-
PDF
- Split View
-
Views
-
Cite
Cite
Ilhan Inci, Ilker Iskender, Jonas Ehrsam, Claudio Caviezel, Sven Hillinger, Isabelle Opitz, Didier Schneiter, Walter Weder, Previous lung volume reduction surgery does not negatively affect survival after lung transplantation, European Journal of Cardio-Thoracic Surgery, Volume 53, Issue 3, March 2018, Pages 596–602, https://doi.org/10.1093/ejcts/ezx318
Close - Share Icon Share
Abstract
Lung volume reduction surgery (LVRS) and lung transplantation (LTx) are the treatments of choice in selected patients with end-stage emphysema. Recently, the history of LVRS has been questioned due to reduced post-transplant survival. We aim to address this question by reviewing our experience, which is the largest single-centre series of LVRS followed by LTx.
We reviewed our prospectively recorded database in patients with emphysema undergoing LTx between 1993 and 2014. Preoperative workup and postoperative outcomes were compared according to previous LVRS status. The Kaplan–Meier test was used for survival analysis and compared with a log-rank test.
One hundred and seventeen patients (66 men; mean age 56 ± 7 years) underwent LTx during the study period, 52 of whom had previous LVRS (LVRS + LTx). The mean time from LVRS to LTx was 45 ± 31 months. Patients were slightly older and had extensive smoking history in the LVRS + LTx group. Overall, in-hospital mortality was 10%, which did not differ significantly regardless of the history of LVRS (P = 0.8). The median survival for the LTx-only and LVRS + LTx groups was 86 [95% confidence interval (CI) 56–116] and 107 (95% CI 77–137) months, respectively (P = 0.6).
Previous LVRS does not negatively affect short-term and long-term outcomes following LTx in patients with end-stage emphysema. The history of LVRS should not preclude the candidacy for LTx. Considering the limited number of donors available, the LVRS option should be kept in mind for the postponement of LTx in carefully selected patients.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) or emphysema is among the most common causes of death worldwide [1]. The disease is characterized by emphysematous destruction of the lung parenchyma, presenting with shortness of breath and progressive decline in exercise capacity and quality of life [2]. The underlying pathogenesis of COPD is multifactorial, yet tobacco smoking and alpha-1 antitrypsin deficiency are important contributors [3]. The majority of these patients are primarily managed with medical treatment options and pulmonary rehabilitation [4]. Surgical options, such as lung volume reduction surgery (LVRS) or lung transplantation (LTx), are considered in carefully selected patients. More recently, bronchoscopic lung volume reduction procedures are emerging, but their role warrants further investigation in the management of end-stage emphysema patients [5].
LVRS and LTx have been shown to provide superior long-term outcomes compared to medical management strategies in selected patients with end-stage emphysema [6]. However, LTx is limited by the donor shortage, and LVRS is not commonly practised, mainly due to procedure-related morbidities [5]. Furthermore, LVRS may be offered as a palliative treatment or bridging procedure to LTx due to the scarcity of donor lungs [7]. LVRS is usually indicated in less advanced disease than LTx candidates. However, there is a group of patients in which both procedures can be offered with improved outcomes [8].
The effect of previous LVRS on post-LTx outcomes remains controversial in patients with end-stage emphysema. Earlier reports indicate comparable outcomes of emphysematous LTx recipients, regardless of the history of LVRS [8–11]. However, it has been recently shown that previous LVRS is correlated with reduced post-LTx survival in a larger cohort [12]. In our study, we hypothesize that previous LVRS does not negatively affect post-LTx survival in patients with end-stage emphysema. Our centre has a long-standing experience in both LVRS and LTx procedures and their combination [13, 14]. The findings are important to guide multidisciplinary teams when assessing patients for LTx with a history of LVRS.
MATERIALS AND METHODS
This study is a retrospective review of a prospectively collected database for patients who underwent LTx due to emphysema at the University Hospital Zurich between September 1993 and December 2014. Patients were discussed in a multidisciplinary joint emphysema and transplantation board meeting prior to surgery. Patients were selected according to the International Society for Heart Lung Transplantation criteria for LTx [14] and our modified inclusion criteria for LVRS [13]. The cohort was divided into 2 groups according to preoperative LVRS status. The study protocol was approved by the institutional review board.
Patient characteristics and complete preoperative workup were compared between the 2 groups. Additionally, preoperative data were presented at the time of LVRS for demonstration purposes. Emphysema distribution was presented as either heterogeneous or homogeneous (intermediate heterogeneous and complete homogeneous) [15]. We have previously shown that the outcomes of intermediate and homogeneous emphysema were comparable after LVRS [15]. The primary end-point of this study was overall survival after LTx. Cumulative survival benefit of combined procedures was also analysed starting from the time of LVRS for the LVRS + LTx group. Retransplantations were censored at the time of retransplantation. Perioperative outcomes were compared as a secondary measure as well. Survival analysis was completed by 31 December 2015 in all patients.
The LTx was performed via clamshell incision or 2 separate anterolateral thoracotomies for sequential procedures [14]. Single LTx was performed using anterolateral thoracotomy. The LVRS was performed either unilaterally or bilaterally using video-assisted thoracoscopic surgery in most of the cases [15]. Patients were followed up at 3 and 6 months after LVRS, and every 6 months thereafter [8]. Follow-up after LTx was performed via regular outpatient clinic visits according to our post-transplant management strategies [14].
