-
PDF
- Split View
-
Views
-
Cite
Cite
Dmitrii Sekhniaidze, Diego Gonzalez-Rivas, Pavel Kononets, Alejandro Garcia, Vladimir Shneider, Malik Agasiev, Ivan Ganzhara, Uniportal video-assisted thoracoscopic carinal resections: technical aspects and outcomes, European Journal of Cardio-Thoracic Surgery, Volume 58, Issue Supplement_1, August 2020, Pages i58–i64, https://doi.org/10.1093/ejcts/ezaa120
Close - Share Icon Share
Abstract
Important benefits in uniportal video-assisted thoracoscopic surgery (VATS) for lung cancer have recently been achieved. However, the use of this technique for complex sleeve procedures is limited. We describe the technical aspects of and patient outcomes following carinal resections using uniportal VATS.
Since 2015, 16 sleeve carinal resections, including 11 right pneumonectomies, 4 right upper lobectomies and 1 lung-sparing carinal resection, have been performed at the Regional Clinic Hospital, Tyumen, Russia.
The mean surgical time was 215.9 ± 67.2 min (range 125–340 min). The mean blood loss volume was 256.3 ± 284.5 ml (range 50–1200 ml). There was 1 case of conversion to thoracotomy. The morbidity rate was 25%, and the mortality rate was 0%. The median overall survival was 38.6 ± 3.5 months.
The use of uniportal VATS for carinal resections in certain patients allows for radical resections with low rates of morbidity and mortality.
INTRODUCTION
Carinal resection is arguably the most challenging procedure in general thoracic surgery and is completed at only a few centres worldwide, likely because of its technical complexity and limited patient benefit. However, good survival results can be expected in patients with pN0- or pN1-stage lung cancer, so these surgical approaches are effective options if performed by experienced surgeons [1].
Today, video-assisted thoracoscopic surgery (VATS) has been widely applied to treat lung cancer. However, guidelines for the treatment of non-small-cell lung cancer only recommend VATS for stage 1 tumours but not for more locally advanced cancers. A few reports from Eastern countries have described the use of VATS for carinal resections [2–4], but few describe the use of uniportal VATS (UVATS) in such procedures. Although the use of VATS and UVATS for carinal resections is reportedly both practical and safe, their utility is limited, with no survival data available [5, 6]. We describe the technical aspects and patient outcomes for carinal resections using UVATS at our hospital since 2015.
PATIENTS AND METHODS
From 2015 to 2018, 16 patients with tumours of the right main bronchus and carina received radical resections at the Regional Clinic Hospital, Tyumen, Russia. All 16 surgeries were performed using UVATS. A central lung tumour requiring carinal resection to enable radical resection was an indicator for minimally invasive carinal resection. Tumour invasion did not extend >2 cm from the lower trachea or 1.5 cm from the opposite main bronchus. The main contraindication for a carinal resection using VATS was a tumour size of >8 cm. In our opinion, lung resection can be performed by UVATS with any tumour size.
Surgical technique
All procedures were performed following oncological principles: tumour-free margins were confirmed by intraoperative frozen section, en bloc resection and systematic lymphadenectomy.
Management of anaesthesiology was comparable to that for open resection. Initially, intubation was performed using a double lumen tube. Once the resection was completed, a high-frequency jet ventilation catheter was inserted into the left main bronchus with the patient positioned in the left lateral decubitus position.
All procedures were performed using UVATS with an incision made in the 4th or 5th intercostal space at the right midaxillary line.
All margins were confirmed to be tumour free by frozen section prior to anastomosis.
Optimal surgical field exposure
The creation of adequate exposure of the surgical field under direct visual control is necessary for the safe execution of all stages of the procedure. After creating a single port between 4 and 6 cm in length, a wound protector was placed to provide optimal exposure. Before dissecting the lung hilum, all adhesions were divided. The azygos vein was transected using vascular clips or ligatures. To create the optimal exposure of the paratracheal space, we fixed the stumps of the azygos vein to the mediastinal and costal pleurae with sutures (Fig. 1).
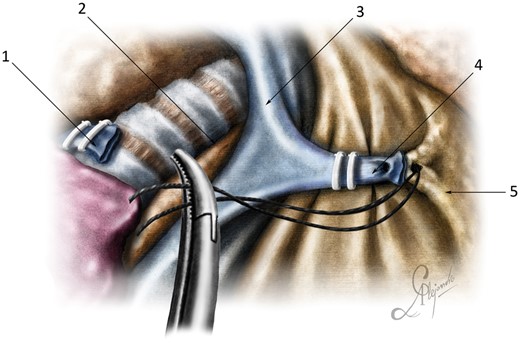
Optimal surgical exposure: (1) posterior stump of the azygos vein; (2) paratracheal tissue; (3) superior vena cava; (4) anterior stump of the azygos vein; and (5) anterior mediastinum.
