-
PDF
- Split View
-
Views
-
Cite
Cite
Jacopo Alfonsi, Giacomo Murana, Henri G Smeenk, Hans Kelder, Marc Schepens, Uday Sonker, Wim J Morshuis, Robin H Heijmen, Open surgical repair of post-dissection thoraco-abdominal aortic aneurysms: early and late outcomes of a single-centre study involving over 200 patients, European Journal of Cardio-Thoracic Surgery, Volume 54, Issue 2, August 2018, Pages 382–388, https://doi.org/10.1093/ejcts/ezy050
Close - Share Icon Share
Abstract
Chronic, post-dissection thoraco-abdominal aortic aneurysms (TAAAs) are increasingly being treated by (hybrid) endovascular means. Although it is less invasive, thoracic endovascular aortic repair is technically complex with the risk of incomplete aneurysm exclusion, necessitating frequent reinterventions with potentially reduced long-term outcomes. The aim of this study was to evaluate contemporary early and late outcomes after open surgical repair of post-dissection TAAA.
At our centre, 633 patients underwent open repair for TAAA over a 20-year period (1994–2015), including 217 (34%) patients for post-dissection TAAA, who were included in this analysis. Circulatory support was obtained by either left heart bypass (173 patients, 79.7%), deep hypothermic circulatory arrest (41 patients, 18.9%) or simple aortic cross-clamping in 3 patients. We analysed all relevant perioperative and intraoperative variables with respect to adverse outcomes. Additionally, long-term survival and the need for aortic reinterventions were studied.
The mean age was 60.2 ± 11.9 years (men 68.2%). We identified 66 Type I (30.4%), 113 Type II (52.1%), 25 Type III (11.5%), 10 Type IV (4.6%) and 3 Type V (1.4%) TAAAs. Early mortality and spinal cord deficit were 5.9% and 5.5%, respectively. Follow-up was 100% complete (mean 6.0 ± 5.8 years), with long-term survival of 71.4% at 10 years, and freedom from death and reoperation was 68.2% at 10 years.
Although it is more invasive than current endovascular approaches for post-dissection TAAA, open surgical repair can be performed safely with acceptable rates of morbidity and mortality when it is done in a specialized aortic centre. Long-term survival and freedom from aortic reintervention are excellent and should also be taken into account when evaluating less invasive alternatives.
INTRODUCTION
Following aortic dissection (both primary Type B and residual after Type A), about one-third of patients develop an aneurysm in the descending thoracic aorta (DTA) or the thoraco-abdominal aorta (TAAA) that requires intervention to prevent a rupture [1–3]. Repair, however, may pose a challenge with regard to the aortic arch proximally or the aneurysmal extent distally (i.e. visceral artery involvement). Open surgical resection (OSR) of the aneurysmal aortic segment is invasive and associated with considerable morbidity and mortality; although in experienced hands, it has been shown to be a valid treatment option [4]. In the search for a less invasive alternative, hybrid options (with debranching of visceral arteries) have been evaluated [5]. The least invasive option may be percutaneous. Thoracic endovascular aortic repair (TEVAR) in the early phase of dissection has gained wide interest for treatment of early complications or for prevention of late dilatation [6]. Although it is gradually becoming a first-line treatment for degenerative aneurysms, its role in chronic post-dissection aneurysm repair is less defined. Apart from the complexity of obtaining an adequate proximal seal, complete exclusion of the dilated false lumen is difficult to achieve due to the rigid intimal membrane and, more importantly, the presence of multiple (large) re-entries distally. Following TEVAR, the need for a secondary intervention (or late conversion to surgery) is common. Additionally, procedural complications are considerable. As an alternative, branched or fenestrated endovascular aneurysm repair (B/FEVAR) using stent grafts is being proposed to overcome the issue of persistent, retrograde false-lumen perfusion. Although this procedure has been shown to be feasible, long-term results are needed [7]. So, a variety of (new) treatment options are used for this complex aortic disease. A recent literature review compared OSR, TEVAR and B/FEVAR [8]. The early survival benefit of TEVAR over OSR was accompanied by higher complication and reintervention rates after endovascular repair, although complications after OSR were usually more serious. Previous reports usually combined data of aneurysmal dilatation and hence resection, restricted to the DTA instead of the truly complex TAAA. In our attempt to identify the optimal mode of repair for chronic, post-dissection TAAA in this endovascular era, we evaluated both the early and long-term results of our large cohort of open surgical patients (>200) using all modern intraoperative adjuncts.
MATERIALS AND METHODS
Between March 1994 and December 2015, 633 consecutive patients underwent TAAA repair at the St. Antonius Hospital in Nieuwegein, Netherlands [9]. Resections limited to the DTA (using a left thoracotomy only) were not included. Retrospectively, we identified from the departmental database 217 patients who were diagnosed with chronic post-dissection aneurysms in the thoraco-abdominal aortic segment. The indication for surgery was either the maximal diameter (>60 mm or >55 mm in the case of Marfan syndrome) or symptoms. Paraplegia or paraparesis was defined as a spinal cord deficit, both immediate (upon awakening) and delayed (with a symptom-free interval). All patients were followed postoperatively at the outpatient clinic at 3 months after discharge and yearly thereafter using computed tomography angiography scanning. Perioperative data were collected through a hospital database, patients’ medical records and contact with the referring physician or general practitioner. The follow-up period ended in February 2016.
Surgical technique
Our technique for open TAAA repair was described previously [9]. Briefly, the left hemidiaphragm was divided circumferentially, and the aorta was exposed using the retroperitoneal route after median rotation of the viscera. Either distal aortic perfusion was established by left heart bypass using a centrifugal pump (173 patients, 79.7%) or extracorporeal circulation with deep hypothermic circulatory arrest was used in the case of the absence of a safe or adequate proximal aortic clamping site (41 patients, 18.9%). Among these 41 cases, 18 patients were treated for residual (post-Type A) post-dissection TAAA. According to the Crawford classification, 20 were Type I, 12 Type II, 3 Type III, 5 Type IV and 1 Type V. Fourteen cases had previous surgery of the distal arch and 10, of the proximal descending aorta. Ten of the surgeries were urgent and 3 were emergency procedures. In only 3 patients, simple cross-clamping without circulatory support was used: 2 of the patients had Type IV TAAA and 1 patient had Type III TAAA. For left heart bypass, the inflow for the centrifugal pump (BioMedicus, Minneapolis, MN, USA) was obtained with cannulation of the left pulmonary vein. Arterial outflow was established through the left common femoral artery or (rarely) the distal aorta. Whenever possible, sequential clamping was used in a craniocaudal fashion to minimize the ischaemic aortic segment and keep the visceral and lower segmental arteries perfused during construction of the proximal anastomosis. We usually directly reattached the patent intercostal and lumbar arteries (between T8 and L2) to the graft through an oval, circular or square opening (occasionally via an interposed 6- to 8-mm Dacron tube when direct reimplantation is technically impossible or in patients with Marfan syndrome). Cerebrospinal fluid drainage and evoked potentials (somatosensory and transcranial motor evoked potentials) were used in 192 (88.5%) and 177 (81.6%) patients, respectively. A neurophysiologist telemonitored the evoked potentials throughout the operation. A reduction of more than 50% (in relation to the baseline signal/amplitude of the tested right lower limb) was considered inadequate. In such a situation, the blood pressure rose immediately, spinal drainage increased and temporarily blocked intercostal arteries (ICA) were reimplanted using Pruitt catheters. When the intercostal arteries were undisturbed throughout the procedure, we occluded them up to Th7 and liberally reimplanted them only between Th7 and L2.