All data were analysed using IBM Statistical Package for Social Sciences (SPSS) Statistics for Windows, Version 24 (IBM Corp., Armonk, NY, USA). Data were presented as continuous or categorical variables. To check the normality of continuous data, the 1-sample Kolmogorov–Smirnov test was performed. Continuous variables were expressed as the mean ± standard deviation for normally distributed variables or median (interquartile range) for non-normally distributed variables. Categorical variables were presented as the total number of patients and percentages. The unpaired t-test and the Mann–Whitney test were performed for normally distributed and non-normally distributed continuous data, respectively. The Pearson’s χ2 or the Fisher’s exact tests were used for categorical data. The Kaplan–Meier survival estimation was applied for survival analysis of the entire cohort. The log-rank test was applied for comparison of cumulative survival estimates. A P-value of ≤0.05 was considered statistically significant.
RESULTS
A total of 117 patients underwent LTx due to emphysema, including 106 bilateral and 11 single procedures, during the study period. Patients with a history of LVRS were allocated to the LVRS + LTx group (n = 52), and the remaining patients comprised the LTx-only group (n = 65). Of note, 377 patients underwent LVRS only during the same period (Fig. 1). The majority of LVRS procedures were performed using video-assisted thoracoscopic surgery (n = 49). In 3 patients, the procedure was completed via thoracotomy due to severe adhesions, and LVRS was performed bilaterally in 38 (73%) patients. Two patients underwent re-LVRS prior to LTx. The number of LTx-only patients versus LVRS + LTx patients throughout the study period (1993–2014) is shown in Fig. 2. The first LTx for emphysema was performed in 1993, followed by the first LTx for a patient with the history of LVRS in 1995. Overall, the number of LTx procedures for emphysema steadily increased over time and a relatively comparable ratio between the 2 groups was maintained (Fig. 2).
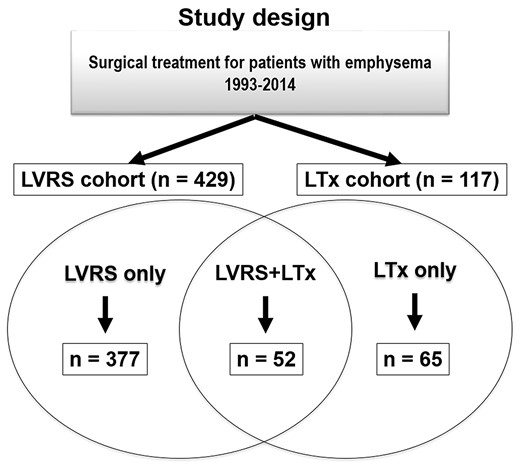
Study design: 117 patients underwent LTx for emphysema between September 1993 and December 2014 at the University Hospital Zurich. Patients were grouped according to pretransplant LVRS status. There were 52 patients in the LVRS + LTx group with LVRS history and the remaining 65 patients were grouped as LTx-only. During the study period, 377 patients underwent LVRS only. LVRS-only patients are not included in the analyses. Of note, in the LVRS-only group, 22 patients underwent re-LVRS, and in the LVRS + LTx group, 2 patients underwent re-LVRS. LTx: lung transplantation; LVRS: lung volume reduction surgery.
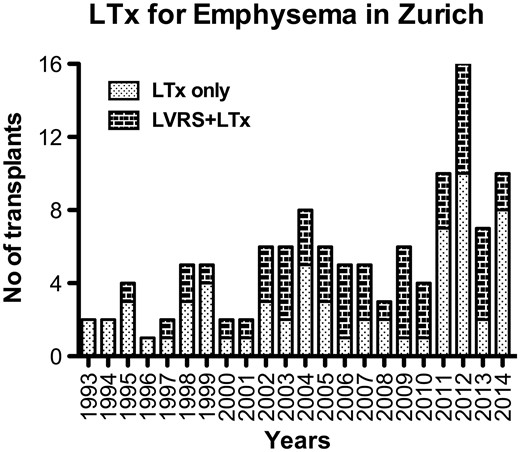
Distribution of the number of lung transplants for emphysema in Zurich according to previous LVRS status during the study period. LTx: lung transplantation; LVRS: lung volume reduction surgery.
The underlying pathogenesis of emphysema was alpha 1-antitrypsin deficiency in 37 (32%) patients. The LVRS + LTx group was slightly older and had a long history of smoking compared with the LTx-only group (Table 1). The mean time from LVRS to LTx was 45 ± 31 months in the LVRS + LTx group. The mean follow-up time did not differ significantly between the 2 groups (Table 1).
