) | 147 (32.7) | NA |
| L (25 mm) | 151 (33.6) | NA |
| XL (27 mm) | 111 (24.7) | NA |
| 19 mm | NA | 22 (4.9) |
| 21 mm | NA | 125 (28.0) |
| 23 mm | NA | 183 (41.0) |
| 25 mm | NA | 104 (23.3) |
| 27 mm | NA | 11 (2.5) |
| 29 mm | NA | 1 (0.2) |
| Concomitant procedures | 136 (30.2) | 127 (28.5) |
| CABG | 108 (24.0) | 98 (22.0) |
| Septal myectomy | 17 (3.8) | 14 (3.1) |
| Aortic annulus enlargement | 0 (0.0) | 4 (0.9) |
| Others | 18 (4.0) | 24 (5.4) |
| . | Perceval (n = 450) . | Stented (n = 446) . |
|---|---|---|
| Operative characteristics, n (%) | ||
| Surgical approach | ||
| Full sternotomy | 222 (49.3) | 236 (52.9) |
| Mini-sternotomy | 228 (50.7) | 210 (47.1) |
| Bicuspid aortic valvea | 47 (10.4) | 54 (12.1) |
| Valve size | ||
| S (21 mm) | 41 (9.1) | NA |
| M (23 mm) | 147 (32.7) | NA |
| L (25 mm) | 151 (33.6) | NA |
| XL (27 mm) | 111 (24.7) | NA |
| 19 mm | NA | 22 (4.9) |
| 21 mm | NA | 125 (28.0) |
| 23 mm | NA | 183 (41.0) |
| 25 mm | NA | 104 (23.3) |
| 27 mm | NA | 11 (2.5) |
| 29 mm | NA | 1 (0.2) |
| Concomitant procedures | 136 (30.2) | 127 (28.5) |
| CABG | 108 (24.0) | 98 (22.0) |
| Septal myectomy | 17 (3.8) | 14 (3.1) |
| Aortic annulus enlargement | 0 (0.0) | 4 (0.9) |
| Others | 18 (4.0) | 24 (5.4) |
Sievers type 1 only allowed per protocol.
CABG: coronary artery bypass graft; NA: not applicable.
The incidence of early PPI was significantly higher in the Su-AVR group in the perioperative phase (10.4%, 47 patients in the Su-AVR group vs 3.1%, 14 patients in the stented group), while the rate after hospital discharge, up to 1-year follow-up, showed no difference (2.3%, 10 patients in the Su-AVR group vs 1.4%, 6 patients in the stented group). The incidence of early PPI in the Perceval group was higher according to the prosthesis size (4.9% in size S; 6.8% in size M; 7.3% in size L; and 21.6% in size XL). The logistic regression analysis in the Perceval group showed a significantly higher risk of PPI with size XL compared to each individual valve sizes (OR 0.272 vs size S, 0.334 vs size M, 0.408 vs size L), but equivalent risk of PPI rates for all other combinations of valve sizes (Fig. 1). The multivariable analysis (Tables 3 and 4) showed that PPI prevalence correlated with valve size XL (P = 0.0119) and preoperative conduction disturbances (P = 0.0179) in the Perceval group. No relevant PPI predictors were found in the SAVR cohort (Tables 5 and 6).
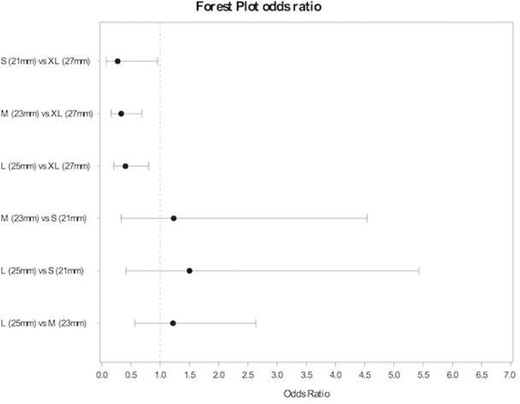
Forest plot. Odds ratio permanent pacemaker implantation early event by valve size (Perceval).
