-
PDF
- Split View
-
Views
-
Cite
Cite
Heinz Jakob, Daniel Dohle, Jaroslav Benedik, Rolf Alexander Jánosi, Thomas Schlosser, Daniel Wendt, Matthias Thielmann, Raimund Erbel, Konstantinos Tsagakis, Long-term experience with the E-vita Open hybrid graft in complex thoracic aortic disease†, European Journal of Cardio-Thoracic Surgery, Volume 51, Issue 2, February 2017, Pages 329–338, https://doi.org/10.1093/ejcts/ezw340
Close - Share Icon Share
Abstract
OBJECTIVES: The E-vita Open hybrid stent graft is intended to achieve one-stage treatment of the proximal and distal thoracic aorta down to the mid-thoracic level in cases of acute (AAD) or chronic (CAD) type I aortic dissection and complex thoracic aortic aneurysm (TAA). We report our long-term results up to 10-year experience.
METHODS: From February 2005 until March 2015, 178 consecutive patients (mean age 59 ± 11 years) underwent surgery using the E-vita Open hybrid graft for AAD (n = 96), CAD (n = 43) or TAA (n = 39). Pre-, intra- and postoperative variables, influential procedural improvements and follow-up data including aortic remodelling analyses are presented.
RESULTS: Overall 30-day mortality was 10%, 10% for AAD, 7% for CAD and 13% for TAA. Univariable analysis identified low left ventricular ejection fraction, peripheral arterial disease, chronic obstructive pulmonary disease and severely compromised haemodynamics as risk factors for in-hospital death. Logistic regression analysis defined compromised haemodynamics and duration of cardiopulmonary bypass as significant. After 7 years, estimated survival was 55% for AAD, 74% for CAD and 73% for TAA patients. Freedom from aorta-related late death was 94%, 91% in AAD, 100% in CAD and 97% in TAA. Positive or stable aortic remodelling down to the stent graft end was achieved in 92% AAD, 82% in CAD and full aneurysmal exclusion in 88%. Further downstream, negative remodelling was observed in 27% of the AAD, 41% of the CAD and 22% of the TAA patients. Freedom from endovascular intervention downstream was 96% in AAD, 75% in CAD and 74% in TAA patients. Freedom from thoraco-abdominal surgery was 97%, 65% and 93%, respectively.
CONCLUSIONS: The E-vita Open hybrid stent graft renders durable long-term performance without any proximal endoleakage or graft failure over time and represents the ideal landing or docking zone for either thoracic endovascular thoracic repair or thoraco-abdominal surgery, if required. No reinterventions were necessary down to the end of the stent graft, proving that the disease is overcome along the hybrid graft down to mid-thoracic level.
INTRODUCTION
The E-vita Open hybrid stent graft is part of a disease management concept designed to possibly avoid a second operation or reintervention down to the mid-thoracic level in complex multisegmental thoracic aortic disease [1]. The so-called frozen elephant trunk (FET) technique [2] combines the classic cut-and-sew surgical technique for replacement of the proximal aorta with endovascular stent-graft treatment of the descending aorta. The FET technique is applicable in acute and chronic aortic disease to stabilize the true lumen and cover the entry/re-entry sites along the proximal half of the descending aorta [3–7]. In the same manner, aneurysmal cavities are excluded from the circulation. In contrast to retrograde endovascular stent graft treatment, the FET graft is fixed proximally at the distal aortic arch using a circular haemostatic surgical suture line, which prevents any proximal endoleak over time. In contrast to this reliable one-stage aortic treatment extending to the mid-thoracic level, one must consider the following two concerns related to the FET technique: first, the FET operation is classified as major surgery, because open arch repair, often in combination with additional cardiac procedures, requires prolonged time on cardiopulmonary bypass (CPB), hypothermic circulatory arrest with selective cerebral perfusion and lower body ischaemia in many cases; second, the FET technique leaves the diseased distal aorta uncovered, thus transferring the area at risk from the upper thoracic level to the thoraco-abdominal areas. It remains to be proven that this manoeuvre reduces the risk of late mortality and morbidity and possibly opens up a good landing/docking zone for distal aortic reintervention when required. This study demonstrates the results from 7 years of experience at a single centre using the E-vita Open principle in acute or chronic aortic dissection (AAD/CAD) and complex thoracic aortic aneurysm (TAA).
Patients and indications
From February 2005 until March 2015, 178 consecutive patients underwent FET surgery using the E-vita Open and E-vita Open Plus (Jotec GmbH, Hechingen, Germany) stent grafts. The underlying disease was AAD in 96 (54%), CAD in 43 (24%) and TAA in 39 cases (22%). Three experienced surgeons out of a total of seven surgeons performed the operation in 153 (86%) patients. Patient characteristics are depicted in Table 1. In AAD, the indications for the FET operation were type I aortic dissection with re-entry sites in the distal arch and/or in the descending aorta, severe true lumen collapse downstream, circular detachment of the intimal layer in the distal arch or rupture in the distal arch or proximal descending aorta. In chronic type I aortic dissection, the FET procedure was performed due to progression of the aneurysm in the arch or descending aorta and to aortic rupture. Retrograde complicated acute or chronic type B (n = 19) dissection with a limited landing zone at least in Zone 2 [8] combined with a proximal aneurysm was similarly treated. The FET technique for TAA was applied in the case of a saccular or fusiform arch aneurysm extending into the proximal descending aorta. In the case of a primary thoraco-abdominal aortic aneurysm, the FET technique was part of a curative two-stage treatment strategy based on the diameter of the mid-descending aorta. The extent of aortic disease was evaluated by computed tomography, angiography, intravascular ultrasound and transoesophageal echocardiography. Discharged patients underwent clinical and computed tomography (CT)/magnetic resonance imaging (MRI) examinations at 6 months, 12 months and annually thereafter.
