-
PDF
- Split View
-
Views
-
Cite
Cite
Tomoyuki Hishida, Hisao Asamura, Kazuo Yoshida, Masahiro Tsuboi, Kohei Yokoi, Shinichi Toyooka, Akihide Matsumura, Tetsuzo Tagawa, Meinoshin Okumura, Clinical features and prognostic impact of coexisting autoimmune disease other than myasthenia gravis in resected thymomas: analysis of a Japanese multi-institutional retrospective database, European Journal of Cardio-Thoracic Surgery, Volume 59, Issue 3, March 2021, Pages 641–649, https://doi.org/10.1093/ejcts/ezaa362
Close - Share Icon Share
Abstract
The purpose of this study was to clarify the prevalence, clinical features and survival of patients with thymoma and non-myasthenia gravis autoimmune disease (NMAD) using a nationwide cohort.
The Japanese Association for Research on the Thymus nationwide database, which includes data from 32 institutions, was examined to clarify the prevalence and characteristics of NMAD associated with thymomas and elucidate the prognostic impact of NMAD for thymoma patients.
Among the 2423 patients with thymomas who were surgically treated between 1991 and 2010, 114 (4.7%) were identified with NMAD. The most frequently observed NMAD was pure red cell aplasia (PRCA) in 44 (1.8%), followed by hypogammaglobulinaemia (0.5%) and rheumatic arthritis (0.5%). Twenty-eight percent of patients with NMAD had concomitant myasthenia gravis. The presence of NMAD was not an independent prognostic factor for overall survival (OS) irrespective of the type of NMAD [PRCA+: hazard ratio (HR) 1.99, 95% confidence interval 0.74–4.47; PRCA− NMAD: HR 1.28, 0.30–3.56]; however, there were more cases with advanced age and disease of the thymoma amongst PRCA+ patients and these showed a worse OS than patients with PRCA− NMAD (P < 0.001), who had an OS similar to those without NMAD (P = 0.489). The 10-year OS rates in PRCA+, PRCA− NMAD and NMAD− groups were 45.5%, 97.4% and 89.5%, respectively. The main causes of death in PRCA+ patients were the progression of thymoma and other diseases including pneumonia.
Although the presence of NMAD itself did not significantly affect survival after surgery for thymoma, the type of NMAD was associated with different clinical features and prognosis. The NMAD+ thymomas should be separately categorized according to the presence or absence of PRCA.
INTRODUCTION
The thymus is a primary lymphoid organ associated with T-cell development. Immature T-cell progenitors enter the thymus where, through interactions with cortical and medullary thymic epithelial cells, they undergo positive and negative selection, and become mature T cells [1, 2]. In thymomas, several abnormalities including a distorted thymic architecture and a reduced expression of major histocompatibility complex class II are observed, and these are considered to induce a defective positive and negative selection of T cells and subsequently are associated with the development of autoimmunity [2, 3]. Myasthenia gravis (MG) is, by far, the most common autoimmune disease (AD), and is observed in 23–47% of thymomas [4–7]. Some thymomas are known to coexist with non-MG AD (NMAD), including pure red cell aplasia (PRCA) and hypogammaglobulinaemia (Good’s syndrome) [2, 8]. Other NMADs, such as systemic lupus erythematosus, polymyositis, thyroiditis, lichen planus and other rare conditions have also been reported sporadically [2, 3, 9]. However, the actual prevalence and prognostic impact of NMAD during the surgical treatment of thymomas are still unknown due to the lack of a comprehensive study using a large cohort.
Therefore, we sought to assess the prevalence, clinical features and survival of patients with thymoma and NMAD using a Japanese nationwide multi-institutional database.
PATIENTS AND METHODS
Study cohort
The Japanese Association for Research on the Thymus (JART) developed a nationwide database in 2012 and retrospectively collected data for 2835 thymic epithelial tumour patients who underwent surgical management between 1991 and 2010 at 32 Japanese institutions. From this database, the data for 2423 patients with thymoma were included in the current study. Patients with thymic carcinoma (n = 306), thymic neuroendocrine tumour (n = 64) and other/unclassified thymic tumour (n = 42) were excluded because AD is generally known to be specific in thymomas. This database included patients who were mainly treated with surgery. The vast majority of patients in this database were from the time period 2005 onward.
In this study, the following parameters in the database were evaluated; patient characteristics, stage, tumour size, histological type, type of resection, resection status, perioperative therapies, recurrence and survival. Histology was reviewed and confirmed by pathologists in each institution per the 2004 World Health Organization (WHO) classification [10], and a central pathology review was not performed. Tumour staging was done initially using the Masaoka stage classification and was further described using the eighth International Thymic Malignancy Interest Group (ITMIG)/International Association for the Study of Lung Cancer (IASLC) staging system. The diagnosis of MG and NMAD was made based on the clinical signs, symptoms, and laboratory data by the decision of individual institutions. Total thymectomy was defined as total resection of the thymus with or without en bloc resection of the anterior mediastinal adipose tissue (extended and simple thymectomy). In the extended thymectomy, the borders of resection were the diaphragm caudally, the thyroid gland orally and the phrenic nerves laterally [11]. Partial thymectomy was a thymomectomy [12], which represented a resection of thymoma with a margin of thymic tissue. The resection status was classified into three groups: R0 (complete resection), R1 (microscopically incomplete resection), R2 (macroscopically incomplete resection, biopsy alone or exploratory thoracotomy). The study was approved by the respective institutional review boards, and the need for obtaining informed consent from each patient was waived.
Statistical analysis
The proportion of subcategories in each variable was calculated by excluding unknown or missing data. Continuous variables were compared using Mann–Whitney's U-test, and categorical variables were analysed using contingency tables and Pearson’s χ2 test or Fisher’s exact test. Postoperative morbidities were evaluated in terms of complications requiring treatments as reported by each institution. Operative mortalities were evaluated in terms of 30- and 90-day mortalities. Survival was evaluated in terms of recurrence-free survival (RFS) after R0 resection and overall survival (OS). RFS was defined as the duration from the date of surgery to the date of death or thymoma recurrence and was censored at the date on which the patient was last known to be alive without recurrence. Survival curves were estimated using the Kaplan–Meier method, and differences between groups were evaluated using a log-rank test. Prognostic factors associated with RFS and OS were evaluated using multivariable Cox regression analysis. The model included the factors based on factors in Table 1 including age, gender, history of malignant disease, mode of resection, pathological tumour size, WHO classification (A/AB, B1, B2/B3), tumour, node and metastasis (TNM) stage (I/II, III, IV), resection status (R0, R1/2), preoperative therapy, postoperative therapy and presence of NMAD [no, yes (PRCA+), yes (PRCA−)], and all factors were forcedly included into the Cox model. Performance status and preoperative use of systemic steroids were excluded from the model because of the small number of positive cases. All statistical analyses were performed using JMP software version 8.02 (SAS Institute, Inc., Cary, NC, USA). All P-values reported were two-sided.
Concomitant non-myasthenia gravis autoimmune disease in patients with thymoma
| . | n = 114, n (%) . |
|---|---|
| Pure red cell aplasia | 44 (39) |
| Hypogammaglobulinaemia (Good’s syndrome) | 11 (10) |
| Rheumatic arthritis | 11 (10) |
| Systemic lupus erythematosus | 10 (9) |
| Sjogren’s syndrome | 9 (8) |
| Thyroid disease | 9 (8) |
| Hyperthyroidism | 7 |
| Hypothyroidism | 2 |
| Ulcerative colitis | 3 (3) |
| Autoimmune neutropenia | 3 (3) |
| Stiff person syndrome | 3 (3) |
| Idiopathic thrombocytopenic purpura | 3 (3) |
| Polymyositis | 3 (3) |
| Behçet's disease | 2 (2) |
| Anti-phospholipid antibody syndrome | 2 (2) |
| Paraneoplastic neurological syndrome | 2 (2) |
| Othersa | 9 (8) |
| Number of coexisting non-myasthenia autoimmune disease | |
| 1 | 106 (93) |
| 2 | 7 (6) |
| 3 | 1 (1) |
| Coexistence of myasthenia gravis | 32 (28) |
| . | n = 114, n (%) . |
|---|---|
| Pure red cell aplasia | 44 (39) |
| Hypogammaglobulinaemia (Good’s syndrome) | 11 (10) |
| Rheumatic arthritis | 11 (10) |
| Systemic lupus erythematosus | 10 (9) |
| Sjogren’s syndrome | 9 (8) |
| Thyroid disease | 9 (8) |
| Hyperthyroidism | 7 |
| Hypothyroidism | 2 |
| Ulcerative colitis | 3 (3) |
| Autoimmune neutropenia | 3 (3) |
| Stiff person syndrome | 3 (3) |
| Idiopathic thrombocytopenic purpura | 3 (3) |
| Polymyositis | 3 (3) |
| Behçet's disease | 2 (2) |
| Anti-phospholipid antibody syndrome | 2 (2) |
| Paraneoplastic neurological syndrome | 2 (2) |
| Othersa | 9 (8) |
| Number of coexisting non-myasthenia autoimmune disease | |
| 1 | 106 (93) |
| 2 | 7 (6) |
| 3 | 1 (1) |
| Coexistence of myasthenia gravis | 32 (28) |
Others included Crohn’s disease, IgA nephropathy, autoimmune hepatitis, Addison’s disease, pemphigus, lichen planus, scleroderma (n = 1 in each) and unspecified autoimmune disease (n = 2).
