-
PDF
- Split View
-
Views
-
Cite
Cite
Paolo Berretta, Luca Di Marco, Davide Pacini, Mariano Cefarelli, Jacopo Alfonsi, Sebastiano Castrovinci, Marco Di Eusanio, Roberto Di Bartolomeo, Reoperations versus primary operation on the aortic root: a propensity score analysis, European Journal of Cardio-Thoracic Surgery, Volume 51, Issue 2, February 2017, Pages 322–328, https://doi.org/10.1093/ejcts/ezw250
Close - Share Icon Share
Abstract
Reported early outcomes for patients undergoing reoperations on the aortic root are worse than those for patients undergoing first-time surgery. The aim of this study was to review our experience with aortic root surgery by stratifying outcomes according to the type of intervention: reoperation versus primary operation on the aortic root.
Of the 1267 patients undergoing aortic root surgery, 180 underwent aortic reoperation with root replacement (ARR) and 1087 underwent primary root replacement (PRR). Treatment bias was addressed by the use of propensity score (PS) matching and multivariate regression analysis. After PS matching, two groups of 116 patients each were created (ARR versus PRR). The primary end-points were in-hospital mortality and occurrence of postoperative complications.
In the unmatched cohort, hospital mortality and postoperative complications rates were higher in the ARR group than in the PRR group (11.1 vs 4.1%, P < 0.001; 22.2 vs 15.1%, P = 0.02). Early results were greatly affected by the type of aortic disease. The in-hospital mortality rate was 3.1% for degenerative aneurysm, 13.3% for chronic dissection, 14% for acute dissection, 16.7% for active endocarditis and 25% for false aneurysm (P < 0.001). In the propensity-matched cohort, no significant differences were observed between the groups in terms of hospital mortality rate (ARR: 6%; PRR: 1.7%; P= 0.2) and postoperative complications (ARR: 16.4%; PRR: 14.7%; P = 0.9). Logistic regression analysis revealed cardiopulmonary bypass (CPB) time [odds ratio (OR): 1.03 per min; P < 0.001] and urgent/emergent status (OR: 6.4; P = 0.04) as independent risk factors for hospital deaths. Age (OR: 1.07 per year; P = 0.03) was the sole independent predictor of postoperative complications.
During root surgery, reintervention did not affect early outcomes and was associated with satisfactory mortality and morbidity rates. In this setting, hospital results were heavily influenced by aortic pathology and the patient's profile.
INTRODUCTION
Over the years, the incidence of reinterventions on the thoracic aorta has risen significantly [1–3]. In the literature, reported early results for patients undergoing reoperations on the aortic root are poorer than those for patients undergoing first-time surgery [4–10]. However, published outcomes of aortic reoperations versus primary operations are based on only a few (although valuable) studies providing data jeopardized by several sources of bias [4, 5].
The aim of this study was to review our experience with aortic root surgery by stratifying outcomes according to the type of intervention: reoperation versus primary operation on the aortic root. Statistical methods were used to control for treatment-selection bias.
PATIENTS AND METHODS
Study population
Our prospectively maintained clinical database was used to identify 1267 consecutive patients who underwent aortic root surgery at Sant'Orsola-Malpighi Hospital (Bologna, Italy) between 1986 and 2014. We divided the 1267 patients into two groups according to the type of intervention for the comparison of hospital outcomes: aortic reoperation with root replacement (ARR, n = 180) versus primary root replacement (PRR, n = 1087). Patients who had previously undergone non-aortic operations (e.g. mitral valve surgery, coronary bypass surgery) were excluded from the study to decrease undesired bias. Prior aortic interventions of ARR patients are depicted in Table 1. At least one prior aortic redo procedure had been performed in 23 (12.8%) patients, and the mean interval between the last previous operation and the actual reintervention was 11 ± 9 years.
| Intervention . | Frequency . | % . |
|---|---|---|
| AVR | 80 | 44.4 |
| AVR + supracoronary aorta replacement | 7 | 3.9 |
| AVR + supracoronary aorta reduction | 5 | 2.8 |
| AVR + supracoronary aorta + arch replacement | 1 | 0.6 |
| AVP | 4 | 2.2 |
| AVR + supracoronary aorta replacement | 8 | 4.4 |
| AVR + supracoronary aorta reduction | 1 | 0.6 |
| Bentall procedure | 24 | 13.3 |
| Supracoronary aorta replacement | 33 | 13.3 |
| Supracoronary aorta reduction | 1 | 0.6 |
| Supracoronary aorta + hemiarch replacement | 11 | 6.1 |
| Supracoronary aorta + arch replacement + ET | 3 | 1.7 |
| Supracoronary aorta + arch replacement + FET | 1 | 0.6 |
| Pulmonary autograft | 1 | 0.6 |
| Associated procedures | ||
| CABG | 9 | 5 |
| MVP | 1 | 0.6 |
| MVR | 4 | 2.2 |
| Intervention . | Frequency . | % . |
|---|---|---|
| AVR | 80 | 44.4 |
| AVR + supracoronary aorta replacement | 7 | 3.9 |
| AVR + supracoronary aorta reduction | 5 | 2.8 |
| AVR + supracoronary aorta + arch replacement | 1 | 0.6 |
| AVP | 4 | 2.2 |
| AVR + supracoronary aorta replacement | 8 | 4.4 |
| AVR + supracoronary aorta reduction | 1 | 0.6 |
| Bentall procedure | 24 | 13.3 |
| Supracoronary aorta replacement | 33 | 13.3 |
| Supracoronary aorta reduction | 1 | 0.6 |
| Supracoronary aorta + hemiarch replacement | 11 | 6.1 |
| Supracoronary aorta + arch replacement + ET | 3 | 1.7 |
| Supracoronary aorta + arch replacement + FET | 1 | 0.6 |
| Pulmonary autograft | 1 | 0.6 |
| Associated procedures | ||
| CABG | 9 | 5 |
| MVP | 1 | 0.6 |
| MVR | 4 | 2.2 |
ARR: aortic root reoperation; AVP: aortic valve plasty; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; hemiarch replacement: partial arch replacement without reimplantation of arch vessels; ET: elephant trunk; FET: frozen elephant trunk; MVP: mitral valve plasty; MVR: mitral valve replacement.