| Parameters . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|
| Age at LTx (years), mean (SD) | 57 (7) | 55 (7) | 0.05* |
| Gender | |||
| Male, n (%) | 27 (52) | 39 (60) | 0.3 |
| Female, n (%) | 25 (48) | 26 (40) | |
| A1AT deficiency, n (%) | 13 (25) | 24 (37) | 0.1 |
| Emphysema distribution,an (%) | |||
| Heterogeneous | 38 (75) | 45 (78) | 0.4 |
| Homogeneous | 13 (25) | 13 (22) | |
| Comorbidities, n (%) | |||
| CAD | 7 (14) | 8 (12) | 0.6 |
| Hypertension | 5 (10) | 9 (14) | |
| No | 22 (42) | 29 (45) | |
| Preoperative steroid, n (%) | 16 (31) | 14 (23) | 0.2 |
| BMI at LTx, mean (SD) | 22 (5) | 22 (5) | 0.6 |
| Smoking pack years, mean (SD) | 45 (27) | 34 (22) | 0.02* |
| Time from LVRS to LTx (months), mean (SD) | 45 (31) | N/A | N/A |
| Time from listing to LTx (months), mean (SD) | 8 (7) | 7 (7) | 0.3 |
| Follow-up time after LTx (months), mean (SD) | 68 (49) | 63 (56) | 0.7 |
| Parameters . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|
| Age at LTx (years), mean (SD) | 57 (7) | 55 (7) | 0.05* |
| Gender | |||
| Male, n (%) | 27 (52) | 39 (60) | 0.3 |
| Female, n (%) | 25 (48) | 26 (40) | |
| A1AT deficiency, n (%) | 13 (25) | 24 (37) | 0.1 |
| Emphysema distribution,an (%) | |||
| Heterogeneous | 38 (75) | 45 (78) | 0.4 |
| Homogeneous | 13 (25) | 13 (22) | |
| Comorbidities, n (%) | |||
| CAD | 7 (14) | 8 (12) | 0.6 |
| Hypertension | 5 (10) | 9 (14) | |
| No | 22 (42) | 29 (45) | |
| Preoperative steroid, n (%) | 16 (31) | 14 (23) | 0.2 |
| BMI at LTx, mean (SD) | 22 (5) | 22 (5) | 0.6 |
| Smoking pack years, mean (SD) | 45 (27) | 34 (22) | 0.02* |
| Time from LVRS to LTx (months), mean (SD) | 45 (31) | N/A | N/A |
| Time from listing to LTx (months), mean (SD) | 8 (7) | 7 (7) | 0.3 |
| Follow-up time after LTx (months), mean (SD) | 68 (49) | 63 (56) | 0.7 |
We do not have the data for emphysema distribution in 8 patients.
P ≤ 0.05.
A1AT: alpha-1 antitrypsin; BMI: body mass index; CAD: coronary artery disease; LTx: lung transplantation; LVRS: lung volume reduction surgery; N/A: not applicable; SD: standard deviation.
| Parameters . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|
| Age at LTx (years), mean (SD) | 57 (7) | 55 (7) | 0.05* |
| Gender | |||
| Male, n (%) | 27 (52) | 39 (60) | 0.3 |
| Female, n (%) | 25 (48) | 26 (40) | |
| A1AT deficiency, n (%) | 13 (25) | 24 (37) | 0.1 |
| Emphysema distribution,an (%) | |||
| Heterogeneous | 38 (75) | 45 (78) | 0.4 |
| Homogeneous | 13 (25) | 13 (22) | |
| Comorbidities, n (%) | |||
| CAD | 7 (14) | 8 (12) | 0.6 |
| Hypertension | 5 (10) | 9 (14) | |
| No | 22 (42) | 29 (45) | |
| Preoperative steroid, n (%) | 16 (31) | 14 (23) | 0.2 |
| BMI at LTx, mean (SD) | 22 (5) | 22 (5) | 0.6 |
| Smoking pack years, mean (SD) | 45 (27) | 34 (22) | 0.02* |
| Time from LVRS to LTx (months), mean (SD) | 45 (31) | N/A | N/A |
| Time from listing to LTx (months), mean (SD) | 8 (7) | 7 (7) | 0.3 |
| Follow-up time after LTx (months), mean (SD) | 68 (49) | 63 (56) | 0.7 |
| Parameters . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|
| Age at LTx (years), mean (SD) | 57 (7) | 55 (7) | 0.05* |
| Gender | |||
| Male, n (%) | 27 (52) | 39 (60) | 0.3 |
| Female, n (%) | 25 (48) | 26 (40) | |
| A1AT deficiency, n (%) | 13 (25) | 24 (37) | 0.1 |
| Emphysema distribution,an (%) | |||
| Heterogeneous | 38 (75) | 45 (78) | 0.4 |
| Homogeneous | 13 (25) | 13 (22) | |
| Comorbidities, n (%) | |||
| CAD | 7 (14) | 8 (12) | 0.6 |
| Hypertension | 5 (10) | 9 (14) | |
| No | 22 (42) | 29 (45) | |
| Preoperative steroid, n (%) | 16 (31) | 14 (23) | 0.2 |
| BMI at LTx, mean (SD) | 22 (5) | 22 (5) | 0.6 |
| Smoking pack years, mean (SD) | 45 (27) | 34 (22) | 0.02* |
| Time from LVRS to LTx (months), mean (SD) | 45 (31) | N/A | N/A |
| Time from listing to LTx (months), mean (SD) | 8 (7) | 7 (7) | 0.3 |
| Follow-up time after LTx (months), mean (SD) | 68 (49) | 63 (56) | 0.7 |
We do not have the data for emphysema distribution in 8 patients.
P ≤ 0.05.
A1AT: alpha-1 antitrypsin; BMI: body mass index; CAD: coronary artery disease; LTx: lung transplantation; LVRS: lung volume reduction surgery; N/A: not applicable; SD: standard deviation.