IgA: immunoglobulin A.
Concomitant non-myasthenia gravis autoimmune disease in patients with thymoma
| . | n = 114, n (%) . |
|---|---|
| Pure red cell aplasia | 44 (39) |
| Hypogammaglobulinaemia (Good’s syndrome) | 11 (10) |
| Rheumatic arthritis | 11 (10) |
| Systemic lupus erythematosus | 10 (9) |
| Sjogren’s syndrome | 9 (8) |
| Thyroid disease | 9 (8) |
| Hyperthyroidism | 7 |
| Hypothyroidism | 2 |
| Ulcerative colitis | 3 (3) |
| Autoimmune neutropenia | 3 (3) |
| Stiff person syndrome | 3 (3) |
| Idiopathic thrombocytopenic purpura | 3 (3) |
| Polymyositis | 3 (3) |
| Behçet's disease | 2 (2) |
| Anti-phospholipid antibody syndrome | 2 (2) |
| Paraneoplastic neurological syndrome | 2 (2) |
| Othersa | 9 (8) |
| Number of coexisting non-myasthenia autoimmune disease | |
| 1 | 106 (93) |
| 2 | 7 (6) |
| 3 | 1 (1) |
| Coexistence of myasthenia gravis | 32 (28) |
| . | n = 114, n (%) . |
|---|---|
| Pure red cell aplasia | 44 (39) |
| Hypogammaglobulinaemia (Good’s syndrome) | 11 (10) |
| Rheumatic arthritis | 11 (10) |
| Systemic lupus erythematosus | 10 (9) |
| Sjogren’s syndrome | 9 (8) |
| Thyroid disease | 9 (8) |
| Hyperthyroidism | 7 |
| Hypothyroidism | 2 |
| Ulcerative colitis | 3 (3) |
| Autoimmune neutropenia | 3 (3) |
| Stiff person syndrome | 3 (3) |
| Idiopathic thrombocytopenic purpura | 3 (3) |
| Polymyositis | 3 (3) |
| Behçet's disease | 2 (2) |
| Anti-phospholipid antibody syndrome | 2 (2) |
| Paraneoplastic neurological syndrome | 2 (2) |
| Othersa | 9 (8) |
| Number of coexisting non-myasthenia autoimmune disease | |
| 1 | 106 (93) |
| 2 | 7 (6) |
| 3 | 1 (1) |
| Coexistence of myasthenia gravis | 32 (28) |
Others included Crohn’s disease, IgA nephropathy, autoimmune hepatitis, Addison’s disease, pemphigus, lichen planus, scleroderma (n = 1 in each) and unspecified autoimmune disease (n = 2).
IgA: immunoglobulin A.
RESULTS
Prevalence and characteristics of non-myasthenia gravis autoimmune disease in thymomas
Among 2423 thymoma patients, a total of 114 (4.7%) in this cohort had a coexisting NMAD. Although various NMADs were identified, the most frequently observed NMADs were PRCA in 44 (39%), which constituted 1.8% of all thymomas, followed by hypogammaglobulinaemia (Good’s syndrome), and rheumatic arthritis in 10% each (0.5% each of all thymomas, Table 1). Almost all patients had one NMAD, but 7 patients had 2 NMADs. Among them, a combination of PRCA and hypogammaglobulinaemia was most common (n = 4). One patient with 3 NMADs had Sjogren’s syndrome, systemic lupus erythematosus and paraneoplastic neurological syndrome. Coexisting MG (NMAD+/MG+) was found in 28%, which was almost the same percentage as the patients without NMAD (NMAD−/MG+, 569/2309, 25%). However, MG was frequently accompanied by PRCA− NMADs [36% (25/70) in PRCA− NMADs vs 16% (7/44) in PRCA, P = 0.022]. The MG severity tended to be higher if the patients had a concomitant NMAD. The percentage of the Myasthenia Gravis Foundation of America (MGFA) classification score of 2 or higher was 85% (23/27) in the NMAD+/MG+ patients and 67% (351/522) in the NMAD−/MG+ patients, among 549 patients who were assessed preoperatively (P = 0.051). Among all NMADs, thyroid disease was observed more in patients with MG than in those without MG [6% (7/32) vs 2% (2/82), P < 0.001].
Characteristics of patients with thymoma and concomitant non-myasthenia gravis autoimmune disease
Characteristics of patients with or without non-myasthenia gravis autoimmune disease
As stated above, the severity of MG tended to be different between patients with and without NMAD in spite of similar proportion of MG. Since this might potentially influence the results of comparison between 2 groups, we excluded MG+ patients (n = 601) from the analysis. Table 2 shows the characteristics of patients with NMAD (NMAD+ group, n = 82) who were compared with those without NMAD (NMAD− group, n = 1740). The NMAD+ group included more patients with poor performance status ≥2 and more patients who were administered systemic steroids before surgery. Surgery was frequently performed using median sternotomy and total thymectomy, mainly extended mode of total thymectomy, in the NMAD+ group than in the NMAD− group. Histologically, major WHO types were AB and B1 in both groups, but type B3 seemed to be infrequent in the NMAD+ group relative to its prevalence in the NMAD− group (7% vs 13%). The NMAD+ group had less stage I and more stage IVA disease than the NMAD− group. Preoperative therapy for thymoma tended to be more frequently conducted in the NMAD+ group possibly due to more advanced stages in this population than in the NMAD− group, and the main modalities for the NMAD+ group included chemotherapy (n = 5), pulse steroid therapy (n = 4) and radiotherapy (n = 1). Postoperative therapy, mainly radiotherapy, was similarly conducted in both groups.
