-
PDF
- Split View
-
Views
-
Cite
Cite
Massimo A Padalino, Nicola Franchetti, Mark Hazekamp, Vladimir Sojak, Thierry Carrel, Alessandro Frigiola, Mauro Lo Rito, Jurgen Horer, Regine Roussin, Julie Cleuziou, Bart Meyns, Jose Fragata, Helena Telles, Anastasios C Polimenakos, Katrien Francois, Altin Veshti, Jukka Salminen, Alvaro Gonzalez Rocafort, Matej Nosal, Luca Vedovelli, Alvise Guariento, Vladimiro L Vida, George E Sarris, Giovanna Boccuzzo, Giovanni Stellin, Surgery for anomalous aortic origin of coronary arteries: a multicentre study from the European Congenital Heart Surgeons Association, European Journal of Cardio-Thoracic Surgery, Volume 56, Issue 4, October 2019, Pages 696–703, https://doi.org/10.1093/ejcts/ezz080
Close - Share Icon Share
Abstract
We sought to describe early and late outcomes in a large surgical series of patients with anomalous aortic origin of coronary arteries.
We performed a retrospective multicentre study including surgical patients with anomalous aortic origin of coronary arteries since 1991. Patients with isolated high coronary takeoff and associated major congenital heart disease were excluded.
We collected 156 surgical patients (median age 39.5 years, interquartile range 15–53) affected by anomalous right (67.9%), anomalous left (22.4%) and other anatomical abnormalities (9.6%). An interarterial course occurred in 86.5%, an intramural course in 62.8% and symptoms in 85.9%. The operations included coronary unroofing (56.4%), reimplantation (19.2%), coronary bypass graft (15.4%) and other (9.0%). Two patients with preoperative cardiac failure died postoperatively (1.3%). All survivors were discharged home in good clinical condition. At a median follow-up of 2 years (interquartile range 1–5, 88.5% complete), there were 3 deaths (2.2%), 9 reinterventions in 8 patients (5 interventional, 3 surgical); 91.2% are in New York Heart Association functional class ≤ II, but symptoms persisted in 14.2%; 48.1% of them returned to sport activity. On Kaplan–Meier analysis, event-free survival at follow-up was 74.6%. Morbidity was not significantly different among age classes, anatomical variants and types of surgical procedures. Furthermore, return to sport activity was significantly higher in younger patients who participated in sports preoperatively.
Surgical repair of anomalous aortic origin of coronary arteries is effective and has few complications. Unroofing and coronary reimplantation are safe and are the most common procedures. The occurrence of late adverse events is not negligible, and long-term surveillance is mandatory. Most young athletes can return to an unrestrained lifestyle.
INTRODUCTION
Anomalous aortic origin of a coronary artery (AAOCA) is a congenital heart disease (CHD) involving a coronary artery of abnormal origin and course, arising from the aorta. It is reported as the second leading cause of sudden cardiac death (SCD) among young athletes when it originates from the opposite sinus of Valsalva [1–4].
An increasing number of children and young adults are being incidentally found to have AAOCA on imaging studies, performed as part of screening campaigns or for other reasons, with transthoracic echocardiography or magnetic resonance imaging (MRI) [5].
Single-centre studies report on limited series, describing surgical repair as safe and effective [6–12], although late effects on symptoms and risk of SCD are undefined and concerns about the surgical effectiveness remain [13–15].
We describe our experience with surgical procedures for AAOCA in a multicentre study from the European Congenital Heart Surgeons Association, to evaluate safety and outcomes in a large cohort of patients.
MATERIALS AND METHODS
We performed a multicentre retrospective study of patients undergoing surgery for AAOCA since 1991. Exclusion criteria were isolated high coronary takeoff and anomalous coronary artery associated with major CHD. Preoperative, intraoperative and postoperative data were retrieved from a common database. Review of medical records was approved by each local hospital committee on clinical investigation. Individual patients were not identified, and the need for patient consent was waived.
Preoperative data included anatomical variants [anomalous aortic origin of right coronary artery (AORCA) vs anomalous aortic origin of left coronary artery (AOLCA) vs ‘other’]; coronary course; and associated cardiac disease and preoperative regular sport activity, both professional and recreational. Diagnostic imaging (transthoracic echocardiography, MRI, computed tomography angiography) was defined as abnormal when AAOCA was diagnosed.
Intraoperative data included surgical technique [intramural coronary course unroofing, coronary reimplantation, coronary artery bypass graft (CABG), other]; associated procedures; cardiopulmonary bypass and cross-clamping times; postoperative complications; and early death.
Follow-up information was collected by referring cardiologists between January 2016 and June 2018. All adverse events (AE) (including death, surgical/interventional cardiology procedures), clinical status [New York Heart Association (NYHA) functional class ≥II, symptoms], postoperative aortic valve (AV) regurgitation and return to sport activity were recorded. We defined ‘coronary related AE’ as those (excluding early or late death) involving coronary perfusion or anatomy (such as coronary surgery or percutaneous procedures and automatic implantable cardioverter defibrillator implant).
Statistical analyses
The results are reported as the number and percentage for categorical variables and median, range and interquartile range (IQR) for quantitative variables. The effect of age on outcomes was evaluated by age class subgroups (<10, 11–30, 31–50, >50 years). Categorical variables were analysed with the Fisher’s exact test, whereas quantitative variables were analysed with the Mann–Whitney test. Event-free survival (including death and surgical/interventional cardiology procedures) was estimated with the Kaplan–Meier curve. Binary logistic regression was used to estimate the occurrence of an event (return to sport activity, adverse events, symptoms and NYHA functional class >II, all at follow-up) depending on related surgical and clinical variables that included AORCA, AOLCA, ‘other’ origin, interarterial and intramural course, preoperative sport activity, symptoms at rest and at effort, type of surgical procedure (unroofing, CABG, coronary reimplantation, other procedures) and age classes (1–10 years; 11–30 years; 31–50 years; 51+ years). Results are presented as P-value, odds ratio (OR) and 95% confidence interval (CI). Statistical analyses were performed with SPSS v23 (IBM corp., Armonk, NY, USA). Prism GraphPad v7 (GraphPad Software, La Jolla, CA, USA) was used to generate the Kaplan–Meier survival curves.
RESULTS
Patients
Surgical repair for AAOCA was performed in 156 patients in 14 centres: AORCA was present in 105 patients (67.3%); AOLCA, in 35 (22.4%); ‘other’ anatomical variants (i.e. left anterior descending/circumflex from anterior sinus, single coronary), in 16 (10.3%). An interarterial course was reported in 86.5%, and an intramural course, in 62.8%. Symptoms were reported in 136 patients (85.9%); 103 (66.0%) were effort-related; noteworthy, 90 symptomatic patients (66.2%) had an intramural course (P = 0.028). Twenty-one patients presented emergently for an aborted SCD, and 1 patient required preoperative extracorporeal membrane oxygenation followed by a successful coronary artery reimplantation. Among associated cardiac anomalies (33 patients, 21.2%), 12 had AV disease. All preoperative data are shown in Table 1.