Preoperative respiratory and cardiac investigations to assess candidacy for LTx and LVRS, including pulmonary function test, blood gas analysis and echocardiography, were comparable between the 2 groups. Of note, a trend towards lower forced expiratory volume in 1 s values was observed in the LVRS + LTx group compared with the LTx-only group (LVRS + LTx versus LTx-only 22.2 ± 5.8% vs 25.1 ± 10.2% of predicted, P = 0.08; Table 2). Data at the time of LVRS in the LVRS + LTx group were presented for demonstration purposes only and were not included in the statistical comparison (Table 2).
| Parameters . | Time of LVRS (n = 52)a . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|---|
| PFT | ||||
| FEV1 (%predicted), mean (SD) | 22.5 (5) | 22.2 (6) | 25.1 (10) | 0.08 |
| FVC (%predicted), mean (SD) | 57.7 (15) | 54.8 (14) | 57.1 (18) | 0.5 |
| RV/TLC (%), mean (SD) | 80.2 (57) | 70.5 (9) | 73.4 (14) | 0.2 |
| DLCO (%predicted), mean (SD) | 30.5 (10) | 28.1 (11) | 28.5 (15) | 0.9 |
| Blood gas (kPa), mean (SD) | ||||
| PaO2 | 8.6 (1) | 9.3 (2) | 8.8 (2) | 0.2 |
| PaCO2 | 5.5 (2) | 5.6 (1) | 5.9 (1) | 0.1 |
| 6-Min walk test (%), mean (SD) | 316 (101) | 274 (97) | 282 (119) | 0.7 |
| NYHA class, mean (SD) | 3.3 (0.5) | 3.5 (0.5) | 3.6 (0.6) | 0.2 |
| Ejection fraction (%), mean (SD) | 62 (10) | 64 (9) | 62 (9) | 0.2 |
| sPAP (mmHg), mean (SD) | 38 (10) | 38 (13) | 40 (17) | 0.5 |
| Parameters . | Time of LVRS (n = 52)a . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|---|
| PFT | ||||
| FEV1 (%predicted), mean (SD) | 22.5 (5) | 22.2 (6) | 25.1 (10) | 0.08 |
| FVC (%predicted), mean (SD) | 57.7 (15) | 54.8 (14) | 57.1 (18) | 0.5 |
| RV/TLC (%), mean (SD) | 80.2 (57) | 70.5 (9) | 73.4 (14) | 0.2 |
| DLCO (%predicted), mean (SD) | 30.5 (10) | 28.1 (11) | 28.5 (15) | 0.9 |
| Blood gas (kPa), mean (SD) | ||||
| PaO2 | 8.6 (1) | 9.3 (2) | 8.8 (2) | 0.2 |
| PaCO2 | 5.5 (2) | 5.6 (1) | 5.9 (1) | 0.1 |
| 6-Min walk test (%), mean (SD) | 316 (101) | 274 (97) | 282 (119) | 0.7 |
| NYHA class, mean (SD) | 3.3 (0.5) | 3.5 (0.5) | 3.6 (0.6) | 0.2 |
| Ejection fraction (%), mean (SD) | 62 (10) | 64 (9) | 62 (9) | 0.2 |
| sPAP (mmHg), mean (SD) | 38 (10) | 38 (13) | 40 (17) | 0.5 |
Data belonging to the time of LVRS were not included in the statistical comparison.
DLCO: diffusion capacity of carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; LTx: lung transplantation; LVRS: lung volume reduction surgery; NYHA: New York Heart Association; PaO2: partial arterial pressure of oxygen; PaCO2: partial arterial pressure of carbon dioxide; PFT: pulmonary function test; RV: residual volume; SD: standard deviation; sPAP: systolic pulmonary arterial pressure; TLC: total lung capacity.
| Parameters . | Time of LVRS (n = 52)a . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|---|
| PFT | ||||
| FEV1 (%predicted), mean (SD) | 22.5 (5) | 22.2 (6) | 25.1 (10) | 0.08 |
| FVC (%predicted), mean (SD) | 57.7 (15) | 54.8 (14) | 57.1 (18) | 0.5 |
| RV/TLC (%), mean (SD) | 80.2 (57) | 70.5 (9) | 73.4 (14) | 0.2 |
| DLCO (%predicted), mean (SD) | 30.5 (10) | 28.1 (11) | 28.5 (15) | 0.9 |
| Blood gas (kPa), mean (SD) | ||||
| PaO2 | 8.6 (1) | 9.3 (2) | 8.8 (2) | 0.2 |
| PaCO2 | 5.5 (2) | 5.6 (1) | 5.9 (1) | 0.1 |
| 6-Min walk test (%), mean (SD) | 316 (101) | 274 (97) | 282 (119) | 0.7 |
| NYHA class, mean (SD) | 3.3 (0.5) | 3.5 (0.5) | 3.6 (0.6) | 0.2 |
| Ejection fraction (%), mean (SD) | 62 (10) | 64 (9) | 62 (9) | 0.2 |
| sPAP (mmHg), mean (SD) | 38 (10) | 38 (13) | 40 (17) | 0.5 |
| Parameters . | Time of LVRS (n = 52)a . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|---|
| PFT | ||||
| FEV1 (%predicted), mean (SD) | 22.5 (5) | 22.2 (6) | 25.1 (10) | 0.08 |
| FVC (%predicted), mean (SD) | 57.7 (15) | 54.8 (14) | 57.1 (18) | 0.5 |
| RV/TLC (%), mean (SD) | 80.2 (57) | 70.5 (9) | 73.4 (14) | 0.2 |
| DLCO (%predicted), mean (SD) | 30.5 (10) | 28.1 (11) | 28.5 (15) | 0.9 |
| Blood gas (kPa), mean (SD) | ||||
| PaO2 | 8.6 (1) | 9.3 (2) | 8.8 (2) | 0.2 |
| PaCO2 | 5.5 (2) | 5.6 (1) | 5.9 (1) | 0.1 |
| 6-Min walk test (%), mean (SD) | 316 (101) | 274 (97) | 282 (119) | 0.7 |
| NYHA class, mean (SD) | 3.3 (0.5) | 3.5 (0.5) | 3.6 (0.6) | 0.2 |
| Ejection fraction (%), mean (SD) | 62 (10) | 64 (9) | 62 (9) | 0.2 |
| sPAP (mmHg), mean (SD) | 38 (10) | 38 (13) | 40 (17) | 0.5 |
Data belonging to the time of LVRS were not included in the statistical comparison.