During exclusion of the visceral segment, selective perfusion to the coeliac and superior mesenteric arteries was obtained using the left heart bypass circuit, whereas a cold (4°C) solution of Ringer’s acetate with mannitol and methylprednisolone was administered intermittently (every 30 min) to protect the renal arteries. Finally, the graft was anastomosed above, on or beyond the aortic bifurcation. If necessary, the left heart bypass was temporarily stopped in order to make an open distal anastomosis. Occasionally, an aorto mono- or bi-iliac or aorto mono- or bifemoral prosthesis was used. After rewarming (rectal temperature of 34°C), bypass was discontinued, and the circuit was emptied through the left atrial cannula. Finally, to promote haemostasis, the prosthesis was covered with the cleaned aneurysmal sac.
Statistical analysis
The study is retrospective. All data are expressed as mean and standard deviation when applicable. Descriptive statistics were computed using standard univariate measures of frequency and central tendency. Univariable analysis of 30-day mortality risk factors was conducted. Multivariable analysis of these risk factors could not be performed due to the low incidence of 30-day deaths. Multivariable risk factor assessments for long-term survival were performed using multiple Cox regression. Survival curves and freedom from reoperation were analysed for all patients who survived the first 30 days at 1, 5, 10 and 15 years using the Kaplan–Meier method. To better separate the effect of the surgical procedure from the underlying disease, we performed a landmark analysis, whereby we analysed only patients who survived the first 30 days. Comparative survival for the reference population was estimated by applying age-specific annualized Dutch population survival rates to the starting value of the patient cohort at the 1st postoperative year. The data for the Dutch reference population was extracted from life tables provided by the Netherlands Central Bureau for Statistics (www.cbs.nl).
RESULTS
During the study period of almost 22 years, 217 patients (34% of the total number of 633 patients operated on for TAAA at our centre) were diagnosed with post-dissection TAAA and included for further analysis. The preoperative characteristics of the patients are reported in Table 1. About one-third of the patients had a previous repair of a Type A aortic dissection (n = 83, 38.2%). Others had primary Type B dissections that were diagnosed during follow-up (n = 134, 61.8%). As part of our 2-stage strategy for post-dissection TAAA post-Type A repair, the majority of our patients had prior aortic arch redo surgery with implantation of an elephant trunk to facilitate distal repair by using left heart bypass instead of deep hypothermic circulatory arrest (DHCA). Fifty-seven patients with a diagnosis of chronic Type A dissection previously underwent a total arch replacement using the elephant trunk technique. Thirty-five (16.1%) patients were diagnosed with Marfan syndrome. The vast majority (91.2%) was treated in an elective setting. Using the classification of Crawford and Safi, we identified 66 cases of Type I (30.4%), 113 Type II (52.1%), 25 Type III (11.5%), 10 Type IV (4.6%) and 3 Type V (1.4%) TAAAs.
| Variables (n = 217) . | Overall (%) . |
|---|---|
| Age (years), mean ± SD | 60.2 ± 11.7 |
| Male, n (%) | 148 (68.2) |
| Hypertension, n (%) | 190 (87.5) |
| Myocardial infarction, n (%) | 29 (13.4) |
| Left ventricular ejection fraction (<50), n (%) | 22 (10.1) |
| Cerebral vascular accident (%), n (%) | 16 (7.4) |
| COPD, n (%) | 32 (14.7) |
| Serum creatinine (mmol), mean ± SD | 96.4 ± 37.3 |
| Previous CABG, n (%) | 15 (6.9) |
| Previous PTCA, n (%) | 13 (5.9) |
| Previous ascending aorta intervention, n (%) | 92 (42.4) |
| Previous arch intervention, n (%) | 69 (31.8) |
| Previous TAA intervention (ET included), n (%) | 50 (23) |
| Previous AAA intervention, n (%) | 30 (13.8) |
| Aortic dissection, n (%) | |
| Type A preoperative | 83 (38.2) |
| Type B preoperative | 134 (61.8) |
| Marfan syndrome, n (%) | 35 (16.1) |
| TAAA I, n (%) | 66 (30.4) |
| TAAA II, n (%) | 113 (52.1) |
| TAAA III, n (%) | 25 (11.5) |
| TAAA IV, n (%) | 10 (4.6) |
| TAAA V, n (%) | 3 (1.4) |
| Elective cases, n (%) | 198 (91.2) |
| Urgent/emergent cases, n (%) | 19 (8.8) |
| Left heart bypass, n (%) | 173 (79.7) |
| DHCA, n (%) | 41 (18.9) |
| Simple cross-clamping, n (%) | 3 (1.4) |
| Variables (n = 217) . | Overall (%) . |
|---|---|
| Age (years), mean ± SD | 60.2 ± 11.7 |
| Male, n (%) | 148 (68.2) |
| Hypertension, n (%) | 190 (87.5) |
| Myocardial infarction, n (%) | 29 (13.4) |
| Left ventricular ejection fraction (<50), n (%) | 22 (10.1) |
| Cerebral vascular accident (%), n (%) | 16 (7.4) |
| COPD, n (%) | 32 (14.7) |
| Serum creatinine (mmol), mean ± SD | 96.4 ± 37.3 |
| Previous CABG, n (%) | 15 (6.9) |
| Previous PTCA, n (%) | 13 (5.9) |
| Previous ascending aorta intervention, n (%) | 92 (42.4) |
| Previous arch intervention, n (%) | 69 (31.8) |
| Previous TAA intervention (ET included), n (%) | 50 (23) |
| Previous AAA intervention, n (%) | 30 (13.8) |
| Aortic dissection, n (%) | |
| Type A preoperative | 83 (38.2) |
| Type B preoperative | 134 (61.8) |
| Marfan syndrome, n (%) | 35 (16.1) |
| TAAA I, n (%) | 66 (30.4) |
| TAAA II, n (%) | 113 (52.1) |
| TAAA III, n (%) | 25 (11.5) |
| TAAA IV, n (%) | 10 (4.6) |
| TAAA V, n (%) | 3 (1.4) |
| Elective cases, n (%) | 198 (91.2) |
| Urgent/emergent cases, n (%) | 19 (8.8) |
| Left heart bypass, n (%) | 173 (79.7) |
| DHCA, n (%) | 41 (18.9) |
| Simple cross-clamping, n (%) | 3 (1.4) |
AAA: abdominal aortic aneurysm; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; DHCA: deep hypothermic circulatory arrest; ET: elephant trunk; PTCA: percutaneous transluminal coronary angioplasty; SD: standard deviation; TAA: thoracic aortic aneurysm; TAAA: thoraco-abdominal aortic aneurysm.