Predictors of permanent pacemaker implantation in the Perceval group: multivariable logistic regression after backward selection in all variables
| . | Intercept . | Valve size S (reference) . | Valve size M . | Valve size L . | Valve size XL . | Age . | Female sex . | Surgical approach full sternotomy . | No concomitant procedure . | No Preoperative Conduction Disorders . |
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | −2.6485 | 0 | −0.2945 | −0.0971 | 0.7762 | 0.0119 | 0.1153 | 0.1561 | 0.1288 | −0.5999 |
| Standard error | 2.0229 | 0.2964 | 0.2775 | 0.3079 | 0.0263 | 0.1913 | 0.1759 | 0.1922 | 0.1705 | |
| P-Value | 0.1904 | 0.3203 | 0.7264 | 0.0117 | 0.6497 | 0.5465 | 0.3749 | 0.5028 | 0.0004 |
| . | Intercept . | Valve size S (reference) . | Valve size M . | Valve size L . | Valve size XL . | Age . | Female sex . | Surgical approach full sternotomy . | No concomitant procedure . | No Preoperative Conduction Disorders . |
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | −2.6485 | 0 | −0.2945 | −0.0971 | 0.7762 | 0.0119 | 0.1153 | 0.1561 | 0.1288 | −0.5999 |
| Standard error | 2.0229 | 0.2964 | 0.2775 | 0.3079 | 0.0263 | 0.1913 | 0.1759 | 0.1922 | 0.1705 | |
| P-Value | 0.1904 | 0.3203 | 0.7264 | 0.0117 | 0.6497 | 0.5465 | 0.3749 | 0.5028 | 0.0004 |
Predictors of permanent pacemaker implantation in the Perceval group: multivariable logistic regression after backward selection in all variables
| . | Intercept . | Valve size S (reference) . | Valve size M . | Valve size L . | Valve size XL . | Age . | Female sex . | Surgical approach full sternotomy . | No concomitant procedure . | No Preoperative Conduction Disorders . |
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | −2.6485 | 0 | −0.2945 | −0.0971 | 0.7762 | 0.0119 | 0.1153 | 0.1561 | 0.1288 | −0.5999 |
| Standard error | 2.0229 | 0.2964 | 0.2775 | 0.3079 | 0.0263 | 0.1913 | 0.1759 | 0.1922 | 0.1705 | |
| P-Value | 0.1904 | 0.3203 | 0.7264 | 0.0117 | 0.6497 | 0.5465 | 0.3749 | 0.5028 | 0.0004 |
| . | Intercept . | Valve size S (reference) . | Valve size M . | Valve size L . | Valve size XL . | Age . | Female sex . | Surgical approach full sternotomy . | No concomitant procedure . | No Preoperative Conduction Disorders . |
|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | −2.6485 | 0 | −0.2945 | −0.0971 | 0.7762 | 0.0119 | 0.1153 | 0.1561 | 0.1288 | −0.5999 |
| Standard error | 2.0229 | 0.2964 | 0.2775 | 0.3079 | 0.0263 | 0.1913 | 0.1759 | 0.1922 | 0.1705 | |
| P-Value | 0.1904 | 0.3203 | 0.7264 | 0.0117 | 0.6497 | 0.5465 | 0.3749 | 0.5028 | 0.0004 |
Predictors of permanent pacemaker implantation in the Perceval group: multivariable logistic regression after backward selection in valves sizes
| . | Intercept . | Valve size reference (S) . | Valve size XL . | No preoperative conduction disorders . |
|---|---|---|---|---|
| Estimate | −1.4105 | 0 | 0.9499 | −1.0846 |
| Standard error | 0.4379 | 0.3778 | 0.4084 | |
| P-Value | 0.0013 | 0.0119 | 0.0079 |
| . | Intercept . | Valve size reference (S) . | Valve size XL . | No preoperative conduction disorders . |
|---|---|---|---|---|
| Estimate | −1.4105 | 0 | 0.9499 | −1.0846 |
| Standard error | 0.4379 | 0.3778 | 0.4084 | |
| P-Value | 0.0013 | 0.0119 | 0.0079 |
Predictors of permanent pacemaker implantation in the Perceval group: multivariable logistic regression after backward selection in valves sizes
| . | Intercept . | Valve size reference (S) . | Valve size XL . | No preoperative conduction disorders . |
|---|---|---|---|---|
| Estimate | −1.4105 | 0 | 0.9499 | −1.0846 |
| Standard error | 0.4379 | 0.3778 | 0.4084 | |
| P-Value | 0.0013 | 0.0119 | 0.0079 |
| . | Intercept . | Valve size reference (S) . | Valve size XL . | No preoperative conduction disorders . |
|---|---|---|---|---|
| Estimate | −1.4105 | 0 | 0.9499 | −1.0846 |
| Standard error | 0.4379 | 0.3778 | 0.4084 | |
| P-Value | 0.0013 | 0.0119 | 0.0079 |
Predictors of permanent pacemaker implantation in the stented valve group: multivariable logistic regression after backward selection in all variables