| . | Total (n = 1822) . | NMAD+ group (n = 82) . | NMAD− group (n = 1740) . | P-value . |
|---|---|---|---|---|
| Age at operation (years), median (range) | 59 (13–87) | 59 (39–83) | 59 (13–87) | 0.086 |
| Gender, n (%) | ||||
| Male | 838 (46) | 32 (39) | 806 (46) | 0.407 |
| Female | 982 (54) | 50 (61) | 932 (54) | |
| Missing | 2 | 0 | 2 | |
| PS, n (%) | ||||
| 0–1 | 1762 (99) | 78 (96) | 1684 (99) | <0.001 |
| ≥2 | 11 (1) | 3 (4) | 8 (1) | |
| Missing | 49 | 1 | 48 | |
| History of malignant disease, n (%) | ||||
| No | 1590 (90) | 78 (96) | 1512 (90) | 0.053 |
| Yes | 177 (10) | 3 (4) | 174 (10) | |
| Missing | 55 | 1 | 54 | |
| Preoperative use of systemic steroids, n (%) | ||||
| No | 1810 (99) | 73 (89) | 1737 (99.8) | <0.001 |
| Yes | 12 (1) | 9 (11) | 3 (0.2) | |
| Mode of surgical approach, n (%) | ||||
| Median sternotomy | 1428 (78) | 72 (88) | 1356 (78) | 0.035 |
| Thoracotomy, VATS | 393 (22) | 10 (12) | 383 (22) | |
| Missing | 1 | 0 | 1 | |
| Mode of resection, n (%) | ||||
| Total thymectomy | 1432 (80) | 69 (86) | 1363 (79) | <0.001 |
| Extended thymectomy | 677 (38) | 45 (56) | 632 (37)a | |
| Simple thymectomy | 755 (42) | 24 (30) | 731 (42) | |
| Partial thymectomy | 349 (19) | 7 (9) | 342 (20) | |
| Biopsy only | 15 (1) | 4 (5) | 11 (1) | |
| Missing | 26 | 2 | 24 | |
| Pathological tumour size (cm), median (range) | 5.7 (0.8–20) | 6.1 (0.8–15) | 5.7 (0.8–20) | 0.124 |
| WHO classification, n (%) | ||||
| A | 170 (9) | 5 (6) | 165 (9) | 0.179 |
| AB | 587 (33) | 28 (34) | 559 (32) | |
| B1 | 436 (24) | 27 (33) | 409 (24) | |
| B2 | 391 (21) | 16 (20) | 375 (22) | |
| B3 | 238 (13) | 6 (7) | 232 (13) | |
| TNM stage (8th), n (%) | ||||
| I | 1446 (80) | 56 (69) | 1390 (80) | 0.034 |
| II | 28 (1.5) | 3 (4) | 25 (1.5) | |
| IIIA | 196 (11) | 10 (12) | 186 (11) | |
| IIIB | 13 (0.5) | 0 | 13 (1) | |
| IVA | 109 (6) | 10 (12) | 99 (5.5) | |
| IVB | 18 (1) | 2 (3) | 16 (1) | |
| Missing | 12 | 1 | 11 | |
| Resection status, n (%) | ||||
| R0 | 1660 (92) | 65 (79) | 1595 (93) | <0.001 |
| R1 | 75 (4) | 8 (10) | 67 (4) | |
| R2 | 69 (4) | 9 (11) | 60 (3) | |
| Missing | 18 | 0 | 18 | |
| Preoperative therapy, n (%) | ||||
| Yes | 127 (7) | 10 (12) | 117 (7) | 0.057 |
| No | 1695 (93) | 72 (88) | 1623 (93) | |
| Postoperative therapy, n (%) | ||||
| Yes | 367 (20) | 19 (23) | 348 (20) | 0.484 |
| No | 1455 (80) | 63 (77) | 1392 (80) | |
| . | Total (n = 1822) . | NMAD+ group (n = 82) . | NMAD− group (n = 1740) . | P-value . |
|---|---|---|---|---|
| Age at operation (years), median (range) | 59 (13–87) | 59 (39–83) | 59 (13–87) | 0.086 |
| Gender, n (%) | ||||
| Male | 838 (46) | 32 (39) | 806 (46) | 0.407 |
| Female | 982 (54) | 50 (61) | 932 (54) | |
| Missing | 2 | 0 | 2 | |
| PS, n (%) | ||||
| 0–1 | 1762 (99) | 78 (96) | 1684 (99) | <0.001 |
| ≥2 | 11 (1) | 3 (4) | 8 (1) | |
| Missing | 49 | 1 | 48 | |
| History of malignant disease, n (%) | ||||
| No | 1590 (90) | 78 (96) | 1512 (90) | 0.053 |
| Yes | 177 (10) | 3 (4) | 174 (10) | |
| Missing | 55 | 1 | 54 | |
| Preoperative use of systemic steroids, n (%) | ||||
| No | 1810 (99) | 73 (89) | 1737 (99.8) | <0.001 |
| Yes | 12 (1) | 9 (11) | 3 (0.2) | |
| Mode of surgical approach, n (%) | ||||
| Median sternotomy | 1428 (78) | 72 (88) | 1356 (78) | 0.035 |
| Thoracotomy, VATS | 393 (22) | 10 (12) | 383 (22) | |
| Missing | 1 | 0 | 1 | |
| Mode of resection, n (%) | ||||
| Total thymectomy | 1432 (80) | 69 (86) | 1363 (79) | <0.001 |
| Extended thymectomy | 677 (38) | 45 (56) | 632 (37)a | |
| Simple thymectomy | 755 (42) | 24 (30) | 731 (42) | |
| Partial thymectomy | 349 (19) | 7 (9) | 342 (20) | |
| Biopsy only | 15 (1) | 4 (5) | 11 (1) | |
| Missing | 26 | 2 | 24 | |
| Pathological tumour size (cm), median (range) | 5.7 (0.8–20) | 6.1 (0.8–15) | 5.7 (0.8–20) | 0.124 |
| WHO classification, n (%) | ||||
| A | 170 (9) | 5 (6) | 165 (9) | 0.179 |
| AB | 587 (33) | 28 (34) | 559 (32) | |
| B1 | 436 (24) | 27 (33) | 409 (24) | |
| B2 | 391 (21) | 16 (20) | 375 (22) | |
| B3 | 238 (13) | 6 (7) | 232 (13) | |
| TNM stage (8th), n (%) | ||||
| I | 1446 (80) | 56 (69) | 1390 (80) | 0.034 |
| II | 28 (1.5) | 3 (4) | 25 (1.5) | |
| IIIA | 196 (11) | 10 (12) | 186 (11) | |
| IIIB | 13 (0.5) | 0 | 13 (1) | |
| IVA | 109 (6) | 10 (12) | 99 (5.5) | |
| IVB | 18 (1) | 2 (3) | 16 (1) | |
| Missing | 12 | 1 | 11 | |
| Resection status, n (%) | ||||
| R0 | 1660 (92) | 65 (79) | 1595 (93) | <0.001 |
| R1 | 75 (4) | 8 (10) | 67 (4) | |
| R2 | 69 (4) | 9 (11) | 60 (3) | |
| Missing | 18 | 0 | 18 | |
| Preoperative therapy, n (%) | ||||
| Yes | 127 (7) | 10 (12) | 117 (7) | 0.057 |
| No | 1695 (93) | 72 (88) | 1623 (93) | |
| Postoperative therapy, n (%) | ||||
| Yes | 367 (20) | 19 (23) | 348 (20) | 0.484 |
| No | 1455 (80) | 63 (77) | 1392 (80) | |
Six cases of extended thymectomy plus extrapleural pneumonectomy are included.
NMAD: non-myasthenia gravis autoimmune disease; PS: performance status; TNM: tumour, node and metastasis; VATS: video-assisted thoracic surgery; WHO: World Health Organization.