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| Nx (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| Age, mean ± SD | 59 ± 11 | 58 ± 11 | 56 ± 13 | 64 ± 10 |
| Female/male | 53/125 | 65/31 | 36/7 | 24 /15 |
| Marfan syndrome | 9 (5) | 3 (3) | 6 (14) | – |
| Emergency <24 h | 97 (55) | 91 (95) | 2 (5) | 4 (10) |
| Previous proximal aortic surgery | 39 (22) | 5 (5) | 27 (63) | 7 (18) |
| Previous descending aortic repair | 16 (9) | 1 (1) | 5 (12) | 10 (26) |
| Previous abdominal aortic repair | 6 (3) | 1 (1) | 2 (5) | 3 (8) |
| Cardiac comorbidities | ||||
| Coronary artery disease | 51 (29) | 25 (26) | 7 (16) | 19 (49) |
| Aortic valve disease | 73 (41) | 59 (62) | 9 (21) | 5 (13) |
| Mitral valve disease | 17 (10) | 9 (9) | 6 (14) | 2 (5) |
| Tricuspid valve disease | 9 (5) | 3 (3) | 3 (7) | 3 (8) |
| EF ≤ 40% | 16 (9) | 9 (9) | 3 (7) | 4 (10) |
| Other comorbidities | ||||
| Peripheral artery disease | 30 (17) | 6 (6) | 7 (16) | 17 (44) |
| Creatinine >1.5 mg/dl | 37 (21) | 21 (22) | 5 (12) | 11 (28) |
| COPD2 | 41 (23) | 14 (15) | 11 (26) | 16 (41) |
| Diabetes mellitus | 16 (9) | 6 (6) | 4 (9) | 6 (15) |
| St/p stroke | 22 (12) | 7 (7) | 7 (16) | 8 (21) |
| Extent of aortic disease | ||||
| Aortic root | 83 (47) | 77 (80) | 4 (9) | 2 (5) |
| Ascending aorta | 140 (79) | 96 (100) | 29 (67) | 15 (39) |
| Aortic arch + descending aorta | 178 (100) | 96 (100) | 43 (100) | 39 (100) |
| Abdominal aorta | 134 (75) | 83 (87) | 37 (86) | 14 (36) |
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| Nx (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| Age, mean ± SD | 59 ± 11 | 58 ± 11 | 56 ± 13 | 64 ± 10 |
| Female/male | 53/125 | 65/31 | 36/7 | 24 /15 |
| Marfan syndrome | 9 (5) | 3 (3) | 6 (14) | – |
| Emergency <24 h | 97 (55) | 91 (95) | 2 (5) | 4 (10) |
| Previous proximal aortic surgery | 39 (22) | 5 (5) | 27 (63) | 7 (18) |
| Previous descending aortic repair | 16 (9) | 1 (1) | 5 (12) | 10 (26) |
| Previous abdominal aortic repair | 6 (3) | 1 (1) | 2 (5) | 3 (8) |
| Cardiac comorbidities | ||||
| Coronary artery disease | 51 (29) | 25 (26) | 7 (16) | 19 (49) |
| Aortic valve disease | 73 (41) | 59 (62) | 9 (21) | 5 (13) |
| Mitral valve disease | 17 (10) | 9 (9) | 6 (14) | 2 (5) |
| Tricuspid valve disease | 9 (5) | 3 (3) | 3 (7) | 3 (8) |
| EF ≤ 40% | 16 (9) | 9 (9) | 3 (7) | 4 (10) |
| Other comorbidities | ||||
| Peripheral artery disease | 30 (17) | 6 (6) | 7 (16) | 17 (44) |
| Creatinine >1.5 mg/dl | 37 (21) | 21 (22) | 5 (12) | 11 (28) |
| COPD2 | 41 (23) | 14 (15) | 11 (26) | 16 (41) |
| Diabetes mellitus | 16 (9) | 6 (6) | 4 (9) | 6 (15) |
| St/p stroke | 22 (12) | 7 (7) | 7 (16) | 8 (21) |
| Extent of aortic disease | ||||
| Aortic root | 83 (47) | 77 (80) | 4 (9) | 2 (5) |
| Ascending aorta | 140 (79) | 96 (100) | 29 (67) | 15 (39) |
| Aortic arch + descending aorta | 178 (100) | 96 (100) | 43 (100) | 39 (100) |
| Abdominal aorta | 134 (75) | 83 (87) | 37 (86) | 14 (36) |
AD: aortic dissection; COPD: chronic obstructive pulmonary disease; EF: left ventricle ejection fraction.
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| Nx (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| Age, mean ± SD | 59 ± 11 | 58 ± 11 | 56 ± 13 | 64 ± 10 |
| Female/male | 53/125 | 65/31 | 36/7 | 24 /15 |
| Marfan syndrome | 9 (5) | 3 (3) | 6 (14) | – |
| Emergency <24 h | 97 (55) | 91 (95) | 2 (5) | 4 (10) |
| Previous proximal aortic surgery | 39 (22) | 5 (5) | 27 (63) | 7 (18) |
| Previous descending aortic repair | 16 (9) | 1 (1) | 5 (12) | 10 (26) |
| Previous abdominal aortic repair | 6 (3) | 1 (1) | 2 (5) | 3 (8) |
| Cardiac comorbidities | ||||
| Coronary artery disease | 51 (29) | 25 (26) | 7 (16) | 19 (49) |
| Aortic valve disease | 73 (41) | 59 (62) | 9 (21) | 5 (13) |
| Mitral valve disease | 17 (10) | 9 (9) | 6 (14) | 2 (5) |
| Tricuspid valve disease | 9 (5) | 3 (3) | 3 (7) | 3 (8) |
| EF ≤ 40% | 16 (9) | 9 (9) | 3 (7) | 4 (10) |
| Other comorbidities | ||||
| Peripheral artery disease | 30 (17) | 6 (6) | 7 (16) | 17 (44) |
| Creatinine >1.5 mg/dl | 37 (21) | 21 (22) | 5 (12) | 11 (28) |
| COPD2 | 41 (23) | 14 (15) | 11 (26) | 16 (41) |
| Diabetes mellitus | 16 (9) | 6 (6) | 4 (9) | 6 (15) |
| St/p stroke | 22 (12) | 7 (7) | 7 (16) | 8 (21) |
| Extent of aortic disease | ||||
| Aortic root | 83 (47) | 77 (80) | 4 (9) | 2 (5) |
| Ascending aorta | 140 (79) | 96 (100) | 29 (67) | 15 (39) |
| Aortic arch + descending aorta | 178 (100) | 96 (100) | 43 (100) | 39 (100) |
| Abdominal aorta | 134 (75) | 83 (87) | 37 (86) | 14 (36) |
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| Nx (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| Age, mean ± SD | 59 ± 11 | 58 ± 11 | 56 ± 13 | 64 ± 10 |
| Female/male | 53/125 | 65/31 | 36/7 | 24 /15 |
| Marfan syndrome | 9 (5) | 3 (3) | 6 (14) | – |
| Emergency <24 h | 97 (55) | 91 (95) | 2 (5) | 4 (10) |
| Previous proximal aortic surgery | 39 (22) | 5 (5) | 27 (63) | 7 (18) |
| Previous descending aortic repair | 16 (9) | 1 (1) | 5 (12) | 10 (26) |
| Previous abdominal aortic repair | 6 (3) | 1 (1) | 2 (5) | 3 (8) |
| Cardiac comorbidities | ||||
| Coronary artery disease | 51 (29) | 25 (26) | 7 (16) | 19 (49) |
| Aortic valve disease | 73 (41) | 59 (62) | 9 (21) | 5 (13) |
| Mitral valve disease | 17 (10) | 9 (9) | 6 (14) | 2 (5) |
| Tricuspid valve disease | 9 (5) | 3 (3) | 3 (7) | 3 (8) |
| EF ≤ 40% | 16 (9) | 9 (9) | 3 (7) | 4 (10) |
| Other comorbidities | ||||
| Peripheral artery disease | 30 (17) | 6 (6) | 7 (16) | 17 (44) |
| Creatinine >1.5 mg/dl | 37 (21) | 21 (22) | 5 (12) | 11 (28) |
| COPD2 | 41 (23) | 14 (15) | 11 (26) | 16 (41) |
| Diabetes mellitus | 16 (9) | 6 (6) | 4 (9) | 6 (15) |
| St/p stroke | 22 (12) | 7 (7) | 7 (16) | 8 (21) |
| Extent of aortic disease | ||||
| Aortic root | 83 (47) | 77 (80) | 4 (9) | 2 (5) |
| Ascending aorta | 140 (79) | 96 (100) | 29 (67) | 15 (39) |
| Aortic arch + descending aorta | 178 (100) | 96 (100) | 43 (100) | 39 (100) |
| Abdominal aorta | 134 (75) | 83 (87) | 37 (86) | 14 (36) |
AD: aortic dissection; COPD: chronic obstructive pulmonary disease; EF: left ventricle ejection fraction.