| . | Intercept . | Valve size 19 (mm) . | Valve size 21 (mm) . | Valve size 23 (mm) . | Valve size 25 (mm) . | Valve size 27 (mm) . | Age . | Female sex . | Surgical approach full sternotomy . | No concomitant procedure . | No preoperative conduction disorders . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | −4.5532 | 4.6357 | 3.7638 | 3.4448 | 3.816 | −7.2384 | −0.0357 | −0.1852 | 0.882 | 0.5488 | −0.241 |
| Standard error | 201.8187 | 201.8006 | 201.7997 | 201.7995 | 201.7997 | 321.9024 | 0.0374 | 0.3153 | 0.2976 | 0.3014 | 0.2773 |
| P-Value | 0.982 | 0.9817 | 0.9851 | 0.9864 | 0.9849 | 0.9821 | 0.3401 | 0.5571 | 0.003 | 0.0686 | 0.3848 |
| . | Intercept . | Valve size 19 (mm) . | Valve size 21 (mm) . | Valve size 23 (mm) . | Valve size 25 (mm) . | Valve size 27 (mm) . | Age . | Female sex . | Surgical approach full sternotomy . | No concomitant procedure . | No preoperative conduction disorders . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | −4.5532 | 4.6357 | 3.7638 | 3.4448 | 3.816 | −7.2384 | −0.0357 | −0.1852 | 0.882 | 0.5488 | −0.241 |
| Standard error | 201.8187 | 201.8006 | 201.7997 | 201.7995 | 201.7997 | 321.9024 | 0.0374 | 0.3153 | 0.2976 | 0.3014 | 0.2773 |
| P-Value | 0.982 | 0.9817 | 0.9851 | 0.9864 | 0.9849 | 0.9821 | 0.3401 | 0.5571 | 0.003 | 0.0686 | 0.3848 |
Predictors of permanent pacemaker implantation in the stented valve group: multivariable logistic regression after backward selection in all variables
| . | Intercept . | Valve size 19 (mm) . | Valve size 21 (mm) . | Valve size 23 (mm) . | Valve size 25 (mm) . | Valve size 27 (mm) . | Age . | Female sex . | Surgical approach full sternotomy . | No concomitant procedure . | No preoperative conduction disorders . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | −4.5532 | 4.6357 | 3.7638 | 3.4448 | 3.816 | −7.2384 | −0.0357 | −0.1852 | 0.882 | 0.5488 | −0.241 |
| Standard error | 201.8187 | 201.8006 | 201.7997 | 201.7995 | 201.7997 | 321.9024 | 0.0374 | 0.3153 | 0.2976 | 0.3014 | 0.2773 |
| P-Value | 0.982 | 0.9817 | 0.9851 | 0.9864 | 0.9849 | 0.9821 | 0.3401 | 0.5571 | 0.003 | 0.0686 | 0.3848 |
| . | Intercept . | Valve size 19 (mm) . | Valve size 21 (mm) . | Valve size 23 (mm) . | Valve size 25 (mm) . | Valve size 27 (mm) . | Age . | Female sex . | Surgical approach full sternotomy . | No concomitant procedure . | No preoperative conduction disorders . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | −4.5532 | 4.6357 | 3.7638 | 3.4448 | 3.816 | −7.2384 | −0.0357 | −0.1852 | 0.882 | 0.5488 | −0.241 |
| Standard error | 201.8187 | 201.8006 | 201.7997 | 201.7995 | 201.7997 | 321.9024 | 0.0374 | 0.3153 | 0.2976 | 0.3014 | 0.2773 |
| P-Value | 0.982 | 0.9817 | 0.9851 | 0.9864 | 0.9849 | 0.9821 | 0.3401 | 0.5571 | 0.003 | 0.0686 | 0.3848 |
Predictors of permanent pacemaker implantation in the stented valve group: multivariable logistic regression after backward selection in valves sizes
| . | Intercept . | Surgical approach full sternotomy . | No concomitant procedure . |
|---|---|---|---|
| Estimate | −5.0826 | 1.7254 | 1.175 |
| Standard error | 0.7693 | 0.5865 | 0.5896 |
| P-Value | 0 | 0.0033 | 0.0463 |
| . | Intercept . | Surgical approach full sternotomy . | No concomitant procedure . |
|---|---|---|---|
| Estimate | −5.0826 | 1.7254 | 1.175 |
| Standard error | 0.7693 | 0.5865 | 0.5896 |
| P-Value | 0 | 0.0033 | 0.0463 |
Predictors of permanent pacemaker implantation in the stented valve group: multivariable logistic regression after backward selection in valves sizes
| . | Intercept . | Surgical approach full sternotomy . | No concomitant procedure . |
|---|---|---|---|
| Estimate | −5.0826 | 1.7254 | 1.175 |
| Standard error | 0.7693 | 0.5865 | 0.5896 |
| P-Value | 0 | 0.0033 | 0.0463 |
| . | Intercept . | Surgical approach full sternotomy . | No concomitant procedure . |
|---|---|---|---|
| Estimate | −5.0826 | 1.7254 | 1.175 |
| Standard error | 0.7693 | 0.5865 | 0.5896 |
| P-Value | 0 | 0.0033 | 0.0463 |
DISCUSSION