| . | Total (n = 1822) . | NMAD+ group (n = 82) . | NMAD− group (n = 1740) . | P-value . |
|---|---|---|---|---|
| Age at operation (years), median (range) | 59 (13–87) | 59 (39–83) | 59 (13–87) | 0.086 |
| Gender, n (%) | ||||
| Male | 838 (46) | 32 (39) | 806 (46) | 0.407 |
| Female | 982 (54) | 50 (61) | 932 (54) | |
| Missing | 2 | 0 | 2 | |
| PS, n (%) | ||||
| 0–1 | 1762 (99) | 78 (96) | 1684 (99) | <0.001 |
| ≥2 | 11 (1) | 3 (4) | 8 (1) | |
| Missing | 49 | 1 | 48 | |
| History of malignant disease, n (%) | ||||
| No | 1590 (90) | 78 (96) | 1512 (90) | 0.053 |
| Yes | 177 (10) | 3 (4) | 174 (10) | |
| Missing | 55 | 1 | 54 | |
| Preoperative use of systemic steroids, n (%) | ||||
| No | 1810 (99) | 73 (89) | 1737 (99.8) | <0.001 |
| Yes | 12 (1) | 9 (11) | 3 (0.2) | |
| Mode of surgical approach, n (%) | ||||
| Median sternotomy | 1428 (78) | 72 (88) | 1356 (78) | 0.035 |
| Thoracotomy, VATS | 393 (22) | 10 (12) | 383 (22) | |
| Missing | 1 | 0 | 1 | |
| Mode of resection, n (%) | ||||
| Total thymectomy | 1432 (80) | 69 (86) | 1363 (79) | <0.001 |
| Extended thymectomy | 677 (38) | 45 (56) | 632 (37)a | |
| Simple thymectomy | 755 (42) | 24 (30) | 731 (42) | |
| Partial thymectomy | 349 (19) | 7 (9) | 342 (20) | |
| Biopsy only | 15 (1) | 4 (5) | 11 (1) | |
| Missing | 26 | 2 | 24 | |
| Pathological tumour size (cm), median (range) | 5.7 (0.8–20) | 6.1 (0.8–15) | 5.7 (0.8–20) | 0.124 |
| WHO classification, n (%) | ||||
| A | 170 (9) | 5 (6) | 165 (9) | 0.179 |
| AB | 587 (33) | 28 (34) | 559 (32) | |
| B1 | 436 (24) | 27 (33) | 409 (24) | |
| B2 | 391 (21) | 16 (20) | 375 (22) | |
| B3 | 238 (13) | 6 (7) | 232 (13) | |
| TNM stage (8th), n (%) | ||||
| I | 1446 (80) | 56 (69) | 1390 (80) | 0.034 |
| II | 28 (1.5) | 3 (4) | 25 (1.5) | |
| IIIA | 196 (11) | 10 (12) | 186 (11) | |
| IIIB | 13 (0.5) | 0 | 13 (1) | |
| IVA | 109 (6) | 10 (12) | 99 (5.5) | |
| IVB | 18 (1) | 2 (3) | 16 (1) | |
| Missing | 12 | 1 | 11 | |
| Resection status, n (%) | ||||
| R0 | 1660 (92) | 65 (79) | 1595 (93) | <0.001 |
| R1 | 75 (4) | 8 (10) | 67 (4) | |
| R2 | 69 (4) | 9 (11) | 60 (3) | |
| Missing | 18 | 0 | 18 | |
| Preoperative therapy, n (%) | ||||
| Yes | 127 (7) | 10 (12) | 117 (7) | 0.057 |
| No | 1695 (93) | 72 (88) | 1623 (93) | |
| Postoperative therapy, n (%) | ||||
| Yes | 367 (20) | 19 (23) | 348 (20) | 0.484 |
| No | 1455 (80) | 63 (77) | 1392 (80) | |
| . | Total (n = 1822) . | NMAD+ group (n = 82) . | NMAD− group (n = 1740) . | P-value . |
|---|---|---|---|---|
| Age at operation (years), median (range) | 59 (13–87) | 59 (39–83) | 59 (13–87) | 0.086 |
| Gender, n (%) | ||||
| Male | 838 (46) | 32 (39) | 806 (46) | 0.407 |
| Female | 982 (54) | 50 (61) | 932 (54) | |
| Missing | 2 | 0 | 2 | |
| PS, n (%) | ||||
| 0–1 | 1762 (99) | 78 (96) | 1684 (99) | <0.001 |
| ≥2 | 11 (1) | 3 (4) | 8 (1) | |
| Missing | 49 | 1 | 48 | |
| History of malignant disease, n (%) | ||||
| No | 1590 (90) | 78 (96) | 1512 (90) | 0.053 |
| Yes | 177 (10) | 3 (4) | 174 (10) | |
| Missing | 55 | 1 | 54 | |
| Preoperative use of systemic steroids, n (%) | ||||
| No | 1810 (99) | 73 (89) | 1737 (99.8) | <0.001 |
| Yes | 12 (1) | 9 (11) | 3 (0.2) | |
| Mode of surgical approach, n (%) | ||||
| Median sternotomy | 1428 (78) | 72 (88) | 1356 (78) | 0.035 |
| Thoracotomy, VATS | 393 (22) | 10 (12) | 383 (22) | |
| Missing | 1 | 0 | 1 | |
| Mode of resection, n (%) | ||||
| Total thymectomy | 1432 (80) | 69 (86) | 1363 (79) | <0.001 |
| Extended thymectomy | 677 (38) | 45 (56) | 632 (37)a | |
| Simple thymectomy | 755 (42) | 24 (30) | 731 (42) | |
| Partial thymectomy | 349 (19) | 7 (9) | 342 (20) | |
| Biopsy only | 15 (1) | 4 (5) | 11 (1) | |
| Missing | 26 | 2 | 24 | |
| Pathological tumour size (cm), median (range) | 5.7 (0.8–20) | 6.1 (0.8–15) | 5.7 (0.8–20) | 0.124 |
| WHO classification, n (%) | ||||
| A | 170 (9) | 5 (6) | 165 (9) | 0.179 |
| AB | 587 (33) | 28 (34) | 559 (32) | |
| B1 | 436 (24) | 27 (33) | 409 (24) | |
| B2 | 391 (21) | 16 (20) | 375 (22) | |
| B3 | 238 (13) | 6 (7) | 232 (13) | |
| TNM stage (8th), n (%) | ||||
| I | 1446 (80) | 56 (69) | 1390 (80) | 0.034 |
| II | 28 (1.5) | 3 (4) | 25 (1.5) | |
| IIIA | 196 (11) | 10 (12) | 186 (11) | |
| IIIB | 13 (0.5) | 0 | 13 (1) | |
| IVA | 109 (6) | 10 (12) | 99 (5.5) | |
| IVB | 18 (1) | 2 (3) | 16 (1) | |
| Missing | 12 | 1 | 11 | |
| Resection status, n (%) | ||||
| R0 | 1660 (92) | 65 (79) | 1595 (93) | <0.001 |
| R1 | 75 (4) | 8 (10) | 67 (4) | |
| R2 | 69 (4) | 9 (11) | 60 (3) | |
| Missing | 18 | 0 | 18 | |
| Preoperative therapy, n (%) | ||||
| Yes | 127 (7) | 10 (12) | 117 (7) | 0.057 |
| No | 1695 (93) | 72 (88) | 1623 (93) | |
| Postoperative therapy, n (%) | ||||
| Yes | 367 (20) | 19 (23) | 348 (20) | 0.484 |
| No | 1455 (80) | 63 (77) | 1392 (80) | |
Six cases of extended thymectomy plus extrapleural pneumonectomy are included.
NMAD: non-myasthenia gravis autoimmune disease; PS: performance status; TNM: tumour, node and metastasis; VATS: video-assisted thoracic surgery; WHO: World Health Organization.
Characteristics of non-myasthenia gravis autoimmune disease patients according to the type of non-myasthenia gravis autoimmune disease
Since the most common NMAD was PRCA, we compared the clinicopathological features between the patients with PRCA (PRCA+ group, n = 37) and those without (PRCA− group, n = 45) among the NMAD+ group. Table 3 shows the comparative features of each group. Patients in the PRCA+ group were older, had more advanced stage III-IV disease resulting in incomplete R1/2 resection, compared with the PRCA− group. Although histological types were not statistically different between the two groups, type AB was most common in the PRCA+ group and the PRCA− group included most patients in the type B1 category. The prevalence of type B3 was lower in the PRCA+ group than in the PRCA− group. No significant differences in clinicopathological features were observed between the patients with or without other major NMADs, including rheumatic arthritis, hypogammaglobulinaemia and systemic lupus erythematosus (data not shown).