Operative setup and operation
All patients with AAD were operated on in a hybrid operative suite equipped with angiography unit and on-site evaluation of the extent of the dissection and the malperfusion treatment [9]. Elective cases were operated on in regular operative rooms. Neuromonitoring was achieved with near infrared spectroscopy. In elective cases, the cerebrospinal fluid pressure was controlled by drainage, 16 G, placed in the fourth to fifth lumbar space 1 day preoperatively. Intraoperatively and for at least 72 h, the cerebrospinal fluid pressure was adjusted to 10 mmHg using the LiquoGuard® system (Moeller Medical, Fulda, Germany) for continuous pressure measurement and drainage. Arterial blood pressure was controlled in both radial arteries and in one femoral artery. An extra stiff 0.035″ guide wire was inserted over a 6F pigtail catheter guided by X-ray or transoesophageal echocardiography in the aortic/true lumen of the proximal descending aorta. The wire was used for the antegrade delivery of the E-vita Open stent. During the study period, several surgical concept modifications were introduced to facilitate the FET procedure, including removal of the distal anastomosis to Zone 2 or proximally [10]. The Zone 2 concept was first introduced in 2009 to avoid time-consuming surgical manoeuvres within the deep chest during hypothermic circulatory arrest distally and to overcome the risk for laryngeal nerve injury. Furthermore, this technique enables the fixation of the graft proximal to the aneurysmal formations in the distal arch, thereby avoiding time-consuming downsizing of the aneurysmal aortic wall during the anastomosis. Using this concept, rerouting of the left subclavian artery (LSA) is required, which is performed prior to sternotomy by an end-to-side anastomosis between the left axillary artery and an 8-mm vascular prosthesis. The graft is tunnelled through the second intercostal space; rerouting is completed by an end-to-side anastomosis at the ascending/arch vascular graft after proximal aortic repair during reperfusion. Thus, the surgical intervention in the distal arch is limited to closure of the origin of the LSA. Otherwise, LSA rerouting is performed intrathoracically during cooling by end-to-end anastomosis between the origin of the LSA and an 8- to 12-mm vascular graft. In both cases, separate cannulation of the graft enables selective perfusion of the LSA controlled by the left radial arterial line. The extracorporeal circulation setup consisted of a centrifugal main pump and a separate roller pump used for selective perfusion of the LSA and the downstream aorta during the arch repair. For the main arterial perfusion, the right axillary artery was the favoured site of cannulation in order to avoid any cerebral circulatory arrest during arch repair. During cooling to a bladder temperature of 28 °C, the ascending aorta was clamped, and cardioplegic arrest was initiated with cold Custodiol solution (Dr Franz Köhler Chemie, Alsbach-Hähnlein, Germany). Bilateral selective cerebral perfusion was usually instituted after opening the arch by inserting a balloon catheter (Gundry® Silicone RCSP Cannulae, Medtronic Inc, USA) into the left carotid artery. The temperature of the solution for cerebral perfusion was 20–22 °C, and the flow was adjusted to 50–60 mmHg pressure continuously measured at the tip of the left carotid artery catheter and the main arterial line. The arch was resected between the left carotid and the subclavian artery. Angioscopy (Olympus®, BF type Q180-AC) was used routinely to evaluate the extent of the aortic disease downstream and to select the landing zone. The E-vita Open stent graft was placed and deployed to the target zone using the wire technique [11]. The haemostatic suture line was created by fixing the stent graft were fixed to the aortic rim, most recently using the collar of the E-vita Open Plus device, with a 3-0 polypropylene continuous suture enforced with external Teflon felt. Then, the integrated vascular prosthesis was pulled back to the arch position. Again, deployment of the stent graft was controlled angioscopically. A 30F Foley balloon catheter was introduced into the stent graft and connected to the separate circuit. After de-airing the downstream aorta, endoclamping was applied, and selective perfusion (1.5–2.5 l/min) of the distal aorta controlled by the femoral artery line was started. Direct reimplantation of the head vessels followed either as a two-vessel island or separately during stepwise rewarming. Rarely, it was necessary to interpose a prosthetic graft because the perfect mobility of the arch prosthesis was not impeded by any clamp. Thus, in some cases, the arch prosthesis was moved upwards 1 or 2 cm. Whole-body reperfusion was established via the right axillary artery after removal of the brachiocephalic trunk clamp, indicating the end of selective cerebral perfusion. The anastomosis between the previously placed ascending aortic graft and the arch prosthesis followed prior to release of the aortic cross-clamp. Finally, the extra-anatomic axillary/subclavian artery graft was reimplanted into any appropriate site of the arch or ascending aortic graft, either via an ante- or retroaortic route during rewarming.
E-vita Open sizing and distal landing zone
The length and diameter of the hybrid stent graft ranged from 100 to 160 mm and 22–40 mm, respectively. In TAA, the stent graft was sized on the basis of the diameter of the aorta at the level of the distal landing zone, and oversizing was strictly limited to 10% or less. In AAD and CAD, the length of the dissection membrane was presumed to be the diameter of the true lumen and was calculated from the axial CT images at the estimated landing zone. In these cases, the stent graft was normal or undersized, especially in CAD. In this series, no patient with a small true lumen and a dissection membrane <22 mm was treated with E-vita Open stent graft. In addition, if calculations based on CT scans were unavailable, intraoperative sizing of the distal arch and downstream aorta was achieved using flexible titanium obturators (Fehling Instruments GmbH&Co.KG, Karlstein, Germany) in order to avoid mismatch. The distal landing zone was selected from preoperative aortic images and controlled intraoperatively using angioscopy. Thereby, intimal and aortic lesions along the descending aorta were visualized, and the stent graft was deployed safely, covering re-entries or other aortic pathologies such as thrombus and atherosclerotic ulcers. In the case of aortic tortuosity, placement of the distal end of the stent graft close to sharp aortic angles was avoided. Care was taken not to land the distal end of the stent graft below the level of the eighth thoracic vertebra.
Definitions
The Penn classification was used to describe the long-term survival in AAD patients in relation to preoperative clinical status [12]. Aorta-related death was defined as any death with direct or indirect connection to the aorta or its branches and any death from unknown cause. An aortic cavity or false lumen was defined as completely excluded in case of complete thrombosis evaluated in late-phase CT slices. Residual disease in the distal aortic segments was defined as partially or completely perfused false lumen or an aneurysmal formation that remained uncovered. The last follow-up CT was used to evaluate the diseased area of the aorta prior to a secondary intervention. Remodelling of the downstream aorta was defined in terms of changes to the diameter or volume as positive, stable or negative with a 10% threshold [13]. First and last postoperative CT examinations were used to interpret the remodelling. The date of the last follow-up examination was used as the cut-off date for long-term analysis.