DLCO: diffusion capacity of carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; LTx: lung transplantation; LVRS: lung volume reduction surgery; NYHA: New York Heart Association; PaO2: partial arterial pressure of oxygen; PaCO2: partial arterial pressure of carbon dioxide; PFT: pulmonary function test; RV: residual volume; SD: standard deviation; sPAP: systolic pulmonary arterial pressure; TLC: total lung capacity.
The intraoperative variables, such as procedure type, the use of extracorporeal life support and estimated blood loss, and postoperative outcomes, including the length of intensive care unit and hospital stays, ventilation days, the use of haemofiltration, chest tube duration and the rate of complications, did not differ significantly between groups. Moreover, in-hospital mortality was also comparable between groups (LVRS + LTx versus LTx-only 9.6% vs 10.8%, P = 0.5; Table 3).
| Parameters . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|
| LTx procedure type, n (%) | |||
| Single | 3 (6) | 8 (12) | 0.2 |
| Bilateral | 49 (94) | 57 (88) | |
| Intraoperative ECLS use, n (%) | 10 (19) | 15 (23) | 0.4 |
| Theatre time (min), median (IQR) | 380 (330–450) | 351 (310–440) | 0.6 |
| Estimated blood loss (ml), median (IQR) | 1000 (600–1500) | 1000 (900–3000) | 0.1 |
| ICU stay (days), median (IQR) | 4 (2–8) | 4 (2-7) | 0.3 |
| CVVH, n (%) | 6 (12) | 3 (5) | 0.2 |
| Length of ventilation (days), median (IQR) | 1 (1–2) | 1 (1–1) | 0.2 |
| Length of chest tube (days), median (IQR) | 14 (11–21) | 14 (10–22) | 0.7 |
| Length of hospital stay (days), median (IQR) | 33 (28–46) | 34 (28–51) | 0.5 |
| Complications, n (%) | |||
| PGD grade 3 | 2 (4) | 6 (9) | 0.2 |
| Tracheostomy | 4 (8) | 9 (14) | 0.2 |
| Lymphocele | 2 (4) | 3 (5) | 0.6 |
| Haemothorax | 8 (15) | 10 (15) | 0.9 |
| Arrhythmias | 13 (25) | 14 (22) | 0.4 |
| Abdominal complication | 8 (15) | 5 (8) | 0.5 |
| Phrenic nerve injury | 0 | 1 (2) | 0.4 |
| 30-Day mortality, n (%) | 4 (7.7) | 2 (3.1) | 0.2 |
| In-hospital mortality, n (%) | 5 (9.6) | 7 (10.8) | 0.5 |
| Parameters . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|
| LTx procedure type, n (%) | |||
| Single | 3 (6) | 8 (12) | 0.2 |
| Bilateral | 49 (94) | 57 (88) | |
| Intraoperative ECLS use, n (%) | 10 (19) | 15 (23) | 0.4 |
| Theatre time (min), median (IQR) | 380 (330–450) | 351 (310–440) | 0.6 |
| Estimated blood loss (ml), median (IQR) | 1000 (600–1500) | 1000 (900–3000) | 0.1 |
| ICU stay (days), median (IQR) | 4 (2–8) | 4 (2-7) | 0.3 |
| CVVH, n (%) | 6 (12) | 3 (5) | 0.2 |
| Length of ventilation (days), median (IQR) | 1 (1–2) | 1 (1–1) | 0.2 |
| Length of chest tube (days), median (IQR) | 14 (11–21) | 14 (10–22) | 0.7 |
| Length of hospital stay (days), median (IQR) | 33 (28–46) | 34 (28–51) | 0.5 |
| Complications, n (%) | |||
| PGD grade 3 | 2 (4) | 6 (9) | 0.2 |
| Tracheostomy | 4 (8) | 9 (14) | 0.2 |
| Lymphocele | 2 (4) | 3 (5) | 0.6 |
| Haemothorax | 8 (15) | 10 (15) | 0.9 |
| Arrhythmias | 13 (25) | 14 (22) | 0.4 |
| Abdominal complication | 8 (15) | 5 (8) | 0.5 |
| Phrenic nerve injury | 0 | 1 (2) | 0.4 |
| 30-Day mortality, n (%) | 4 (7.7) | 2 (3.1) | 0.2 |
| In-hospital mortality, n (%) | 5 (9.6) | 7 (10.8) | 0.5 |
CVVH: continuous venovenous haemofiltration; ECLS: extracorporeal life support; ICU: intensive care unit; IQR: interquartile range; LTx: lung transplantation; LVRS: lung volume reduction surgery; PGD: primary graft dysfunction at 72 h.