In cases of sleeve right upper lobectomies and lung-sparing resections, a vascular tourniquet was used on the pulmonary artery, to provided more space for manipulation during reconstruction (Fig. 2).

Optimal surgical exposure: (1) lung; (2) bronchus intermedius; (3) left main bronchus; (4) trachea; (5) anterior stump of azygos vein; (6) right pulmonary artery; and (7) tourniquet.
Nodes first technique
After the division of all adhesions and optimal exposure of the surgical field, a systematic mediastinal lymphadenectomy (the nodes first technique) was performed. During lymphadenectomy, all structures of the mediastinum and the lung hilum were exposed and the ability to perform radical resection was confirmed. Mediastinal lymphadenectomy included removing the hilar (#10), subcarinal (#7) and right paratracheal (#2R and #4R) lymph nodes. When removing a block of nodes, it is important to preserve the blood supply to the tracheal wall and main bronchi for optimal healing of the tracheobronchial anastomosis.
The mediastinal pleura was opened along the vagus nerve. And the block of subcarinal lymph nodes was separated from the wall of the oesophagus. In this case, it was necessary to ensure that the active branch of the energy device scalpel did not contact the wall of the oesophagus and the membrane of the bronchi. A nasogastric tube was not used as it would prevent adequate visualization during UVATS subcarinal lymph node dissection. The middle chest oesophagus was partially mobilized from the main bronchi and moved posteriorly for better visualization of the subcarinal space. As a result, the branches of the bronchial artery could be isolated and clipped, creating additional conditions for working in a ‘dry’ surgical field.
Then, right paratracheal lymph node dissection was performed. The mediastinal pleura was dissected in the cranial direction along the border of the superior vena cava (along the phrenic nerve) and the right vagus nerve. After cutting the azygos vein and exposure, lymph node dissection was straightforward. The lower semicircle of the brachiocephalic artery was the upper point of the dissection.
Mediastinal lymphadenectomy performed during the first stage of the surgery allowed free access to the mobilized bronchial structures and permitted visual assessment of the border of the tumour when evaluating the possibility of resection. In addition, after dissecting the lymph nodes, all structures of the lung hilum and trachea become mobile, resulting in safer intersection.
Anastomosis
Two types of anastomotic reconstructions were used. The first was relatively simple, consisting of an end-to-end, tracheobronchial anastomosis with sleeve carinal pneumonectomy. The second type was double-barrel reconstruction and was required for sleeve carinal right upper lobectomy and sleeve carinal lung-sparing resection.
After carinal resection, a mismatch of stump diameter often occurred. This could be overcome using telescopic techniques invaginating the smaller diameter into the larger.
When performing tracheobronchial suturing using UVATS, it was very important to position the camera in the posterior part of the incision, so that both hands were visible beneath the camera. Then, the same principle was applied as for an open anterior thoracotomy: the surgeon had a direct view of their hands. The dimensions of the reconstruction were important to consider when planning the use of only a single incision [7].
A single tracheobronchial anastomosis was always performed in an end-to-end fashion using a running suture. Monofilament suture material (both non-absorbable and absorbable) such as Prolene 3/0 and polydioxanone sutures 3/0 with 2 needles (Ethicon, Somerville, NJ, USA) were typically used. The running suture began at the left cartilage–membranous junction of the trachea and the bronchus. The suture first joined the left side wall of the trachea to the left side wall of the left main bronchus. Then, the cartilaginous and membranous sections were sutured continuously with both ends of the thread directed towards each other and tied on the right wall of the tracheobronchial anastomosis (Fig. 3 and Video 1).
End-to-end tracheobronchial anastomosis.
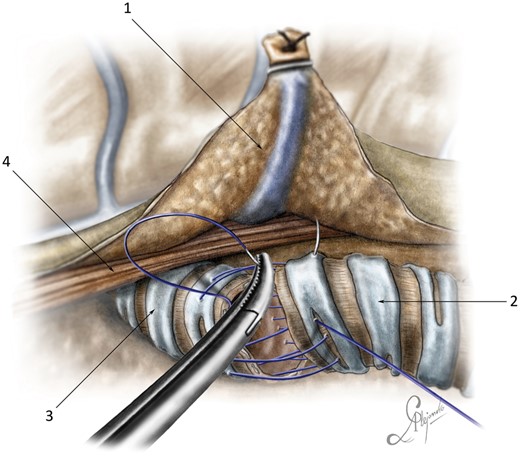
End-to-end tracheobronchial anastomosis: (1) posterior stump of the azygos vein; (2) trachea; (3) left main bronchus; and (4) oesophagus.