| Intervention . | Frequency . | % . |
|---|---|---|
| AVR | 80 | 44.4 |
| AVR + supracoronary aorta replacement | 7 | 3.9 |
| AVR + supracoronary aorta reduction | 5 | 2.8 |
| AVR + supracoronary aorta + arch replacement | 1 | 0.6 |
| AVP | 4 | 2.2 |
| AVR + supracoronary aorta replacement | 8 | 4.4 |
| AVR + supracoronary aorta reduction | 1 | 0.6 |
| Bentall procedure | 24 | 13.3 |
| Supracoronary aorta replacement | 33 | 13.3 |
| Supracoronary aorta reduction | 1 | 0.6 |
| Supracoronary aorta + hemiarch replacement | 11 | 6.1 |
| Supracoronary aorta + arch replacement + ET | 3 | 1.7 |
| Supracoronary aorta + arch replacement + FET | 1 | 0.6 |
| Pulmonary autograft | 1 | 0.6 |
| Associated procedures | ||
| CABG | 9 | 5 |
| MVP | 1 | 0.6 |
| MVR | 4 | 2.2 |
| Intervention . | Frequency . | % . |
|---|---|---|
| AVR | 80 | 44.4 |
| AVR + supracoronary aorta replacement | 7 | 3.9 |
| AVR + supracoronary aorta reduction | 5 | 2.8 |
| AVR + supracoronary aorta + arch replacement | 1 | 0.6 |
| AVP | 4 | 2.2 |
| AVR + supracoronary aorta replacement | 8 | 4.4 |
| AVR + supracoronary aorta reduction | 1 | 0.6 |
| Bentall procedure | 24 | 13.3 |
| Supracoronary aorta replacement | 33 | 13.3 |
| Supracoronary aorta reduction | 1 | 0.6 |
| Supracoronary aorta + hemiarch replacement | 11 | 6.1 |
| Supracoronary aorta + arch replacement + ET | 3 | 1.7 |
| Supracoronary aorta + arch replacement + FET | 1 | 0.6 |
| Pulmonary autograft | 1 | 0.6 |
| Associated procedures | ||
| CABG | 9 | 5 |
| MVP | 1 | 0.6 |
| MVR | 4 | 2.2 |
ARR: aortic root reoperation; AVP: aortic valve plasty; AVR: aortic valve replacement; CABG: coronary artery bypass grafting; hemiarch replacement: partial arch replacement without reimplantation of arch vessels; ET: elephant trunk; FET: frozen elephant trunk; MVP: mitral valve plasty; MVR: mitral valve replacement.
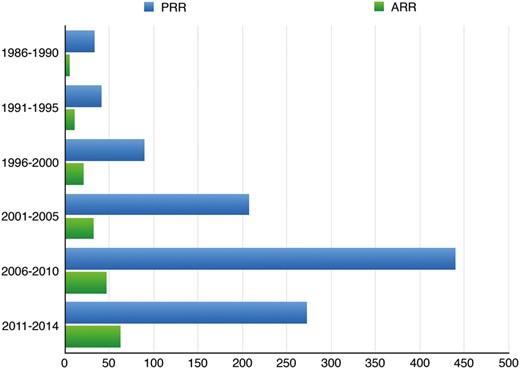
Distribution of reoperations (ARR) and primary operations (PRR) throughout the study period (P = 0.1). ARR: aortic reoperation with root replacement; PRR: primary root replacement.
Approval from the institutional review board was obtained.
Surgical technique
The aorta was exposed through a standard sternotomy in all patients. Vessel selection for arterial cannulation for cardiopulmonary bypass (CPB) depended upon the presence of aortic dissection, atheromatous disease, the extent of the aortic replacement and the degree of safety at chest re-entry. In general, when surgery was limited to the root/ascending aorta and a low-risk resternotomy was anticipated, CPB arterial inflow was obtained by cannulating the distal ascending aorta or the aortic arch. In patients who required some degree of aortic arch replacement, our favourite site for arterial cannulation was the right axillary artery or the innominate artery, mainly because they facilitate antegrade cerebral perfusion. The femoral artery was cannulated in patients who required CPB before resternotomy when an axillary artery was unavailable for cannulation or in unstable patients when prompt initiation of CPB became necessary. The right atrium, the common femoral vein or both venae cavae were used for venous return. The left heart was vented through insertion of a catheter in the right superior pulmonary vein, in the pulmonary artery trunk or in the left ventricular apex. Cold crystalloid cardioplegic solution was used in all cases. In ARR patients determined to be at high risk of chest re-entry lesion [2], resternotomy was performed on CPB (n = 7; 3.9%) or under deep hypothermic circulatory arrest (nasopharyngeal 18°C) (n = 3; 1.7%). In these patients, if significant aortic insufficiency was present, the apex of the left ventricle was cannulated before resternotomy (through a left anterior minithoracotomy) to avoid left ventricle distension (n = 5; 2.8%). In all cases involving partial or total aortic arch replacement or in patients in whom resternotomy was performed under circulatory arrest, antegrade selective cerebral perfusion (ASCP) was used for cerebral protection [11].
Aortic root operations included the Bentall procedure [12, 13], the Cabrol procedure [14] or prosthesis-sparing intervention [15]. The latter was performed in ARR patients with a prior well-functioning aortic valve prosthesis. In patients who underwent aortic arch reconstruction, the ‘en bloc technique’ or the ‘separated graft technique’ was used as a method of arch vessel reimplantation [16]. A conventional or a frozen elephant trunk procedure [17] was performed in patients with a diseased descending thoracic aorta.
Objectives of the study and analytic plan
The main purpose of this study was to compare hospital outcomes after aortic root surgery in patients who underwent aortic reoperation with root replacement versus those who underwent primary root replacement. Treatment bias was addressed by the use of propensity score (PS) matching and multivariate regression analysis. Second, using multivariable logistic models, we sought to identify the independent predictors of hospital deaths and major postoperative complications [permanent neurological deficit (PND), mechanical ventilation >72 h, acute myocardial infarction, temporary/permanent dialysis, bleeding requiring chest reopening and sepsis]. Covariate balance was measured using the standardized differences between the two treatment groups, which were calculated as the differences in the means divided by the pooled standard deviation and expressed as a percentage. A standardized difference greater than 10% is suggested to represent a meaningful covariate imbalance [18]. The primary end-points were all-cause mortality and occurrence of postoperative complications during hospitalization. In accordance with recent recommendations [18], differences in mortality rates and occurrence of postoperative complications between treatment groups were reported in terms of absolute risk reduction (AbRR) and odds ratios (OR). A two-sided probability value less than 0.05 was considered statistically significant. All analyses were performed with the SPSS 20.0 software (SPSS, Chicago, IL).