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Total patients | 156 (100) | 105 (67.3) | 35 (22.4) | 16 (10.3) | NS |
| Female | 72/156 (46.2) | 47/105 (44.8) | 18/35 (51.4) | 7/16 (43.8) | NS |
| Anomalous course of coronary artery | NS | ||||
| Interarterial | 135/156 (86.5) | 96/105 (91.4) | 29/35 (82.9) | 10/16 (62.5) | NS |
| Intramural | 98/156 (62.8) | 77/105 (73.3)a | 18/35 (51.4)b | 3/16 (18.8)c | 0.022; 0.035; <0.0001 |
| Intraseptal or retroaortic | 4/156 (2.6) | 1/105 (1.0) | 2/35 (5.7) | 1/16 (6.3) | NS |
| Anterior to pulmonary artery | 1/156 (0.6) | 0/105 (0) | 0/35 (0) | 1/16 (6.3) | NS |
| Other | 16/156 (10.3) | 8/105 (7.6) | 4/35 (11.4) | 4/16 (25.0) | NS |
| Symptoms at diagnosis | 136/156 (87.2) | 94/105 (89.5)a | 30/35 (85.7) | 12/16 (75.0)b | 0.010 |
| Symptoms at effort | 103/156 (66.0) | 72/105 (68.6) | 22/35 (62.9) | 9/16 (56.3) | NS |
| Chest pain | 42/156 (26.9) | 31/105 (29.5) | 8/35 (22.9) | 3/16 (18.8) | NS |
| Cardiac arrest/low cardiac output | 21/156 (13.5) | 15/105 (14.3) | 5/35 (14.3) | 1/16 (6.6) | NS |
| Dyspnoea | 10/156 (6.4) | 4/105 (3.8) | 4/35 (11.4) | 2/16 (12.5) | NS |
| Palpitations | 7/156 (4.5) | 2/105 (1.9) | 3/35 (8.6) | 2/16 (12.5) | NS |
| Syncope | 14/156 (9.0) | 5/105 (4.8)a | 8/35 (22.9)b | 1/16 (6.3) | NS |
| Fatigue | 4/156 (2.6) | 3/105 (2.9) | 0/35 (0) | 1/16 (6.3) | NS |
| Not specified | 36/156 (23.1) | 35/105 (33.3)a | 1/35 (2.9)b | 2/16 (12.5)b | 0.003; <0.001 |
| No symptoms | 20/156 (12.8) | 11/105 (10.5)a | 5/35 (14.3) | 4/16 (25.0)b | 0.010 |
| Preoperative sport activity | 44/156 (28.2) | 29/105 (27.6) | 13/35 (37.1) | 2/16 (12.5) | NS |
| Age at procedure (years) | 39.5 (15–53) | 41 (19–53)a | 15 (12–44)b | 46 (16–57) | 0.070 |
| Percutaneous/CABG | 6/156 (3.8) | 6/105 (5.7) | 0/35 (0) | 0/16 (0) | NS |
| Associated cardiac disease | 33/156 (21.2) | 23/105 (21.9) | 11/35 (31.4) | 5/16 (31.3) | NS |
| Atrial septal defect | 4/156 (2.6) | 4/105 (3.8) | 0/35 (0) | 0/16 (0) | NS |
| Aortic valve anomaly | 12/156 (7.7) | 4/105 (3.8) | 5/35 (14.3) | 3/16 (18.8) | NS |
| Mitral valve anomaly | 7/156 (4.5) | 5/105 (4.8) | 2/35 (5.7) | 0/16 (0) | NS |
| Other | 10/156 (6.4) | 3/105 (2.9) | 4/35 (11.4) | 2/16 (12.5) | NS |
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Total patients | 156 (100) | 105 (67.3) | 35 (22.4) | 16 (10.3) | NS |
| Female | 72/156 (46.2) | 47/105 (44.8) | 18/35 (51.4) | 7/16 (43.8) | NS |
| Anomalous course of coronary artery | NS | ||||
| Interarterial | 135/156 (86.5) | 96/105 (91.4) | 29/35 (82.9) | 10/16 (62.5) | NS |
| Intramural | 98/156 (62.8) | 77/105 (73.3)a | 18/35 (51.4)b | 3/16 (18.8)c | 0.022; 0.035; <0.0001 |
| Intraseptal or retroaortic | 4/156 (2.6) | 1/105 (1.0) | 2/35 (5.7) | 1/16 (6.3) | NS |
| Anterior to pulmonary artery | 1/156 (0.6) | 0/105 (0) | 0/35 (0) | 1/16 (6.3) | NS |
| Other | 16/156 (10.3) | 8/105 (7.6) | 4/35 (11.4) | 4/16 (25.0) | NS |
| Symptoms at diagnosis | 136/156 (87.2) | 94/105 (89.5)a | 30/35 (85.7) | 12/16 (75.0)b | 0.010 |
| Symptoms at effort | 103/156 (66.0) | 72/105 (68.6) | 22/35 (62.9) | 9/16 (56.3) | NS |
| Chest pain | 42/156 (26.9) | 31/105 (29.5) | 8/35 (22.9) | 3/16 (18.8) | NS |
| Cardiac arrest/low cardiac output | 21/156 (13.5) | 15/105 (14.3) | 5/35 (14.3) | 1/16 (6.6) | NS |
| Dyspnoea | 10/156 (6.4) | 4/105 (3.8) | 4/35 (11.4) | 2/16 (12.5) | NS |
| Palpitations | 7/156 (4.5) | 2/105 (1.9) | 3/35 (8.6) | 2/16 (12.5) | NS |
| Syncope | 14/156 (9.0) | 5/105 (4.8)a | 8/35 (22.9)b | 1/16 (6.3) | NS |
| Fatigue | 4/156 (2.6) | 3/105 (2.9) | 0/35 (0) | 1/16 (6.3) | NS |
| Not specified | 36/156 (23.1) | 35/105 (33.3)a | 1/35 (2.9)b | 2/16 (12.5)b | 0.003; <0.001 |
| No symptoms | 20/156 (12.8) | 11/105 (10.5)a | 5/35 (14.3) | 4/16 (25.0)b | 0.010 |
| Preoperative sport activity | 44/156 (28.2) | 29/105 (27.6) | 13/35 (37.1) | 2/16 (12.5) | NS |
| Age at procedure (years) | 39.5 (15–53) | 41 (19–53)a | 15 (12–44)b | 46 (16–57) | 0.070 |
| Percutaneous/CABG | 6/156 (3.8) | 6/105 (5.7) | 0/35 (0) | 0/16 (0) | NS |
| Associated cardiac disease | 33/156 (21.2) | 23/105 (21.9) | 11/35 (31.4) | 5/16 (31.3) | NS |
| Atrial septal defect | 4/156 (2.6) | 4/105 (3.8) | 0/35 (0) | 0/16 (0) | NS |
| Aortic valve anomaly | 12/156 (7.7) | 4/105 (3.8) | 5/35 (14.3) | 3/16 (18.8) | NS |
| Mitral valve anomaly | 7/156 (4.5) | 5/105 (4.8) | 2/35 (5.7) | 0/16 (0) | NS |
| Other | 10/156 (6.4) | 3/105 (2.9) | 4/35 (11.4) | 2/16 (12.5) | NS |
Numbers represent median (interquartile range) for continuous variables and n (%) for categorical variables.
Values in the same row that have different superscript letters are significantly different from each other.
AOLCA: aortic origin of left coronary artery; AORCA: aortic origin of right coronary artery; CABG: coronary artery bypass graft; NS, not significant.
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Total patients | 156 (100) | 105 (67.3) | 35 (22.4) | 16 (10.3) | NS |
| Female | 72/156 (46.2) | 47/105 (44.8) | 18/35 (51.4) | 7/16 (43.8) | NS |
| Anomalous course of coronary artery | NS | ||||
| Interarterial | 135/156 (86.5) | 96/105 (91.4) | 29/35 (82.9) | 10/16 (62.5) | NS |
| Intramural | 98/156 (62.8) | 77/105 (73.3)a | 18/35 (51.4)b | 3/16 (18.8)c | 0.022; 0.035; <0.0001 |
| Intraseptal or retroaortic | 4/156 (2.6) | 1/105 (1.0) | 2/35 (5.7) | 1/16 (6.3) | NS |
| Anterior to pulmonary artery | 1/156 (0.6) | 0/105 (0) | 0/35 (0) | 1/16 (6.3) | NS |
| Other | 16/156 (10.3) | 8/105 (7.6) | 4/35 (11.4) | 4/16 (25.0) | NS |
| Symptoms at diagnosis | 136/156 (87.2) | 94/105 (89.5)a | 30/35 (85.7) | 12/16 (75.0)b | 0.010 |
| Symptoms at effort | 103/156 (66.0) | 72/105 (68.6) | 22/35 (62.9) | 9/16 (56.3) | NS |
| Chest pain | 42/156 (26.9) | 31/105 (29.5) | 8/35 (22.9) | 3/16 (18.8) | NS |
| Cardiac arrest/low cardiac output | 21/156 (13.5) | 15/105 (14.3) | 5/35 (14.3) | 1/16 (6.6) | NS |
| Dyspnoea | 10/156 (6.4) | 4/105 (3.8) | 4/35 (11.4) | 2/16 (12.5) | NS |
| Palpitations | 7/156 (4.5) | 2/105 (1.9) | 3/35 (8.6) | 2/16 (12.5) | NS |
| Syncope | 14/156 (9.0) | 5/105 (4.8)a | 8/35 (22.9)b | 1/16 (6.3) | NS |
| Fatigue | 4/156 (2.6) | 3/105 (2.9) | 0/35 (0) | 1/16 (6.3) | NS |
| Not specified | 36/156 (23.1) | 35/105 (33.3)a | 1/35 (2.9)b | 2/16 (12.5)b | 0.003; <0.001 |
| No symptoms | 20/156 (12.8) | 11/105 (10.5)a | 5/35 (14.3) | 4/16 (25.0)b | 0.010 |
| Preoperative sport activity | 44/156 (28.2) | 29/105 (27.6) | 13/35 (37.1) | 2/16 (12.5) | NS |
| Age at procedure (years) | 39.5 (15–53) | 41 (19–53)a | 15 (12–44)b | 46 (16–57) | 0.070 |
| Percutaneous/CABG | 6/156 (3.8) | 6/105 (5.7) | 0/35 (0) | 0/16 (0) | NS |
| Associated cardiac disease | 33/156 (21.2) | 23/105 (21.9) | 11/35 (31.4) | 5/16 (31.3) | NS |
| Atrial septal defect | 4/156 (2.6) | 4/105 (3.8) | 0/35 (0) | 0/16 (0) | NS |
| Aortic valve anomaly | 12/156 (7.7) | 4/105 (3.8) | 5/35 (14.3) | 3/16 (18.8) | NS |
| Mitral valve anomaly | 7/156 (4.5) | 5/105 (4.8) | 2/35 (5.7) | 0/16 (0) | NS |
| Other | 10/156 (6.4) | 3/105 (2.9) | 4/35 (11.4) | 2/16 (12.5) | NS |
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Total patients | 156 (100) | 105 (67.3) | 35 (22.4) | 16 (10.3) | NS |