The double-barrel tracheobronchial reconstruction is technically more difficult. It involves the reimplantation of the left main bronchus and the bronchus intermedius to the trachea with construction of a neo-carina. Anastomosis began from the left wall of the trachea and the left main bronchus. Cartilaginous and membranous regions were sequentially joined using a continuous suture. Next, a neo-carina between the medial walls of the left main bronchus and the intermediate bronchi (or the right main bronchus in the case of lung sparing) was created using interrupted sutures (Fig. 4).

Double-barrel carinal reconstruction: (1) right main bronchus; (2) trachea; (3) left main bronchus; and (4) suction.
Then, a continuous suture was used between the trachea and the bronchus intermedius as for single tracheobronchial anastomosis.
Covering
The anastomosis was always covered to prevent leakage. In cases of a short and limited resection without tension or risk of leakage, a mediastinal flap could be used. In this case, a flap of mediastinal pleura and fat was mobilized with an energy device from the anterior mediastinum and attached to the anastomosis with separate sutures.
In cases of a high risk of anastomotic leakage, the diaphragm was used to cover the anastomosis. A flap was cut so that the lower diaphragmatic vessels maintained vascularity (Fig. 5).
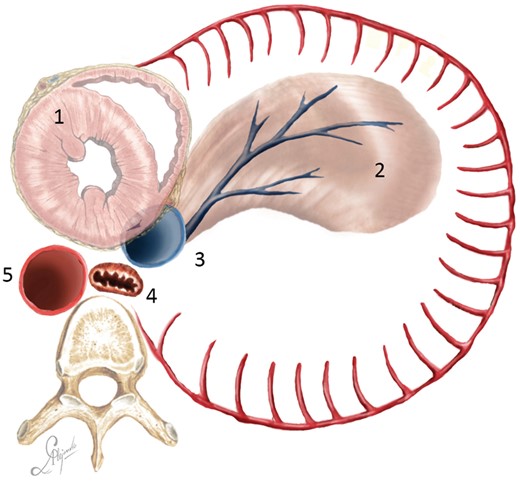
Diaphragmatic flap cutting scheme (bird view): (1) heart; (2) diaphragmatic flap; (3) inferior vena cava; (4) oesophagus; and (5) descending aorta.
Any part of the diaphragm that was sufficiently wide and long enough could be used. After cutting the flap, the defect in the diaphragm was closed with a continuous suture. In all cases, we prepared the flap so that the apical section extended 2–4 cm higher than the tracheobronchial anastomosis.
The flap was fixed with 4 U-shaped relaxation sutures around the ipsilateral semicircle of the tracheobronchial anastomosis. The first suture was passed through the para-aortic fascia at the level of the tracheobronchial anastomosis. The lower and front sutures were passed through the medial wall of the contralateral main bronchus and the anterior wall of the anastomosis. The upper suture was passed through all layers of the lateral tracheal wall. Finally, the tracheobronchial anastomosis was tightly wrapped (Fig. 6 and Video 2).
Diaphragmatic flap fixation.
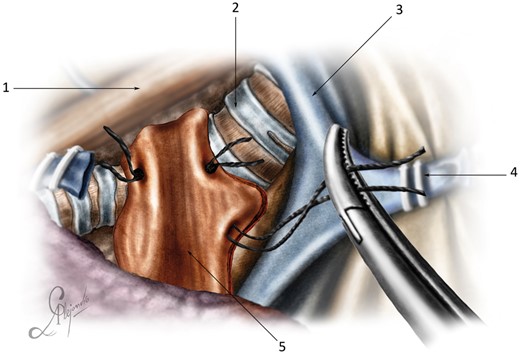
Diaphragmatic flap fixation: (1) oesophagus; (2) trachea; (3) superior vena cava; (4) anterior stump of the azygos vein; and (5) diaphragmatic flap.
Statistical analysis
All statistical tests were performed using IBM SPSS Statistics 20.0 for Windows (USA). Measurement data were shown as the mean (standard deviation). For the survival analysis, we used the Kaplan–Meier method and compared by the log-rank test.