| Variables (n = 217) . | Overall (%) . |
|---|---|
| Age (years), mean ± SD | 60.2 ± 11.7 |
| Male, n (%) | 148 (68.2) |
| Hypertension, n (%) | 190 (87.5) |
| Myocardial infarction, n (%) | 29 (13.4) |
| Left ventricular ejection fraction (<50), n (%) | 22 (10.1) |
| Cerebral vascular accident (%), n (%) | 16 (7.4) |
| COPD, n (%) | 32 (14.7) |
| Serum creatinine (mmol), mean ± SD | 96.4 ± 37.3 |
| Previous CABG, n (%) | 15 (6.9) |
| Previous PTCA, n (%) | 13 (5.9) |
| Previous ascending aorta intervention, n (%) | 92 (42.4) |
| Previous arch intervention, n (%) | 69 (31.8) |
| Previous TAA intervention (ET included), n (%) | 50 (23) |
| Previous AAA intervention, n (%) | 30 (13.8) |
| Aortic dissection, n (%) | |
| Type A preoperative | 83 (38.2) |
| Type B preoperative | 134 (61.8) |
| Marfan syndrome, n (%) | 35 (16.1) |
| TAAA I, n (%) | 66 (30.4) |
| TAAA II, n (%) | 113 (52.1) |
| TAAA III, n (%) | 25 (11.5) |
| TAAA IV, n (%) | 10 (4.6) |
| TAAA V, n (%) | 3 (1.4) |
| Elective cases, n (%) | 198 (91.2) |
| Urgent/emergent cases, n (%) | 19 (8.8) |
| Left heart bypass, n (%) | 173 (79.7) |
| DHCA, n (%) | 41 (18.9) |
| Simple cross-clamping, n (%) | 3 (1.4) |
| Variables (n = 217) . | Overall (%) . |
|---|---|
| Age (years), mean ± SD | 60.2 ± 11.7 |
| Male, n (%) | 148 (68.2) |
| Hypertension, n (%) | 190 (87.5) |
| Myocardial infarction, n (%) | 29 (13.4) |
| Left ventricular ejection fraction (<50), n (%) | 22 (10.1) |
| Cerebral vascular accident (%), n (%) | 16 (7.4) |
| COPD, n (%) | 32 (14.7) |
| Serum creatinine (mmol), mean ± SD | 96.4 ± 37.3 |
| Previous CABG, n (%) | 15 (6.9) |
| Previous PTCA, n (%) | 13 (5.9) |
| Previous ascending aorta intervention, n (%) | 92 (42.4) |
| Previous arch intervention, n (%) | 69 (31.8) |
| Previous TAA intervention (ET included), n (%) | 50 (23) |
| Previous AAA intervention, n (%) | 30 (13.8) |
| Aortic dissection, n (%) | |
| Type A preoperative | 83 (38.2) |
| Type B preoperative | 134 (61.8) |
| Marfan syndrome, n (%) | 35 (16.1) |
| TAAA I, n (%) | 66 (30.4) |
| TAAA II, n (%) | 113 (52.1) |
| TAAA III, n (%) | 25 (11.5) |
| TAAA IV, n (%) | 10 (4.6) |
| TAAA V, n (%) | 3 (1.4) |
| Elective cases, n (%) | 198 (91.2) |
| Urgent/emergent cases, n (%) | 19 (8.8) |
| Left heart bypass, n (%) | 173 (79.7) |
| DHCA, n (%) | 41 (18.9) |
| Simple cross-clamping, n (%) | 3 (1.4) |
AAA: abdominal aortic aneurysm; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; DHCA: deep hypothermic circulatory arrest; ET: elephant trunk; PTCA: percutaneous transluminal coronary angioplasty; SD: standard deviation; TAA: thoracic aortic aneurysm; TAAA: thoraco-abdominal aortic aneurysm.
Early morbidity
Perioperative myocardial infarction occurred in 4 (1.8%) patients, in whom we used either left heart bypass (n = 1/173, 0.6%) or DHCA (n = 3/41, 7.3%) (Table 2). One died in the operating room because he could not be weaned from the extracorporeal circulation. Postoperative stroke was observed in 5 (2.3%) patients in whom we used either left heart bypass (n = 3/173, 1.7%) or DHCA (n = 2/41, 4.8%). Spinal cord deficit was diagnosed in 12 (5.5%) patients in whom we used left heart bypass (n = 8/173, 4.6%) or DHCA (n = 4/41, 9.7%). Five cases of permanent paraplegia (2.3%) and 7 cases of paraparesis (3.2%) occurred. Paraparesis was transient and was resolved at discharge in 3 of 7 cases. The overall postoperative rate of acute kidney injury necessitating haemodialysis and tracheostomy was, respectively, 5.1% [left heart bypass, 8/173 (4.6%) and DHCA, 3/41 (7.3%)] and 6.4% [left heart bypass, 11/173 (6.3%), DHCA 3/41 (7.3%)] (Table 2).
| Variables (n = 217) . | Type I (n = 66) . | Type II (n = 113) . | Type III (n = 25) . | Type IV (n = 10) . | Type V (n = 3) . | Overall (n = 217) . |
|---|---|---|---|---|---|---|
| Overall in-hospital mortality, n (%) | 4 (6.1) | 6 (5.3) | 3 (12) | 13 (5.9) | ||
| Permanent spinal cord deficit, n (%) | 4 (6.1) | 7 (6.2) | 1 (4) | 12 (5.5) | ||
| Paraplegia | 1 (1.5) | 3 (2.6) | 1 (4) | 5 (2.3) | ||
| Paraparesis | 3 (4.5) | 4 (3.5) | 7 (3.2) | |||
| Stroke, n (%) | 3 (4.5) | 2 (1.8) | 5 (2.3) | |||
| AMI, n (%) | 3 (4.5) | 1 (0.9) | 4 (1.8) | |||
| AKI necessitating haemodialysis, n (%) | 3 (4.5) | 7 (6.2) | 1 (4) | 11 (5.1) | ||
| Creatinine serum level postoperative peak (mmol), mean ± SD | 125 ± 95 | 153 ± 93 | 140 ± 90 | 143 ± 81 | 142.3 ± 94 | |
| Tracheostomy, n (%) | 5 (7.6) | 7 (6.2) | 2 (8) | 14 (6.4) |
| Variables (n = 217) . | Type I (n = 66) . | Type II (n = 113) . | Type III (n = 25) . | Type IV (n = 10) . | Type V (n = 3) . | Overall (n = 217) . |
|---|---|---|---|---|---|---|
| Overall in-hospital mortality, n (%) | 4 (6.1) | 6 (5.3) | 3 (12) | 13 (5.9) | ||
| Permanent spinal cord deficit, n (%) | 4 (6.1) | 7 (6.2) | 1 (4) | 12 (5.5) | ||
| Paraplegia | 1 (1.5) | 3 (2.6) | 1 (4) | 5 (2.3) | ||
| Paraparesis | 3 (4.5) | 4 (3.5) | 7 (3.2) | |||
| Stroke, n (%) | 3 (4.5) | 2 (1.8) | 5 (2.3) | |||
| AMI, n (%) | 3 (4.5) | 1 (0.9) | 4 (1.8) | |||
| AKI necessitating haemodialysis, n (%) | 3 (4.5) | 7 (6.2) | 1 (4) | 11 (5.1) | ||
| Creatinine serum level postoperative peak (mmol), mean ± SD | 125 ± 95 | 153 ± 93 | 140 ± 90 | 143 ± 81 | 142.3 ± 94 | |
| Tracheostomy, n (%) | 5 (7.6) | 7 (6.2) | 2 (8) | 14 (6.4) |
AKI: acute kidney injury; AMI: acute myocardial infarction; SD: standard deviation.