Statistical analysis
Categorical variables are presented as percents. Continuous variables are presented as mean ± SD or median (range), if appropriate. The Fisher’s two-sided exact test for categorical and the Student’s t-test for continuous variables were used. Independent predictors (P ≤ 0.05) for in-hospital mortality were calculated using multivariable logistic regression analysis based on the preoperative and intraoperative variables. Overall survival and freedom from reintervention are presented as Kaplan–Meier curves and log-rank statistical test for significance. Eight-year and 5-year cutoffs were used to estimate survival and secondary aortic interventions during the follow-up period. Interim results are given after 2, 5 and 8 years. Due to the addition of new surgical methods during the study period, evaluation of interim parameters was adjusted to the fourth postoperative year. Correlations with mortality and reintervention were calculated by Cox univariable regression analysis and are given as hazard ratio (HR), 95% confidence interval (CI), P-value. Determinants of overall mortality and secondary distal aortic reintervention were calculated using Cox hazards backward stepwise regression analysis. SPSS Statistic 20.0 was used for statistical analysis.
RESULTS
In-hospital stay
Intraoperative results are presented in Table 2.
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| Primary cannulation | ||||
| Axillary artery right | 96 (54) | 33 (34) | 32 (75) | 31 (79) |
| Axillary artery left | 4 (2) | 4 (4) | – | – |
| Brachiocephalic trunk | 3 (2) | 1 (1) | – | 2 (5) |
| Ascending/arch | 72 (40) | 57 (59) | 10 (23) | 5 (13) |
| Femoral | 3 (2) | 1 (1) | 1 (2) | 1 (3) |
| Perfusion | ||||
| Selective cerebral | 178 (100) | 96 (100) | 43 (100) | 39 (100) |
| Selective left axillary artery | 73 (41) | 32 (33) | 20 (47) | 21 (54) |
| Selective distal | 51 (29) | 25 (26) | 12 (28) | 14 (36) |
| Aortic valve | ||||
| Repair | 65 (37) | 60 (63) | 3 (7) | 2 (5) |
| Replacement | 24 (14) | 13 (14) | 9 (21) | 2 (5) |
| Root replacement | ||||
| Partial | 39 (22) | 37 (39) | 2 (5) | – |
| Total | 18 (10) | 11 (12) | 7 (16) | – |
| Ascending aorta replacement | 163 (92) | 96 (100) | 37 (86) | 30 (77) |
| E-vita Open stent graft, mm | ||||
| Diameter, mean ± SD | 5 (3) | 26 ±3 | 27 ±4 | 32 ±6 |
| Length | 112 (63) | 1 (1) | 1 (2) | 3 (8) |
| <130 | 61 (34) | 67 (70) | 25 (58) | 20 (51) |
| 130 | 28 (29) | 17 (40) | 16 (41) | |
| >130 | ||||
| Proximal E-vita fixation | ||||
| Zone 3 | 95 (53) | 58 (60) | 20 (47) | 17 (44) |
| Zone 2 | 76 (43) | 34 (35) | 21 (49) | 21 (54) |
| Zone 1 | 4 (2) | 2 (2) | 1 (2) | 1 (3) |
| Zone 0 | 3 (2) | 2 (2) | 1 (2) | – |
| Head-vessel reimplantation | ||||
| Enbloc | 95 (53) | 49 (51) | 22 (51) | 24 (62) |
| Separately | 83 (47) | 47 (49) | 21 (49) | 15 (38) |
| Left subclavian artery rerouting | ||||
| Aortic–subclavian graft | 42 (24) | 19 (45) | 12 (29) | 11 (28) |
| Aortic–axillary graft | 39 (22) | 20 (21) | 11 (26) | 8 (21) |
| Carotid–subclavian graft | 3 (2) | – | – | 3 (8) |
| Combined procedures | ||||
| Endovascular aortic/arterial repair | 29 (16) | 19 (20) | 9 (21) | 1 (3) |
| Coronary artery bypass graft | 46 (26) | 27 (28) | 5 (12) | 14 (36) |
| Mitral valve repair | 8 (5) | 3 (3) | 3 (7) | 2 (5) |
| Tricuspid valve repair | 2 (1) | — | 2 (4) | — |
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| Primary cannulation | ||||
| Axillary artery right | 96 (54) | 33 (34) | 32 (75) | 31 (79) |
| Axillary artery left | 4 (2) | 4 (4) | – | – |
| Brachiocephalic trunk | 3 (2) | 1 (1) | – | 2 (5) |
| Ascending/arch | 72 (40) | 57 (59) | 10 (23) | 5 (13) |
| Femoral | 3 (2) | 1 (1) | 1 (2) | 1 (3) |
| Perfusion | ||||
| Selective cerebral | 178 (100) | 96 (100) | 43 (100) | 39 (100) |
| Selective left axillary artery | 73 (41) | 32 (33) | 20 (47) | 21 (54) |
| Selective distal | 51 (29) | 25 (26) | 12 (28) | 14 (36) |
| Aortic valve | ||||
| Repair | 65 (37) | 60 (63) | 3 (7) | 2 (5) |
| Replacement | 24 (14) | 13 (14) | 9 (21) | 2 (5) |
| Root replacement | ||||
| Partial | 39 (22) | 37 (39) | 2 (5) | – |
| Total | 18 (10) | 11 (12) | 7 (16) | – |
| Ascending aorta replacement | 163 (92) | 96 (100) | 37 (86) | 30 (77) |
| E-vita Open stent graft, mm | ||||
| Diameter, mean ± SD | 5 (3) | 26 ±3 | 27 ±4 | 32 ±6 |
| Length | 112 (63) | 1 (1) | 1 (2) | 3 (8) |
| <130 | 61 (34) | 67 (70) | 25 (58) | 20 (51) |
| 130 | 28 (29) | 17 (40) | 16 (41) | |
| >130 | ||||
| Proximal E-vita fixation | ||||
| Zone 3 | 95 (53) | 58 (60) | 20 (47) | 17 (44) |
| Zone 2 | 76 (43) | 34 (35) | 21 (49) | 21 (54) |
| Zone 1 | 4 (2) | 2 (2) | 1 (2) | 1 (3) |
| Zone 0 | 3 (2) | 2 (2) | 1 (2) | – |
| Head-vessel reimplantation | ||||
| Enbloc | 95 (53) | 49 (51) | 22 (51) | 24 (62) |
| Separately | 83 (47) | 47 (49) | 21 (49) | 15 (38) |
| Left subclavian artery rerouting | ||||
| Aortic–subclavian graft | 42 (24) | 19 (45) | 12 (29) | 11 (28) |
| Aortic–axillary graft | 39 (22) | 20 (21) | 11 (26) | 8 (21) |
| Carotid–subclavian graft | 3 (2) | – | – | 3 (8) |
| Combined procedures | ||||
| Endovascular aortic/arterial repair | 29 (16) | 19 (20) | 9 (21) | 1 (3) |
| Coronary artery bypass graft | 46 (26) | 27 (28) | 5 (12) | 14 (36) |
| Mitral valve repair | 8 (5) | 3 (3) | 3 (7) | 2 (5) |
| Tricuspid valve repair | 2 (1) | — | 2 (4) | — |
AD: aortic dissection; SD: standard deviation.