| Female | 72/156 (46.2) | 47/105 (44.8) | 18/35 (51.4) | 7/16 (43.8) | NS |
| Anomalous course of coronary artery | NS | ||||
| Interarterial | 135/156 (86.5) | 96/105 (91.4) | 29/35 (82.9) | 10/16 (62.5) | NS |
| Intramural | 98/156 (62.8) | 77/105 (73.3)a | 18/35 (51.4)b | 3/16 (18.8)c | 0.022; 0.035; <0.0001 |
| Intraseptal or retroaortic | 4/156 (2.6) | 1/105 (1.0) | 2/35 (5.7) | 1/16 (6.3) | NS |
| Anterior to pulmonary artery | 1/156 (0.6) | 0/105 (0) | 0/35 (0) | 1/16 (6.3) | NS |
| Other | 16/156 (10.3) | 8/105 (7.6) | 4/35 (11.4) | 4/16 (25.0) | NS |
| Symptoms at diagnosis | 136/156 (87.2) | 94/105 (89.5)a | 30/35 (85.7) | 12/16 (75.0)b | 0.010 |
| Symptoms at effort | 103/156 (66.0) | 72/105 (68.6) | 22/35 (62.9) | 9/16 (56.3) | NS |
| Chest pain | 42/156 (26.9) | 31/105 (29.5) | 8/35 (22.9) | 3/16 (18.8) | NS |
| Cardiac arrest/low cardiac output | 21/156 (13.5) | 15/105 (14.3) | 5/35 (14.3) | 1/16 (6.6) | NS |
| Dyspnoea | 10/156 (6.4) | 4/105 (3.8) | 4/35 (11.4) | 2/16 (12.5) | NS |
| Palpitations | 7/156 (4.5) | 2/105 (1.9) | 3/35 (8.6) | 2/16 (12.5) | NS |
| Syncope | 14/156 (9.0) | 5/105 (4.8)a | 8/35 (22.9)b | 1/16 (6.3) | NS |
| Fatigue | 4/156 (2.6) | 3/105 (2.9) | 0/35 (0) | 1/16 (6.3) | NS |
| Not specified | 36/156 (23.1) | 35/105 (33.3)a | 1/35 (2.9)b | 2/16 (12.5)b | 0.003; <0.001 |
| No symptoms | 20/156 (12.8) | 11/105 (10.5)a | 5/35 (14.3) | 4/16 (25.0)b | 0.010 |
| Preoperative sport activity | 44/156 (28.2) | 29/105 (27.6) | 13/35 (37.1) | 2/16 (12.5) | NS |
| Age at procedure (years) | 39.5 (15–53) | 41 (19–53)a | 15 (12–44)b | 46 (16–57) | 0.070 |
| Percutaneous/CABG | 6/156 (3.8) | 6/105 (5.7) | 0/35 (0) | 0/16 (0) | NS |
| Associated cardiac disease | 33/156 (21.2) | 23/105 (21.9) | 11/35 (31.4) | 5/16 (31.3) | NS |
| Atrial septal defect | 4/156 (2.6) | 4/105 (3.8) | 0/35 (0) | 0/16 (0) | NS |
| Aortic valve anomaly | 12/156 (7.7) | 4/105 (3.8) | 5/35 (14.3) | 3/16 (18.8) | NS |
| Mitral valve anomaly | 7/156 (4.5) | 5/105 (4.8) | 2/35 (5.7) | 0/16 (0) | NS |
| Other | 10/156 (6.4) | 3/105 (2.9) | 4/35 (11.4) | 2/16 (12.5) | NS |
Numbers represent median (interquartile range) for continuous variables and n (%) for categorical variables.
Values in the same row that have different superscript letters are significantly different from each other.
AOLCA: aortic origin of left coronary artery; AORCA: aortic origin of right coronary artery; CABG: coronary artery bypass graft; NS, not significant.
Normal baseline electrocardiogram results at presentation were present in 130 patients (86.1%). The most frequent electrocardiogram abnormalities were ST elevation (7), Q waves (6) and right bundle branch block (2). The most common imaging procedures were transthoracic echocardiography and computed tomography angiography multislice (95.5% and 72.4%, respectively), which were diagnostic in 37.6% and 86%, respectively. Provocative exercise and nuclear stress tests were performed occasionally (Supplementary Material, Table S1).
Early outcomes
All intraoperative and postoperative data are summarized in Table 2. The median age at surgery was 39.5 years (IQR 15–53; range 3 months–70 years); patients with AOLCA were operated on at a younger age (P = 0.07).
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Type of surgical procedure* | Median age at procedure* | ||||
| Unroofinga | 88/156 (56.4)32 years (IQR 14–50; range 0.5–67) | 66/105 (62.9) | 19/35 (54.3) | 3/16 (18.8) | NS |
| Coronary reimplantationa | 30/156 (19.2)37 years (IQR 16.5–47; range 0.5–64) | 26/105 (24.8) | 2/35 (5.7) | 2/16 (12.5) | NS |
| CABGb | 24/156 (15.4)58.5 years (IQR 47–66; range 1–70) | 8/105 (7.6) | 7/35 (20.0) | 9/16 (56.3) | NS |
| Othera | 14/156 (9.0)32.5 years (IQR 15–43; range 3–63) | 5/105 (4.8) | 7/35 (20.0) | 2/16 (12.5) | NS |
| CPB time (min) | 73 (54–103) | 68 (52–91) | 84 (68–116) | 88 (47–137) | NS |
| CC time (min) | 47 (35–69) | 43 (34–66) | 53 (41–70) | 58 (37–78) | NS |
| ICU (days) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 2 (1–3) | NS |
| Major postoperative complications | |||||
| Low cardiac output syndrome | 9/156 (5.8) | 2/105 (1.9)a | 5/35 (14.3)b | 2/16 (12.5) | 0.010 |
| Early reintervention | 7/156 (4.5) | 3/105 (2.9) | 3/35 (8.6) | 1/16 (6.3) | NS |
| Right coronary stenting | 2/156 (1.3) | 2/105 (1.9) | 0/35 (0) | 0/16 (0) | NS |
| Balloon dilatation of pulmonary stenosis | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Removal of thrombus from left main coronary | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Coronary artery bypass graft | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Heart transplant | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Sinus Valsalva aneurysm resection | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Other | 1/156 (0.6) | 0/105 (0) | 0/35 (0) | 1/16 (6.3) | NS |
| Mechanical support | 6/156 (3.8) | 1/105 (1.0)a | 3/35 (8.6)b | 2/16 (12.5)b | 0.050; 0.050 |
| ECMO | 2/156 (1.3) | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Intra-aortic balloon pump | 2/156 (1.3 | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Ventricular assist device | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Impella device | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Hospital deaths | 2/156 (1.3) | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Minor postoperative complications | |||||
| Pericardial/pleural effusion | 6/156 (3.8) | 5/105 (4.8) | 0/35 (0) | 1/16 (6.3) | NS |
| Arrhythmia | 4/156 (2.6) | 2/105 (1.9) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Sepsis/infection | 3/156 (1.9) | 3/105 (2.9) | 0/35 (0) | 0/16 (0) | NS |
| Respiratory insufficiency | 2/156 (1.3) | 1/105 (1.0) | 1/35 (2.9) | 0/16 (0) | NS |
| Aortic insufficiency (mild-moderate) | 2/156 (1.3) | 2/105 (1.9) | 0/35 (0) | 0/16 (0) | NS |
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Type of surgical procedure* | Median age at procedure* | ||||
| Unroofinga | 88/156 (56.4)32 years (IQR 14–50; range 0.5–67) | 66/105 (62.9) | 19/35 (54.3) | 3/16 (18.8) | NS |
| Coronary reimplantationa | 30/156 (19.2)37 years (IQR 16.5–47; range 0.5–64) | 26/105 (24.8) | 2/35 (5.7) | 2/16 (12.5) | NS |
| CABGb | 24/156 (15.4)58.5 years (IQR 47–66; range 1–70) | 8/105 (7.6) | 7/35 (20.0) | 9/16 (56.3) | NS |
| Othera | 14/156 (9.0)32.5 years (IQR 15–43; range 3–63) | 5/105 (4.8) | 7/35 (20.0) | 2/16 (12.5) | NS |
| CPB time (min) | 73 (54–103) | 68 (52–91) | 84 (68–116) | 88 (47–137) | NS |
| CC time (min) | 47 (35–69) | 43 (34–66) | 53 (41–70) | 58 (37–78) | NS |
| ICU (days) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 2 (1–3) | NS |
| Major postoperative complications | |||||
| Low cardiac output syndrome | 9/156 (5.8) | 2/105 (1.9)a | 5/35 (14.3)b | 2/16 (12.5) | 0.010 |
| Early reintervention | 7/156 (4.5) | 3/105 (2.9) | 3/35 (8.6) | 1/16 (6.3) | NS |
| Right coronary stenting | 2/156 (1.3) | 2/105 (1.9) | 0/35 (0) | 0/16 (0) | NS |