Characteristics of patients with NMAD according to the presence or absence of PRCA
| . | NMAD+ (PRCA+) group (n = 37) . | NMAD+ (PRCA−) group (n = 45) . | P-value . |
|---|---|---|---|
| Age at operation (years), median (range) | 66 (46–80) | 56 (39–83) | <0.001 |
| Gender, n (%) | |||
| Male | 17 (46) | 15 (33) | 0.244 |
| Female | 20 (54) | 30 (67) | |
| PS, n (%) | |||
| ≥2 | 2 (5) | 1 (2) | 0.457 |
| History of malignant disease, n (%) | |||
| Yes | 1 (3) | 2 (5) | 0.662 |
| Preoperative use of systemic steroids, n (%) | |||
| Yes | 3 (8) | 6 (13) | 0.451 |
| Mode of resection, n (%) | |||
| Total thymectomy | 31 (84) | 38 (88) | 0.935 |
| Extended thymectomy | 23 (62) | 22 (51) | |
| Simple thymectomy | 8 (22) | 16 (37) | |
| Pathological tumour size (cm), median (range) | 7.0 (1.4–15) | 5.5 (0.8–14) | 0.096 |
| WHO classification, n (%) | |||
| A | 1 (3) | 4 (9) | 0.172 |
| AB | 16 (43) | 12 (27) | |
| B1 | 10 (27) | 17 (38) | |
| B2 | 9 (24) | 7 (15) | |
| B3 | 1 (3) | 5 (11) | |
| TNM stage (8th),an (%) | |||
| I | 19 (51) | 37 (84) | 0.004 |
| II | 2 (6) | 1 (2) | |
| III | 7 (19) | 3 (7) | |
| IVa | 9 (24) | 1 (2) | |
| IVb | 0 | 2 (5) | |
| Resection status, n (%) | |||
| R0 | 25 (68) | 40 (89) | 0.018 |
| R1/2 | 12 (32) | 5 (11) | |
| . | NMAD+ (PRCA+) group (n = 37) . | NMAD+ (PRCA−) group (n = 45) . | P-value . |
|---|---|---|---|
| Age at operation (years), median (range) | 66 (46–80) | 56 (39–83) | <0.001 |
| Gender, n (%) | |||
| Male | 17 (46) | 15 (33) | 0.244 |
| Female | 20 (54) | 30 (67) | |
| PS, n (%) | |||
| ≥2 | 2 (5) | 1 (2) | 0.457 |
| History of malignant disease, n (%) | |||
| Yes | 1 (3) | 2 (5) | 0.662 |
| Preoperative use of systemic steroids, n (%) | |||
| Yes | 3 (8) | 6 (13) | 0.451 |
| Mode of resection, n (%) | |||
| Total thymectomy | 31 (84) | 38 (88) | 0.935 |
| Extended thymectomy | 23 (62) | 22 (51) | |
| Simple thymectomy | 8 (22) | 16 (37) | |
| Pathological tumour size (cm), median (range) | 7.0 (1.4–15) | 5.5 (0.8–14) | 0.096 |
| WHO classification, n (%) | |||
| A | 1 (3) | 4 (9) | 0.172 |
| AB | 16 (43) | 12 (27) | |
| B1 | 10 (27) | 17 (38) | |
| B2 | 9 (24) | 7 (15) | |
| B3 | 1 (3) | 5 (11) | |
| TNM stage (8th),an (%) | |||
| I | 19 (51) | 37 (84) | 0.004 |
| II | 2 (6) | 1 (2) | |
| III | 7 (19) | 3 (7) | |
| IVa | 9 (24) | 1 (2) | |
| IVb | 0 | 2 (5) | |
| Resection status, n (%) | |||
| R0 | 25 (68) | 40 (89) | 0.018 |
| R1/2 | 12 (32) | 5 (11) | |
One patient in PRCA− group had unknown TNM stage.
NMAD: non-myasthenia gravis autoimmune disease; PRCA: pure red cell aplasia; PS: performance status; TNM: tumour, node and metastasis; WHO: World Health Organization.
Characteristics of patients with NMAD according to the presence or absence of PRCA
| . | NMAD+ (PRCA+) group (n = 37) . | NMAD+ (PRCA−) group (n = 45) . | P-value . |
|---|---|---|---|
| Age at operation (years), median (range) | 66 (46–80) | 56 (39–83) | <0.001 |
| Gender, n (%) | |||
| Male | 17 (46) | 15 (33) | 0.244 |
| Female | 20 (54) | 30 (67) | |
| PS, n (%) | |||
| ≥2 | 2 (5) | 1 (2) | 0.457 |
| History of malignant disease, n (%) | |||
| Yes | 1 (3) | 2 (5) | 0.662 |
| Preoperative use of systemic steroids, n (%) | |||
| Yes | 3 (8) | 6 (13) | 0.451 |
| Mode of resection, n (%) | |||
| Total thymectomy | 31 (84) | 38 (88) | 0.935 |
| Extended thymectomy | 23 (62) | 22 (51) | |
| Simple thymectomy | 8 (22) | 16 (37) | |
| Pathological tumour size (cm), median (range) | 7.0 (1.4–15) | 5.5 (0.8–14) | 0.096 |
| WHO classification, n (%) | |||
| A | 1 (3) | 4 (9) | 0.172 |
| AB | 16 (43) | 12 (27) | |
| B1 | 10 (27) | 17 (38) | |
| B2 | 9 (24) | 7 (15) | |
| B3 | 1 (3) | 5 (11) | |
| TNM stage (8th),an (%) | |||
| I | 19 (51) | 37 (84) | 0.004 |
| II | 2 (6) | 1 (2) | |
| III | 7 (19) | 3 (7) | |
| IVa | 9 (24) | 1 (2) | |
| IVb | 0 | 2 (5) | |
| Resection status, n (%) | |||
| R0 | 25 (68) | 40 (89) | 0.018 |
| R1/2 | 12 (32) | 5 (11) | |
| . | NMAD+ (PRCA+) group (n = 37) . | NMAD+ (PRCA−) group (n = 45) . | P-value . |
|---|---|---|---|
| Age at operation (years), median (range) | 66 (46–80) | 56 (39–83) | <0.001 |
| Gender, n (%) | |||
| Male | 17 (46) | 15 (33) | 0.244 |
| Female | 20 (54) | 30 (67) | |
| PS, n (%) | |||
| ≥2 | 2 (5) | 1 (2) | 0.457 |
| History of malignant disease, n (%) | |||
| Yes | 1 (3) | 2 (5) | 0.662 |
| Preoperative use of systemic steroids, n (%) | |||
| Yes | 3 (8) | 6 (13) | 0.451 |
| Mode of resection, n (%) | |||
| Total thymectomy | 31 (84) | 38 (88) | 0.935 |
| Extended thymectomy | 23 (62) | 22 (51) | |
| Simple thymectomy | 8 (22) | 16 (37) | |
| Pathological tumour size (cm), median (range) | 7.0 (1.4–15) | 5.5 (0.8–14) | 0.096 |
| WHO classification, n (%) | |||
| A | 1 (3) | 4 (9) | 0.172 |
| AB | 16 (43) | 12 (27) | |
| B1 | 10 (27) | 17 (38) | |
| B2 | 9 (24) | 7 (15) | |
| B3 | 1 (3) | 5 (11) | |
| TNM stage (8th),an (%) | |||
| I | 19 (51) | 37 (84) | 0.004 |
| II | 2 (6) | 1 (2) | |
| III | 7 (19) | 3 (7) | |
| IVa | 9 (24) | 1 (2) | |
| IVb | 0 | 2 (5) | |
| Resection status, n (%) | |||
| R0 | 25 (68) | 40 (89) | 0.018 |
| R1/2 | 12 (32) | 5 (11) | |
One patient in PRCA− group had unknown TNM stage.
NMAD: non-myasthenia gravis autoimmune disease; PRCA: pure red cell aplasia; PS: performance status; TNM: tumour, node and metastasis; WHO: World Health Organization.
Perioperative course of the patients with or without non-myasthenia gravis autoimmune disease
There was no significant inter-group difference in morbidities with any grade (n = 11, 13% in the NMAD+ group and n = 181, 10% in the NMAD− group, P = 0.385). Major reported morbidities in the NMAD+ group included wound infection (n = 2) and arrhythmia (n = 2). Within 30 and 90 days postoperatively, 4 (0.2%) and 5 (0.3%) patients died. The cause of death within 90 days was due to possibly unrecovered postoperative complications (n = 4) and tumour progression (n = 1) but all of the patients were in the NMAD− group.
Survival and prognostic factors of patients with thymoma and non-myasthenia gravis autoimmune disease
First, we evaluated the survival of patients with or without NMAD excluding MG+ patients. The RFS after R0 resection and OS curves according to the presence or absence of NMAD are shown in Fig. 1A and B. Although there was no significant difference in RFS between the groups, the NMAD+ group had a worse OS than the NMAD− group (n = 0.034). Next, the survival of the patients with NMAD was compared between the patients with or without PRCA. Figure 2A and B shows RFS curves after R0 and OS curves of each group. As seen in Fig. 2B, patients with PRCA [NMAD+ (PRCA+) group] demonstrated a worse OS than patients with PRCA− NMAD [NMAD+ (PRCA−) group, P < 0.001], who showed an OS similar to those without NMAD (NMAD− group, P = 0.489). The 10-year OS was 45.5% in the NMAD+ (PRCA+) group, 97.4% in the NMAD+ (PRCA−) group and 89.5% in the NMAD− group. The median OS time in the NMAD+ (PRCA+) group was 8.4 years. However, the PRCA was linked with advanced age and stage of thymoma. Multivariate Cox regression analysis showed that the presence of NMAD itself (PRCA+ or PRCA−) was not a statistically independent prognostic factor for RFS after R0 and OS (Table 4). Table 5 summarizes the cause of death according to the status of concomitant NMAD. In the NMAD+ (PRCA+) group, the common causes of death were a progression of thymoma and other diseases including pneumonia, which were more frequent than the remaining groups.