RESULTS
The mean age of the patients (15 men, 1 woman) was 59 ± 4.0 years (range 54–69 years). All tumours were diagnosed as pathologically malignant using a fibre bronchoscope prior to surgery. Seven (40%) patients in our study were subjected to neoadjuvant chemotherapy (via tumour board decision). The mean number of neoadjuvant chemotherapy courses was 3.8 ± 1.1 courses (range 3–6 courses) (Table 1).
| Variables . | Age . | Gender . | Smoking history (years) . | FEV1 (%) . | FVC (%) . | VC (%) . | Histology . | Neoadjuvant chemotherapy . | Clinical stage . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 55 | Male | 40 | 60 | 75 | 87 | SC | 0 | T3N0M0 |
| Case 2 | 55 | Male | 48 | 74 | 77 | 99 | SC | 0 | T3N0M0 |
| Case 3 | 60 | Male | 38 | 72 | 80 | 80 | SC | 0 | T3N0M0 |
| Case 4 | 61 | Female | 0 | 72 | 88 | 88 | CR | 6 EC | T4N0M0 |
| Case 5 | 69 | Male | 50 | 64 | 100 | 123 | SC | 0 | T3N0M0 |
| Case 6 | 65 | Male | 50 | 48 | 44 | 64 | SC | 4 EP | T3N0M0 |
| Case 7 | 57 | Male | 42 | 78 | 66 | 92 | SC | 0 | T3N0M0 |
| Case 8 | 57 | Male | 40 | 88 | 75 | 86 | SC | 3 EP | T2N2M0 |
| Case 9 | 61 | Male | 40 | 54 | 50 | 51 | SC | 4 EP | T4N0M0 |
| Case 10 | 60 | Male | 40 | 79 | 61 | 79 | SC | 3 EP | T4N1M0 |
| Case 11 | 55 | Male | 40 | 80 | 84 | 84 | SC | 0 | T4N0M0 |
| Case 12 | 59 | Male | 40 | 48 | 66 | 75 | ADC | 0 | T2N0M0 |
| Case 13 | 54 | Male | 35 | 62 | 86 | 88 | SC | 4 GC | T4N2M0 |
| Case 14 | 57 | Male | 38 | 88 | 78 | 101 | SC | 0 | T3N0M0 |
| Case 15 | 62 | Male | 40 | 50 | 70 | 86 | ADC | 3 GC | T3N2M0 |
| Case 16 | 57 | Male | 45 | 88 | 78 | 101 | SC | 0 | T3N0M0 |
| Variables . | Age . | Gender . | Smoking history (years) . | FEV1 (%) . | FVC (%) . | VC (%) . | Histology . | Neoadjuvant chemotherapy . | Clinical stage . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 55 | Male | 40 | 60 | 75 | 87 | SC | 0 | T3N0M0 |
| Case 2 | 55 | Male | 48 | 74 | 77 | 99 | SC | 0 | T3N0M0 |
| Case 3 | 60 | Male | 38 | 72 | 80 | 80 | SC | 0 | T3N0M0 |
| Case 4 | 61 | Female | 0 | 72 | 88 | 88 | CR | 6 EC | T4N0M0 |
| Case 5 | 69 | Male | 50 | 64 | 100 | 123 | SC | 0 | T3N0M0 |
| Case 6 | 65 | Male | 50 | 48 | 44 | 64 | SC | 4 EP | T3N0M0 |
| Case 7 | 57 | Male | 42 | 78 | 66 | 92 | SC | 0 | T3N0M0 |
| Case 8 | 57 | Male | 40 | 88 | 75 | 86 | SC | 3 EP | T2N2M0 |
| Case 9 | 61 | Male | 40 | 54 | 50 | 51 | SC | 4 EP | T4N0M0 |
| Case 10 | 60 | Male | 40 | 79 | 61 | 79 | SC | 3 EP | T4N1M0 |
| Case 11 | 55 | Male | 40 | 80 | 84 | 84 | SC | 0 | T4N0M0 |
| Case 12 | 59 | Male | 40 | 48 | 66 | 75 | ADC | 0 | T2N0M0 |
| Case 13 | 54 | Male | 35 | 62 | 86 | 88 | SC | 4 GC | T4N2M0 |
| Case 14 | 57 | Male | 38 | 88 | 78 | 101 | SC | 0 | T3N0M0 |
| Case 15 | 62 | Male | 40 | 50 | 70 | 86 | ADC | 3 GC | T3N2M0 |
| Case 16 | 57 | Male | 45 | 88 | 78 | 101 | SC | 0 | T3N0M0 |
ADC: adenocarcinoma; CR: carcinoid tumour; EC: etoposide + carboplatin; EP: etoposide + paclitaxel; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GC: gemzar + cisplatin; SC: squamous cell carcinoma; VC: vital capacity.