| Variables (n = 217) . | Type I (n = 66) . | Type II (n = 113) . | Type III (n = 25) . | Type IV (n = 10) . | Type V (n = 3) . | Overall (n = 217) . |
|---|---|---|---|---|---|---|
| Overall in-hospital mortality, n (%) | 4 (6.1) | 6 (5.3) | 3 (12) | 13 (5.9) | ||
| Permanent spinal cord deficit, n (%) | 4 (6.1) | 7 (6.2) | 1 (4) | 12 (5.5) | ||
| Paraplegia | 1 (1.5) | 3 (2.6) | 1 (4) | 5 (2.3) | ||
| Paraparesis | 3 (4.5) | 4 (3.5) | 7 (3.2) | |||
| Stroke, n (%) | 3 (4.5) | 2 (1.8) | 5 (2.3) | |||
| AMI, n (%) | 3 (4.5) | 1 (0.9) | 4 (1.8) | |||
| AKI necessitating haemodialysis, n (%) | 3 (4.5) | 7 (6.2) | 1 (4) | 11 (5.1) | ||
| Creatinine serum level postoperative peak (mmol), mean ± SD | 125 ± 95 | 153 ± 93 | 140 ± 90 | 143 ± 81 | 142.3 ± 94 | |
| Tracheostomy, n (%) | 5 (7.6) | 7 (6.2) | 2 (8) | 14 (6.4) |
| Variables (n = 217) . | Type I (n = 66) . | Type II (n = 113) . | Type III (n = 25) . | Type IV (n = 10) . | Type V (n = 3) . | Overall (n = 217) . |
|---|---|---|---|---|---|---|
| Overall in-hospital mortality, n (%) | 4 (6.1) | 6 (5.3) | 3 (12) | 13 (5.9) | ||
| Permanent spinal cord deficit, n (%) | 4 (6.1) | 7 (6.2) | 1 (4) | 12 (5.5) | ||
| Paraplegia | 1 (1.5) | 3 (2.6) | 1 (4) | 5 (2.3) | ||
| Paraparesis | 3 (4.5) | 4 (3.5) | 7 (3.2) | |||
| Stroke, n (%) | 3 (4.5) | 2 (1.8) | 5 (2.3) | |||
| AMI, n (%) | 3 (4.5) | 1 (0.9) | 4 (1.8) | |||
| AKI necessitating haemodialysis, n (%) | 3 (4.5) | 7 (6.2) | 1 (4) | 11 (5.1) | ||
| Creatinine serum level postoperative peak (mmol), mean ± SD | 125 ± 95 | 153 ± 93 | 140 ± 90 | 143 ± 81 | 142.3 ± 94 | |
| Tracheostomy, n (%) | 5 (7.6) | 7 (6.2) | 2 (8) | 14 (6.4) |
AKI: acute kidney injury; AMI: acute myocardial infarction; SD: standard deviation.
Early mortality
Early mortality (both in-hospital and 30-day mortality) was 5.9% [13 patients, left heart bypass 9/173 (5.2%), DHCA 4/41 (9.7%)] (Table 2). When the surgery was emergent/urgent, 2 of 19 (10.5%) patients died. When the surgery was elective, 11 of 198 (5.6%) patients died. Two patients died intraoperatively of uncontrollable bleeding or heart failure. Three (1.4%) patients died of multiorgan failure, 3 (1.4%) of intestinal ischaemia and 3 (1.4%) of respiratory failure. The perioperative risk factors for 30-day mortality are described with univariable analysis in Table 3. Only postoperative acute kidney injury and postoperative respiratory insufficiency were identified as significant risk factors for 30-day mortality. Preoperative chronic renal insufficiency and chronic obstructive pulmonary disease did not demonstrate a statistically significant difference. Also, none of the intraoperative variables analysed proved to be significantly related to in-hospital death. Due to the small number of events (13 deaths at 30 days), multivariable analysis was not possible.
| Variables . | In-hospital survival (n = 204) . | In-hospital deaths (n = 13) . | Odds ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| Preoperative | ||||
| Female, n (%) | 65 (31.9) | 4 (30.8) | 0.97 (0.25–3.16) | 1.000 |
| Age (years), mean ± SD | 59.9 ± 11.8 | 65.1 ± 9.23 | 1.05 (0.99–1.11) | 0.075 |
| Hypertension, n (%) | 178 (87.3) | 12 (92.3) | 1.56 (0.28–39.2) | 1.000 |
| Prior AMI, n (%) | 26 (12.7) | 3 (23.1) | 2.11 (0.43–7.60) | 0.390 |
| Prior CABG, n (%) | 15 (7.35) | 0 (0.00) | 0.607 | |
| Prior PCI, n (%) | 12 (5.88) | 1 (7.69) | 1.49 (0.06–8.78) | 0.563 |
| LVEF (%), n (%) | 1.000 | |||
| >50 | 182 (89.7) | 12 (92.3) | Ref. | |
| 30–50 | 19 (9.36) | 1 (7.69) | ||
| <30 | 2 (0.99) | 0 (0.00) | ||
| Prior stroke | 13 (6.37) | 3 (23.1) | 4.48 (0.87–17.3) | 0.060 |
| Prior TIA | 6 (2.94) | 0 (0.00) | 1.000 | |
| History of COPD | 30 (14.7) | 2 (15.4) | 1.11 (0.15–4.51) | 1.000 |
| Preoperative creatinine level (μmol/l), median (IQR) | 88.5 (78.0–106) | 89.0 (82.0–100) | 1.01 (1.00–1.02) | 0.951 |
| Preoperatively on dialysis | 204 (100) | 13 (100) | Ref. | |
| History of diabetes | 8 (3.92) | 0 (0.00) | 1.000 | |
| History of claudication | 5 (2.45) | 2 (15.4) | 7.35 (0.87–40.6) | 0.059 |
| History of GE disease | 6 (2.94) | 1 (7.69) | 3.02 (0.11–20.6) | 0.355 |
| Ascending aortic aneurysm | 50 (24.5) | 4 (30.8) | 1.39 (0.35–4.57) | 0.741 |
| Arch aortic aneurysm | 38 (18.6) | 2 (15.4) | 0.84 (0.12–3.37) | 1.000 |
| Descending aortic aneurysm | 93 (45.6) | 5 (38.5) | 0.76 (0.22–2.39) | 0.831 |
| Abdominal aortic aneurysm | 64 (31.4) | 3 (23.1) | 0.68 (0.14–2.36) | 0.759 |
| Prior ascending aortic surgery | 87 (42.6) | 5 (38.5) | 0.85 (0.24–2.69) | 0.995 |
| Prior arch aortic surgery | 67 (32.8) | 2 (15.4) | 0.40 (0.05–1.55) | 0.234 |
| Prior descending aortic surgery | 47 (23.0) | 3 (23.1) | 1.04 (0.21–3.62) | 1.000 |
| Prior abdominal aortic surgery | 29 (14.2) | 1 (7.69) | 0.57 (0.02–3.09) | 1.000 |
| Prior Type A dissection | 77 (37.7) | 6 (46.2) | 1.42 (0.43–4.51) | 0.567 |
| Prior Type B dissection | 127 (62.3) | 7 (53.8) | 0.71 (0.22–2.32) | 0.567 |
| Rupture with pain | 11 (5.39) | 1 (7.69) | 1.63 (0.06–9.73) | 0.533 |
| Rupture with shock | 1 (0.49) | 1 (7.69) | 16.3 (0.40–657) | 0.116 |
| Rupture with haemothorax | 5 (2.45) | 1 (7.69) | 3.62 (0.13–26.2) | 0.313 |