Propensity score matching
A PS was calculated for each patient using a non-parsimonious multivariable logistic regression, with reoperation as the dependent variable; it incorporated 20 preoperative covariates and 1 intraoperative relevant covariate as the independent variable (Table 2). Patients undergoing reoperative surgery were matched on a one-to-one basis with patients undergoing first-time surgery on the basis of the PS, by the use of nearest-neighbour matching without replacement, and a matching tolerance (caliper) of 0.2.
| Characteristics . | Overall cohort . | Propensity-matched cohort . | ||||
|---|---|---|---|---|---|---|
| ARR (n = 180) . | PRR (n = 1087) . | Standardized differencea . | ARR (n = 116) . | PRR (n = 116) . | Standardized differencea . | |
| Female gender | 20.6 | 19.8 | 1.9 | 21.6 | 22.4 | −2.1 |
| Age (mean, years) | 57.4 | 59.7 | −16.9 | 56.8 | 56.9 | −0.7 |
| Hypertension | 60.6 | 66.5 | −12.2 | 56 | 51.7 | 8.8 |
| Diabetes | 6.7 | 12.8 | −24.5 | 6 | 8.6 | −9.3 |
| Obesity | 4.4 | 8.1 | −17.1 | 4.3 | 4.9 | −6.4 |
| Smoking | 33 | 45.3 | −26.1 | 32.2 | 31 | 2.6 |
| Chronic pulmonary disease | 9.5 | 7.8 | 5.7 | 8.7 | 7.8 | 3.2 |
| Renal failure | 4.4 | 3.1 | 6.3 | 2.6 | 2.6 | 0 |
| Bicuspid aortic valve | 11.7 | 34.4 | −70.1 | 16.4 | 14.7 | 5.4 |
| Marfan syndrome | 5.6 | 3.9 | 7.3 | 5.2 | 3.5 | 7.4 |
| NYHA III–IV | 22.6 | 28.2 | −13.1 | 22.4 | 20.2 | 4.3 |
| Coronary artery disease | 15.6 | 14.8 | 2 | 13.8 | 10.6 | 9.2 |
| Cerebrovascular disease | 3.9 | 4.2 | −1.4 | 5.2 | 3.5 | 8.5 |
| Peripheral vascular disease | 1.1 | 3.1 | −18.2 | 1.7 | 4.1 | −9.1 |
| Urgent/emergency surgery | 18.9 | 10.7 | 20.9 | 11.2 | 16.4 | −9.2 |
| Indications for reoperation | ||||||
| Degenerative aneurysm | 49.4 | 88.7 | −78.3 | 74.1 | 74.1 | 0 |
| TAAD | 3.9 | 9.2 | −27.4 | 6 | 8.6 | −9.3 |
| Chronic post-dissection aneurysm | 25 | 1.4 | 54.4 | 14.7 | 12.9 | 4 |
| False aneurysm | 10 | – | 33.2 | 0.9 | – | 2.9 |
| Endocarditis | 12.2 | 0.7 | 35 | 4.3 | 4.3 | 0 |
| Extent of aortic repair | ||||||
| Root | 66.1 | 85.9 | 41.7 | 71.6 | 69.8 | −3.6 |
| Root + arch | 33.9 | 14.1 | 41.7 | 28.4 | 30.2 | −3.6 |
| Characteristics . | Overall cohort . | Propensity-matched cohort . | ||||
|---|---|---|---|---|---|---|
| ARR (n = 180) . | PRR (n = 1087) . | Standardized differencea . | ARR (n = 116) . | PRR (n = 116) . | Standardized differencea . | |
| Female gender | 20.6 | 19.8 | 1.9 | 21.6 | 22.4 | −2.1 |
| Age (mean, years) | 57.4 | 59.7 | −16.9 | 56.8 | 56.9 | −0.7 |
| Hypertension | 60.6 | 66.5 | −12.2 | 56 | 51.7 | 8.8 |
| Diabetes | 6.7 | 12.8 | −24.5 | 6 | 8.6 | −9.3 |
| Obesity | 4.4 | 8.1 | −17.1 | 4.3 | 4.9 | −6.4 |
| Smoking | 33 | 45.3 | −26.1 | 32.2 | 31 | 2.6 |
| Chronic pulmonary disease | 9.5 | 7.8 | 5.7 | 8.7 | 7.8 | 3.2 |
| Renal failure | 4.4 | 3.1 | 6.3 | 2.6 | 2.6 | 0 |
| Bicuspid aortic valve | 11.7 | 34.4 | −70.1 | 16.4 | 14.7 | 5.4 |
| Marfan syndrome | 5.6 | 3.9 | 7.3 | 5.2 | 3.5 | 7.4 |
| NYHA III–IV | 22.6 | 28.2 | −13.1 | 22.4 | 20.2 | 4.3 |
| Coronary artery disease | 15.6 | 14.8 | 2 | 13.8 | 10.6 | 9.2 |
| Cerebrovascular disease | 3.9 | 4.2 | −1.4 | 5.2 | 3.5 | 8.5 |
| Peripheral vascular disease | 1.1 | 3.1 | −18.2 | 1.7 | 4.1 | −9.1 |
| Urgent/emergency surgery | 18.9 | 10.7 | 20.9 | 11.2 | 16.4 | −9.2 |
| Indications for reoperation | ||||||
| Degenerative aneurysm | 49.4 | 88.7 | −78.3 | 74.1 | 74.1 | 0 |
| TAAD | 3.9 | 9.2 | −27.4 | 6 | 8.6 | −9.3 |
| Chronic post-dissection aneurysm | 25 | 1.4 | 54.4 | 14.7 | 12.9 | 4 |
| False aneurysm | 10 | – | 33.2 | 0.9 | – | 2.9 |
| Endocarditis | 12.2 | 0.7 | 35 | 4.3 | 4.3 | 0 |
| Extent of aortic repair | ||||||
| Root | 66.1 | 85.9 | 41.7 | 71.6 | 69.8 | −3.6 |
| Root + arch | 33.9 | 14.1 | 41.7 | 28.4 | 30.2 | −3.6 |
ARR: aortic root reoperation; NYHA: New York Heart Association; PRR: primary root replacement; TAAD: type A acute aortic dissection.
aStandardized difference is the mean difference divided by the pooled standard deviation, expressed as percentage. Values are percentages unless otherwise indicated.