| Variables . | Age . | Gender . | Smoking history (years) . | FEV1 (%) . | FVC (%) . | VC (%) . | Histology . | Neoadjuvant chemotherapy . | Clinical stage . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 55 | Male | 40 | 60 | 75 | 87 | SC | 0 | T3N0M0 |
| Case 2 | 55 | Male | 48 | 74 | 77 | 99 | SC | 0 | T3N0M0 |
| Case 3 | 60 | Male | 38 | 72 | 80 | 80 | SC | 0 | T3N0M0 |
| Case 4 | 61 | Female | 0 | 72 | 88 | 88 | CR | 6 EC | T4N0M0 |
| Case 5 | 69 | Male | 50 | 64 | 100 | 123 | SC | 0 | T3N0M0 |
| Case 6 | 65 | Male | 50 | 48 | 44 | 64 | SC | 4 EP | T3N0M0 |
| Case 7 | 57 | Male | 42 | 78 | 66 | 92 | SC | 0 | T3N0M0 |
| Case 8 | 57 | Male | 40 | 88 | 75 | 86 | SC | 3 EP | T2N2M0 |
| Case 9 | 61 | Male | 40 | 54 | 50 | 51 | SC | 4 EP | T4N0M0 |
| Case 10 | 60 | Male | 40 | 79 | 61 | 79 | SC | 3 EP | T4N1M0 |
| Case 11 | 55 | Male | 40 | 80 | 84 | 84 | SC | 0 | T4N0M0 |
| Case 12 | 59 | Male | 40 | 48 | 66 | 75 | ADC | 0 | T2N0M0 |
| Case 13 | 54 | Male | 35 | 62 | 86 | 88 | SC | 4 GC | T4N2M0 |
| Case 14 | 57 | Male | 38 | 88 | 78 | 101 | SC | 0 | T3N0M0 |
| Case 15 | 62 | Male | 40 | 50 | 70 | 86 | ADC | 3 GC | T3N2M0 |
| Case 16 | 57 | Male | 45 | 88 | 78 | 101 | SC | 0 | T3N0M0 |
| Variables . | Age . | Gender . | Smoking history (years) . | FEV1 (%) . | FVC (%) . | VC (%) . | Histology . | Neoadjuvant chemotherapy . | Clinical stage . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | 55 | Male | 40 | 60 | 75 | 87 | SC | 0 | T3N0M0 |
| Case 2 | 55 | Male | 48 | 74 | 77 | 99 | SC | 0 | T3N0M0 |
| Case 3 | 60 | Male | 38 | 72 | 80 | 80 | SC | 0 | T3N0M0 |
| Case 4 | 61 | Female | 0 | 72 | 88 | 88 | CR | 6 EC | T4N0M0 |
| Case 5 | 69 | Male | 50 | 64 | 100 | 123 | SC | 0 | T3N0M0 |
| Case 6 | 65 | Male | 50 | 48 | 44 | 64 | SC | 4 EP | T3N0M0 |
| Case 7 | 57 | Male | 42 | 78 | 66 | 92 | SC | 0 | T3N0M0 |
| Case 8 | 57 | Male | 40 | 88 | 75 | 86 | SC | 3 EP | T2N2M0 |
| Case 9 | 61 | Male | 40 | 54 | 50 | 51 | SC | 4 EP | T4N0M0 |
| Case 10 | 60 | Male | 40 | 79 | 61 | 79 | SC | 3 EP | T4N1M0 |
| Case 11 | 55 | Male | 40 | 80 | 84 | 84 | SC | 0 | T4N0M0 |
| Case 12 | 59 | Male | 40 | 48 | 66 | 75 | ADC | 0 | T2N0M0 |
| Case 13 | 54 | Male | 35 | 62 | 86 | 88 | SC | 4 GC | T4N2M0 |
| Case 14 | 57 | Male | 38 | 88 | 78 | 101 | SC | 0 | T3N0M0 |
| Case 15 | 62 | Male | 40 | 50 | 70 | 86 | ADC | 3 GC | T3N2M0 |
| Case 16 | 57 | Male | 45 | 88 | 78 | 101 | SC | 0 | T3N0M0 |
ADC: adenocarcinoma; CR: carcinoid tumour; EC: etoposide + carboplatin; EP: etoposide + paclitaxel; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GC: gemzar + cisplatin; SC: squamous cell carcinoma; VC: vital capacity.
Four patients were considered at high risk for anastomotic fistula formation due to a positive water probe test at the end of the anastomosis, high anastomotic tension after extended pneumonectomy and >4 courses of chemotherapy. These patients all underwent diaphragmoplasty of the tracheobronchial anastomosis.