We report the results of a prospective, randomized, open-label, non-inferiority trial comparing patients with severe symptomatic aortic valve stenosis undergoing surgical aortic valve replacement, with or without concomitant procedures treated with conventional stented tissue valves versus Perceval sutureless valves, with respect to postoperative conduction disturbances requiring PPI. The findings of the present study can be summarized as follows: (i) perioperative PPI rate was significantly higher in the Su-AVR group, (ii) no difference was found for PPI in the post-hospital discharge period up to 1-year follow-up, (iii) preoperative conduction disturbances and valve size XL were independent predictors of postoperative PPI in the Su-AVR group and (iv) other combinations of valve size did not show statistical difference for PPI rates in the Su-AVR group.
In our cohort, the rate of PPI after Su-AVR was in accordance with previously published experiences [18, 19]. Notwithstanding, in the SAVR cohort, postoperative PPI was rather low, if compared with available data in the literature [10, 20, 21]. Recently, Berretta et al. [22], in their comparison of 243 patients undergoing rapid-deployment valve replacement versus conventional SAVR, showed that the rate of PPI was more than four-fold higher in the rapid-deployment group (10.5% vs 2.1%). The mechanisms of atrioventricular conduction disturbances after Su-AVR leading to PPI are not definitively elucidated yet. Lam et al. [23] investigated a potential learning-curve effect leading to more PPI after Su-AVR. However, the recent series of Mikus et al. [24] emphasized the role of the surgeon’s experience in the postoperative need for PPI after Perceval implant.
Preoperative conduction disorders have already been shown as important predictive factors for PPI after Su-AVR. Specifically, Coti et al. [25] identified a right bundle branch block as a risk factor for postoperative PPI in patients receiving a rapid-deployment aortic valve. In the present trial, preoperative conduction disturbances were predictive factors for postoperative PPI in the Su-AVR group. Also, in the recent retrospective series of Szecel et al. [26], involving 468 patients receiving Perceval valve, the PPI rate was 7.9% in the overall population while it was only 3.9% in the subgroup of patients without pre-existing conduction or rhythm disorders. In addition, Paparella et al. [27], in their analysis of a centralized database involving 11 centres from Italy, found no increased risk of PPI in the Perceval group with respect to the conventional SAVR after adjustment for the presence of preoperative rhythm disturbances. This emphasizes the potential key role of baseline conduction disturbances in developing further atrioventricular conduction defects leading to PPI.
In our study, the use of a valve size XL in the Su-AVR group was an independent predictor of postoperative PPI, while the other valve sizes in the Su-AVR group did not show statistical difference for PPI rates compared to stented valves. This finding is in accordance with the findings by Toledano et al (18), who observed, in their analysis of 140 patients receiving a Perceval implant, a trend towards higher new-onset atrioventricular block with greater sutureless prosthesis size. Indeed, larger valve sizes may have larger sealing collars compared to smaller size, leading to more postoperative PPI [28]. Moreover, the depth of the guiding suture for placing the valve may have a negative impact on post-Su-AVR PPI, as a recent modified insertion of the guiding suture at the base of the aortic annulus has shown to confer lower PPI when using a Perceval valve [29]. Indeed, the greatest sub-annular protrusion when using a Perceval valve size XL with respect to smaller valve sizes may explain the compression of the conduction systems during the deployment of such valve size and the consequent postoperative need for PPI [28, 29]. In addition, results from a European multicentre experience [30] showed a lower incidence of PPI after Su-AVR when using a Perceval valve size S. As the increased PPI rate for sutureless appears to be size dependent with a higher rate for XL size (showing the greatest sub-annular protrusion), the next-generation design (Perceval PLUS), with adapted design to reduce sub-annular valve collar protrusion, should be able to address this crucial aspect. Further clinical investigations are therefore required to evaluate the influence of the new Perceval valve design on this peculiar aspect.