| Characteristics . | Overall cohort . | Propensity-matched cohort . | ||||
|---|---|---|---|---|---|---|
| ARR (n = 180) . | PRR (n = 1087) . | Standardized differencea . | ARR (n = 116) . | PRR (n = 116) . | Standardized differencea . | |
| Female gender | 20.6 | 19.8 | 1.9 | 21.6 | 22.4 | −2.1 |
| Age (mean, years) | 57.4 | 59.7 | −16.9 | 56.8 | 56.9 | −0.7 |
| Hypertension | 60.6 | 66.5 | −12.2 | 56 | 51.7 | 8.8 |
| Diabetes | 6.7 | 12.8 | −24.5 | 6 | 8.6 | −9.3 |
| Obesity | 4.4 | 8.1 | −17.1 | 4.3 | 4.9 | −6.4 |
| Smoking | 33 | 45.3 | −26.1 | 32.2 | 31 | 2.6 |
| Chronic pulmonary disease | 9.5 | 7.8 | 5.7 | 8.7 | 7.8 | 3.2 |
| Renal failure | 4.4 | 3.1 | 6.3 | 2.6 | 2.6 | 0 |
| Bicuspid aortic valve | 11.7 | 34.4 | −70.1 | 16.4 | 14.7 | 5.4 |
| Marfan syndrome | 5.6 | 3.9 | 7.3 | 5.2 | 3.5 | 7.4 |
| NYHA III–IV | 22.6 | 28.2 | −13.1 | 22.4 | 20.2 | 4.3 |
| Coronary artery disease | 15.6 | 14.8 | 2 | 13.8 | 10.6 | 9.2 |
| Cerebrovascular disease | 3.9 | 4.2 | −1.4 | 5.2 | 3.5 | 8.5 |
| Peripheral vascular disease | 1.1 | 3.1 | −18.2 | 1.7 | 4.1 | −9.1 |
| Urgent/emergency surgery | 18.9 | 10.7 | 20.9 | 11.2 | 16.4 | −9.2 |
| Indications for reoperation | ||||||
| Degenerative aneurysm | 49.4 | 88.7 | −78.3 | 74.1 | 74.1 | 0 |
| TAAD | 3.9 | 9.2 | −27.4 | 6 | 8.6 | −9.3 |
| Chronic post-dissection aneurysm | 25 | 1.4 | 54.4 | 14.7 | 12.9 | 4 |
| False aneurysm | 10 | – | 33.2 | 0.9 | – | 2.9 |
| Endocarditis | 12.2 | 0.7 | 35 | 4.3 | 4.3 | 0 |
| Extent of aortic repair | ||||||
| Root | 66.1 | 85.9 | 41.7 | 71.6 | 69.8 | −3.6 |
| Root + arch | 33.9 | 14.1 | 41.7 | 28.4 | 30.2 | −3.6 |
| Characteristics . | Overall cohort . | Propensity-matched cohort . | ||||
|---|---|---|---|---|---|---|
| ARR (n = 180) . | PRR (n = 1087) . | Standardized differencea . | ARR (n = 116) . | PRR (n = 116) . | Standardized differencea . | |
| Female gender | 20.6 | 19.8 | 1.9 | 21.6 | 22.4 | −2.1 |
| Age (mean, years) | 57.4 | 59.7 | −16.9 | 56.8 | 56.9 | −0.7 |
| Hypertension | 60.6 | 66.5 | −12.2 | 56 | 51.7 | 8.8 |
| Diabetes | 6.7 | 12.8 | −24.5 | 6 | 8.6 | −9.3 |
| Obesity | 4.4 | 8.1 | −17.1 | 4.3 | 4.9 | −6.4 |
| Smoking | 33 | 45.3 | −26.1 | 32.2 | 31 | 2.6 |
| Chronic pulmonary disease | 9.5 | 7.8 | 5.7 | 8.7 | 7.8 | 3.2 |
| Renal failure | 4.4 | 3.1 | 6.3 | 2.6 | 2.6 | 0 |
| Bicuspid aortic valve | 11.7 | 34.4 | −70.1 | 16.4 | 14.7 | 5.4 |
| Marfan syndrome | 5.6 | 3.9 | 7.3 | 5.2 | 3.5 | 7.4 |
| NYHA III–IV | 22.6 | 28.2 | −13.1 | 22.4 | 20.2 | 4.3 |
| Coronary artery disease | 15.6 | 14.8 | 2 | 13.8 | 10.6 | 9.2 |
| Cerebrovascular disease | 3.9 | 4.2 | −1.4 | 5.2 | 3.5 | 8.5 |
| Peripheral vascular disease | 1.1 | 3.1 | −18.2 | 1.7 | 4.1 | −9.1 |
| Urgent/emergency surgery | 18.9 | 10.7 | 20.9 | 11.2 | 16.4 | −9.2 |
| Indications for reoperation | ||||||
| Degenerative aneurysm | 49.4 | 88.7 | −78.3 | 74.1 | 74.1 | 0 |
| TAAD | 3.9 | 9.2 | −27.4 | 6 | 8.6 | −9.3 |
| Chronic post-dissection aneurysm | 25 | 1.4 | 54.4 | 14.7 | 12.9 | 4 |
| False aneurysm | 10 | – | 33.2 | 0.9 | – | 2.9 |
| Endocarditis | 12.2 | 0.7 | 35 | 4.3 | 4.3 | 0 |
| Extent of aortic repair | ||||||
| Root | 66.1 | 85.9 | 41.7 | 71.6 | 69.8 | −3.6 |
| Root + arch | 33.9 | 14.1 | 41.7 | 28.4 | 30.2 | −3.6 |
ARR: aortic root reoperation; NYHA: New York Heart Association; PRR: primary root replacement; TAAD: type A acute aortic dissection.
aStandardized difference is the mean difference divided by the pooled standard deviation, expressed as percentage. Values are percentages unless otherwise indicated.
RESULTS
Patients' characteristics and intraoperative data
In the unmatched cohort, the ARR patients were younger than the PRR patients (57.4 vs 59.7 years; P = 0.03) and were more likely to undergo urgent/emergent operations (18.9 vs 10.7%; P = 0.003). When compared with PRR patients, ARR patients were more likely to have acute endocarditis (12.2 vs 0.7%; P < 0.001), a false aneurysm (10 vs 0%; P < 0.001) and a chronic post-dissection aneurysm (25 vs 1.4%; P < 0.001) and less likely to have a degenerative aneurysm (49.4 vs 88.7%; P < 0.001) and an acute type A aortic dissection (3.9 vs 9.2%; P = 0.01) (Table 2). Intraoperatively, the PRR group was more frequently associated with a less extensive aortic replacement and a shorter period of CPB, myocardial ischaemia and ASCP (Table 3). Total arch replacement using the elephant trunk or the frozen elephant trunk technique was performed in 18 (10%) ARR patients and 15 (1.4%) PRR patients (P < 0.001).