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| Primary cannulation | ||||
| Axillary artery right | 96 (54) | 33 (34) | 32 (75) | 31 (79) |
| Axillary artery left | 4 (2) | 4 (4) | – | – |
| Brachiocephalic trunk | 3 (2) | 1 (1) | – | 2 (5) |
| Ascending/arch | 72 (40) | 57 (59) | 10 (23) | 5 (13) |
| Femoral | 3 (2) | 1 (1) | 1 (2) | 1 (3) |
| Perfusion | ||||
| Selective cerebral | 178 (100) | 96 (100) | 43 (100) | 39 (100) |
| Selective left axillary artery | 73 (41) | 32 (33) | 20 (47) | 21 (54) |
| Selective distal | 51 (29) | 25 (26) | 12 (28) | 14 (36) |
| Aortic valve | ||||
| Repair | 65 (37) | 60 (63) | 3 (7) | 2 (5) |
| Replacement | 24 (14) | 13 (14) | 9 (21) | 2 (5) |
| Root replacement | ||||
| Partial | 39 (22) | 37 (39) | 2 (5) | – |
| Total | 18 (10) | 11 (12) | 7 (16) | – |
| Ascending aorta replacement | 163 (92) | 96 (100) | 37 (86) | 30 (77) |
| E-vita Open stent graft, mm | ||||
| Diameter, mean ± SD | 5 (3) | 26 ±3 | 27 ±4 | 32 ±6 |
| Length | 112 (63) | 1 (1) | 1 (2) | 3 (8) |
| <130 | 61 (34) | 67 (70) | 25 (58) | 20 (51) |
| 130 | 28 (29) | 17 (40) | 16 (41) | |
| >130 | ||||
| Proximal E-vita fixation | ||||
| Zone 3 | 95 (53) | 58 (60) | 20 (47) | 17 (44) |
| Zone 2 | 76 (43) | 34 (35) | 21 (49) | 21 (54) |
| Zone 1 | 4 (2) | 2 (2) | 1 (2) | 1 (3) |
| Zone 0 | 3 (2) | 2 (2) | 1 (2) | – |
| Head-vessel reimplantation | ||||
| Enbloc | 95 (53) | 49 (51) | 22 (51) | 24 (62) |
| Separately | 83 (47) | 47 (49) | 21 (49) | 15 (38) |
| Left subclavian artery rerouting | ||||
| Aortic–subclavian graft | 42 (24) | 19 (45) | 12 (29) | 11 (28) |
| Aortic–axillary graft | 39 (22) | 20 (21) | 11 (26) | 8 (21) |
| Carotid–subclavian graft | 3 (2) | – | – | 3 (8) |
| Combined procedures | ||||
| Endovascular aortic/arterial repair | 29 (16) | 19 (20) | 9 (21) | 1 (3) |
| Coronary artery bypass graft | 46 (26) | 27 (28) | 5 (12) | 14 (36) |
| Mitral valve repair | 8 (5) | 3 (3) | 3 (7) | 2 (5) |
| Tricuspid valve repair | 2 (1) | — | 2 (4) | — |
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| Primary cannulation | ||||
| Axillary artery right | 96 (54) | 33 (34) | 32 (75) | 31 (79) |
| Axillary artery left | 4 (2) | 4 (4) | – | – |
| Brachiocephalic trunk | 3 (2) | 1 (1) | – | 2 (5) |
| Ascending/arch | 72 (40) | 57 (59) | 10 (23) | 5 (13) |
| Femoral | 3 (2) | 1 (1) | 1 (2) | 1 (3) |
| Perfusion | ||||
| Selective cerebral | 178 (100) | 96 (100) | 43 (100) | 39 (100) |
| Selective left axillary artery | 73 (41) | 32 (33) | 20 (47) | 21 (54) |
| Selective distal | 51 (29) | 25 (26) | 12 (28) | 14 (36) |
| Aortic valve | ||||
| Repair | 65 (37) | 60 (63) | 3 (7) | 2 (5) |
| Replacement | 24 (14) | 13 (14) | 9 (21) | 2 (5) |
| Root replacement | ||||
| Partial | 39 (22) | 37 (39) | 2 (5) | – |
| Total | 18 (10) | 11 (12) | 7 (16) | – |
| Ascending aorta replacement | 163 (92) | 96 (100) | 37 (86) | 30 (77) |
| E-vita Open stent graft, mm | ||||
| Diameter, mean ± SD | 5 (3) | 26 ±3 | 27 ±4 | 32 ±6 |
| Length | 112 (63) | 1 (1) | 1 (2) | 3 (8) |
| <130 | 61 (34) | 67 (70) | 25 (58) | 20 (51) |
| 130 | 28 (29) | 17 (40) | 16 (41) | |
| >130 | ||||
| Proximal E-vita fixation | ||||
| Zone 3 | 95 (53) | 58 (60) | 20 (47) | 17 (44) |
| Zone 2 | 76 (43) | 34 (35) | 21 (49) | 21 (54) |
| Zone 1 | 4 (2) | 2 (2) | 1 (2) | 1 (3) |
| Zone 0 | 3 (2) | 2 (2) | 1 (2) | – |
| Head-vessel reimplantation | ||||
| Enbloc | 95 (53) | 49 (51) | 22 (51) | 24 (62) |
| Separately | 83 (47) | 47 (49) | 21 (49) | 15 (38) |
| Left subclavian artery rerouting | ||||
| Aortic–subclavian graft | 42 (24) | 19 (45) | 12 (29) | 11 (28) |
| Aortic–axillary graft | 39 (22) | 20 (21) | 11 (26) | 8 (21) |
| Carotid–subclavian graft | 3 (2) | – | – | 3 (8) |
| Combined procedures | ||||
| Endovascular aortic/arterial repair | 29 (16) | 19 (20) | 9 (21) | 1 (3) |
| Coronary artery bypass graft | 46 (26) | 27 (28) | 5 (12) | 14 (36) |
| Mitral valve repair | 8 (5) | 3 (3) | 3 (7) | 2 (5) |
| Tricuspid valve repair | 2 (1) | — | 2 (4) | — |
AD: aortic dissection; SD: standard deviation.
Overall 30-day and in-hospital mortality rates were 10% and 11%, respectively; AAD 10% and 12%, CAD 7% and 7%, TAA 13% and 15%. In univariable analysis within the pre- and intraoperative variables, low left ventricular ejection fraction (P = 0.021), peripheral artery disease (P = 0.050), chronic obstructive pulmonary disease (P = 0.022), severely compromised haemodynamics (P = 0.003) and duration of CPB (P = 0.012) correlated significantly with the number of in-hospital deaths. Compromised haemodynamics (OR 6.952, 95% CI 2.175–22.213, P = 0.001) and CPB duration (OR 2.096, 95% CI 1.147–3.828, P = 0.016) were significant independent risk factors for in-hospital deaths. The operative times are presented in Table 3. Causes for in-hospital deaths were cardiac failure (n = 6), visceral complications (n= 8) including intestinal/pancreas/liver ischaemia, pulmonary failure (n = 2), pulmonary embolism (n = 1), ischaemic stroke (n = 1), intracerebral bleeding (n = 1) and aorto-oesophageal fistula (n = 1). Postoperative results are presented in Table 4.