| Balloon dilatation of pulmonary stenosis | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Removal of thrombus from left main coronary | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Coronary artery bypass graft | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Heart transplant | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Sinus Valsalva aneurysm resection | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Other | 1/156 (0.6) | 0/105 (0) | 0/35 (0) | 1/16 (6.3) | NS |
| Mechanical support | 6/156 (3.8) | 1/105 (1.0)a | 3/35 (8.6)b | 2/16 (12.5)b | 0.050; 0.050 |
| ECMO | 2/156 (1.3) | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Intra-aortic balloon pump | 2/156 (1.3 | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Ventricular assist device | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Impella device | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Hospital deaths | 2/156 (1.3) | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Minor postoperative complications | |||||
| Pericardial/pleural effusion | 6/156 (3.8) | 5/105 (4.8) | 0/35 (0) | 1/16 (6.3) | NS |
| Arrhythmia | 4/156 (2.6) | 2/105 (1.9) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Sepsis/infection | 3/156 (1.9) | 3/105 (2.9) | 0/35 (0) | 0/16 (0) | NS |
| Respiratory insufficiency | 2/156 (1.3) | 1/105 (1.0) | 1/35 (2.9) | 0/16 (0) | NS |
| Aortic insufficiency (mild-moderate) | 2/156 (1.3) | 2/105 (1.9) | 0/35 (0) | 0/16 (0) | NS |
Numbers represent median (interquartile range) for continuous variables and n (%) for categorical variables.
Values in the same row that have different superscript letters are significantly different from each other.
Median ages at surgical procedures were compared, and age at CABG was significantly higher than that with all other techniques (P < 0.01).
AOLCA: aortic origin of left coronary artery; AORCA: aortic origin of right coronary artery; CABG: coronary artery bypass graft; CC: cross-clamping; CPB: cardiopulmonary bypass; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; IQR: interquartile range; NS, not significant.
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Type of surgical procedure* | Median age at procedure* | ||||
| Unroofinga | 88/156 (56.4)32 years (IQR 14–50; range 0.5–67) | 66/105 (62.9) | 19/35 (54.3) | 3/16 (18.8) | NS |
| Coronary reimplantationa | 30/156 (19.2)37 years (IQR 16.5–47; range 0.5–64) | 26/105 (24.8) | 2/35 (5.7) | 2/16 (12.5) | NS |
| CABGb | 24/156 (15.4)58.5 years (IQR 47–66; range 1–70) | 8/105 (7.6) | 7/35 (20.0) | 9/16 (56.3) | NS |
| Othera | 14/156 (9.0)32.5 years (IQR 15–43; range 3–63) | 5/105 (4.8) | 7/35 (20.0) | 2/16 (12.5) | NS |
| CPB time (min) | 73 (54–103) | 68 (52–91) | 84 (68–116) | 88 (47–137) | NS |
| CC time (min) | 47 (35–69) | 43 (34–66) | 53 (41–70) | 58 (37–78) | NS |
| ICU (days) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 2 (1–3) | NS |
| Major postoperative complications | |||||
| Low cardiac output syndrome | 9/156 (5.8) | 2/105 (1.9)a | 5/35 (14.3)b | 2/16 (12.5) | 0.010 |
| Early reintervention | 7/156 (4.5) | 3/105 (2.9) | 3/35 (8.6) | 1/16 (6.3) | NS |
| Right coronary stenting | 2/156 (1.3) | 2/105 (1.9) | 0/35 (0) | 0/16 (0) | NS |
| Balloon dilatation of pulmonary stenosis | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Removal of thrombus from left main coronary | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Coronary artery bypass graft | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Heart transplant | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Sinus Valsalva aneurysm resection | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Other | 1/156 (0.6) | 0/105 (0) | 0/35 (0) | 1/16 (6.3) | NS |
| Mechanical support | 6/156 (3.8) | 1/105 (1.0)a | 3/35 (8.6)b | 2/16 (12.5)b | 0.050; 0.050 |
| ECMO | 2/156 (1.3) | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Intra-aortic balloon pump | 2/156 (1.3 | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Ventricular assist device | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Impella device | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Hospital deaths | 2/156 (1.3) | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Minor postoperative complications | |||||
| Pericardial/pleural effusion | 6/156 (3.8) | 5/105 (4.8) | 0/35 (0) | 1/16 (6.3) | NS |
| Arrhythmia | 4/156 (2.6) | 2/105 (1.9) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Sepsis/infection | 3/156 (1.9) | 3/105 (2.9) | 0/35 (0) | 0/16 (0) | NS |
| Respiratory insufficiency | 2/156 (1.3) | 1/105 (1.0) | 1/35 (2.9) | 0/16 (0) | NS |
| Aortic insufficiency (mild-moderate) | 2/156 (1.3) | 2/105 (1.9) | 0/35 (0) | 0/16 (0) | NS |
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Type of surgical procedure* | Median age at procedure* | ||||
| Unroofinga | 88/156 (56.4)32 years (IQR 14–50; range 0.5–67) | 66/105 (62.9) | 19/35 (54.3) | 3/16 (18.8) | NS |
| Coronary reimplantationa | 30/156 (19.2)37 years (IQR 16.5–47; range 0.5–64) | 26/105 (24.8) | 2/35 (5.7) | 2/16 (12.5) | NS |
| CABGb | 24/156 (15.4)58.5 years (IQR 47–66; range 1–70) | 8/105 (7.6) | 7/35 (20.0) | 9/16 (56.3) | NS |
| Othera | 14/156 (9.0)32.5 years (IQR 15–43; range 3–63) | 5/105 (4.8) | 7/35 (20.0) | 2/16 (12.5) | NS |
| CPB time (min) | 73 (54–103) | 68 (52–91) | 84 (68–116) | 88 (47–137) | NS |
| CC time (min) | 47 (35–69) | 43 (34–66) | 53 (41–70) | 58 (37–78) | NS |
| ICU (days) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 2 (1–3) | NS |
| Major postoperative complications | |||||
| Low cardiac output syndrome | 9/156 (5.8) | 2/105 (1.9)a | 5/35 (14.3)b | 2/16 (12.5) | 0.010 |
| Early reintervention | 7/156 (4.5) | 3/105 (2.9) | 3/35 (8.6) | 1/16 (6.3) | NS |
| Right coronary stenting | 2/156 (1.3) | 2/105 (1.9) | 0/35 (0) | 0/16 (0) | NS |
| Balloon dilatation of pulmonary stenosis | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Removal of thrombus from left main coronary | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Coronary artery bypass graft | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Heart transplant | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Sinus Valsalva aneurysm resection | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Other | 1/156 (0.6) | 0/105 (0) | 0/35 (0) | 1/16 (6.3) | NS |
| Mechanical support | 6/156 (3.8) | 1/105 (1.0)a | 3/35 (8.6)b | 2/16 (12.5)b | 0.050; 0.050 |
| ECMO | 2/156 (1.3) | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Intra-aortic balloon pump | 2/156 (1.3 | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Ventricular assist device | 1/156 (0.6) | 0/105 (0) | 1/35 (2.9) | 0/16 (0) | NS |
| Impella device | 1/156 (0.6) | 1/105 (1.0) | 0/35 (0) | 0/16 (0) | NS |
| Hospital deaths | 2/156 (1.3) | 0/105 (0) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Minor postoperative complications | |||||
| Pericardial/pleural effusion | 6/156 (3.8) | 5/105 (4.8) | 0/35 (0) | 1/16 (6.3) | NS |
| Arrhythmia | 4/156 (2.6) | 2/105 (1.9) | 1/35 (2.9) | 1/16 (6.3) | NS |
| Sepsis/infection | 3/156 (1.9) | 3/105 (2.9) | 0/35 (0) | 0/16 (0) | NS |
| Respiratory insufficiency | 2/156 (1.3) | 1/105 (1.0) | 1/35 (2.9) | 0/16 (0) | NS |
| Aortic insufficiency (mild-moderate) | 2/156 (1.3) | 2/105 (1.9) | 0/35 (0) | 0/16 (0) | NS |
Numbers represent median (interquartile range) for continuous variables and n (%) for categorical variables.