| Parameters . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|
| LTx procedure type, n (%) | |||
| Single | 3 (6) | 8 (12) | 0.2 |
| Bilateral | 49 (94) | 57 (88) | |
| Intraoperative ECLS use, n (%) | 10 (19) | 15 (23) | 0.4 |
| Theatre time (min), median (IQR) | 380 (330–450) | 351 (310–440) | 0.6 |
| Estimated blood loss (ml), median (IQR) | 1000 (600–1500) | 1000 (900–3000) | 0.1 |
| ICU stay (days), median (IQR) | 4 (2–8) | 4 (2-7) | 0.3 |
| CVVH, n (%) | 6 (12) | 3 (5) | 0.2 |
| Length of ventilation (days), median (IQR) | 1 (1–2) | 1 (1–1) | 0.2 |
| Length of chest tube (days), median (IQR) | 14 (11–21) | 14 (10–22) | 0.7 |
| Length of hospital stay (days), median (IQR) | 33 (28–46) | 34 (28–51) | 0.5 |
| Complications, n (%) | |||
| PGD grade 3 | 2 (4) | 6 (9) | 0.2 |
| Tracheostomy | 4 (8) | 9 (14) | 0.2 |
| Lymphocele | 2 (4) | 3 (5) | 0.6 |
| Haemothorax | 8 (15) | 10 (15) | 0.9 |
| Arrhythmias | 13 (25) | 14 (22) | 0.4 |
| Abdominal complication | 8 (15) | 5 (8) | 0.5 |
| Phrenic nerve injury | 0 | 1 (2) | 0.4 |
| 30-Day mortality, n (%) | 4 (7.7) | 2 (3.1) | 0.2 |
| In-hospital mortality, n (%) | 5 (9.6) | 7 (10.8) | 0.5 |
| Parameters . | LVRS + LTx (n = 52) . | LTx-only (n = 65) . | P-value . |
|---|---|---|---|
| LTx procedure type, n (%) | |||
| Single | 3 (6) | 8 (12) | 0.2 |
| Bilateral | 49 (94) | 57 (88) | |
| Intraoperative ECLS use, n (%) | 10 (19) | 15 (23) | 0.4 |
| Theatre time (min), median (IQR) | 380 (330–450) | 351 (310–440) | 0.6 |
| Estimated blood loss (ml), median (IQR) | 1000 (600–1500) | 1000 (900–3000) | 0.1 |
| ICU stay (days), median (IQR) | 4 (2–8) | 4 (2-7) | 0.3 |
| CVVH, n (%) | 6 (12) | 3 (5) | 0.2 |
| Length of ventilation (days), median (IQR) | 1 (1–2) | 1 (1–1) | 0.2 |
| Length of chest tube (days), median (IQR) | 14 (11–21) | 14 (10–22) | 0.7 |
| Length of hospital stay (days), median (IQR) | 33 (28–46) | 34 (28–51) | 0.5 |
| Complications, n (%) | |||
| PGD grade 3 | 2 (4) | 6 (9) | 0.2 |
| Tracheostomy | 4 (8) | 9 (14) | 0.2 |
| Lymphocele | 2 (4) | 3 (5) | 0.6 |
| Haemothorax | 8 (15) | 10 (15) | 0.9 |
| Arrhythmias | 13 (25) | 14 (22) | 0.4 |
| Abdominal complication | 8 (15) | 5 (8) | 0.5 |
| Phrenic nerve injury | 0 | 1 (2) | 0.4 |
| 30-Day mortality, n (%) | 4 (7.7) | 2 (3.1) | 0.2 |
| In-hospital mortality, n (%) | 5 (9.6) | 7 (10.8) | 0.5 |
CVVH: continuous venovenous haemofiltration; ECLS: extracorporeal life support; ICU: intensive care unit; IQR: interquartile range; LTx: lung transplantation; LVRS: lung volume reduction surgery; PGD: primary graft dysfunction at 72 h.
Overall survival rates did not differ significantly regardless of preoperative LVRS status in patients undergoing LTx for emphysema (P = 0.5) (Fig. 3). The 1-year and 5-year survival rates for the LVRS + LTx versus the LTx-only groups were 87% and 66% vs 83% and 61%, respectively. The median survival for the LVRS + LTx and LTx-only groups was 107 [95% confidence interval (CI) 77–137] and 86 (95% CI 56–116) months, respectively. The cumulative survival benefit of LVRS followed by LTx (median 143 months, 95% CI 110–177 months) was markedly better than LTx alone (P < 0.001; Fig. 4).