| Characteristics . | Overall cohort . | Propensity-matched cohort . | ||||
|---|---|---|---|---|---|---|
| ARR (n = 180) . | PRR (n = 1087) . | P-value . | ARR (n = 116) . | PRR (n = 116) . | P-value . | |
| Extent of aortic repair | ||||||
| Root | 66.1 | 85.9 | <0.001 | 71.6 | 69.8 | 0.9 |
| Root + arch | 33.9 | 14.1 | <0.001 | 28.4 | 30.2 | 0.9 |
| Root procedures | ||||||
| Bentall | 77.8 | 98.7 | <0.001 | 71.6 | 98.4 | <0.001 |
| Cabrol | 2.2 | 1.3 | 0.3 | 1.7 | 1.7 | 1 |
| Prosthesis sparing | 18.3 | - | <0.001 | 26.7 | - | <0.001 |
| Associated procedures | 15.6 | 19.1 | 0.3 | 12.9 | 15.5 | 0.7 |
| Unplanned CABG | 2.8 | 2.4 | 0.8 | 1.7 | 2.6 | 1 |
| CPB time (mean; minutes) | 213.7 | 151.7 | <0.001 | 192 | 160.9 | <0.001 |
| Cross-clamp time (mean; minutes) | 153.5 | 117.3 | <0.001 | 142.3 | 122.7 | <0.001 |
| ASCP time | 62.6 | 44.2 | <0.001 | 55.4 | 44.6 | 0.1 |
| Characteristics . | Overall cohort . | Propensity-matched cohort . | ||||
|---|---|---|---|---|---|---|
| ARR (n = 180) . | PRR (n = 1087) . | P-value . | ARR (n = 116) . | PRR (n = 116) . | P-value . | |
| Extent of aortic repair | ||||||
| Root | 66.1 | 85.9 | <0.001 | 71.6 | 69.8 | 0.9 |
| Root + arch | 33.9 | 14.1 | <0.001 | 28.4 | 30.2 | 0.9 |
| Root procedures | ||||||
| Bentall | 77.8 | 98.7 | <0.001 | 71.6 | 98.4 | <0.001 |
| Cabrol | 2.2 | 1.3 | 0.3 | 1.7 | 1.7 | 1 |
| Prosthesis sparing | 18.3 | - | <0.001 | 26.7 | - | <0.001 |
| Associated procedures | 15.6 | 19.1 | 0.3 | 12.9 | 15.5 | 0.7 |
| Unplanned CABG | 2.8 | 2.4 | 0.8 | 1.7 | 2.6 | 1 |
| CPB time (mean; minutes) | 213.7 | 151.7 | <0.001 | 192 | 160.9 | <0.001 |
| Cross-clamp time (mean; minutes) | 153.5 | 117.3 | <0.001 | 142.3 | 122.7 | <0.001 |
| ASCP time | 62.6 | 44.2 | <0.001 | 55.4 | 44.6 | 0.1 |
ARR: aortic root reoperation; ASCP: antegrade selective cerebral perfusion; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; PRR: primary root replacement.
| Characteristics . | Overall cohort . | Propensity-matched cohort . | ||||
|---|---|---|---|---|---|---|
| ARR (n = 180) . | PRR (n = 1087) . | P-value . | ARR (n = 116) . | PRR (n = 116) . | P-value . | |
| Extent of aortic repair | ||||||
| Root | 66.1 | 85.9 | <0.001 | 71.6 | 69.8 | 0.9 |
| Root + arch | 33.9 | 14.1 | <0.001 | 28.4 | 30.2 | 0.9 |
| Root procedures | ||||||
| Bentall | 77.8 | 98.7 | <0.001 | 71.6 | 98.4 | <0.001 |
| Cabrol | 2.2 | 1.3 | 0.3 | 1.7 | 1.7 | 1 |
| Prosthesis sparing | 18.3 | - | <0.001 | 26.7 | - | <0.001 |
| Associated procedures | 15.6 | 19.1 | 0.3 | 12.9 | 15.5 | 0.7 |
| Unplanned CABG | 2.8 | 2.4 | 0.8 | 1.7 | 2.6 | 1 |
| CPB time (mean; minutes) | 213.7 | 151.7 | <0.001 | 192 | 160.9 | <0.001 |
| Cross-clamp time (mean; minutes) | 153.5 | 117.3 | <0.001 | 142.3 | 122.7 | <0.001 |
| ASCP time | 62.6 | 44.2 | <0.001 | 55.4 | 44.6 | 0.1 |
| Characteristics . | Overall cohort . | Propensity-matched cohort . | ||||
|---|---|---|---|---|---|---|
| ARR (n = 180) . | PRR (n = 1087) . | P-value . | ARR (n = 116) . | PRR (n = 116) . | P-value . | |
| Extent of aortic repair | ||||||
| Root | 66.1 | 85.9 | <0.001 | 71.6 | 69.8 | 0.9 |
| Root + arch | 33.9 | 14.1 | <0.001 | 28.4 | 30.2 | 0.9 |
| Root procedures | ||||||
| Bentall | 77.8 | 98.7 | <0.001 | 71.6 | 98.4 | <0.001 |
| Cabrol | 2.2 | 1.3 | 0.3 | 1.7 | 1.7 | 1 |
| Prosthesis sparing | 18.3 | - | <0.001 | 26.7 | - | <0.001 |
| Associated procedures | 15.6 | 19.1 | 0.3 | 12.9 | 15.5 | 0.7 |
| Unplanned CABG | 2.8 | 2.4 | 0.8 | 1.7 | 2.6 | 1 |
| CPB time (mean; minutes) | 213.7 | 151.7 | <0.001 | 192 | 160.9 | <0.001 |
| Cross-clamp time (mean; minutes) | 153.5 | 117.3 | <0.001 | 142.3 | 122.7 | <0.001 |
| ASCP time | 62.6 | 44.2 | <0.001 | 55.4 | 44.6 | 0.1 |
ARR: aortic root reoperation; ASCP: antegrade selective cerebral perfusion; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; PRR: primary root replacement.
Overall in-hospital outcomes
Sixty-five patients died during hospitalization, for an overall in-hospital mortality rate of 5.1%. The mortality rates among the elective and emergency cases were 3.4 (38/1117) and 18% (27/150), respectively (P < 0.001). The type of aortic disease greatly affected the in-hospital mortality rate, being 3.1% for degenerative aneurysm, 13.3% for chronic dissection, 14% for acute dissection, 16.7% for active endocarditis and 25% for false aneurysm (P < 0.001). At least one major postoperative complication occurred in 204 patients (16%). A mechanical ventilation of >72 h was required in 7.8% (n = 79) of patients, and a definitive and a temporary dialysis was performed in 0.2% (n = 3) and in 4% (n = 51) of cases, respectively. A postoperative myocardial infarction occurred in 2.9% (n = 37) of patients, and a chest reopening for bleeding was performed in 5.3% (n = 67). Distribution of deaths and postoperative complications across type of operation (ARR versuss PRR) is illustrated in Table 4.