General perioperative data are presented in Table 2. The most common surgical procedure performed in this study was sleeve carinal pneumonectomy (69%). In addition, 4 (25%) sleeve carinal right upper lobectomies and 1 (6%) lung-sparing sleeve carinal resection were performed. The mean surgical time was 215.9 ± 67.2 min (range 125–340 min), and the mean blood loss volume was 256.3 ± 284.5 ml (range 50–1200 ml). There was 1 case of conversion to thoracotomy due to uncontrolled bleeding. We registered postoperative complications in 4 cases (25%), 1 case of pneumonia and 3 cases requiring surgery. In 1 case, chylothorax was observed on postoperative day 15 after sleeve pneumonectomy, which required repeat UVATS, debridement and clipping of the thoracic duct. One case of postoperative haemothorax was observed on postoperative day 4, requiring repeat UVATS and debridement. In 1 case, anastomotic leakage was observed on postoperative day 3, which required emergency thoracotomy, suturing of the defect of the anastomosis, and diaphragmoplasty. After the surgical correction of these complications, no other postoperative complications were observed. There was no postoperative mortality. The median survival was 38.6 ± 3.5 months.
| Variable . | Procedure . | Operation time . | Blood loss . | Duration of thoracic drainage (days) . | Pathological stage . | Adjuvant chemotherapy . | Follow-up duration (months) . | Recurrence-free duration (months) . | Status . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | SPE | 190 | 1200 | 5 | T3N2M0 | 0 | 12 | 6 | Died |
| Case 2 | SPE | 150 | 100 | 7 | T4N0M0 | 5 EC | 24 | 14 | Died |
| Case 3 | SPE | 125 | 100 | 5 | T4N2M0 | 6 EP | 30 | 26 | Died |
| Case 4 | LSSCR | 280 | 250 | 6 | T4N0M0 | 0 EC | 37 | 37 | Alive |
| Case 5 | SPE | 140 | 50 | 7 | T3N0M0 | 6 EP | 36 | 36 | Alive |
| Case 6 | SPE | 235 | 100 | 3 | T3N0M0 | 0 | 24 | 24 | Alive |
| Case 7 | SCRUL | 340 | 250 | 7 | T3N0M0 | 3 EP | 23 | 23 | Alive |
| Case 8 | SPE | 175 | 600 | 2 | T2N2M0 | 1 EP | 19 | 10 | Died |
| Case 9 | SPE | 245 | 350 | 9 | T4N0M0 | 4 EP | 28 | 26 | Alive |
| Case 10 | SPE | 220 | 200 | 15 | T4N1M0 | 6 EP | 18 | 18 | Alive |
| Case 11 | SCRUL | 305 | 200 | 9 | T3N2M0 | 1 EP | 12 | 12 | Alive |
| Case 12 | SPE | 130 | 200 | 3 | T4N2M0 | 0 | 6 | 6 | Alive |
| Case 13 | SPE | 220 | 150 | 4 | T4N0M0 | 3 EP | 46 | 46 | Alive |
| Case 14 | SCRUL | 310 | 100 | 6 | T3N0M0 | 0 | 47 | 47 | Alive |
| Case 15 | SPE | 220 | 150 | 4 | T4N0M0 | 3 EP | 43 | 43 | Alive |
| Case 16 | SCRUL | 270 | 100 | 6 | T3N0M0 | 0 | 44 | 44 | Alive |
| Variable . | Procedure . | Operation time . | Blood loss . | Duration of thoracic drainage (days) . | Pathological stage . | Adjuvant chemotherapy . | Follow-up duration (months) . | Recurrence-free duration (months) . | Status . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | SPE | 190 | 1200 | 5 | T3N2M0 | 0 | 12 | 6 | Died |
| Case 2 | SPE | 150 | 100 | 7 | T4N0M0 | 5 EC | 24 | 14 | Died |
| Case 3 | SPE | 125 | 100 | 5 | T4N2M0 | 6 EP | 30 | 26 | Died |
| Case 4 | LSSCR | 280 | 250 | 6 | T4N0M0 | 0 EC | 37 | 37 | Alive |
| Case 5 | SPE | 140 | 50 | 7 | T3N0M0 | 6 EP | 36 | 36 | Alive |
| Case 6 | SPE | 235 | 100 | 3 | T3N0M0 | 0 | 24 | 24 | Alive |
| Case 7 | SCRUL | 340 | 250 | 7 | T3N0M0 | 3 EP | 23 | 23 | Alive |
| Case 8 | SPE | 175 | 600 | 2 | T2N2M0 | 1 EP | 19 | 10 | Died |
| Case 9 | SPE | 245 | 350 | 9 | T4N0M0 | 4 EP | 28 | 26 | Alive |
| Case 10 | SPE | 220 | 200 | 15 | T4N1M0 | 6 EP | 18 | 18 | Alive |
| Case 11 | SCRUL | 305 | 200 | 9 | T3N2M0 | 1 EP | 12 | 12 | Alive |
| Case 12 | SPE | 130 | 200 | 3 | T4N2M0 | 0 | 6 | 6 | Alive |
| Case 13 | SPE | 220 | 150 | 4 | T4N0M0 | 3 EP | 46 | 46 | Alive |
| Case 14 | SCRUL | 310 | 100 | 6 | T3N0M0 | 0 | 47 | 47 | Alive |
| Case 15 | SPE | 220 | 150 | 4 | T4N0M0 | 3 EP | 43 | 43 | Alive |
| Case 16 | SCRUL | 270 | 100 | 6 | T3N0M0 | 0 | 44 | 44 | Alive |
EC: etoposide + carboplatin; EP: etoposide + paclitaxel; LSSCR: lung-sparing sleeve carinal resection; SCRUL: sleeve carinal right upper lobectomy; SPE: sleeve pneumonectomy.