Values in the same row that have different superscript letters are significantly different from each other.
Median ages at surgical procedures were compared, and age at CABG was significantly higher than that with all other techniques (P < 0.01).
AOLCA: aortic origin of left coronary artery; AORCA: aortic origin of right coronary artery; CABG: coronary artery bypass graft; CC: cross-clamping; CPB: cardiopulmonary bypass; ECMO: extracorporeal membrane oxygenation; ICU: intensive care unit; IQR: interquartile range; NS, not significant.
The most common surgical procedure was coronary unroofing in 88 patients (56.4%), followed by coronary reimplantation and CABG (which was performed in older patients). Other techniques (14 patients) included coronary osteoplasty (9); Vouhè reimplantation [16] (3); and pulmonary artery translocation (2). Thirty-four patients (21.8%) underwent an additional surgical procedure (Supplementary Material, Table S2).
Operative mortality was 1.3% (2): a 44-year-old patient (intramural AOLCA) with aborted SCD during exercising died 15 days after Vouhè reimplantation of low cardiac output syndrome; another 1-year-old infant (left anterior descending from right coronary sinus, with preoperative ejection fraction <20%) died 61 days after CABG, of multiorgan failure following mechanical cardiac support.
Twenty-two postoperative major complications occurred in 14 patients (9%): low cardiac output syndrome in 9 (6 required mechanical cardiac support); 7 patients (4.5%) required an early reintervention, 1 of whom (11-year-old patient with AOLCA, interarterial course and severe preoperative congestive heart failure, after unroofing) underwent a successful heart transplant after mechanical cardiac support for 4 months. All 154 survivors were discharged home on antiaggregant therapy in good clinical condition; 2 patients (1.3%) were discharged home with mild-moderate AV regurgitation after unroofing, determined by echocardiography.
Late outcomes
At a median follow-up time of 2 years (IQR 1–5, range 1 month to 23 years, 88.5% complete, Table 3), among 133 early survivors, there were 3 late deaths (2.2%) in 3 septuagenarians (AORCA and interarterial course, 2; other, 1) after 1, 60 and 102 months from CABG.
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Follow-up completeness | 138/156 (88.5) | 91/105 (86.7) | 32/35 (91.4) | 14/16 (100) | NS |
| Alive | 133/156 (86.4) | 89/105 (84.8) | 31/35 (88.6) | 13/16 (81.3) | NS |
| Lost | 18/156 (11.5) | 17/105 (16.2) | 3/35 (8.6) | 1/16 (12.5) | NS |
| Age (years) | 38 (18–54.5) | 42 (20–53)a | 25 (17–53)b | 53 (17.5–57.5) | 0.020 |
| Presence of symptoms | 24/133 (14.2) | 17/89 (19.1) | 3/31 (9.7) | 4/13 (30.8) | NS |
| Chest pain | 10/133 (7.5) | 8/89 (9.0) | 2/31 (6.5) | 0/13 (0) | NS |
| Dyspnoea | 3/133 (2.3) | 3/89 (3.4) | 0/31 (0) | 0/13 (0) | NS |
| Fatigue | 3/133 (2.3) | 2/89 (2.2) | 0/31 (0) | 1/13 (7.7) | NS |
| Syncope | 1/133 (0.8) | 0/89 (0) | 0/31 (0) | 1/13 (7.7) | NS |
| Palpitations | 1/133 (0.8) | 0/89 (0) | 1/31 (3.2) | 0/13 (0) | NS |
| Vertigo/discomfort | 1/133 (0.8) | 1/89 (1.1) | 0/31 (0) | 0/13 (0) | NS |
| Not specified | 5/133 (3.8) | 3/89 (3.4) | 0/31 (0) | 2/13 (0) | NS |
| Sport activity | 64/133 (48.1) | 45/89 (50.6) | 12/31 (38.7) | 7/13 (53.8) | NS |
| Time of follow-up (years), min–max | 2 (1–5) [0–23] | 2 (1–4)a [0–8] | 5 (2–11)b [0–23] | 1 (0–5.5) [0–9] | 0.001 |
| NYHA | 13/133 (9.8) | 8/89 (9.0) | 3/31 (9.7) | 2/13 (15.4) | NS |
| Adverse events | 12/133 (9.0) | 7/89 (7.9) | 1/31 (3.2)a | 4/13 (30.8)b | 0.086 |
| Cardiac interventional procedure | 6/133 (4.5) | 4/89 (4.5) | 0/31 (0) | 2/13 (15.4) | NS |
| Surgical reinterventional procedure | 3/133 (2.3) | 1/89 (1.1) | 1/31 (3.3) | 1/13 (7.7) | NS |
| Late deaths | 3/154 (1.9) | 2/105 (1.9) | 0/31 (0) | 1/13 (7.7) | NS |
| *Coronary-related AE | 5/133 (3.7) | 3/89 (3.3) | 1/31 (3.2) | 1/13 (7.7) | NS |
| Overall deaths | 5/138 (3.6) | 2/91 (2.2) | 1/32 (3.1) | 2/15 (13.3) | NS |
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Follow-up completeness | 138/156 (88.5) | 91/105 (86.7) | 32/35 (91.4) | 14/16 (100) | NS |
| Alive | 133/156 (86.4) | 89/105 (84.8) | 31/35 (88.6) | 13/16 (81.3) | NS |
| Lost | 18/156 (11.5) | 17/105 (16.2) | 3/35 (8.6) | 1/16 (12.5) | NS |
| Age (years) | 38 (18–54.5) | 42 (20–53)a | 25 (17–53)b | 53 (17.5–57.5) | 0.020 |
| Presence of symptoms | 24/133 (14.2) | 17/89 (19.1) | 3/31 (9.7) | 4/13 (30.8) | NS |
| Chest pain | 10/133 (7.5) | 8/89 (9.0) | 2/31 (6.5) | 0/13 (0) | NS |
| Dyspnoea | 3/133 (2.3) | 3/89 (3.4) | 0/31 (0) | 0/13 (0) | NS |
| Fatigue | 3/133 (2.3) | 2/89 (2.2) | 0/31 (0) | 1/13 (7.7) | NS |
| Syncope | 1/133 (0.8) | 0/89 (0) | 0/31 (0) | 1/13 (7.7) | NS |
| Palpitations | 1/133 (0.8) | 0/89 (0) | 1/31 (3.2) | 0/13 (0) | NS |
| Vertigo/discomfort | 1/133 (0.8) | 1/89 (1.1) | 0/31 (0) | 0/13 (0) | NS |
| Not specified | 5/133 (3.8) | 3/89 (3.4) | 0/31 (0) | 2/13 (0) | NS |
| Sport activity | 64/133 (48.1) | 45/89 (50.6) | 12/31 (38.7) | 7/13 (53.8) | NS |
| Time of follow-up (years), min–max | 2 (1–5) [0–23] | 2 (1–4)a [0–8] | 5 (2–11)b [0–23] | 1 (0–5.5) [0–9] | 0.001 |
| NYHA | 13/133 (9.8) | 8/89 (9.0) | 3/31 (9.7) | 2/13 (15.4) | NS |
| Adverse events | 12/133 (9.0) | 7/89 (7.9) | 1/31 (3.2)a | 4/13 (30.8)b | 0.086 |
| Cardiac interventional procedure | 6/133 (4.5) | 4/89 (4.5) | 0/31 (0) | 2/13 (15.4) | NS |
| Surgical reinterventional procedure | 3/133 (2.3) | 1/89 (1.1) | 1/31 (3.3) | 1/13 (7.7) | NS |
| Late deaths | 3/154 (1.9) | 2/105 (1.9) | 0/31 (0) | 1/13 (7.7) | NS |
| *Coronary-related AE | 5/133 (3.7) | 3/89 (3.3) | 1/31 (3.2) | 1/13 (7.7) | NS |
| Overall deaths | 5/138 (3.6) | 2/91 (2.2) | 1/32 (3.1) | 2/15 (13.3) | NS |
Numbers represent median (interquartile range) for continuous variable and n (%) for categorical variable. Values in the same row that have different superscript letters are significantly different from each other.