| Symptom pain | 32 (15.7) | 5 (38.5) | 3.37 (0.94–11.0) | 0.050 |
| Symptom hoarseness | 2 (0.98) | 1 (7.69) | 8.70 (0.26–115) | 0.170 |
| Symptom dyspnoea | 11 (5.39) | 0 (0.00) | 1.000 | |
| Marfan syndrome | 33 (16.2) | 2 (15.4) | 1.00 (0.14–4.01) | 1.000 |
| Urgency | 0.318 | |||
| Elective | 187 (91.7) | 11 (84.6) | Ref. | |
| Urgent | 11 (5.39) | 2 (15.4) | ||
| Emergent | 6 (2.94) | 0 (0.00) | ||
| Type of TAAA | 0.656 | |||
| 1 | 62 (30.4) | 4 (30.8) | Ref. | |
| 2 | 107 (52.5) | 6 (46.2) | ||
| 3 | 22 (10.8) | 3 (23.1) | ||
| 4 | 10 (4.90) | 0 (0.00) | ||
| 5 | 3 (1.47) | 0 (0.00) | ||
| Intraoperative | ||||
| Left bypass | 165 (80.9) | 8 (61.5) | 0.38 (0.12–1.34) | 0.145 |
| DHCA | 37 (18.1) | 4 (30.8) | 2.04 (0.51–6.76) | 0.274 |
| Cross-clamp | 2 (0.98) | 1 (7.69) | 8.70 (0.26–115) | 0.170 |
| Postoperative | ||||
| MI | 3 (1.49) | 1 (7.69) | 5.88 (0.19–55.2) | 0.222 |
| Stroke | 4 (1.96) | 1 (7.69) | 4.50 (0.16–35.8) | 0.268 |
| Paraplegia | 4 (1.96) | 1 (7.69) | 4.50 (0.16–35.8) | 0.268 |
| Paraparesis | 7 (3.43) | 0 (0.00) | 1.000 | |
| Dialysis | 8 (3.92) | 3 (23.1) | 7.37 (1.37–31.2) | 0.021 |
| Tracheostomy | 11 (5.39) | 3 (23.1) | 5.33 (1.02–21.1) | 0.042 |
| Variables . | In-hospital survival (n = 204) . | In-hospital deaths (n = 13) . | Odds ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| Preoperative | ||||
| Female, n (%) | 65 (31.9) | 4 (30.8) | 0.97 (0.25–3.16) | 1.000 |
| Age (years), mean ± SD | 59.9 ± 11.8 | 65.1 ± 9.23 | 1.05 (0.99–1.11) | 0.075 |
| Hypertension, n (%) | 178 (87.3) | 12 (92.3) | 1.56 (0.28–39.2) | 1.000 |
| Prior AMI, n (%) | 26 (12.7) | 3 (23.1) | 2.11 (0.43–7.60) | 0.390 |
| Prior CABG, n (%) | 15 (7.35) | 0 (0.00) | 0.607 | |
| Prior PCI, n (%) | 12 (5.88) | 1 (7.69) | 1.49 (0.06–8.78) | 0.563 |
| LVEF (%), n (%) | 1.000 | |||
| >50 | 182 (89.7) | 12 (92.3) | Ref. | |
| 30–50 | 19 (9.36) | 1 (7.69) | ||
| <30 | 2 (0.99) | 0 (0.00) | ||
| Prior stroke | 13 (6.37) | 3 (23.1) | 4.48 (0.87–17.3) | 0.060 |
| Prior TIA | 6 (2.94) | 0 (0.00) | 1.000 | |
| History of COPD | 30 (14.7) | 2 (15.4) | 1.11 (0.15–4.51) | 1.000 |
| Preoperative creatinine level (μmol/l), median (IQR) | 88.5 (78.0–106) | 89.0 (82.0–100) | 1.01 (1.00–1.02) | 0.951 |
| Preoperatively on dialysis | 204 (100) | 13 (100) | Ref. | |
| History of diabetes | 8 (3.92) | 0 (0.00) | 1.000 | |
| History of claudication | 5 (2.45) | 2 (15.4) | 7.35 (0.87–40.6) | 0.059 |
| History of GE disease | 6 (2.94) | 1 (7.69) | 3.02 (0.11–20.6) | 0.355 |
| Ascending aortic aneurysm | 50 (24.5) | 4 (30.8) | 1.39 (0.35–4.57) | 0.741 |
| Arch aortic aneurysm | 38 (18.6) | 2 (15.4) | 0.84 (0.12–3.37) | 1.000 |
| Descending aortic aneurysm | 93 (45.6) | 5 (38.5) | 0.76 (0.22–2.39) | 0.831 |
| Abdominal aortic aneurysm | 64 (31.4) | 3 (23.1) | 0.68 (0.14–2.36) | 0.759 |
| Prior ascending aortic surgery | 87 (42.6) | 5 (38.5) | 0.85 (0.24–2.69) | 0.995 |
| Prior arch aortic surgery | 67 (32.8) | 2 (15.4) | 0.40 (0.05–1.55) | 0.234 |
| Prior descending aortic surgery | 47 (23.0) | 3 (23.1) | 1.04 (0.21–3.62) | 1.000 |
| Prior abdominal aortic surgery | 29 (14.2) | 1 (7.69) | 0.57 (0.02–3.09) | 1.000 |
| Prior Type A dissection | 77 (37.7) | 6 (46.2) | 1.42 (0.43–4.51) | 0.567 |
| Prior Type B dissection | 127 (62.3) | 7 (53.8) | 0.71 (0.22–2.32) | 0.567 |
| Rupture with pain | 11 (5.39) | 1 (7.69) | 1.63 (0.06–9.73) | 0.533 |
| Rupture with shock | 1 (0.49) | 1 (7.69) | 16.3 (0.40–657) | 0.116 |
| Rupture with haemothorax | 5 (2.45) | 1 (7.69) | 3.62 (0.13–26.2) | 0.313 |
| Symptom pain | 32 (15.7) | 5 (38.5) | 3.37 (0.94–11.0) | 0.050 |
| Symptom hoarseness | 2 (0.98) | 1 (7.69) | 8.70 (0.26–115) | 0.170 |
| Symptom dyspnoea | 11 (5.39) | 0 (0.00) | 1.000 | |
| Marfan syndrome | 33 (16.2) | 2 (15.4) | 1.00 (0.14–4.01) | 1.000 |
| Urgency | 0.318 | |||
| Elective | 187 (91.7) | 11 (84.6) | Ref. | |
| Urgent | 11 (5.39) | 2 (15.4) | ||
| Emergent | 6 (2.94) | 0 (0.00) | ||
| Type of TAAA | 0.656 | |||
| 1 | 62 (30.4) | 4 (30.8) | Ref. | |
| 2 | 107 (52.5) | 6 (46.2) | ||
| 3 | 22 (10.8) | 3 (23.1) | ||
| 4 | 10 (4.90) | 0 (0.00) | ||
| 5 | 3 (1.47) | 0 (0.00) | ||
| Intraoperative | ||||
| Left bypass | 165 (80.9) | 8 (61.5) | 0.38 (0.12–1.34) | 0.145 |
| DHCA | 37 (18.1) | 4 (30.8) | 2.04 (0.51–6.76) | 0.274 |
| Cross-clamp | 2 (0.98) | 1 (7.69) | 8.70 (0.26–115) | 0.170 |
| Postoperative | ||||
| MI | 3 (1.49) | 1 (7.69) | 5.88 (0.19–55.2) | 0.222 |
| Stroke | 4 (1.96) | 1 (7.69) | 4.50 (0.16–35.8) | 0.268 |
| Paraplegia | 4 (1.96) | 1 (7.69) | 4.50 (0.16–35.8) | 0.268 |
| Paraparesis | 7 (3.43) | 0 (0.00) | 1.000 | |
| Dialysis | 8 (3.92) | 3 (23.1) | 7.37 (1.37–31.2) | 0.021 |
| Tracheostomy | 11 (5.39) | 3 (23.1) | 5.33 (1.02–21.1) | 0.042 |
AMI: acute myocardial infarction; CABG: coronary artery bypass graft; CI: confidence interval; COPD: chronic obstructive pulmonary disease; DHCA: deep hypothermic circulatory arrest; GE: gastroenteric; IQR: interquartile range; LVEF: left ventricle ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; TAAA: thoraco-abdominal aortic aneurysm; TIA: transitory ischaemic attack.