Limitations
Several limitations of this study have to be underlined. This study was performed in a selected, non-consecutive study population, leading to potentials bias. The statistical regression was performed on the 2 separated cohorts and not on the entire population, as the ‘same valve size’ is hardly comparable in the 2 cohorts. The decision about valve size was left to the discretion of the performing surgeon and the indication for PPI was decided by the treating physician from each centres, without consensus across centres. Also, the surgical technique may differ across involved centres and surgeons.
CONCLUSIONS
In conclusion, the increased PPI rate for Su-AVR appears to be size dependent with a higher rate for size XL. The combination of preoperative conduction disorder and a size XL can lead to a higher probability of early PPI in Su-AVR.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
This research project was funded by CORCYM S.r.l.
Conflict of interest: Dr. Roberto Lorusso is a consultant for Medtronic, Getinge and LivaNova and an Advisory Board Member of Eurosets; all honoraria are paid to the University for research support. Martin Andreas has received institutional research funding (Edwards, Abbott, Medtronic, LSI) and has served as a proctor/speaker/consultant (Edwards, Abbott, Medtronic). The other authors report no conflicts of interest.
Data Availability Statement
The data underlying this article are available in the article.
Author contributions
Roberto Lorusso: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization. Justine M. Ravaux: Conceptualization; Data curation; Methodology; Validation; Visualization; Writing—original draft; Writing—review & editing. Francesco Pollari: Conceptualization; Funding acquisition; Investigation; Resources; Validation; Visualization. Thierry A. Folliguet: Conceptualization; Methodology; Resources; Validation; Visualization. Utz Kappert: Conceptualization; Funding acquisition; Methodology; Validation; Visualization. Bart Meuris: Conceptualization; Funding acquisition; Methodology; Validation; Visualization. Malakh L. Shrestha: Conceptualization; Funding acquisition; Methodology; Validation; Visualization. Eric E. Roselli: Conceptualization; Funding acquisition; Methodology; Validation; Visualization. Nikolaos Bonaros: Conceptualization; Funding acquisition; Methodology; Validation; Visualization. Olivier Fabre: Conceptualization; Funding acquisition; Methodology; Validation; Visualization. Pierre Corbi: Conceptualization; Funding acquisition; Methodology; Validation; Visualization. Giovanni Troise: Conceptualization; Funding acquisition; Investigation; Methodology; Validation; Visualization. Martin Andreas: Conceptualization; Funding acquisition; Investigation; Methodology; Validation; Visualization; Writing—review & editing. Frederic Pinaud: Conceptualization; Funding acquisition; Methodology; Project administration; Validation; Visualization. Steffen Pfeiffer: Conceptualization; Methodology; Project administration; Validation; Visualization. Sami Kueri: Conceptualization; Methodology; Resources; Validation; Visualization. Erwin Tan: Conceptualization; Investigation; Methodology; Validation; Visualization. Pierre Voisine: Conceptualization; Funding acquisition; Methodology; Project administration; Validation; Visualization. Evaldas Girdauskas: Conceptualization; Data curation; Methodology; Validation; Visualization. Filip Rega: Conceptualization; Funding acquisition; Methodology; Project administration; Resources; Validation; Visualization. Julio Garcia-Puente: Conceptualization; Funding acquisition; Investigation; Methodology; Validation; Visualization. Theodor Fischlein: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Corey Adams, Michel Carrier, Massimo Meco and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the Society of Thoracic Surgeons 57th Annual Meeting, 29–31 January 2021, Austin, TX, USA.
REFERENCES
ABBREVIATIONS
Author notes
The list of PERSIST-AVR Investigators is available in the Supplementary Material.
- aortic valve
- artificial cardiac pacemaker
- perioperative cardiovascular risk
- cardiac pacemaker implantation
- aortic valve replacement
- bioprosthesis
- cardiac conduction system disorders
- cardiovascular system
- follow-up
- objective (goal)
- preoperative care
- sutures
- brain
- proton pump inhibitors
- pacemaker, permanent
- prostheses
- clinical trial registration