Three coronary stenting; 1 surgical resection of intramyocardial course in 1 AOLCA; 1 automatic implantable cardioverter defibrillator implant in 1 AORCA.
AE: adverse events; AOLCA: aortic origin of left coronary artery; AORCA: aortic origin of right coronary artery; NS, not significant; NYHA: New York Heart Association.
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Follow-up completeness | 138/156 (88.5) | 91/105 (86.7) | 32/35 (91.4) | 14/16 (100) | NS |
| Alive | 133/156 (86.4) | 89/105 (84.8) | 31/35 (88.6) | 13/16 (81.3) | NS |
| Lost | 18/156 (11.5) | 17/105 (16.2) | 3/35 (8.6) | 1/16 (12.5) | NS |
| Age (years) | 38 (18–54.5) | 42 (20–53)a | 25 (17–53)b | 53 (17.5–57.5) | 0.020 |
| Presence of symptoms | 24/133 (14.2) | 17/89 (19.1) | 3/31 (9.7) | 4/13 (30.8) | NS |
| Chest pain | 10/133 (7.5) | 8/89 (9.0) | 2/31 (6.5) | 0/13 (0) | NS |
| Dyspnoea | 3/133 (2.3) | 3/89 (3.4) | 0/31 (0) | 0/13 (0) | NS |
| Fatigue | 3/133 (2.3) | 2/89 (2.2) | 0/31 (0) | 1/13 (7.7) | NS |
| Syncope | 1/133 (0.8) | 0/89 (0) | 0/31 (0) | 1/13 (7.7) | NS |
| Palpitations | 1/133 (0.8) | 0/89 (0) | 1/31 (3.2) | 0/13 (0) | NS |
| Vertigo/discomfort | 1/133 (0.8) | 1/89 (1.1) | 0/31 (0) | 0/13 (0) | NS |
| Not specified | 5/133 (3.8) | 3/89 (3.4) | 0/31 (0) | 2/13 (0) | NS |
| Sport activity | 64/133 (48.1) | 45/89 (50.6) | 12/31 (38.7) | 7/13 (53.8) | NS |
| Time of follow-up (years), min–max | 2 (1–5) [0–23] | 2 (1–4)a [0–8] | 5 (2–11)b [0–23] | 1 (0–5.5) [0–9] | 0.001 |
| NYHA | 13/133 (9.8) | 8/89 (9.0) | 3/31 (9.7) | 2/13 (15.4) | NS |
| Adverse events | 12/133 (9.0) | 7/89 (7.9) | 1/31 (3.2)a | 4/13 (30.8)b | 0.086 |
| Cardiac interventional procedure | 6/133 (4.5) | 4/89 (4.5) | 0/31 (0) | 2/13 (15.4) | NS |
| Surgical reinterventional procedure | 3/133 (2.3) | 1/89 (1.1) | 1/31 (3.3) | 1/13 (7.7) | NS |
| Late deaths | 3/154 (1.9) | 2/105 (1.9) | 0/31 (0) | 1/13 (7.7) | NS |
| *Coronary-related AE | 5/133 (3.7) | 3/89 (3.3) | 1/31 (3.2) | 1/13 (7.7) | NS |
| Overall deaths | 5/138 (3.6) | 2/91 (2.2) | 1/32 (3.1) | 2/15 (13.3) | NS |
| . | All (%) . | AORCA (%) . | AOLCA (%) . | Other (%) . | P-value . |
|---|---|---|---|---|---|
| Follow-up completeness | 138/156 (88.5) | 91/105 (86.7) | 32/35 (91.4) | 14/16 (100) | NS |
| Alive | 133/156 (86.4) | 89/105 (84.8) | 31/35 (88.6) | 13/16 (81.3) | NS |
| Lost | 18/156 (11.5) | 17/105 (16.2) | 3/35 (8.6) | 1/16 (12.5) | NS |
| Age (years) | 38 (18–54.5) | 42 (20–53)a | 25 (17–53)b | 53 (17.5–57.5) | 0.020 |
| Presence of symptoms | 24/133 (14.2) | 17/89 (19.1) | 3/31 (9.7) | 4/13 (30.8) | NS |
| Chest pain | 10/133 (7.5) | 8/89 (9.0) | 2/31 (6.5) | 0/13 (0) | NS |
| Dyspnoea | 3/133 (2.3) | 3/89 (3.4) | 0/31 (0) | 0/13 (0) | NS |
| Fatigue | 3/133 (2.3) | 2/89 (2.2) | 0/31 (0) | 1/13 (7.7) | NS |
| Syncope | 1/133 (0.8) | 0/89 (0) | 0/31 (0) | 1/13 (7.7) | NS |
| Palpitations | 1/133 (0.8) | 0/89 (0) | 1/31 (3.2) | 0/13 (0) | NS |
| Vertigo/discomfort | 1/133 (0.8) | 1/89 (1.1) | 0/31 (0) | 0/13 (0) | NS |
| Not specified | 5/133 (3.8) | 3/89 (3.4) | 0/31 (0) | 2/13 (0) | NS |
| Sport activity | 64/133 (48.1) | 45/89 (50.6) | 12/31 (38.7) | 7/13 (53.8) | NS |
| Time of follow-up (years), min–max | 2 (1–5) [0–23] | 2 (1–4)a [0–8] | 5 (2–11)b [0–23] | 1 (0–5.5) [0–9] | 0.001 |
| NYHA | 13/133 (9.8) | 8/89 (9.0) | 3/31 (9.7) | 2/13 (15.4) | NS |
| Adverse events | 12/133 (9.0) | 7/89 (7.9) | 1/31 (3.2)a | 4/13 (30.8)b | 0.086 |
| Cardiac interventional procedure | 6/133 (4.5) | 4/89 (4.5) | 0/31 (0) | 2/13 (15.4) | NS |
| Surgical reinterventional procedure | 3/133 (2.3) | 1/89 (1.1) | 1/31 (3.3) | 1/13 (7.7) | NS |
| Late deaths | 3/154 (1.9) | 2/105 (1.9) | 0/31 (0) | 1/13 (7.7) | NS |
| *Coronary-related AE | 5/133 (3.7) | 3/89 (3.3) | 1/31 (3.2) | 1/13 (7.7) | NS |
| Overall deaths | 5/138 (3.6) | 2/91 (2.2) | 1/32 (3.1) | 2/15 (13.3) | NS |
Numbers represent median (interquartile range) for continuous variable and n (%) for categorical variable. Values in the same row that have different superscript letters are significantly different from each other.
Three coronary stenting; 1 surgical resection of intramyocardial course in 1 AOLCA; 1 automatic implantable cardioverter defibrillator implant in 1 AORCA.
AE: adverse events; AOLCA: aortic origin of left coronary artery; AORCA: aortic origin of right coronary artery; NS, not significant; NYHA: New York Heart Association.
Nine major AE occurred in 8 patients (6.0% of survivors) after a median time of 9 months (IQR 6–69; range 1–80 months): 3 patients (2.3%) required a surgical reoperation (AV replacement, followed by pacemaker implantation; ascending aorta replacement; and resection of an intramyocardial bridge); 5 (3.7%) had a non-surgical procedure (coronary artery stenting in 3, cryoablation procedure for Wolff Parkinson White syndrome in 1; automatic implantable cardioverter defibrillator implantation in 1); overall, there were 5 coronary-related AE (Table 3).
Most late survivors (120, 91.2%) were in NYHA functional class ≤ II, and 48.1% (mostly younger patients) returned to sport activity, according to international guidelines [17]. Patients were followed up using standard electrocardiogram and echocardiography; provocative stress tests were performed rarely (Supplementary Material, Table S3). Symptoms were present in 24 (14.2%) patients, mostly undefined chest pain, which was correlated to positive stress test results in 1 patient only, whereas 2 patients had concomitant arrhythmias and hypertension. Of note, 23 patients were symptomatic at diagnosis.