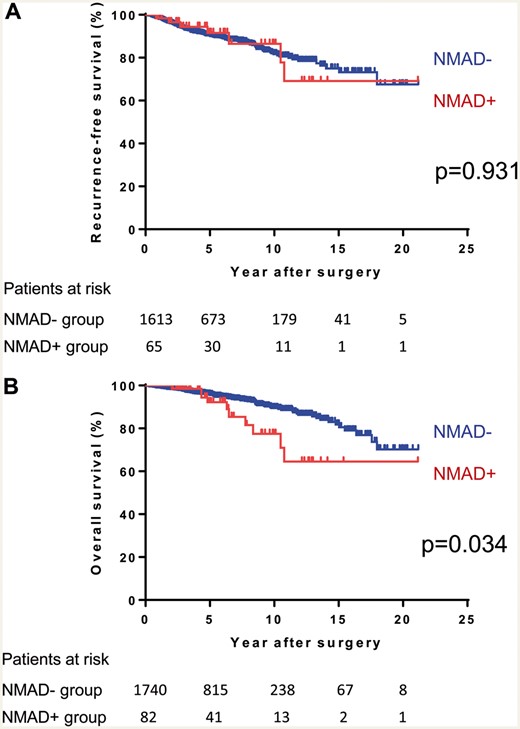
(A) Recurrence-free survival after R0 resection and (B) overall survival curves of thymoma patients with or without NMAD. NMAD: non-myasthenia gravis autoimmune disease.
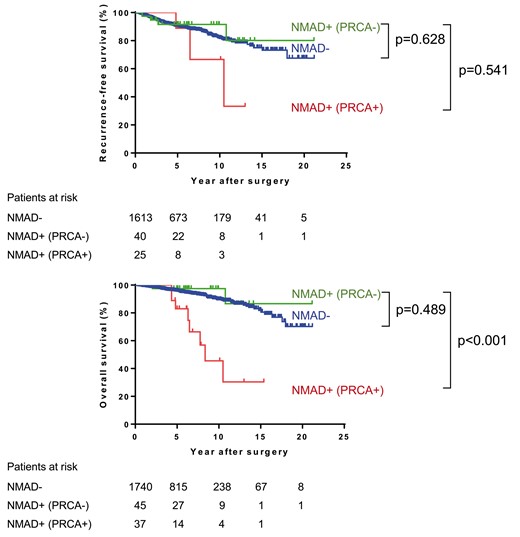
(A) Recurrence-free survival after R0 resection and (B) overall survival curves of thymoma patients according to the status and the type of NMAD. NMAD: non-myasthenia gravis autoimmune disease; PRCA: pure red cell aplasia.
Results of multivariate analyses for prognostic factors associated with RFS after R0 resection and OS
| Variables . |
| P-value . |
| P-value . |
|---|---|---|---|---|
| Age, 1 year up | 1.01 (0.99–1.03) | 0.075 | 1.05 (1.02–1.07) | <0.001 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.29 (0.93–1.78) | 0.115 | 1.27 (0.85–1.89) | 0.230 |
| History of malignant disease | ||||
| No | 1 | 1 | ||
| Yes | 2.55 (1.54–4.05) | <0.001 | 2.57 (1.43–4.36) | 0.002 |
| Mode of resection | ||||
| Total thymectomy | 1 | 1 | ||
| Partial thymectomy | 0.88 (0.53–1.38) | 0.584 | 0.83 (0.45–1.42) | 0.512 |
| Biopsy alone | 0.99 (0.05–5.26) | 0.991 | ||
| Pathological tumour size, 1 cm up | 1.06 (1.00–1.12) | 0.021 | 1.07 (0.99–1.15) | 0.057 |
| WHO classification | ||||
| A/AB | 1 | 1 | ||
| B1 | 1.63 (0.96–2.72) | 0.065 | 1.48 (0.78–2.76) | 0.220 |
| B2/B3 | 3.28 (2.18–5.06) | <0.001 | 2.10 (1.27–3.56) | 0.004 |
| TNM stage | ||||
| I/II | 1 | 1 | ||
| III | 3.77 (2.46–5.74) | <0.001 | 1.97 (1.09–3.46) | 0.024 |
| IV | 6.70 (3.52–12.24) | <0.001 | 3.28 (1.55–6.82) | 0.002 |
| Resection status | ||||
| R0 | 1 | |||
| R1/2 | 1.42 (0.72–2.77) | 0.306 | ||
| Preoperative therapy | ||||
| No | 1 | 1 | ||
| Yes | 1.76 (1.01–2.96) | 0.046 | 2.45 (1.33–4.42) | 0.004 |
| Postoperative therapy | ||||
| No | 1 | 1 | ||
| Yes | 0.93 (0.63–1.36) | 0.715 | 0.88 (0.54–1.43) | 0.620 |
| Presence of NMAD | ||||
| No | 1 | 1 | ||
| Yes (PRCA+) | 1.48 (0.36–3.99) | 0.531 | 1.99 (0.74–4.47) | 0.157 |
| Yes (PRCA−) | 1.52 (0.52–3.42) | 0.399 | 1.28 (0.30–3.56) | 0.693 |
| Variables . |
| P-value . |
| P-value . |
|---|---|---|---|---|
| Age, 1 year up | 1.01 (0.99–1.03) | 0.075 | 1.05 (1.02–1.07) | <0.001 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.29 (0.93–1.78) | 0.115 | 1.27 (0.85–1.89) | 0.230 |
| History of malignant disease | ||||
| No | 1 | 1 | ||
| Yes | 2.55 (1.54–4.05) | <0.001 | 2.57 (1.43–4.36) | 0.002 |
| Mode of resection | ||||
| Total thymectomy | 1 | 1 | ||
| Partial thymectomy | 0.88 (0.53–1.38) | 0.584 | 0.83 (0.45–1.42) | 0.512 |
| Biopsy alone | 0.99 (0.05–5.26) | 0.991 | ||
| Pathological tumour size, 1 cm up | 1.06 (1.00–1.12) | 0.021 | 1.07 (0.99–1.15) | 0.057 |
| WHO classification | ||||
| A/AB | 1 | 1 | ||
| B1 | 1.63 (0.96–2.72) | 0.065 | 1.48 (0.78–2.76) | 0.220 |
| B2/B3 | 3.28 (2.18–5.06) | <0.001 | 2.10 (1.27–3.56) | 0.004 |
| TNM stage | ||||
| I/II | 1 | 1 | ||
| III | 3.77 (2.46–5.74) | <0.001 | 1.97 (1.09–3.46) | 0.024 |
| IV | 6.70 (3.52–12.24) | <0.001 | 3.28 (1.55–6.82) | 0.002 |
| Resection status | ||||
| R0 | 1 | |||
| R1/2 | 1.42 (0.72–2.77) | 0.306 | ||
| Preoperative therapy | ||||
| No | 1 | 1 | ||
| Yes | 1.76 (1.01–2.96) | 0.046 | 2.45 (1.33–4.42) | 0.004 |
| Postoperative therapy | ||||
| No | 1 | 1 | ||
| Yes | 0.93 (0.63–1.36) | 0.715 | 0.88 (0.54–1.43) | 0.620 |
| Presence of NMAD | ||||
| No | 1 | 1 | ||
| Yes (PRCA+) | 1.48 (0.36–3.99) | 0.531 | 1.99 (0.74–4.47) | 0.157 |
| Yes (PRCA−) | 1.52 (0.52–3.42) | 0.399 | 1.28 (0.30–3.56) | 0.693 |
CI: confidence interval; HR: hazard ratio; NMAD: non-myasthenia gravis autoimmune disease; OS: overall survival; PRCA: pure red cell aplasia; PS: performance status; RFS: recurrence-free survival; TNM: tumour, node and metastasis; WHO: World Health Organization.