| Variable . | Procedure . | Operation time . | Blood loss . | Duration of thoracic drainage (days) . | Pathological stage . | Adjuvant chemotherapy . | Follow-up duration (months) . | Recurrence-free duration (months) . | Status . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | SPE | 190 | 1200 | 5 | T3N2M0 | 0 | 12 | 6 | Died |
| Case 2 | SPE | 150 | 100 | 7 | T4N0M0 | 5 EC | 24 | 14 | Died |
| Case 3 | SPE | 125 | 100 | 5 | T4N2M0 | 6 EP | 30 | 26 | Died |
| Case 4 | LSSCR | 280 | 250 | 6 | T4N0M0 | 0 EC | 37 | 37 | Alive |
| Case 5 | SPE | 140 | 50 | 7 | T3N0M0 | 6 EP | 36 | 36 | Alive |
| Case 6 | SPE | 235 | 100 | 3 | T3N0M0 | 0 | 24 | 24 | Alive |
| Case 7 | SCRUL | 340 | 250 | 7 | T3N0M0 | 3 EP | 23 | 23 | Alive |
| Case 8 | SPE | 175 | 600 | 2 | T2N2M0 | 1 EP | 19 | 10 | Died |
| Case 9 | SPE | 245 | 350 | 9 | T4N0M0 | 4 EP | 28 | 26 | Alive |
| Case 10 | SPE | 220 | 200 | 15 | T4N1M0 | 6 EP | 18 | 18 | Alive |
| Case 11 | SCRUL | 305 | 200 | 9 | T3N2M0 | 1 EP | 12 | 12 | Alive |
| Case 12 | SPE | 130 | 200 | 3 | T4N2M0 | 0 | 6 | 6 | Alive |
| Case 13 | SPE | 220 | 150 | 4 | T4N0M0 | 3 EP | 46 | 46 | Alive |
| Case 14 | SCRUL | 310 | 100 | 6 | T3N0M0 | 0 | 47 | 47 | Alive |
| Case 15 | SPE | 220 | 150 | 4 | T4N0M0 | 3 EP | 43 | 43 | Alive |
| Case 16 | SCRUL | 270 | 100 | 6 | T3N0M0 | 0 | 44 | 44 | Alive |
| Variable . | Procedure . | Operation time . | Blood loss . | Duration of thoracic drainage (days) . | Pathological stage . | Adjuvant chemotherapy . | Follow-up duration (months) . | Recurrence-free duration (months) . | Status . |
|---|---|---|---|---|---|---|---|---|---|
| Case 1 | SPE | 190 | 1200 | 5 | T3N2M0 | 0 | 12 | 6 | Died |
| Case 2 | SPE | 150 | 100 | 7 | T4N0M0 | 5 EC | 24 | 14 | Died |
| Case 3 | SPE | 125 | 100 | 5 | T4N2M0 | 6 EP | 30 | 26 | Died |
| Case 4 | LSSCR | 280 | 250 | 6 | T4N0M0 | 0 EC | 37 | 37 | Alive |
| Case 5 | SPE | 140 | 50 | 7 | T3N0M0 | 6 EP | 36 | 36 | Alive |
| Case 6 | SPE | 235 | 100 | 3 | T3N0M0 | 0 | 24 | 24 | Alive |
| Case 7 | SCRUL | 340 | 250 | 7 | T3N0M0 | 3 EP | 23 | 23 | Alive |
| Case 8 | SPE | 175 | 600 | 2 | T2N2M0 | 1 EP | 19 | 10 | Died |
| Case 9 | SPE | 245 | 350 | 9 | T4N0M0 | 4 EP | 28 | 26 | Alive |
| Case 10 | SPE | 220 | 200 | 15 | T4N1M0 | 6 EP | 18 | 18 | Alive |
| Case 11 | SCRUL | 305 | 200 | 9 | T3N2M0 | 1 EP | 12 | 12 | Alive |
| Case 12 | SPE | 130 | 200 | 3 | T4N2M0 | 0 | 6 | 6 | Alive |
| Case 13 | SPE | 220 | 150 | 4 | T4N0M0 | 3 EP | 46 | 46 | Alive |
| Case 14 | SCRUL | 310 | 100 | 6 | T3N0M0 | 0 | 47 | 47 | Alive |
| Case 15 | SPE | 220 | 150 | 4 | T4N0M0 | 3 EP | 43 | 43 | Alive |
| Case 16 | SCRUL | 270 | 100 | 6 | T3N0M0 | 0 | 44 | 44 | Alive |
EC: etoposide + carboplatin; EP: etoposide + paclitaxel; LSSCR: lung-sparing sleeve carinal resection; SCRUL: sleeve carinal right upper lobectomy; SPE: sleeve pneumonectomy.