| Variables . | In-hospital survival (n = 204) . | In-hospital deaths (n = 13) . | Odds ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| Preoperative | ||||
| Female, n (%) | 65 (31.9) | 4 (30.8) | 0.97 (0.25–3.16) | 1.000 |
| Age (years), mean ± SD | 59.9 ± 11.8 | 65.1 ± 9.23 | 1.05 (0.99–1.11) | 0.075 |
| Hypertension, n (%) | 178 (87.3) | 12 (92.3) | 1.56 (0.28–39.2) | 1.000 |
| Prior AMI, n (%) | 26 (12.7) | 3 (23.1) | 2.11 (0.43–7.60) | 0.390 |
| Prior CABG, n (%) | 15 (7.35) | 0 (0.00) | 0.607 | |
| Prior PCI, n (%) | 12 (5.88) | 1 (7.69) | 1.49 (0.06–8.78) | 0.563 |
| LVEF (%), n (%) | 1.000 | |||
| >50 | 182 (89.7) | 12 (92.3) | Ref. | |
| 30–50 | 19 (9.36) | 1 (7.69) | ||
| <30 | 2 (0.99) | 0 (0.00) | ||
| Prior stroke | 13 (6.37) | 3 (23.1) | 4.48 (0.87–17.3) | 0.060 |
| Prior TIA | 6 (2.94) | 0 (0.00) | 1.000 | |
| History of COPD | 30 (14.7) | 2 (15.4) | 1.11 (0.15–4.51) | 1.000 |
| Preoperative creatinine level (μmol/l), median (IQR) | 88.5 (78.0–106) | 89.0 (82.0–100) | 1.01 (1.00–1.02) | 0.951 |
| Preoperatively on dialysis | 204 (100) | 13 (100) | Ref. | |
| History of diabetes | 8 (3.92) | 0 (0.00) | 1.000 | |
| History of claudication | 5 (2.45) | 2 (15.4) | 7.35 (0.87–40.6) | 0.059 |
| History of GE disease | 6 (2.94) | 1 (7.69) | 3.02 (0.11–20.6) | 0.355 |
| Ascending aortic aneurysm | 50 (24.5) | 4 (30.8) | 1.39 (0.35–4.57) | 0.741 |
| Arch aortic aneurysm | 38 (18.6) | 2 (15.4) | 0.84 (0.12–3.37) | 1.000 |
| Descending aortic aneurysm | 93 (45.6) | 5 (38.5) | 0.76 (0.22–2.39) | 0.831 |
| Abdominal aortic aneurysm | 64 (31.4) | 3 (23.1) | 0.68 (0.14–2.36) | 0.759 |
| Prior ascending aortic surgery | 87 (42.6) | 5 (38.5) | 0.85 (0.24–2.69) | 0.995 |
| Prior arch aortic surgery | 67 (32.8) | 2 (15.4) | 0.40 (0.05–1.55) | 0.234 |
| Prior descending aortic surgery | 47 (23.0) | 3 (23.1) | 1.04 (0.21–3.62) | 1.000 |
| Prior abdominal aortic surgery | 29 (14.2) | 1 (7.69) | 0.57 (0.02–3.09) | 1.000 |
| Prior Type A dissection | 77 (37.7) | 6 (46.2) | 1.42 (0.43–4.51) | 0.567 |
| Prior Type B dissection | 127 (62.3) | 7 (53.8) | 0.71 (0.22–2.32) | 0.567 |
| Rupture with pain | 11 (5.39) | 1 (7.69) | 1.63 (0.06–9.73) | 0.533 |
| Rupture with shock | 1 (0.49) | 1 (7.69) | 16.3 (0.40–657) | 0.116 |
| Rupture with haemothorax | 5 (2.45) | 1 (7.69) | 3.62 (0.13–26.2) | 0.313 |
| Symptom pain | 32 (15.7) | 5 (38.5) | 3.37 (0.94–11.0) | 0.050 |
| Symptom hoarseness | 2 (0.98) | 1 (7.69) | 8.70 (0.26–115) | 0.170 |
| Symptom dyspnoea | 11 (5.39) | 0 (0.00) | 1.000 | |
| Marfan syndrome | 33 (16.2) | 2 (15.4) | 1.00 (0.14–4.01) | 1.000 |
| Urgency | 0.318 | |||
| Elective | 187 (91.7) | 11 (84.6) | Ref. | |
| Urgent | 11 (5.39) | 2 (15.4) | ||
| Emergent | 6 (2.94) | 0 (0.00) | ||
| Type of TAAA | 0.656 | |||
| 1 | 62 (30.4) | 4 (30.8) | Ref. | |
| 2 | 107 (52.5) | 6 (46.2) | ||
| 3 | 22 (10.8) | 3 (23.1) | ||
| 4 | 10 (4.90) | 0 (0.00) | ||
| 5 | 3 (1.47) | 0 (0.00) | ||
| Intraoperative | ||||
| Left bypass | 165 (80.9) | 8 (61.5) | 0.38 (0.12–1.34) | 0.145 |
| DHCA | 37 (18.1) | 4 (30.8) | 2.04 (0.51–6.76) | 0.274 |
| Cross-clamp | 2 (0.98) | 1 (7.69) | 8.70 (0.26–115) | 0.170 |
| Postoperative | ||||
| MI | 3 (1.49) | 1 (7.69) | 5.88 (0.19–55.2) | 0.222 |
| Stroke | 4 (1.96) | 1 (7.69) | 4.50 (0.16–35.8) | 0.268 |
| Paraplegia | 4 (1.96) | 1 (7.69) | 4.50 (0.16–35.8) | 0.268 |
| Paraparesis | 7 (3.43) | 0 (0.00) | 1.000 | |
| Dialysis | 8 (3.92) | 3 (23.1) | 7.37 (1.37–31.2) | 0.021 |
| Tracheostomy | 11 (5.39) | 3 (23.1) | 5.33 (1.02–21.1) | 0.042 |
| Variables . | In-hospital survival (n = 204) . | In-hospital deaths (n = 13) . | Odds ratio (95% CI) . | P-value . |
|---|---|---|---|---|
| Preoperative | ||||
| Female, n (%) | 65 (31.9) | 4 (30.8) | 0.97 (0.25–3.16) | 1.000 |
| Age (years), mean ± SD | 59.9 ± 11.8 | 65.1 ± 9.23 | 1.05 (0.99–1.11) | 0.075 |
| Hypertension, n (%) | 178 (87.3) | 12 (92.3) | 1.56 (0.28–39.2) | 1.000 |
| Prior AMI, n (%) | 26 (12.7) | 3 (23.1) | 2.11 (0.43–7.60) | 0.390 |
| Prior CABG, n (%) | 15 (7.35) | 0 (0.00) | 0.607 | |
| Prior PCI, n (%) | 12 (5.88) | 1 (7.69) | 1.49 (0.06–8.78) | 0.563 |
| LVEF (%), n (%) | 1.000 | |||
| >50 | 182 (89.7) | 12 (92.3) | Ref. | |
| 30–50 | 19 (9.36) | 1 (7.69) | ||
| <30 | 2 (0.99) | 0 (0.00) | ||
| Prior stroke | 13 (6.37) | 3 (23.1) | 4.48 (0.87–17.3) | 0.060 |
| Prior TIA | 6 (2.94) | 0 (0.00) | 1.000 | |
| History of COPD | 30 (14.7) | 2 (15.4) | 1.11 (0.15–4.51) | 1.000 |
| Preoperative creatinine level (μmol/l), median (IQR) | 88.5 (78.0–106) | 89.0 (82.0–100) | 1.01 (1.00–1.02) | 0.951 |
| Preoperatively on dialysis | 204 (100) | 13 (100) | Ref. | |
| History of diabetes | 8 (3.92) | 0 (0.00) | 1.000 | |
| History of claudication | 5 (2.45) | 2 (15.4) | 7.35 (0.87–40.6) | 0.059 |
| History of GE disease | 6 (2.94) | 1 (7.69) | 3.02 (0.11–20.6) | 0.355 |
| Ascending aortic aneurysm | 50 (24.5) | 4 (30.8) | 1.39 (0.35–4.57) | 0.741 |
| Arch aortic aneurysm | 38 (18.6) | 2 (15.4) | 0.84 (0.12–3.37) | 1.000 |
| Descending aortic aneurysm | 93 (45.6) | 5 (38.5) | 0.76 (0.22–2.39) | 0.831 |
| Abdominal aortic aneurysm | 64 (31.4) | 3 (23.1) | 0.68 (0.14–2.36) | 0.759 |
| Prior ascending aortic surgery | 87 (42.6) | 5 (38.5) | 0.85 (0.24–2.69) | 0.995 |