Statistical analyses
Analyses of categorical variables with the Fisher’s exact test (Table 4) and binary logistic regression analysis (Table 5) showed that the prevalence of AE was not significantly different among age classes, anatomical variants and surgical procedures, whereas return to sport activity was significantly higher in younger patients, in those with an AOLCA and those who exhibited an intramural course. Unroofing and an interarterial anatomical course of AAOCA seemed to protect the patient from AE (P = 0.021; OR 0.305, 95% CI 0.111–0.836) and recurrence of symptoms at follow-up (P = 0.024; OR 0.211, 95% CI 0.055–0.836), respectively. At Kaplan–Meier analysis, event free survival at follow-up was 74.6% (Fig. 1).
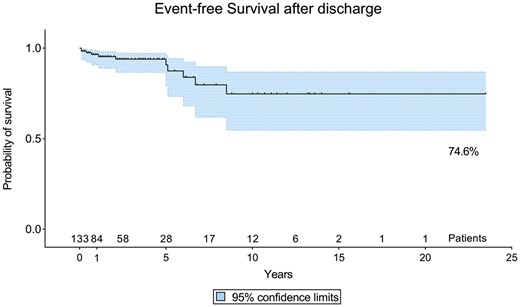
Kaplan–Meier curve showing event-free survival after discharge; the solid line is the survival curve; the dashed lines represent the 95% confidence interval; and the dots are censored observations.
| Type of surgical procedure . | All adverse events . | Sport activity at follow-up . | Symptoms at follow-up . | Overall mortality . |
|---|---|---|---|---|
| Unroofing—CABG | ||||
| P-value | 0.032 | 0.043 | 0.054 | 0.030 |
| OR | 0.259 | 3.250 | 0.319 | 0.080 |
| 95% CI | 0.078–0.864 | 1.072–9.851 | 0.104–0.978 | 0.008–0.813 |
| Unroofing–other | ||||
| P-value | 0.011 | |||
| OR | 0.156 | |||
| 95% CI | 0.041–0.593 | |||
| CABG–other | ||||
| P-value | 0.045 | |||
| OR | 0.143 | |||
| 95% CI | 0.026–0.774 | |||
| Age class at procedure (years)a | ||||
| 11–30; 31–50 | ||||
| P-value | 0.017 | |||
| OR | 3.021 | |||
| 95% CI | 1.247–7.316 | |||
| 11–30; 51+ | ||||
| P-value | 0.003 | |||
| OR | 4.218 | |||
| 95% CI | 1.635–10.880 | |||
| Preoperative sport activity | ||||
| P-value | <0.001 | |||
| OR | 7.815 | |||
| 95% CI | 3.105–19.668 | |||
| Type of surgical procedure . | All adverse events . | Sport activity at follow-up . | Symptoms at follow-up . | Overall mortality . |
|---|---|---|---|---|
| Unroofing—CABG | ||||
| P-value | 0.032 | 0.043 | 0.054 | 0.030 |
| OR | 0.259 | 3.250 | 0.319 | 0.080 |
| 95% CI | 0.078–0.864 | 1.072–9.851 | 0.104–0.978 | 0.008–0.813 |
| Unroofing–other | ||||
| P-value | 0.011 | |||
| OR | 0.156 | |||
| 95% CI | 0.041–0.593 | |||
| CABG–other | ||||
| P-value | 0.045 | |||
| OR | 0.143 | |||
| 95% CI | 0.026–0.774 | |||
| Age class at procedure (years)a | ||||
| 11–30; 31–50 | ||||
| P-value | 0.017 | |||
| OR | 3.021 | |||
| 95% CI | 1.247–7.316 | |||
| 11–30; 51+ | ||||
| P-value | 0.003 | |||
| OR | 4.218 | |||
| 95% CI | 1.635–10.880 | |||
| Preoperative sport activity | ||||
| P-value | <0.001 | |||
| OR | 7.815 | |||
| 95% CI | 3.105–19.668 | |||
Analysis was performed in all classes; we report here only significant results.
CABG: coronary artery bypass graft; CI: confidence interval; OR: odd ratio.
| Type of surgical procedure . | All adverse events . | Sport activity at follow-up . | Symptoms at follow-up . | Overall mortality . |
|---|---|---|---|---|
| Unroofing—CABG | ||||
| P-value | 0.032 | 0.043 | 0.054 | 0.030 |
| OR | 0.259 | 3.250 | 0.319 | 0.080 |
| 95% CI | 0.078–0.864 | 1.072–9.851 | 0.104–0.978 | 0.008–0.813 |
| Unroofing–other | ||||
| P-value | 0.011 | |||
| OR | 0.156 | |||
| 95% CI | 0.041–0.593 | |||
| CABG–other | ||||
| P-value | 0.045 | |||
| OR | 0.143 | |||
| 95% CI | 0.026–0.774 | |||
| Age class at procedure (years)a | ||||
| 11–30; 31–50 | ||||
| P-value | 0.017 | |||
| OR | 3.021 | |||
| 95% CI | 1.247–7.316 | |||
| 11–30; 51+ | ||||
| P-value | 0.003 | |||
| OR | 4.218 | |||
| 95% CI | 1.635–10.880 | |||
| Preoperative sport activity | ||||
| P-value | <0.001 | |||
| OR | 7.815 | |||
| 95% CI | 3.105–19.668 | |||
| Type of surgical procedure . | All adverse events . | Sport activity at follow-up . | Symptoms at follow-up . | Overall mortality . |
|---|---|---|---|---|
| Unroofing—CABG | ||||
| P-value | 0.032 | 0.043 | 0.054 | 0.030 |
| OR | 0.259 | 3.250 | 0.319 | 0.080 |
| 95% CI | 0.078–0.864 | 1.072–9.851 | 0.104–0.978 | 0.008–0.813 |
| Unroofing–other | ||||
| P-value | 0.011 | |||
| OR | 0.156 | |||
| 95% CI | 0.041–0.593 | |||
| CABG–other | ||||
| P-value | 0.045 | |||
| OR | 0.143 | |||
| 95% CI | 0.026–0.774 | |||
| Age class at procedure (years)a | ||||
| 11–30; 31–50 | ||||
| P-value | 0.017 | |||
| OR | 3.021 | |||
| 95% CI | 1.247–7.316 | |||
| 11–30; 51+ | ||||
| P-value | 0.003 | |||
| OR | 4.218 | |||
| 95% CI | 1.635–10.880 | |||
| Preoperative sport activity | ||||
| P-value | <0.001 | |||
| OR | 7.815 | |||
| 95% CI | 3.105–19.668 | |||
Analysis was performed in all classes; we report here only significant results.
CABG: coronary artery bypass graft; CI: confidence interval; OR: odd ratio.
| . | P-value . | OR (95% CI) . |
|---|---|---|
| Return to sport activity | ||
| AOLCA | 0.012 | 0.095 (0.015–0.591) |
| Intramural course | 0.025 | 2.839 (1.137–7.087) |
| Preoperative sport activity | <0.0001 | 9.690 (3.111–30.181) |
| Age class (1–10 years vs 51+) | 0.013 | 9.375 (1.615–54.423) |
| Age class (11–30 years vs 51+) | 0.009 | 5.310 (1.526–18.481) |
| Adverse events at follow-up | ||
| Unroofing | 0.021 | 0.305 (0.111–0.836) |
| Symptoms at follow-up | ||
| Interarterial course | 0.024 | 0.211 (0.055–0.811) |
| NYHA functional class >II at follow-up | ||
| Interarterial course | 0.029 | 0.224 (0.059–0.855) |
| . | P-value . | OR (95% CI) . |
|---|---|---|
| Return to sport activity | ||
| AOLCA | 0.012 | 0.095 (0.015–0.591) |
| Intramural course | 0.025 | 2.839 (1.137–7.087) |
| Preoperative sport activity | <0.0001 | 9.690 (3.111–30.181) |
| Age class (1–10 years vs 51+) | 0.013 | 9.375 (1.615–54.423) |
| Age class (11–30 years vs 51+) | 0.009 | 5.310 (1.526–18.481) |
| Adverse events at follow-up | ||
| Unroofing | 0.021 | 0.305 (0.111–0.836) |
| Symptoms at follow-up | ||
| Interarterial course | 0.024 | 0.211 (0.055–0.811) |
| NYHA functional class >II at follow-up | ||
| Interarterial course | 0.029 | 0.224 (0.059–0.855) |
Considered variables were AORCA, AOLCA, ‘other’ origin, interarterial and intramural course, preoperative sport activity, symptoms at rest and at effort, type of surgical procedure and age classes (1–10 years; 11–30 years; 31–50 years; 51+ years).