Operative times (mean ± SD) according to level of frozen elephant trunk fixation
| Arch level . | Zone 3 versus Zone ≤ 2 . | Zone ≤ 2 versus Zone ≤ 2 + SDP . | |||
|---|---|---|---|---|---|
| Minutes, mean ± SD . | Zone 3 . | Zone ≤ 2 . | P-value . | Zone ≤ 2 + SDP . | P-value . |
| (N = 95) . | (N = 40) . | . | (N = 43) . | . | |
| Cardiopulmonary bypass | 252 ± 61 | 254 ± 52 | 0.823 | 239 ± 34 | 0.135 |
| Cardioplegic arrest | 147 ± 35 | 126 ± 43 | 0.003 | 120 ± 30 | 0.509 |
| Selective cerebral perfusion | 68 ± 18 | 57 ± 13 | <0.001 | 58 ± 13 | 0.561 |
| Visceral ischaemia | 72 ± 23 | 59 ± 15 | <0.001 | 35 ± 13 | <0.001 |
| Arch level . | Zone 3 versus Zone ≤ 2 . | Zone ≤ 2 versus Zone ≤ 2 + SDP . | |||
|---|---|---|---|---|---|
| Minutes, mean ± SD . | Zone 3 . | Zone ≤ 2 . | P-value . | Zone ≤ 2 + SDP . | P-value . |
| (N = 95) . | (N = 40) . | . | (N = 43) . | . | |
| Cardiopulmonary bypass | 252 ± 61 | 254 ± 52 | 0.823 | 239 ± 34 | 0.135 |
| Cardioplegic arrest | 147 ± 35 | 126 ± 43 | 0.003 | 120 ± 30 | 0.509 |
| Selective cerebral perfusion | 68 ± 18 | 57 ± 13 | <0.001 | 58 ± 13 | 0.561 |
| Visceral ischaemia | 72 ± 23 | 59 ± 15 | <0.001 | 35 ± 13 | <0.001 |
SD: standard deviation; SDP: selective distal perfusion.
Operative times (mean ± SD) according to level of frozen elephant trunk fixation
| Arch level . | Zone 3 versus Zone ≤ 2 . | Zone ≤ 2 versus Zone ≤ 2 + SDP . | |||
|---|---|---|---|---|---|
| Minutes, mean ± SD . | Zone 3 . | Zone ≤ 2 . | P-value . | Zone ≤ 2 + SDP . | P-value . |
| (N = 95) . | (N = 40) . | . | (N = 43) . | . | |
| Cardiopulmonary bypass | 252 ± 61 | 254 ± 52 | 0.823 | 239 ± 34 | 0.135 |
| Cardioplegic arrest | 147 ± 35 | 126 ± 43 | 0.003 | 120 ± 30 | 0.509 |
| Selective cerebral perfusion | 68 ± 18 | 57 ± 13 | <0.001 | 58 ± 13 | 0.561 |
| Visceral ischaemia | 72 ± 23 | 59 ± 15 | <0.001 | 35 ± 13 | <0.001 |
| Arch level . | Zone 3 versus Zone ≤ 2 . | Zone ≤ 2 versus Zone ≤ 2 + SDP . | |||
|---|---|---|---|---|---|
| Minutes, mean ± SD . | Zone 3 . | Zone ≤ 2 . | P-value . | Zone ≤ 2 + SDP . | P-value . |
| (N = 95) . | (N = 40) . | . | (N = 43) . | . | |
| Cardiopulmonary bypass | 252 ± 61 | 254 ± 52 | 0.823 | 239 ± 34 | 0.135 |
| Cardioplegic arrest | 147 ± 35 | 126 ± 43 | 0.003 | 120 ± 30 | 0.509 |
| Selective cerebral perfusion | 68 ± 18 | 57 ± 13 | <0.001 | 58 ± 13 | 0.561 |
| Visceral ischaemia | 72 ± 23 | 59 ± 15 | <0.001 | 35 ± 13 | <0.001 |
SD: standard deviation; SDP: selective distal perfusion.
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| New stroke | 17 (10) | 7 (7) | 5 (12) | 5 (13) |
| Permanent | 12 (7) | 5 (5) | 3 (7) | 4 (10) |
| Transient | 5 (3) | 2 (2) | 2 (5) | 1 (3) |
| Spinal injury | 11 (6) | 5 (5) | 5 (12) | 1 (3) |
| Paraplegia | 5 (3) | 2 (2) | 2 (5) | 1 (3) |
| Paraparesis | 4 (2) | 2 (2) | 2 (5) | — |
| No residuals | 2 (1) | 1 (1) | 1 (2) | — |
| Temporary continuous venovenous haemodiafiltration | 56 (32) | 39 (41) | 11 (26) | 6 (15) |
| Re-exploration | 21 (12) | 12 (13) | 6 (14) | 3 (8) |
| Distal ischaemiaa | 22 (12) | 18 (19) | 3 (7) | 1 (3) |
| Ventilation > 72 h | 77 (43) | 48 (50) | 16 (37) | 13 (33) |
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| New stroke | 17 (10) | 7 (7) | 5 (12) | 5 (13) |
| Permanent | 12 (7) | 5 (5) | 3 (7) | 4 (10) |
| Transient | 5 (3) | 2 (2) | 2 (5) | 1 (3) |
| Spinal injury | 11 (6) | 5 (5) | 5 (12) | 1 (3) |
| Paraplegia | 5 (3) | 2 (2) | 2 (5) | 1 (3) |
| Paraparesis | 4 (2) | 2 (2) | 2 (5) | — |
| No residuals | 2 (1) | 1 (1) | 1 (2) | — |
| Temporary continuous venovenous haemodiafiltration | 56 (32) | 39 (41) | 11 (26) | 6 (15) |
| Re-exploration | 21 (12) | 12 (13) | 6 (14) | 3 (8) |
| Distal ischaemiaa | 22 (12) | 18 (19) | 3 (7) | 1 (3) |
| Ventilation > 72 h | 77 (43) | 48 (50) | 16 (37) | 13 (33) |
Only two patients required dialysis following long-term visceral and/or peripheral ischaemia.