| Variables . | Overall cohort . | Propensity-matched cohort . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Redo (n = 180) . | Primary operation (n = 1087) . | AbRR%a . | OR (95% CI) . | P-value . | Redo (n = 116) . | Primary operation (n = 116) . | AbRR%a . | OR (95% CI) . | P-value . | |
| In-hospital mortality rate | 11.1 | 4.1 | 7 | 2.9 (1.666–5.029) | <0.001 | 6 | 1.7 | 4.3 | 2.7 (0.744–18.009) | 0.2 |
| Major complications | 22.2 | 15.1 | 7.1 | 1.6 (1.090–2.372) | 0.02 | 16.4 | 14.7 | 1.7 | 1.1 (0.569–2.324) | 0.9 |
| Mechanical ventilation >72 h | 11.1 | 7.1 | 4 | 1.6 (0.944–2.804) | 0.08 | 5.3 | 4.9 | 0.4 | 1.1 (0.295–3.955) | 0.6 |
| Acute myocardial infarction | 3.9 | 2.7 | 1.2 | 2.4 (1.471–3.978) | 0.001 | 3.1 | 2 | 1.1 | 1.6 (0.612–3.965) | 1 |
| Temporary/permanent dialysis | 6.7 | 3.9 | 2.8 | 1.9 (1.203–3.248) | 0.01 | 1.7 | 1.7 | 0 | 0.7 (0.074–6.922) | 1 |
| Permanent neurological deficit | 4.4 | 1.7 | 2.7 | 2.6 (1.127–6.066) | 0.04 | 0.9 | 2.6 | −1.7 | 0.3 (0.034–3.196) | 0.6 |
| Bleeding | 8.9 | 4.7 | 4.2 | 1.9 (1.104–3.558) | 0.03 | 5.2 | 4.3 | 0.9 | 1.2 (0.359–4.084) | 1 |
| Sepsis | 3.9 | 4.7 | −0.8 | 0.8 (0.367–1.841) | 0.8 | 3.4 | 3.9 | −0.5 | 0.9 (0.207–3.030) | 1 |
| Variables . | Overall cohort . | Propensity-matched cohort . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Redo (n = 180) . | Primary operation (n = 1087) . | AbRR%a . | OR (95% CI) . | P-value . | Redo (n = 116) . | Primary operation (n = 116) . | AbRR%a . | OR (95% CI) . | P-value . | |
| In-hospital mortality rate | 11.1 | 4.1 | 7 | 2.9 (1.666–5.029) | <0.001 | 6 | 1.7 | 4.3 | 2.7 (0.744–18.009) | 0.2 |
| Major complications | 22.2 | 15.1 | 7.1 | 1.6 (1.090–2.372) | 0.02 | 16.4 | 14.7 | 1.7 | 1.1 (0.569–2.324) | 0.9 |
| Mechanical ventilation >72 h | 11.1 | 7.1 | 4 | 1.6 (0.944–2.804) | 0.08 | 5.3 | 4.9 | 0.4 | 1.1 (0.295–3.955) | 0.6 |
| Acute myocardial infarction | 3.9 | 2.7 | 1.2 | 2.4 (1.471–3.978) | 0.001 | 3.1 | 2 | 1.1 | 1.6 (0.612–3.965) | 1 |
| Temporary/permanent dialysis | 6.7 | 3.9 | 2.8 | 1.9 (1.203–3.248) | 0.01 | 1.7 | 1.7 | 0 | 0.7 (0.074–6.922) | 1 |
| Permanent neurological deficit | 4.4 | 1.7 | 2.7 | 2.6 (1.127–6.066) | 0.04 | 0.9 | 2.6 | −1.7 | 0.3 (0.034–3.196) | 0.6 |
| Bleeding | 8.9 | 4.7 | 4.2 | 1.9 (1.104–3.558) | 0.03 | 5.2 | 4.3 | 0.9 | 1.2 (0.359–4.084) | 1 |
| Sepsis | 3.9 | 4.7 | −0.8 | 0.8 (0.367–1.841) | 0.8 | 3.4 | 3.9 | −0.5 | 0.9 (0.207–3.030) | 1 |
Values are percentages unless otherwise indicated.
AbRR: absolute risk reduction; CI: confidence interval; OR: odds ratio.
aAbRR negative value represents the per cent difference in mortality between patients undergoing reoperation and primary operation, in favour of reoperation group.
| Variables . | Overall cohort . | Propensity-matched cohort . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Redo (n = 180) . | Primary operation (n = 1087) . | AbRR%a . | OR (95% CI) . | P-value . | Redo (n = 116) . | Primary operation (n = 116) . | AbRR%a . | OR (95% CI) . | P-value . | |
| In-hospital mortality rate | 11.1 | 4.1 | 7 | 2.9 (1.666–5.029) | <0.001 | 6 | 1.7 | 4.3 | 2.7 (0.744–18.009) | 0.2 |
| Major complications | 22.2 | 15.1 | 7.1 | 1.6 (1.090–2.372) | 0.02 | 16.4 | 14.7 | 1.7 | 1.1 (0.569–2.324) | 0.9 |
| Mechanical ventilation >72 h | 11.1 | 7.1 | 4 | 1.6 (0.944–2.804) | 0.08 | 5.3 | 4.9 | 0.4 | 1.1 (0.295–3.955) | 0.6 |
| Acute myocardial infarction | 3.9 | 2.7 | 1.2 | 2.4 (1.471–3.978) | 0.001 | 3.1 | 2 | 1.1 | 1.6 (0.612–3.965) | 1 |
| Temporary/permanent dialysis | 6.7 | 3.9 | 2.8 | 1.9 (1.203–3.248) | 0.01 | 1.7 | 1.7 | 0 | 0.7 (0.074–6.922) | 1 |
| Permanent neurological deficit | 4.4 | 1.7 | 2.7 | 2.6 (1.127–6.066) | 0.04 | 0.9 | 2.6 | −1.7 | 0.3 (0.034–3.196) | 0.6 |
| Bleeding | 8.9 | 4.7 | 4.2 | 1.9 (1.104–3.558) | 0.03 | 5.2 | 4.3 | 0.9 | 1.2 (0.359–4.084) | 1 |
| Sepsis | 3.9 | 4.7 | −0.8 | 0.8 (0.367–1.841) | 0.8 | 3.4 | 3.9 | −0.5 | 0.9 (0.207–3.030) | 1 |
| Variables . | Overall cohort . | Propensity-matched cohort . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Redo (n = 180) . | Primary operation (n = 1087) . | AbRR%a . | OR (95% CI) . | P-value . | Redo (n = 116) . | Primary operation (n = 116) . | AbRR%a . | OR (95% CI) . | P-value . | |
| In-hospital mortality rate | 11.1 | 4.1 | 7 | 2.9 (1.666–5.029) | <0.001 | 6 | 1.7 | 4.3 | 2.7 (0.744–18.009) | 0.2 |
| Major complications | 22.2 | 15.1 | 7.1 | 1.6 (1.090–2.372) | 0.02 | 16.4 | 14.7 | 1.7 | 1.1 (0.569–2.324) | 0.9 |
| Mechanical ventilation >72 h | 11.1 | 7.1 | 4 | 1.6 (0.944–2.804) | 0.08 | 5.3 | 4.9 | 0.4 | 1.1 (0.295–3.955) | 0.6 |
| Acute myocardial infarction | 3.9 | 2.7 | 1.2 | 2.4 (1.471–3.978) | 0.001 | 3.1 | 2 | 1.1 | 1.6 (0.612–3.965) | 1 |
| Temporary/permanent dialysis | 6.7 | 3.9 | 2.8 | 1.9 (1.203–3.248) | 0.01 | 1.7 | 1.7 | 0 | 0.7 (0.074–6.922) | 1 |
| Permanent neurological deficit | 4.4 | 1.7 | 2.7 | 2.6 (1.127–6.066) | 0.04 | 0.9 | 2.6 | −1.7 | 0.3 (0.034–3.196) | 0.6 |
| Bleeding | 8.9 | 4.7 | 4.2 | 1.9 (1.104–3.558) | 0.03 | 5.2 | 4.3 | 0.9 | 1.2 (0.359–4.084) | 1 |
| Sepsis | 3.9 | 4.7 | −0.8 | 0.8 (0.367–1.841) | 0.8 | 3.4 | 3.9 | −0.5 | 0.9 (0.207–3.030) | 1 |
Values are percentages unless otherwise indicated.