AOLCA: aortic origin of left coronary artery; AORCA: aortic origin of right coronary artery; CI: confidence interval; NYHA: New York Heart Association; OR: odds ratio.
| . | P-value . | OR (95% CI) . |
|---|---|---|
| Return to sport activity | ||
| AOLCA | 0.012 | 0.095 (0.015–0.591) |
| Intramural course | 0.025 | 2.839 (1.137–7.087) |
| Preoperative sport activity | <0.0001 | 9.690 (3.111–30.181) |
| Age class (1–10 years vs 51+) | 0.013 | 9.375 (1.615–54.423) |
| Age class (11–30 years vs 51+) | 0.009 | 5.310 (1.526–18.481) |
| Adverse events at follow-up | ||
| Unroofing | 0.021 | 0.305 (0.111–0.836) |
| Symptoms at follow-up | ||
| Interarterial course | 0.024 | 0.211 (0.055–0.811) |
| NYHA functional class >II at follow-up | ||
| Interarterial course | 0.029 | 0.224 (0.059–0.855) |
| . | P-value . | OR (95% CI) . |
|---|---|---|
| Return to sport activity | ||
| AOLCA | 0.012 | 0.095 (0.015–0.591) |
| Intramural course | 0.025 | 2.839 (1.137–7.087) |
| Preoperative sport activity | <0.0001 | 9.690 (3.111–30.181) |
| Age class (1–10 years vs 51+) | 0.013 | 9.375 (1.615–54.423) |
| Age class (11–30 years vs 51+) | 0.009 | 5.310 (1.526–18.481) |
| Adverse events at follow-up | ||
| Unroofing | 0.021 | 0.305 (0.111–0.836) |
| Symptoms at follow-up | ||
| Interarterial course | 0.024 | 0.211 (0.055–0.811) |
| NYHA functional class >II at follow-up | ||
| Interarterial course | 0.029 | 0.224 (0.059–0.855) |
Considered variables were AORCA, AOLCA, ‘other’ origin, interarterial and intramural course, preoperative sport activity, symptoms at rest and at effort, type of surgical procedure and age classes (1–10 years; 11–30 years; 31–50 years; 51+ years).
AOLCA: aortic origin of left coronary artery; AORCA: aortic origin of right coronary artery; CI: confidence interval; NYHA: New York Heart Association; OR: odds ratio.
DISCUSSION
This study is the largest surgical series ever reported that evaluated surgical outcomes of AAOCA and demonstrated an excellent early survival, with low operative risk and no significant difference in survival or reintervention with different surgical techniques and age classes.
As reported in the literature and in other surgical series [6–12], in our study AORCA was the most frequent anomaly (67.3%).
Despite the lack of true surgical guidelines for intervention in AAOCA, there are some recommendations and expert consensus guidelines [18, 19] that can help with management. In our experience, surgical treatment was indicated in all patients with AOLCA despite the absence of symptoms, especially in active patients, in those < 30 years old and in symptomatic patients with AORCA. All patients with AORCA who were asymptomatic and who had surgery presented with positive results from the preoperative diagnostic tests, or a history of silent ischaemia, or associated cardiac disease, which justified the operation.
As reported elsewhere [6–12], we confirmed that surgical repair is safe, with low operative mortality (1.3%) and that, in our series, death was associated with very poor preoperative conditions. Unroofing of the intramural segment was the most common procedure, with no early deaths. It was a simple procedure, more congenial to congenital surgeons [20], that was performed in 88/98 cases of AAOCA with an intramural course. Its protective effect on late-onset AE is due to the excision of the intervening roof of the intramural segment, whose removal prevents coronary compression and ischaemia during effort, relocates the functional orifice to the appropriate sinus, enlarges the orifice significantly and eliminates the portion of the vessel that lies between the great arteries. Significant postoperative aortic regurgitation late after unroofing has been reported sporadically [12, 19, 21]. In our series, 2 patients were discharged home with mild AV regurgitation after an unroofing procedure for AORCA but were lost to follow-up, whereas another patient underwent late AV replacement after CABG.
Coronary reimplantation was favoured when the intramural segment was too short or absent. In contrast to what has been suggested elsewhere [12], in our series, when the anomalous coronary intramural course was close to or below the aortic commissure, it was not the preferred technique. In such cases, the commissure was usually taken down and resuspended after unroofing.
In 2 cases, translocation of the pulmonary artery was performed as an additional technique. Relocating the pulmonary artery away from the coronaries may be a safe adjunct to other procedures when the abnormal coronary may still have some interarterial residual course.
In this study, CABG was performed in 24 patients at a significantly older age compared to that reported for other procedures (Table 2). Davies et al. [10] reported a successful series of 36 adults (mean age 47 years) with AAOCA, in whom 1/3 received CABG. However, CABG may be suboptimal in AAOCA because most patients have a preoperative normal coronary blood flow, and competitive flow may jeopardize the long-term patency of the internal mammary artery. In fact, Fedoruk et al. [22] described 40% late graft occlusion in 5 patients with AORCA treated with a right internal mammary artery graft, whereas Tavaf-Motamen et al. [23] reported 2 patients treated with CABG for AORCA who had both early recurrence of symptoms and graft failure. Thus, CABG alone may be best suited when other procedures are contraindicated, such as in AAOCA with severe proximal narrowing or in older patients with diffuse atherosclerosis.
Because no deaths were associated with unroofing in our series, we believe that it is the procedure of choice for young patients with risky anomalous coronary anatomy with an intramural course (mainly AOLCA), even in the absence of symptoms, whereas different surgical strategies may be used in addition. The Vouhè technique and the recently described ‘unflooring’ technique [24] (that couples patch augmentation of the intramural course with standard unroofing) show an increasing technical effort to optimize clinical results. In addition, only 4 patients (aged 12, 47, 58 and 64 years) required a reintervention on the coronaries (3 stenting, 1 resection of the intramyocardial bridge), which demonstrates the anatomical effectiveness of surgery.
In our study, the median age at operation was 39 years, which seems to underline the fact that AAOCA is not only a surgical problem of the young or teenagers, as described elsewhere [6–12]. We could not demonstrate any difference in onset of AE at follow-up in different age classes. Because the physiopathological process causing SCD seems to be triggered by transitory ischaemia through the anomalous coronaries on effort or soon after, we posit that this event may occur at any age, even in asymptomatic active adults, especially nowadays when elderly people are more active and in better shape than in the past.
There is a significant variation between adult and paediatric hospitals, both in terms of training of caregivers and surgical abilities. In his series of 29 patients (20 at a paediatric centre, 9 at an adult centre), Herrman [20] noticed that the unroofing procedure was most commonly performed in paediatric centres and that the 2 isolated unroofing procedures in adult centres were performed by a congenitally trained cardiac surgeon. In our study, since all surgeons in the European Congenital Heart Surgeons Association are congenitally trained surgeons, the surgical choice was dictated by anatomical variants and the age of the patients rather than by specific technical abilities. We concluded that AAOCA should be treated by a congenitally trained cardiac surgeon regardless of the patient’s age at presentation.
Nearly half of the patients in our series returned to preoperative sport activity and an unrestricted lifestyle (Table 5). This result shows that surgical repair of AAOCA allows the potential for patients to resume desirable activities and the lifestyle of their choice, critically important from the perspective of their perception for quality of life. This observation is in accordance with the current recommendations of the American Heart Association and American College of Cardiology [17], which suggest that ‘after successful surgical repair of an anomalous origin from the wrong sinus, athletes may consider participation in all sports 3 months after surgery if free of symptoms and exercise stress test shows no evidence of ischaemia or cardiac arrhythmias’.
Despite an acceptable rate of AE at follow-up (9%), with 3 late deaths in elderly patients, and surgical reoperations/reinterventions in 8 patients, mostly >50 years of age, 14.2% of patients continued to have symptoms postoperatively. Thus, this result justifies a close and accurate clinical and instrumental follow-up, as recommended elsewhere [18].
Limitations
Major limitations are the intrinsic retrospective nature of the study (with evident intercentre/intracentre variability regarding indications, choice of surgical technique and possible omissions of certain data points at follow-up data collection), lack of objective evidence for elimination of ischaemia in most patients, the short duration of the follow-up period and the rarity of the feared adverse outcome (SCD) in the natural history of this condition.
CONCLUSIONS
Surgery for AAOCA is a safe procedure, with very low operative mortality and rate of early complications, which may be related to compromised preoperative conditions. Unroofing and coronary reimplantation are the most common and safest procedures, with no operative deaths, whereas CABG may be an option in older patients with diffuse vasculopathy.
Most patients are rendered asymptomatic postoperatively, and athletes, especially if young, can return to sports after surgery. However, occurrence of late adverse events (surgical, non-surgical, SCD) is not negligible, and long-term surveillance is mandatory.
Conflict of interest: none declared.
Footnotes
Presented at the 32nd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Milan, Italy, 18–20 October 2018.