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| New stroke | 17 (10) | 7 (7) | 5 (12) | 5 (13) |
| Permanent | 12 (7) | 5 (5) | 3 (7) | 4 (10) |
| Transient | 5 (3) | 2 (2) | 2 (5) | 1 (3) |
| Spinal injury | 11 (6) | 5 (5) | 5 (12) | 1 (3) |
| Paraplegia | 5 (3) | 2 (2) | 2 (5) | 1 (3) |
| Paraparesis | 4 (2) | 2 (2) | 2 (5) | — |
| No residuals | 2 (1) | 1 (1) | 1 (2) | — |
| Temporary continuous venovenous haemodiafiltration | 56 (32) | 39 (41) | 11 (26) | 6 (15) |
| Re-exploration | 21 (12) | 12 (13) | 6 (14) | 3 (8) |
| Distal ischaemiaa | 22 (12) | 18 (19) | 3 (7) | 1 (3) |
| Ventilation > 72 h | 77 (43) | 48 (50) | 16 (37) | 13 (33) |
| . | Overall . | Acute AD . | Chronic AD . | Aneurysm . |
|---|---|---|---|---|
| N (%) . | N (%) . | N (%) . | N (%) . | |
| (N = 178) . | (N = 96) . | (N = 43) . | (N = 39) . | |
| New stroke | 17 (10) | 7 (7) | 5 (12) | 5 (13) |
| Permanent | 12 (7) | 5 (5) | 3 (7) | 4 (10) |
| Transient | 5 (3) | 2 (2) | 2 (5) | 1 (3) |
| Spinal injury | 11 (6) | 5 (5) | 5 (12) | 1 (3) |
| Paraplegia | 5 (3) | 2 (2) | 2 (5) | 1 (3) |
| Paraparesis | 4 (2) | 2 (2) | 2 (5) | — |
| No residuals | 2 (1) | 1 (1) | 1 (2) | — |
| Temporary continuous venovenous haemodiafiltration | 56 (32) | 39 (41) | 11 (26) | 6 (15) |
| Re-exploration | 21 (12) | 12 (13) | 6 (14) | 3 (8) |
| Distal ischaemiaa | 22 (12) | 18 (19) | 3 (7) | 1 (3) |
| Ventilation > 72 h | 77 (43) | 48 (50) | 16 (37) | 13 (33) |
Only two patients required dialysis following long-term visceral and/or peripheral ischaemia.
Follow-up
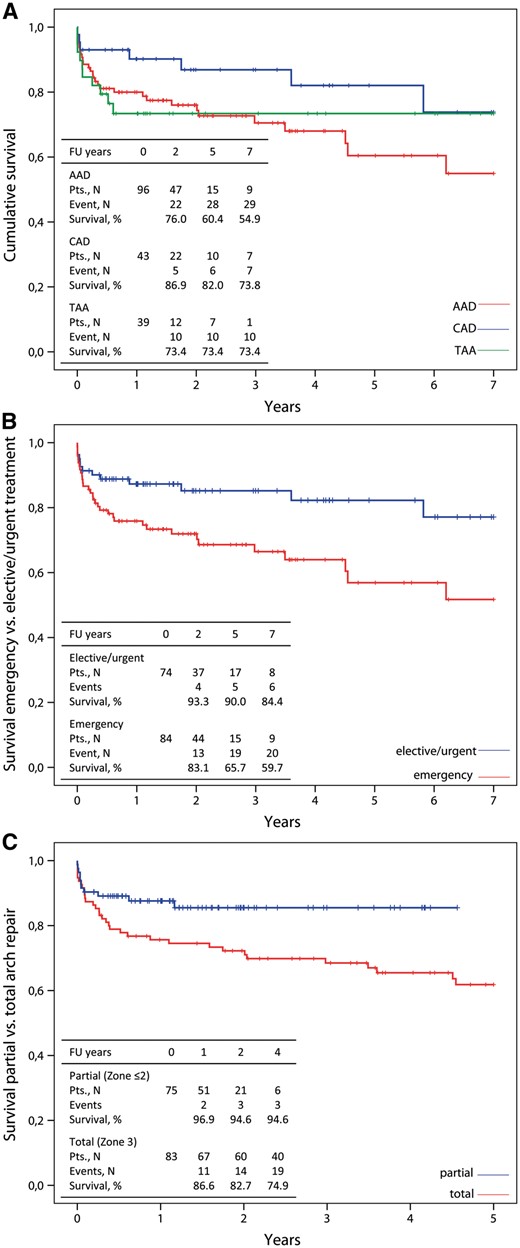
Kaplan–Meier curves showing cumulative survival (A) in acute aortic dissection (AAD), chronic aortic dissection (CAD) and thoracic aortic aneurysm (TAA) and with emergency treatment (B) and with the arch repair technique (C) in follow-up.
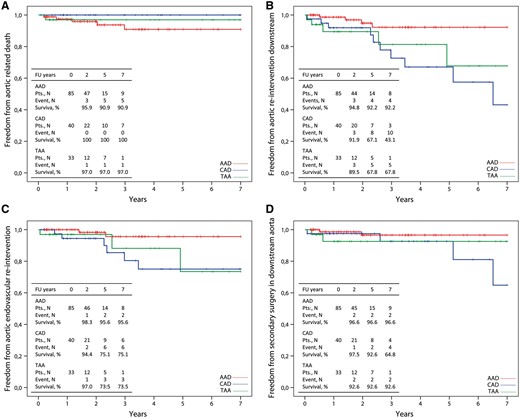
Kaplan–Meier curves of freedom from aortic-related death (A), freedom from reintervention in the downstream aorta (B), endovascularly (C) or surgically (D) during the follow-up period.
Aortic results
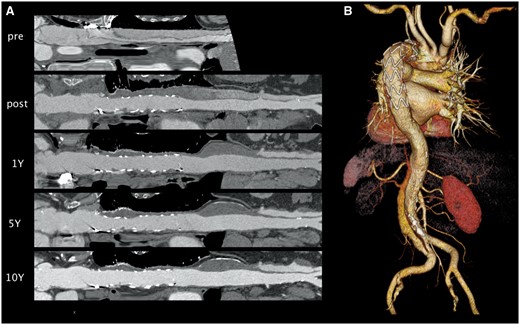
3D-multiplanar reconstruction (A) analysis illustrates the durable aortic treatment combined with positive remodelling in acute aortic dissection 10 years after implantation of the E-vita Open stent graft (B).
Freedom from secondary intervention in the downstream aorta was significantly improved in AAD (92%) compared with CAD (43%, P = 0.002) and TAA (68%, P = 0.031) patients (Fig. 2B). Freedom from endovascular intervention was 96% in AAD, 75% in CAD and 74% in TAA cases (Fig. 2C). Freedom from endovascular intervention in AAD was significantly better compared with CAD (P = 0.016) but not to TAA (P = 0.067). Freedom from secondary surgery along the downstream aorta was increased in AAD (97%) and TAA (93%) compared with CAD (65%) but was not significant (P = 0.091 and P = 0.634, respectively) (Fig. 2D). Type B AD, Marfan syndrome, stent graft diameter and negative remodelling at the level of the stent graft were identified as independent risk factors for reintervention during follow-up.
DISCUSSION
The classic surgical treatment of multisegmental thoracic aortic disease provides durability but requires two major operations via median sternotomy and left lateral thoracotomy [14, 15] that are associated with substantial morbidity and mortality [16]. In contrast, minimally invasive endovascular techniques allow the exclusion of descending aortic pathologies at low risk, but bear the risk of aorta-related complications over time such as endoleakage, stent graft migration, aortic penetration caused by bare springs or dissection and the necessity of reinterventions due to the progression of aortic disease [17, 18]. Commonly, the short proximal landing zone in the distal arch represents a limitation for retrograde endovascular treatment. The combination of both techniques led to the evolution of the FET procedure, which has the advantage of relying only on a median sternotomy.