| Prior arch aortic surgery | 67 (32.8) | 2 (15.4) | 0.40 (0.05–1.55) | 0.234 |
| Prior descending aortic surgery | 47 (23.0) | 3 (23.1) | 1.04 (0.21–3.62) | 1.000 |
| Prior abdominal aortic surgery | 29 (14.2) | 1 (7.69) | 0.57 (0.02–3.09) | 1.000 |
| Prior Type A dissection | 77 (37.7) | 6 (46.2) | 1.42 (0.43–4.51) | 0.567 |
| Prior Type B dissection | 127 (62.3) | 7 (53.8) | 0.71 (0.22–2.32) | 0.567 |
| Rupture with pain | 11 (5.39) | 1 (7.69) | 1.63 (0.06–9.73) | 0.533 |
| Rupture with shock | 1 (0.49) | 1 (7.69) | 16.3 (0.40–657) | 0.116 |
| Rupture with haemothorax | 5 (2.45) | 1 (7.69) | 3.62 (0.13–26.2) | 0.313 |
| Symptom pain | 32 (15.7) | 5 (38.5) | 3.37 (0.94–11.0) | 0.050 |
| Symptom hoarseness | 2 (0.98) | 1 (7.69) | 8.70 (0.26–115) | 0.170 |
| Symptom dyspnoea | 11 (5.39) | 0 (0.00) | 1.000 | |
| Marfan syndrome | 33 (16.2) | 2 (15.4) | 1.00 (0.14–4.01) | 1.000 |
| Urgency | 0.318 | |||
| Elective | 187 (91.7) | 11 (84.6) | Ref. | |
| Urgent | 11 (5.39) | 2 (15.4) | ||
| Emergent | 6 (2.94) | 0 (0.00) | ||
| Type of TAAA | 0.656 | |||
| 1 | 62 (30.4) | 4 (30.8) | Ref. | |
| 2 | 107 (52.5) | 6 (46.2) | ||
| 3 | 22 (10.8) | 3 (23.1) | ||
| 4 | 10 (4.90) | 0 (0.00) | ||
| 5 | 3 (1.47) | 0 (0.00) | ||
| Intraoperative | ||||
| Left bypass | 165 (80.9) | 8 (61.5) | 0.38 (0.12–1.34) | 0.145 |
| DHCA | 37 (18.1) | 4 (30.8) | 2.04 (0.51–6.76) | 0.274 |
| Cross-clamp | 2 (0.98) | 1 (7.69) | 8.70 (0.26–115) | 0.170 |
| Postoperative | ||||
| MI | 3 (1.49) | 1 (7.69) | 5.88 (0.19–55.2) | 0.222 |
| Stroke | 4 (1.96) | 1 (7.69) | 4.50 (0.16–35.8) | 0.268 |
| Paraplegia | 4 (1.96) | 1 (7.69) | 4.50 (0.16–35.8) | 0.268 |
| Paraparesis | 7 (3.43) | 0 (0.00) | 1.000 | |
| Dialysis | 8 (3.92) | 3 (23.1) | 7.37 (1.37–31.2) | 0.021 |
| Tracheostomy | 11 (5.39) | 3 (23.1) | 5.33 (1.02–21.1) | 0.042 |
AMI: acute myocardial infarction; CABG: coronary artery bypass graft; CI: confidence interval; COPD: chronic obstructive pulmonary disease; DHCA: deep hypothermic circulatory arrest; GE: gastroenteric; IQR: interquartile range; LVEF: left ventricle ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention; SD: standard deviation; TAAA: thoraco-abdominal aortic aneurysm; TIA: transitory ischaemic attack.
Even though we found higher rates of mortality and nearly every major postoperative complication when using DHCA, the difference compared with using left heart bypass was not statistically significant; the long-term survival was also not significantly different (log-rank P-value 0.9). Of note, in our cohort, DHCA was only used when a left heart bypass was not deemed safe or technically possible.
Late follow-up
Follow-up was 100% complete, with a mean duration of 6.0 ± 5.8 years, including clinical examinations and computed tomography imaging at regular intervals at our outpatient clinic. During the follow-up period, 33 hospital survivors died (33/204, 16.2%). Of these, 3 patients died of cardiac causes and 1 of an aortic-related event (non-treated Type A dissection). The Kaplan–Meier estimate of survival at 1, 5, 10 and 15 years was 91.9%, 81.2%, 71.4% and 66.0%, respectively (Fig. 1). According to the landmark analysis, late survival at 10 years for patients surviving the first 30 days was not significantly different than that for the Dutch population matched for age and sex (Fig. 2). Using Cox regression for all patients and age at increments of 1 year [hazards ratio = 1.05, confidence interval (CI) 1.01–1.08; P = 0.0041], preoperative coronary artery disease (acute myocardial infarction, percutaneous coronary intervention, previous coronary artery bypass graft) (hazards ratio = 2.33, CI 1.28–4.25; P = 0.0058) and previous cerebrovascular accident (stroke or transient ischaemic attack) (hazards ratio = 2.83, CI 1.30–6.16; P = 0.0085) emerged as independent predictors of late death. Preoperative chronic renal failure and chronic obstructive pulmonary disease were not independent risk factors.
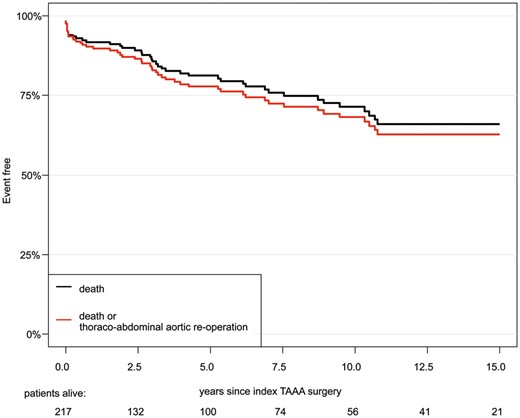
Kaplan–Meier estimate of overall survival, freedom from death and reoperation on the descending aorta. TAAA: thoraco-abdominal aortic aneurysm.
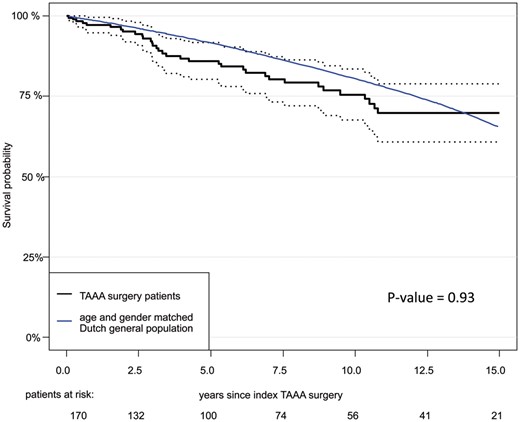
Landmark analysis of patients who survived the first 30 postoperative days.
Freedom from reoperation
During the initial postoperative period, 4 (1.8%) patients required early surgical reintervention, including re-exploration for haemothorax in 3 patients and superficial wound infection in 1 patient. During the follow-up period, 6 (2.8%) patients underwent reintervention at the descending and/or abdominal aortic segment (Table 4). Overall freedom from death and aortic reintervention at the thoraco-abdominal aorta at 1, 5, 10 and 15 years was therefore 89.8%, 77.8%, 68.2% and 62.8%, respectively, with a CI of 95% (Fig. 1). The reoperation rate for progressive disease at another location was 4.4% (9 patients); 5 were due to acute Type A dissection and 4 were due to aneurysm formation requiring an elective Bentall procedure with concomitant coronary artery bypass grafting in 2 of them. As a result, 15 (6.9%) patients underwent an aortic reoperation during the follow-up period.