Results of multivariate analyses for prognostic factors associated with RFS after R0 resection and OS
| Variables . |
| P-value . |
| P-value . |
|---|---|---|---|---|
| Age, 1 year up | 1.01 (0.99–1.03) | 0.075 | 1.05 (1.02–1.07) | <0.001 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.29 (0.93–1.78) | 0.115 | 1.27 (0.85–1.89) | 0.230 |
| History of malignant disease | ||||
| No | 1 | 1 | ||
| Yes | 2.55 (1.54–4.05) | <0.001 | 2.57 (1.43–4.36) | 0.002 |
| Mode of resection | ||||
| Total thymectomy | 1 | 1 | ||
| Partial thymectomy | 0.88 (0.53–1.38) | 0.584 | 0.83 (0.45–1.42) | 0.512 |
| Biopsy alone | 0.99 (0.05–5.26) | 0.991 | ||
| Pathological tumour size, 1 cm up | 1.06 (1.00–1.12) | 0.021 | 1.07 (0.99–1.15) | 0.057 |
| WHO classification | ||||
| A/AB | 1 | 1 | ||
| B1 | 1.63 (0.96–2.72) | 0.065 | 1.48 (0.78–2.76) | 0.220 |
| B2/B3 | 3.28 (2.18–5.06) | <0.001 | 2.10 (1.27–3.56) | 0.004 |
| TNM stage | ||||
| I/II | 1 | 1 | ||
| III | 3.77 (2.46–5.74) | <0.001 | 1.97 (1.09–3.46) | 0.024 |
| IV | 6.70 (3.52–12.24) | <0.001 | 3.28 (1.55–6.82) | 0.002 |
| Resection status | ||||
| R0 | 1 | |||
| R1/2 | 1.42 (0.72–2.77) | 0.306 | ||
| Preoperative therapy | ||||
| No | 1 | 1 | ||
| Yes | 1.76 (1.01–2.96) | 0.046 | 2.45 (1.33–4.42) | 0.004 |
| Postoperative therapy | ||||
| No | 1 | 1 | ||
| Yes | 0.93 (0.63–1.36) | 0.715 | 0.88 (0.54–1.43) | 0.620 |
| Presence of NMAD | ||||
| No | 1 | 1 | ||
| Yes (PRCA+) | 1.48 (0.36–3.99) | 0.531 | 1.99 (0.74–4.47) | 0.157 |
| Yes (PRCA−) | 1.52 (0.52–3.42) | 0.399 | 1.28 (0.30–3.56) | 0.693 |
| Variables . |
| P-value . |
| P-value . |
|---|---|---|---|---|
| Age, 1 year up | 1.01 (0.99–1.03) | 0.075 | 1.05 (1.02–1.07) | <0.001 |
| Gender | ||||
| Female | 1 | 1 | ||
| Male | 1.29 (0.93–1.78) | 0.115 | 1.27 (0.85–1.89) | 0.230 |
| History of malignant disease | ||||
| No | 1 | 1 | ||
| Yes | 2.55 (1.54–4.05) | <0.001 | 2.57 (1.43–4.36) | 0.002 |
| Mode of resection | ||||
| Total thymectomy | 1 | 1 | ||
| Partial thymectomy | 0.88 (0.53–1.38) | 0.584 | 0.83 (0.45–1.42) | 0.512 |
| Biopsy alone | 0.99 (0.05–5.26) | 0.991 | ||
| Pathological tumour size, 1 cm up | 1.06 (1.00–1.12) | 0.021 | 1.07 (0.99–1.15) | 0.057 |
| WHO classification | ||||
| A/AB | 1 | 1 | ||
| B1 | 1.63 (0.96–2.72) | 0.065 | 1.48 (0.78–2.76) | 0.220 |
| B2/B3 | 3.28 (2.18–5.06) | <0.001 | 2.10 (1.27–3.56) | 0.004 |
| TNM stage | ||||
| I/II | 1 | 1 | ||
| III | 3.77 (2.46–5.74) | <0.001 | 1.97 (1.09–3.46) | 0.024 |
| IV | 6.70 (3.52–12.24) | <0.001 | 3.28 (1.55–6.82) | 0.002 |
| Resection status | ||||
| R0 | 1 | |||
| R1/2 | 1.42 (0.72–2.77) | 0.306 | ||
| Preoperative therapy | ||||
| No | 1 | 1 | ||
| Yes | 1.76 (1.01–2.96) | 0.046 | 2.45 (1.33–4.42) | 0.004 |
| Postoperative therapy | ||||
| No | 1 | 1 | ||
| Yes | 0.93 (0.63–1.36) | 0.715 | 0.88 (0.54–1.43) | 0.620 |
| Presence of NMAD | ||||
| No | 1 | 1 | ||
| Yes (PRCA+) | 1.48 (0.36–3.99) | 0.531 | 1.99 (0.74–4.47) | 0.157 |
| Yes (PRCA−) | 1.52 (0.52–3.42) | 0.399 | 1.28 (0.30–3.56) | 0.693 |
CI: confidence interval; HR: hazard ratio; NMAD: non-myasthenia gravis autoimmune disease; OS: overall survival; PRCA: pure red cell aplasia; PS: performance status; RFS: recurrence-free survival; TNM: tumour, node and metastasis; WHO: World Health Organization.
| Cause of death . | NMAD− group (n = 1740), n (%) . | NMAD+ (PRCA−) group (n = 45), n (%) . | NMAD+ (PRCA+) group (n = 37), n (%) . | P-valuea . |
|---|---|---|---|---|
| All deaths | 105 (6.0) | 2 (4.4) | 8 (21.6) | <0.001 |
| Progression of thymoma | 35 (2.0) | 1 (2.2) | 3 (8.1) | 0.011 |
| Other malignancies | 21 (1.2) | 0 | 0 | |
| Deterioration of AD | 2 (0.1)b | 0 | 1 (2.7)c | |
| Other disease/unknown | 47 (2.7)d | 1 (2.2)e | 4 (10.8)f | 0.003 |
| Cause of death . | NMAD− group (n = 1740), n (%) . | NMAD+ (PRCA−) group (n = 45), n (%) . | NMAD+ (PRCA+) group (n = 37), n (%) . | P-valuea . |
|---|---|---|---|---|
| All deaths | 105 (6.0) | 2 (4.4) | 8 (21.6) | <0.001 |
| Progression of thymoma | 35 (2.0) | 1 (2.2) | 3 (8.1) | 0.011 |
| Other malignancies | 21 (1.2) | 0 | 0 | |
| Deterioration of AD | 2 (0.1)b | 0 | 1 (2.7)c | |
| Other disease/unknown | 47 (2.7)d | 1 (2.2)e | 4 (10.8)f | 0.003 |
NMAD+ (PRCA+) group was compared with NMAD− and NMAD+ (PRCA−) groups.
Two patients died of late-onset MG developed during postoperative course.
Deterioration of PRCA.
Pneumonia (n = 6), sepsis (n = 1), empyema (n = 1), postoperative infection (n = 1), suicide (n = 1) and unknown disease (n = 37).
Unknown disease.
Pneumonia (n = 1) and unknown disease (n = 3).
AD, autoimmune disease; MG, myasthenia gravis; NMAD, non-myasthenia gravis autoimmune disease; PRCA, pure red cell aplasia.
| Cause of death . | NMAD− group (n = 1740), n (%) . | NMAD+ (PRCA−) group (n = 45), n (%) . | NMAD+ (PRCA+) group (n = 37), n (%) . | P-valuea . |
|---|---|---|---|---|
| All deaths | 105 (6.0) | 2 (4.4) | 8 (21.6) | <0.001 |
| Progression of thymoma | 35 (2.0) | 1 (2.2) | 3 (8.1) | 0.011 |
| Other malignancies | 21 (1.2) | 0 | 0 | |
| Deterioration of AD | 2 (0.1)b | 0 | 1 (2.7)c | |
| Other disease/unknown | 47 (2.7)d | 1 (2.2)e | 4 (10.8)f | 0.003 |
| Cause of death . | NMAD− group (n = 1740), n (%) . | NMAD+ (PRCA−) group (n = 45), n (%) . | NMAD+ (PRCA+) group (n = 37), n (%) . | P-valuea . |
|---|---|---|---|---|
| All deaths | 105 (6.0) | 2 (4.4) | 8 (21.6) | <0.001 |
| Progression of thymoma | 35 (2.0) | 1 (2.2) | 3 (8.1) | 0.011 |
| Other malignancies | 21 (1.2) | 0 | 0 | |
| Deterioration of AD | 2 (0.1)b | 0 | 1 (2.7)c | |
| Other disease/unknown | 47 (2.7)d | 1 (2.2)e | 4 (10.8)f | 0.003 |
NMAD+ (PRCA+) group was compared with NMAD− and NMAD+ (PRCA−) groups.