AbRR: absolute risk reduction; CI: confidence interval; OR: odds ratio.
aAbRR negative value represents the per cent difference in mortality between patients undergoing reoperation and primary operation, in favour of reoperation group.
In-hospital mortality and morbidity rates: reoperation versus primary operation
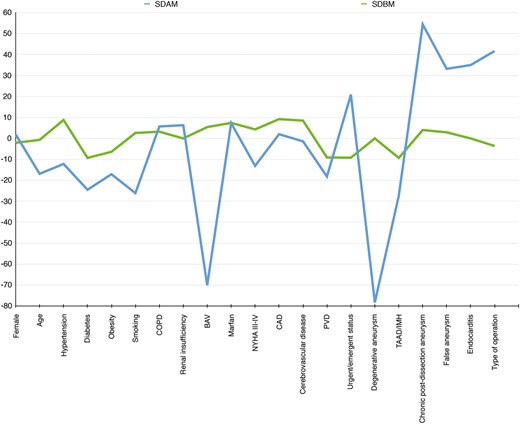
Balance in covariates before and after propensity matching. BAV: bicuspid aortic valve; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; IMH: intramural haematoma; NYHA: New York Heart Association; PVD: peripheral vascular disease; SDAM: standardized difference after matching; SDBM: standardized difference before matching; TAAD: type A acute aortic dissection.
The unadjusted comparison of hospital mortality and morbidity rates showed significant differences between groups (AbRR for hospital death: 7%, P < 0.001; AbRR for major postoperative complications: 7.1%, P = 0.02). Conversely, in the matched cohort, reoperation and primary operation were associated with a similar risk of in-hospital mortality and major postoperative complications (AbRR for hospital death: 4.3%, P = 0.2; AbRR for major postoperative complications: 1.7%, P = 0.9) (Table 4). This finding was confirmed after controlling for all measured covariates by standard logistic regression and for the estimated probability of reoperation (Table 5). After matching, all measured postoperative complications were similar in the two treatment groups (Table 4).
Unadjusted and adjusted odds ratio for hospital mortality rate and major postoperative complications: aortic root reoperation versus primary root replacement
| Risk adjustment method for hospital mortality rate and major complications (ARR versus PRR) . | P-value . | Odds ratio . | 95% confidence interval . |
|---|---|---|---|
| Hospital mortality | |||
| Unadjusted | <0.001 | 2.9 | 1.666–5.029 |
| Standard logistic regressiona | 0.2 | 3.7 | 0.519–26.251 |
| Propensity-adjusted logistic regressionb | 0.5 | 1.4 | 0.599–3.079 |
| Postoperative complications | |||
| Unadjusted | 0.002 | 1.6 | 1.090–2.372 |
| Standard logistic regressiona | 0.5 | 1.1 | 0.071–3.677 |
| Propensity-adjusted logistic regressionb | 0.9 | 1.1 | 0.614–1.779 |
| Risk adjustment method for hospital mortality rate and major complications (ARR versus PRR) . | P-value . | Odds ratio . | 95% confidence interval . |
|---|---|---|---|
| Hospital mortality | |||
| Unadjusted | <0.001 | 2.9 | 1.666–5.029 |
| Standard logistic regressiona | 0.2 | 3.7 | 0.519–26.251 |
| Propensity-adjusted logistic regressionb | 0.5 | 1.4 | 0.599–3.079 |
| Postoperative complications | |||
| Unadjusted | 0.002 | 1.6 | 1.090–2.372 |
| Standard logistic regressiona | 0.5 | 1.1 | 0.071–3.677 |
| Propensity-adjusted logistic regressionb | 0.9 | 1.1 | 0.614–1.779 |
ARR: aortic root reoperation; PRR: primary root replacement.
aLogistic regression against 15 measured covariates including type of operation (reoperation versus primary operation).
bLogistic regression against type of operation (reoperation versus primary operation) adjusted for the estimated probability of reoperation.
Unadjusted and adjusted odds ratio for hospital mortality rate and major postoperative complications: aortic root reoperation versus primary root replacement
| Risk adjustment method for hospital mortality rate and major complications (ARR versus PRR) . | P-value . | Odds ratio . | 95% confidence interval . |
|---|---|---|---|
| Hospital mortality | |||
| Unadjusted | <0.001 | 2.9 | 1.666–5.029 |
| Standard logistic regressiona | 0.2 | 3.7 | 0.519–26.251 |
| Propensity-adjusted logistic regressionb | 0.5 | 1.4 | 0.599–3.079 |
| Postoperative complications | |||
| Unadjusted | 0.002 | 1.6 | 1.090–2.372 |
| Standard logistic regressiona | 0.5 | 1.1 | 0.071–3.677 |
| Propensity-adjusted logistic regressionb | 0.9 | 1.1 | 0.614–1.779 |
| Risk adjustment method for hospital mortality rate and major complications (ARR versus PRR) . | P-value . | Odds ratio . | 95% confidence interval . |
|---|---|---|---|
| Hospital mortality | |||
| Unadjusted | <0.001 | 2.9 | 1.666–5.029 |
| Standard logistic regressiona | 0.2 | 3.7 | 0.519–26.251 |
| Propensity-adjusted logistic regressionb | 0.5 | 1.4 | 0.599–3.079 |
| Postoperative complications | |||
| Unadjusted | 0.002 | 1.6 | 1.090–2.372 |
| Standard logistic regressiona | 0.5 | 1.1 | 0.071–3.677 |
| Propensity-adjusted logistic regressionb | 0.9 | 1.1 | 0.614–1.779 |
ARR: aortic root reoperation; PRR: primary root replacement.
aLogistic regression against 15 measured covariates including type of operation (reoperation versus primary operation).
bLogistic regression against type of operation (reoperation versus primary operation) adjusted for the estimated probability of reoperation.
Predictors of all-cause in-hospital death and major postoperative complications
Using multivariate analysis, independent risk factors for hospital death were determined to be CPB time (OR: 1.03 per min; 95% confidence interval, CI: 1.013–1.041; P < 0.001) and urgent/emergent status (OR: 6.4; 95% CI: 1.093–37.684 P = 0.04). Age (OR: 1.07 per year; 95% CI: 1.006–1.132; P = 0.03) was the only risk factor for major postoperative complications.