Reinterventions in the operated-on aortic segment (thoraco-abdominal aorta)
| Aetiology for reintervention (n = 6) . | Number . | Surgical procedure . |
|---|---|---|
| Reoperation of treated segment | 3 | Pseudoaneurysm island visceral vessels |
| Coiling intercostal island aneurysm | ||
| Pseudoaneurysm distal suture | ||
| Disease progression to other aortic segments | 3 | Infrarenal residual aortic dissection |
| TEVAR | ||
| TAA open (from LSA to T8) |
| Aetiology for reintervention (n = 6) . | Number . | Surgical procedure . |
|---|---|---|
| Reoperation of treated segment | 3 | Pseudoaneurysm island visceral vessels |
| Coiling intercostal island aneurysm | ||
| Pseudoaneurysm distal suture | ||
| Disease progression to other aortic segments | 3 | Infrarenal residual aortic dissection |
| TEVAR | ||
| TAA open (from LSA to T8) |
LSA: left subclavian artery; TAA: thoracic aortic aneurysm; TEVAR: transluminal endovascular aortic repair.
Reinterventions in the operated-on aortic segment (thoraco-abdominal aorta)
| Aetiology for reintervention (n = 6) . | Number . | Surgical procedure . |
|---|---|---|
| Reoperation of treated segment | 3 | Pseudoaneurysm island visceral vessels |
| Coiling intercostal island aneurysm | ||
| Pseudoaneurysm distal suture | ||
| Disease progression to other aortic segments | 3 | Infrarenal residual aortic dissection |
| TEVAR | ||
| TAA open (from LSA to T8) |
| Aetiology for reintervention (n = 6) . | Number . | Surgical procedure . |
|---|---|---|
| Reoperation of treated segment | 3 | Pseudoaneurysm island visceral vessels |
| Coiling intercostal island aneurysm | ||
| Pseudoaneurysm distal suture | ||
| Disease progression to other aortic segments | 3 | Infrarenal residual aortic dissection |
| TEVAR | ||
| TAA open (from LSA to T8) |
LSA: left subclavian artery; TAA: thoracic aortic aneurysm; TEVAR: transluminal endovascular aortic repair.
DISCUSSION
We describe one of the largest series of open surgical repair for post-dissection TAAA to date. Although the results of the study are limited because it is retrospective, our early and long-term data clearly show open repair to be a safe and durable treatment option when performed in a high-volume centre with surgeons experienced with aortic procedures. We found better early outcomes in post-dissection TAAA compared to our previously reported series of patients with degenerative TAAA [9]. In-hospital mortality was 5.9% vs 10.9%; permanent spinal cord deficits occurred in 5.5% vs 5.9%. These results are in line with the results recently published by Coselli et al. [4]; early mortality in their series was 5.7%. They suggested that this outcome could be due to the young age of the patients; in fact, their patients were approximately a decade younger than is typical for distal aortic repair. Our data confirm this hypothesis; in fact, the patients in our series were younger than those in the series of Murana (65 years) [9] in the complete series of TAAA and also than those in the series reported by Coselli et al. [4] (67 years). In the current study, excluding resections that were limited to the DTA, we presented one of the largest series of patients undergoing post-dissection TAAA open repair. Fujikawa et al. [10] and Estrera et al. [11] published results of 234 and 209 patients, respectively, including true TAAAs in only 107 (46%) and 141 (67%) of them, respectively. Short-term mortality rates for post-dissection TAAA were reported by van Bogerijen et al. [6] and Kouchoukos et al. [12]: 5.6% and 5.8%, respectively. We found an early mortality rate of 5.9%. Coselli et al. [4] in the largest series of TAAA (3309 patients) reported an overall early mortality rate of 7.5% and in particular of 5.7% among those who had chronic dissection repairs. The rate of spinal cord injury in our series was 5.5%, with a permanent paraplegia rate of 2.3%. The lowest rates of spinal cord injury reported in contemporary series are 1.1% [13] and 2.3% [14]. Coselli et al. [4] described permanent paraplegia and paraparesis rates of 2.9% and 2.4%, respectively. According to these results, we can conclude that modern perfusion strategies, cerebral spinal fluid drainage, the routine use of neurophysiological monitoring and careful haemodynamic regulation perioperatively are likely to account for the improvements in total outcome after surgical repair of post-dissection TAAA. Our follow-up was complete for all patients. A mean follow-up period of 6 years and freedom from mortality at 10 and 15 years were 68.2% and 62.8%, respectively. Long-term follow-up for this type of aortic surgery is rare, and we identified only 3 papers presenting 10-year survival rates—32–60% [11, 12, 15]. According to our experience after post-dissection TAAA repair, the reoperation rate for an uninvolved aortic segment (progression of aortic disease in another location) was low (4.41%). In recent reviews, reoperations after open surgical repair were common, ranging between 5.8% and 29.0% [13, 14, 16, 17]. The absolute rate of reintervention reported by Estrera et al. [11] was 7.7%. As has been demonstrated previously, long-term survival of patients who survive open repair is not significantly different from the survival of the Dutch population, matched for age and sex. This result means that the operation is still worthwhile despite the comorbidities that can accompany this extensive surgery. The endovascular approach to TAAA is becoming an appealing alternative to the traditional open repair due to better early outcomes including lower perioperative mortality and morbidity rates especially for TAAA with a degenerative aetiology. In comparison, after TEVAR, the need for a secondary intervention is common, and the complication rate after the procedure is high despite the less invasive procedure. Recent guidelines recommended medical management for cases of acute uncomplicated descending thoracic aortic dissection and endovascular intervention, if anatomically suitable, for complicated cases involving rupture and malperfusion [2]. However, secondary interventions after initial optimal medical therapy are common, with intervention rates ranging between 9.0% and 40.6% [3]. The most common indications for secondary interventions for post-dissection TAAA include aneurysm formation, rapid aneurysmal sac enlargement, extension of dissection and malperfusion [3–8]. In this context, the outcomes of surgical repair of a thoraco-abdominal aortic aneurysm should be compared with those of TEVAR and B/FEVAR. With TEVAR, the early mortality rates were 0.0–13.7% [16, 18–20] and for B/FEVAR, they ranged between 0.0% and 9.7% [8, 21]. Patterson et al. [20] in 2013, using the Medtronic Thoracic Endovascular Registry, reported the largest series of 197 patients. However, the other TEVAR and B/FEVAR studies did not present large series of patients, and they were all retrospective studies. A 10-year survival rate of 63.0% with TEVAR was reported in 1 study [22]; a 5-year survival rate for B/FEVAR was not yet available. For TEVAR, reinterventions were reported to be between 4.3% and 47.4%; after B/FEVAR, they were between 0.0% and 53.3% [8]. According to these results, thoracic endovascular aortic repair does appear to be a suitable, less invasive option. Van Bogerijen et al. [6] compared OSR and TEVAR and identified similar results between treatment strategies for the primary outcome of late mortality, but late treatment failure was higher in the endovascular strategy group. Currently, no randomized controlled clinical trials exist, mainly due to the rarity of the disease, to provide definitive evidence on the optimal management strategy for post-dissection TAAA. However, before definitive conclusions can be drawn, more evidence is needed, preferably randomized controlled trials. Until then, surgery will play an important role in the treatment of patients with thoraco-abdominal aorta aneurysms.
CONCLUSION
Our data suggest that open surgery for chronic, post-dissection TAAA, when it is performed at an experienced centre using all modern adjuncts, can produce good outcomes with excellent long-term survival. In the current endovascular era (with increasing experience in complex branched stent grafts for degenerative diseases), open surgery should still be considered a valid treatment option, specifically in the extensively dilated, complex post-dissection state.
Conflict of interest: none declared.
Presented at the 31st Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 7–10 October 2017.