Two patients died of late-onset MG developed during postoperative course.
Deterioration of PRCA.
Pneumonia (n = 6), sepsis (n = 1), empyema (n = 1), postoperative infection (n = 1), suicide (n = 1) and unknown disease (n = 37).
Unknown disease.
Pneumonia (n = 1) and unknown disease (n = 3).
AD, autoimmune disease; MG, myasthenia gravis; NMAD, non-myasthenia gravis autoimmune disease; PRCA, pure red cell aplasia.
DISCUSSION
This study is the current largest comprehensive study of NMADs in patients with thymomas. NMAD was found in 4.7% of all patients with thymomas who were candidates for surgery and many types of AD were included in addition to previously known PRCA and hypogammaglobulinaemia (Good’s syndrome). The NMAD+ thymomas had unique clinical features compared with NMAD− thymomas. Histologically, among the NMAD+ thymomas, type B3 thymomas, which is an aggressive form of thymoma [13], tended to be infrequent; however, there were more advanced disease and subsequent R1–2 cases in the NMAD+ population, especially in PRCA+ patients. Multivariate analysis did not identify the presence of NMAD itself as a significant prognostic factor irrespective of the type of NMAD but the PRCA+ thymomas were related to worse survival than other thymomas.
PRCA is a rare disorder but is the second most common AD associated with thymomas, as in the current study (1.8% of all thymomas) [14]. Thymoma-associated PRCA was first described in 1928 by Matras and Priesel and accounted for 8.5–22% of all PRCA cases in recent series [8, 15]. As for its mechanism, several hypotheses have been proposed including direct or indirect T-cell proliferation by thymomas that inhibits erythroid differentiation [15, 16]; however, the detailed pathophysiology has not been clarified. Histologically, PRCA+ thymomas often contain spindle-shaped epithelial cells [16] and a recent Japanese multicentre survey showed that type AB was the predominant histology, as demonstrated in our results [15]. In this study, it was remarkable that PRCA+ thymomas were linked with advanced stages and subsequent incomplete resection. Frequent advanced stage and subsequent incomplete resection in patients with thymoma-associated PRCA have been reported in previous single institutional studies and our results supported these findings [17, 18]. This implied that some thymoma-associated PRCAs developed as a result of accumulated autoimmune abnormalities caused by the progression of thymoma.
In this study, the main causes of death in the PRCA+ patients were progression/recurrence of thymoma and other diseases including pneumonia, which were more frequent than in PRCA-negative patients. Increased thymoma progression/recurrence can be explained by more advanced disease at surgery. The actual reason for the increase in other diseases was unknown because of unavailable detailed information but these might be associated with immunodeficient conditions potentially induced by the treatment of PRCA and secondary AD during postoperative follow-up. For thymoma-associated PRCA, thymectomy is usually insufficient for the normalization of erythropoiesis. Repeated blood transfusion and immunosuppressive therapy are usually required even after complete resection with total thymectomy [8, 15]. A recent study showed that induction therapy using cyclosporine induced an excellent response rate of 95% and contributed to a favourable median OS of 142.1 months [15, 19]. However, many patients received long-term maintenance immunosuppressive therapy, which might potentially cause immunodeficient conditions and infectious diseases including pneumonia in the late period [19]. A recent systemic review reported that 24% of NMAD+ thymoma patients had a relapse or new onset of AD after surgery [20], and several investigators showed that concomitant hypogammaglobulinaemia arising in PRCA patients might induce a severe immunocompromised state [9, 21]. For PRCA+ thymoma patients, postoperative close follow-up of immune status including periodic measurement of immune-related test values such as plasma gamma globulin might be desirable. In addition, prevention and appropriate management of infectious complications might be crucial for further survival improvement of thymoma-associated PRCA.
This study showed that NMAD and MG were not mutually exclusive, and coexisting MG was found especially in patients with NMADs other than PRCA (36%). Histologically, the NMAD+ and the NMAD− group showed a predominance of type AB and B1, whereas many previous studies have identified type B2 as the most common histology in thymomas with MG [1, 4, 5]. These studies suggested that the mechanism by which NMAD develops was different from that of MG in thymomas. However, the severity of MG, defined as an MGFA score of 2 or higher, tended to be higher in the NMAD+/MG+ patients than in the NMAD−/MG+ patients. These indicated that some NMADs might be related to MG in terms of these occurrences.
As for the mode of resection, total thymectomy by extended thymectomy is often indicated in MG patients but its utility in the NMAD patients is unclear. In this series, extended thymectomy was still the main mode of resection in thymoma patients with NMAD. A previous study hypothesized that thymectomy may decrease the activity of T helper cells or it may enhance the activity of suppressor T cells, whose function was depressed in ADs [22]. However, the therapeutic value of maximal removal of thymic tissue has not been proven in NMAD+ patients. Masaoka et al. pathologically examined resected thymic tissue adjacent to thymoma in 14 PRCA+ thymomas [16]. In all cases, the thymus showed involution with disappeared cortex and no germinal centre, which marked a distinct contrast with the myasthenic thymus. These findings might be an age-related involution but this suggested PRCA+ thymus was non-functional. Masaoka et al. also showed no therapeutic difference for PRCA between extended and simple thymectomy among PRCA+ thymomas. Although further studies are needed, extended thymectomy is presumably not required for NMAD+/MG− thymomas.
Limitations
This study is the largest comprehensive series to analyse the clinical profiles and survival of thymoma patients with NMAD in detail. One of the strengths of this study is that a nationwide large database with little missing information was used, although the patient enrolment was finished in 2010. The current results may be used as a reference for the background and surgical outcomes of thymoma patients with NMAD in clinical practice at multiple institutions. Limitations included a retrospective design with a selection bias. Thus, the current results should be interpreted as descriptive. Data on the preoperative grade of clinical symptoms and postoperative course for NMAD were also not available because of no unified criteria to evaluate these for various NMADs. As for PRCA, we could not analyse the association between the administration of cyclosporin and prognosis. Postoperative NMAD status and treatment should be evaluated in future prospective studies of thymomas.
CONCLUSION
In conclusion, this nationwide retrospective study showed that NMAD was found in 4.7% of surgically treated thymomas. The NMAD+ thymomas showed unique clinical and histological features compared with NMAD− thymomas. Although the presence of NMAD itself was not a significant prognostic factor after thymoma resection, the type of NMAD (PRCA+ or PRCA−) was associated with different clinical features and prognosis. Thymoma patients with NMAD can be treated using the same surgical indication as those without NMAD but the PRCA+ thymoma patients should be categorized and managed separately from the PRCA− patients. Careful postoperative monitoring for immune status and infectious disease might be crucial for improving the survival of PRCA+ thymoma patients.
ACKNOWLEDGEMENTS
The authors thank all of the institutions that participated in the Japanese Association for Research on the Thymus (JART) project to develop a nationwide database for thymic epithelial tumours.
Conflict of interest: none declared.
Author contributions
Tomoyuki Hishida: Conceptualization; Formal analysis; Investigation; Methodology; Visualization; Writing—original draft. Hisao Asamura: Conceptualization; Supervision; Writing—review & editing. Kazuo Yoshida: Writing—review & editing. Masahiro Tsuboi: Writing—review & editing. Kohei Yokoi: Writing—review & editing. Shinichi Toyooka: Writing—review & editing. Akihide Matsumura: Writing—review & editing. Tetsuzo Tagawa: Writing—review & editing. Meinoshin Okumura: Project administration; Resources; Supervision; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thank the anonymous reviewer(s) for their contribution to the peer review process of this article.
Presented at the 33rd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 3–5 October 2019.
REFERENCES
1
2
3
4
5
6
7
8
9
11
12
13
14
15
16
17
18
19
20
21
22
ABBREVIATIONS
- AD
Autoimmune disease
- HR
Hazard ratio
- MG
Myasthenia gravis
- NMAD
Non-myasthenia gravis autoimmune disease
- OS
Overall survival
- PRCA
Pure red cell aplasia
- RFS
Recurrence-free survival
- WHO
World Health Organization