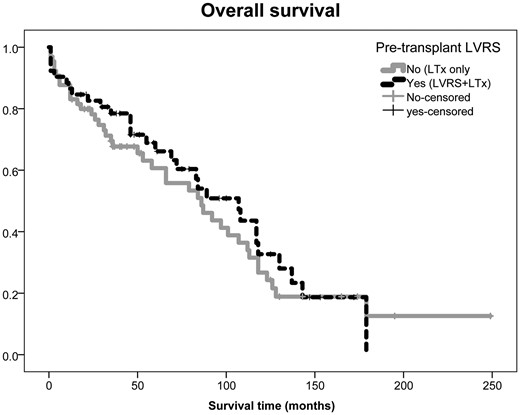
Overall survival did not differ significantly regardless of pretransplant LVRS status between the study groups (P = 0.6). The 5-year survival rate for LVRS + LTx versus LTx-only groups was 66% vs 61%, respectively. LTx: lung transplantation; LVRS: lung volume reduction surgery.
![Cumulative survival benefit of LVRS followed by LTx [median 143 months, 95% confidence interval (CI) 110–177 months] was markedly better than LTx alone (median 86 months, 95% CI 56–116 months; P < 0.001). LTx: lung transplantation; LVRS: lung volume reduction surgery.](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/53/3/10.1093_ejcts_ezx318/1/m_ezx318f4.jpeg?Expires=1747981472&Signature=tqddy~g5rNdJMqP9RVdJjidGfE7G~r-UKBDpxA5f0sAzW7aHz~-XOC2vOAoGUXG-0FYN2f0UYFu0EFNymwc3TzD9kpQoECM3OEvimxk8t6LqpStYKaa8brAnOAhmXzVDvZ4SuoA6QZFEkz5gYJMoYw8BOl~KnD4v-j5twP4VkQEmjj2Q5PYX~D76JGSIW0ss5tMo5ISZg~tMooToJAebTRYzLcXEX5tnS0sPzdHUJ5ZWsaNj2GVZTwPHkhDxWs9VNbs7pGUgKvFrD9WFXn0Nj~Adzs5DA6PwMWm2xrP9dyBrYwMmsQGXXUzzf~ucgipY6GfbrpvI8HiKQ6FtgYsXGQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Cumulative survival benefit of LVRS followed by LTx [median 143 months, 95% confidence interval (CI) 110–177 months] was markedly better than LTx alone (median 86 months, 95% CI 56–116 months; P < 0.001). LTx: lung transplantation; LVRS: lung volume reduction surgery.
DISCUSSION
This retrospective analysis of a large-volume single-centre experience related to surgical treatment of end-stage emphysema demonstrates that the history of LVRS does not negatively affect short-term and long-term outcomes following LTx.
Although the history of LVRS dates back to the mid-20th century, the beneficial effects of the procedure was not shown until the end of the 20th century [16]. Shortly after, the success in the open technique has led to the development of a minimally invasive approach for LVRS [17]. The National Emphysema Treatment Trial was the landmark study to confirm that LVRS might be a better option than medical treatment in carefully selected patients [18]. Since then, LVRS procedures have become popular and adopted by many centres worldwide. Indications for LVRS further evolved to include homogenous type disease as well [15]. In general, LVRS does not replace the need for LTx in younger patients. However, LVRS is a definitive treatment option for patients around the age of 60 years and with significant comorbidities, which means that transplantation is not recommended. We summarized our selection criteria for LVRS and/or LTx in the management of end stage based on patients’ age, pulmonary function tests, radiological findings, pulmonary hypertension and steroid intake in Fig. 5.
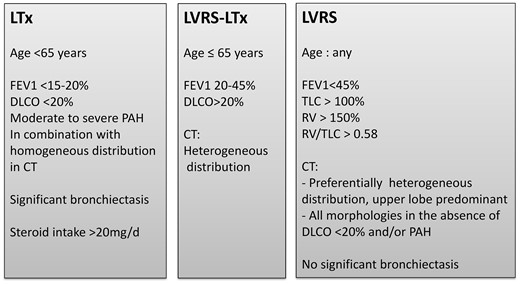
Selection criteria for LVRS and/or LTx in the surgical management of end-stage emphysema at the University Hospital Zurich based on patients’ age, pulmonary function tests, radiological findings, pulmonary hypertension and steroid intake. CT: computed tomography; DLCO: diffusion capacity of carbon monoxide; FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; LTx: lung transplantation; LVRS: lung volume reduction surgery; PAH: pulmonary arterial hypertension; RV: residual volume; TLC: total lung capacity.
In addition, LTx is a more curative option than LVRS in the surgical management of end-stage emphysema and is generally offered for more advanced disease in younger patients with less comorbidities [8]. Although LTx markedly improves the quality of life and exercise capacity, the procedure carries significant perioperative risks, and the recipient is required to use lifelong immunosuppression [5]. According to the most recent International Society for Heart Lung Transplantation registry, COPD together with alpha-1 antitrypsin deficiency continues to be the leading indication for LTx, accounting for approximately 36% of transplantations performed worldwide [19]. However, donor shortage remains the main limiting factor for transplantation. Therefore, LVRS has been suggested as an option for the postponement of transplantation [8]. More recently, bronchoscopic LVRS has also been suggested as a bridging procedure prior to LTx [20].