DISCUSSION
Since 1968, aortic root replacement using the Bentall technique [12] has proved to be a safe and effective treatment for thoracic aortic disease [19–21]. Over the last decades, the reinterventions on the thoracic aorta have risen quickly as a result of the increased incidence of aortic diseases in an ageing population, the increased use of biological aortic valves and root substitutes, and the increased use of techniques for valve-sparing aortic root reconstruction. Our series strongly confirms such a trend because the number of aortic reoperations has quadrupled during the study period (Fig. 1). As previously reported by others [5, 6], the main indications for surgery in ARR patients were progression of the aortic pathology and mechanical (non-infective false aneurysm) or infectious complications. Taking into account the long mean interval time between the last previous procedure and the actual reintervention (11 ± 9 years), and the fact that degenerative aneurysm and chronic dissection accounted for 75% of all ARR procedures, we determined that regular clinical and imaging follow-ups are mandatory in patients undergoing operations on the proximal thoracic aorta.
Published early results of patients undergoing aortic reoperation with root replacement are worse than those of patients undergoing first-time surgery. Hospital mortality rates range from 6.5 to 15% in patients undergoing reoperative aortic surgery [4–10] and from 2 to 4% in patients undergoing first-time aortic root replacement [19–22]. Recently, Silva et al. [5] compared the impact of ascending aorta and aortic reoperation with root replacement on early and mid-term outcomes in patients undergoing aortic surgery. The hospital mortality rate was 12.1% in reoperative patients and 6.8% in patients undergoing a primary operation; major postoperative complications occurred in 24.1 and 16.9% of patients, respectively. Similarly, Etz et al. [4] reported on 435 patients undergoing aortic root repair, stratifying outcomes according to the type of intervention (reoperation versus primary operation). The hospital mortality rate was 7% in the reoperative group and 3% in the first-time surgery group; the postoperative complication rate was 21 and 17%, respectively. In our study, we assessed outcomes in a selected group of patients undergoing aortic root surgery. Unlike the above-mentioned series, patients underwent aortic valve and ascending aorta replacement; patients who had previously undergone non-aortic surgery were excluded from the analysis. Hence, the substantial differences in the study populations do not allow us to compare our outcomes with those of the Mount Sinai [4] and Madrid [5] groups.
In our unmatched cohort, the outcomes of patients undergoing reoperations were poorer than those of patients having a primary operation. Hospital mortality (AbRR: 7%, P < 0.001) and postoperative complication rates (AbRR: 7%, P = 0.002) were worse in ARR patients than in PRR patients, which highlights the complexity of referred patients and of contemplated procedures in the ARR group. Conversely, in the matched cohort, reoperation and primary operation were associated with a similar risk of in-hospital mortality rates (AbRR: 4%, P = 0.2) and major postoperative complications (AbRR: 2%, P = 0.9). These findings were strengthened after controlling for all other measured covariates by standard logistic regression and by the estimated probability (PS) of reoperation. We may speculate that the different hospital outcomes between ARR and PRR patients were mostly influenced by diversity in patients' profiles and type of aortic disease. In this setting, the early mortality rate was very low in patients who presented with degenerative aneurysm (3.1%), and dismal in patients with false aneurysm (25%) or active endocarditis (16.7%) (P < 0.001). In the ARR group, the cumulative incidence of active endocarditis and false aneurysm was 22.2%, compared with only 0.7% in the PRR group (P < 0001). In contrast, considering that urgent/emergent status was the most relevant predictor of hospital deaths (OR: 6.4), almost 90% of PRR patients had a degenerative aneurysm and underwent elective surgery. Furthermore, CPB time was confirmed to be an intraoperative predictor of mortality (OR: 1.03 per min; P < 0.001) and likely acted as a surrogate for demanding reinterventions such as those performed in patients with active endocarditis and aortic dissection. Our findings confirm that aortic reoperation in patients with active endocarditis remains a major concern [23], but, in this setting, current evidence is not yet strong enough to demonstrate what is the most effective management technique—surgical or medical therapy.
In aortic reoperations with root replacement, likely more than in other cardiac interventions, an effective method of myocardial protection can improve postoperative outcomes. In recent series, myocardial failure was the major cause of death in patients undergoing reoperations on the aortic root [8], and unplanned coronary artery bypass graft (CABG) was associated with operative mortality due to myocardial failure [24]. The main concern involves patients with significant aortic insufficiency, in which preventing (or delaying) ventricular fibrillation, and therefore left ventricle distension, decreases the risk of myocardial dysfunction at the end of the operation. In this setting, in ARR patients in whom the ascending aorta (or graft) could not be isolated and clamped quickly, distension of the left ventricle was avoided by venting the apex of the heart through a small left thoracotomy. In patients in whom the coronary artery ostia are detached, dissected or involved in the infectious process, selective antegrade administration of cardioplegia should be carried out with care, and retrograde or percutaneous infusion of cardioplegia through the coronary sinus should be considered [25]. Moreover, if difficulties in administering cardioplegia are expected with all methods, a lower core temperature provides additional time for myocardial protection to address the complex situation more effectively. Using these strategies, in our matched cohort, we did not observe a higher rate of acute myocardial infarction or unplanned CABG in ARR patients.
Limitations
The present study has several limitations of which retrospective design and statistical methods represent the most critical. Only randomized controlled trials may simultaneously guarantee prospective data and account for all differences between treatment groups. Propensity analysis is a powerful statistical technique, but it is limited by the number and accuracy of the assessed variables. Furthermore, PS analysis dismissed the sample size of the series and a type II error may have been generated. The statistical power of combined end-point ‘postoperative complications’ may be limited by the low event rate for each variable included. However, it is worth noting that in our analysis a considerable number of plausible preoperative and intraoperative covariates were used to compute the PS, and post-matching covariate balance was excellent. This study assesses the effectiveness of our surgical treatment in patients undergoing aortic root reoperations with root replacement and provides relevant evidence in this setting. Nevertheless, our findings cannot be generalized to the whole cardiac surgery community. There is growing evidence supporting the observation that outcomes following complex aortic procedures are improved at high-volume centres [26]. Patients who require aortic reoperations with root replacement should be referred to these centres.
CONCLUSION
Our study indicated that reoperation did not affect outcomes in patients undergoing aortic root surgery operated on at a high-volume aortic centre by experienced surgeons. In this setting, hospital results were heavily influenced by aortic pathology and the patient's profile. Although patients who presented with degenerative aneurysm were associated with satisfactory outcomes, those affected by false aneurysm and active endocarditis remain associated with high rates of postoperative mortality and morbidity.
Conflict of interest: none declared.