Survival analysis was dependent on the stage of the tumour. Of the 16 study patients, 6 had stage N1–2 tumours and a median overall survival of 22.0 ± 4.7 months; the median overall survival of the remaining 10 patients without metastases to the mediastinal lymph nodes was higher 44.4 ± 2.4 months (P = 0.003).
There were 5 cases of cancer recurrence. In all cases, these were distant metastases that caused death.
Adjuvant chemotherapy was administered to 10 patients (60%). The mean number of adjuvant chemotherapy courses was 3.8 ± 1.9 courses (range 1–6).
DISCUSSION
The rapid development of minimally invasive technologies over the past 25 years has allowed for the performance of very complex procedures such as bronchovascular resection and carinal resection [8, 9]. The advent of UVATS has accelerated this evolution. The first UVATS lobectomy was reported in 2011 [10], and the first UVATS carinal resection was performed in 2016 [5].
In the series of patients presented above, there was no mortality during the postoperative period. In addition, the percentage of postoperative complications was acceptable. Good postoperative performance is largely due to the careful selection of relatively young patients. Although one can perform any procedure using UVATS, there is some restriction on the number of instruments inserted into the wound. To overcome this limitation, we used various retraction sutures and tourniquets, which were completely located in the pleural cavity and did not hinder the actions of the surgeon.
We also used the nodes first technique, a systematic mediastinal lymphatic dissection [11]. Via lymphadenectomy, we determine the degree of resection of the lung parenchyma (with carinal lobectomy), confirmed the possibility of performing radical resection and prepared all anatomical structures for resection.
We prefer to perform anastomosis using a continuous suture, which has been shown to be safe and practical for bronchial anastomoses [12–14].
A continuous seam improves visibility and provides more space for manipulation by the surgeon.
In a large series of sleeve carinal resections, Porhanov et al. [15] reported a high frequency of anastomotic problems and related mortality.
A diaphragmatic flap is one of the most reliable materials for covering an anastomosis, as its axis vessels are well marked and provide good circulation. The diaphragm has a strong, bilateral, serous cover that guarantees mechanical strength and integrity, and it cannot be stratified or torn. Its dimensions and form are varied, and the flap can reach the tracheobronchial anastomosis easily. The diaphragm is also thin, elastic and flat, so it can be modelled to yield a dense, airtight and leak-proof cover. The flap should be fixed to normal tissues beyond the area of the anastomosis so that destructive inflammation cannot spread to the site of the sutures [16]. In our series, 4 patients underwent diaphragmoplasty of the tracheobronchial anastomosis without any leakage during the subsequent postoperative period.
Many researchers have described the dependence of postoperative survival on the stage of the tumour [17, 18]. In our series, we observed significant differences in survival depending on metastasis to the mediastinal lymph nodes. The procedures described here are accompanied by less trauma and an acceptable number of complications. These advantages allow us to further use and develop this method for complex procedures such as carinal resection.
CONCLUSION
In conclusion, UVATS carinal resection is safe and practical for use in certain patients and results in acceptable postoperative outcomes. Further research is needed to evaluate long-term outcomes for these patients.
Conflict of interest: none declared.
Author contributions
Dmitrii Sekhniaidze: Writing—original draft; Writing—review & editing. Diego Gonzalez-Rivas: Conceptualization; Investigation; Methodology. Pavel Kononets: Writing—original draft. Alejandro Garcia: Resources; Writing—original draft. Vladimir Shneider: Methodology; Supervision. Malik Agasiev: Data curation; Formal analysis. Ivan Ganzhara: Data curation; Formal analysis.
Presented at the 7th Asian Single Port VATS Symposium, Nagoya, Japan, 24–25 May 2019.