The impact of previous LVRS on post-transplant outcomes remains controversial. Earlier publications reported comparable outcomes after LTx regardless of the history of LVRS [9–11]. However, these studies were limited by the lack of defined selection criteria for patients, small numbers and high frequency of unilateral procedures in both arms. We reported our initial experience in patients undergoing LTx after LVRS in 2006 [8]. Although the numbers were limited to 8, we did not observe any adverse impact of LVRS on outcomes after LTx. All the procedures were performed bilaterally in a well-defined group of patients in that study [8]. More recently, Backhus et al. [12] have shown reduced survival in patients undergoing LTx after LVRS compared with LTx alone. In this single-centre retrospective study, the authors reported 174 patients undergoing LTx due to COPD between 1995 and 2010. Among these, 36 of them had previous LVRS with a mean follow-up time of 50 months. The median survival was 96 months in the LTx-only group, which was significantly decreased to 49 months for combined LVRS and LTx. The authors concluded that extra care should be given when selecting patients for LTx with a history of LVRS due to reduced post-transplant survival [12].
To our knowledge, the present series is the largest single-centre experience so far for patients undergoing LVRS followed by LTx (n = 52). We did not observe significant differences between short-term and long-term outcomes for patients undergoing combined procedures when compared with patients undergoing LTx-only procedure, although one might expect to find difficulties during transplant surgery in patients with a history of LVRS. Similar to earlier reports [9–11], we did not find differences in perioperative outcomes, such as prolonged surgery time, ventilation days and intensive care unit stay and increased complication rates, in our cohort. In the series by Backhus et al. [12], they observed longer operation times and hospital stays during LTx in patients with a history of LVRS. They did not report the exact number of patients undergoing open versus thoracoscopic procedures during LVRS [12]. In our series, the majority of LVRS procedures have been performed with video-assisted thoracoscopic surgery since the initiation of our programme [17]. We believe that the use of a minimally invasive approach during LVRS could have prevented the likelihood of increased perioperative risks related to extensive adhesions during transplantation. In the case of upper lobe-predominant emphysema, the resection often starts at the level of the vena azygos or the aortic arch for the right and left hemithorax, respectively. It follows a hockey stick-like pattern from anterior to posterior to keep the dome-shaped form of the lung. Adhesions that we observed during LTx were generally loose adhesions and were located in the apex. Similarly, we also encountered mediastinal adhesions that could be easily released from the adjacent structures. The use of buttressed staplers, especially with bovine pericardium, has been suggested to correlate with dense adhesions, thereby increasing the complication risk in patients undergoing LTx after LVRS [21]. We did use buttressed staplers in the early 2000s, but, currently, it is not routine in our practice. We did not observe any phrenic nerve injury during LTx related to previous LVRS. We did not have any specific strategy to prevent direct contact between the stapling line and the phrenic nerve. In case of severe adhesions around the phrenic nerve during the LTx surgery, we release the lung by cutting from the lateral edges of the adhesion and leave a minimal tissue (visceral pleura) over the phrenic nerve.
More importantly, in line with our earlier experience [8], previous LVRS did not affect long-term survival after LTx, with a median survival of 107 months in the LVRS + LTx group and of 86 months in the LTx-only group. We believe that achieving a relatively comparable perioperative period is the crucial element in this success. Backhus et al. [12] also emphasized the importance of an early postoperative course on long-term outcomes for patients undergoing combined procedures. Of note, we achieved these outcomes in patients who had relatively more severe illness in the LVRS + LTx group, determined by relatively older age, extensive smoking history and forced expiratory volume in 1 s values that were more severely deteriorated before transplantation. Not surprisingly, the cumulative survival benefit of LVRS followed by LTx was markedly better than LTx alone. We acknowledge that not all the patients met the listing criteria for LTx at the time of LVRS and therefore providing the cumulative survival benefit may not be accurate. Nevertheless, LVRS has led to the postponement of LTx with a mean time of 45 months in our cohort. We believe that patient selection is the crucial element in this success. One might question whether patients were considered for LTx after an unsuccessful LVRS or not. Senbaklavaci et al. [11] addressed the importance of successful LVRS on outcomes following LTx. Unfortunately, we do not have data on our initial decision as to whether LVRS would be performed as a definitive or a bridging procedure on all patients at our institution. We decided not to include LVRS-only patients in this study, mainly because we wanted to concentrate on the effect of LVRS on short-term and long-term outcomes after LTx. The management of patients undergoing LVRS differs significantly from patients undergoing LTx regarding the perioperative period and long-term follow-up. This is the main limitation of this study. The first successful long-term survival after LTx was achieved in 1983 [22]. However, LVRS came into practice again approximately 10 years after this achievement [16]. A decade-long experience gained in LTx prior to initiation of LVRS programmes may be considered beneficial for the LVRS + LTx group. As illustrated in Fig. 2, we performed the first LTx in the LVRS + LTx group 2 years after the first LTx for emphysema in our programme. We think that the effect of historical bias was limited in this study.
CONCLUSION
In conclusion, previous LVRS does not negatively affect long-term outcomes following LTx in patients with end-stage emphysema. Therefore, a history of LVRS should not preclude the candidacy for LTx. Considering the limited number of donor organs available, the LVRS option should be kept in mind for the postponement of LTx in selected cases. Multi-institutional reports are warranted to understand the effect of previous LVRS on long-term outcomes following LTx.
Funding
This work was supported by the Zurich Lung League with a grant.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 24th European Conference on General Thoracic Surgery, Naples, Italy, 29 May-1 June 2016.
Ilhan Inci and Ilker Iskender contributed equally to this work.