The FET technique combines the safe and durable replacement of the ascending aortic arch with a polyester prosthesis, secured by a surgical suture line in the distal arch at the seamless transition to the integrated covered stent graft for exclusion of descending thoracic aortic disease. This procedure provides a stable reconstruction as demonstrated by our experience with the E-vita Open stent graft in our institution without any complications from the proximal ascending aorta down to the stent graft end, except for one type II endoleak in one chronic dissection, in which the connection between the dissected LSA and the descending aorta was left in place after semicircular arch resection [19]. Durable exclusion of the descending aorta disease by the stent-grafted FET portion was achieved in almost 90% of cases throughout a mean follow-up period of 3 years. Results were better in patients with acute AD and aneurysm, whereas the false lumen remained partially patent around the distal stent graft portion in 23% of CAD patients. However, the progression of false lumen enlargement in such patients could be stopped in 82% by moving the main residual chronic diseased area into the distal half of the descending aorta.
In acute AD, the immediate interruption of perfusion of the false lumen led not only to false lumen thrombosis but also to positive remodelling of the aorta up to complete obliteration of the false lumen in 92% and to freedom from endovascular or surgical reintervention downstream in more than 95%. Comparing this result with the reported 90% incidence of residual false lumen perfusion after isolated proximal aortic repair in acute type I AD patients and the resulting risk for downstream reintervention and aneurysmal growth of >55 mm in up to 40% [20] of patients, we suggest a more liberal use of extensive thoracic aortic repair, taking into account our low in-hospital death rate in such patients and the 91% freedom from aortic-related death. Due to the complexity of our patient population, the 7-year survival rate of 55% is satisfactory though not ideal [21, 22]. According to the Penn classification [13], the long-term survival rate varied from 85% in Penn A to 27% in Penn BC patients.
In chronic AD, residual false lumen perfusion distally has to be preserved in the case of false lumen-dependant organ perfusion and thus represents a risk for reintervention [6]. The remodelling process looks different compared with that with acute AD. The intimal wall is thicker and stiff, limiting the expansion of the true lumen. Over time, the entry margins degenerate to orifices like communication leaks feeding an established pathway for the false lumen and organ perfusion. In addition, the expansion of the false lumen cavity and the stiffness of the outer aortic wall limit the shrinkage of the false lumen. However, the exclusion of proximal entries interrupts antegrade perfusion of the false lumen in several cases, thus reducing the blood pathway to side re-entries distally with reduction of the pressure within the false lumen [23], as indicated by stable, positive remodelling distally to the stent graft in 60% of chronic AD patients and avoidance of aortic expansion. Residual false lumen perfusion close to the distal stent graft portion indicates the intensity of retrograde flow, which may increase the risk of enlargement of the false lumen. Thus, the indication for distal aortic reintervention in chronic AD arises from enlargement of the false lumen in the distal descending aorta in 8 out of 10 patients. In the case of enlargement and indication for reintervention, the E-vita Open stent graft could be elegantly used as a safe, easy way to handle the docking place for either endovascular or surgical reintervention, allowing for a simple end-to-end anastomosis with clamping of the proximal stent graft.
In aortic aneurysm, differentiation of the aneurysmal type is essential to estimate the prognosis after the FET technique. A durable exclusion of aneurysmal cavities close to the arch was achieved in 100% of cases. However, sealing the distal stent graft transition to the middle thoracic aorta depends on the ratio of graft to aortic diameter, which cannot always be matched with equal diameter or at a maximum of 10% oversizing, especially in mega-aortic disease. In addition, it depends on the ongoing aortic enlargement. Secondary type Ib endoleaks occurred in two patients treated by stent graft extension. Patients who had the FET procedure as the first treatment for thoraco-abdominal aneurysm are not representative of FET efficacy. Three patients successfully underwent a two-part approach; one died in the interim of aortic rupture. In patients with thoraco-abdominal aortic aneurysm, the FET procedure offers a long landing zone and thereby facilitates a second endovascular treatment. In the case of thoraco-abdominal surgery, the presence of an FET graft allows placement of the cross-clamp at the mid-thoracic level, thus avoiding the complex preparation of the distal arch without endangering the vagus and recurrent nerves. The polyester fabric of the stent graft enables end-to-end anastomosis with a conventional aortic prosthesis similar to that of the classic elephant trunk procedure. Thus, we favour the use of the FET procedure in mega-aortic disease and limit the use of the classic elephant trunk procedure only to aortic dissection patients with a strong dependence on coeliac and superior mesenteric artery perfusion from an entry in the proximal descending aorta or to patients with a small true lumen of <20 mm.
The remaining risk for distal progression of the aortic disease makes close patient follow-up mandatory [24]. Changes in aortic diameters and luminal volumes provide important information about the dynamics of the disease and enable timely planning of reintervention. Thus, freedom from aorta-related death was over 90% including one aortic rupture and two unknown events. Occurrence of aorto-oesophageal or aortopulmonary fistula is associated with poor prognosis, so we strongly recommend avoidance of oversizing.
Although the FET procedure seems to be a promising option for durable one-stage treatment of multisegmental aortic disease and creation of a safe docking zone far removed from the arch for secondary distal reintervention, the results demonstrate that the FET technique represents major surgery with significant complications. The complexity of the operation is demonstrated by the fact that the duration of CPB was associated with worse in-hospital outcome. As a consequence, stepwise acceleration of the arch procedure was achieved during recent years by moving the distal fixation to Zone 2 or even proximally, combined with rerouting of the LSA by an extra-anatomic prosthetic bypass to the left axillary artery and early selective perfusion of the whole body during cardioplegic arrest. Thus, visceral ischaemic time could be reduced to 30 min, and selective antegrade cerebral perfusion time to <60 min. Using this concept, the survival rate could be improved but not to a satisfactory level because of the prolonged duration of CPB. To further reduce this duration, the core temperature for the procedure has been increased from 24 to 28 °C with a cerebral perfusate temperature ranging from 20 to 22 °C during circulatory arrest, thus achieving the required hypothermic protection for the cerebral, spinal and visceral ischaemia period but shortening the required rewarming period by one-quarter. The acceleration of the arch repair using the Zone 2 concept improved the 5-year mid-term survival rate significantly and indicated that the distal anastomosis in Zone 3 is an independent risk factor for mortality. Furthermore, the proximalization of the distal anastomosis resulted in the elimination of recurrent nerve injury. The fact that the FET technique is less invasive makes it safer for high-risk patients such as octogenarians, those with multiple chronic medical conditions and Penn BC acute AD patients. The development of a new three-zone hybrid graft for the treatment of an ascending arch and descending aorta that comprises a proximal polyester graft for anastomosis in the distal ascending aorta followed by insertion of a non-covered stent in one section of the arch and a covered stent in the descending aorta could facilitate and accelerate the treatment of acute type I aortic dissection and complicated type III dissection. This procedure is currently undergoing clinical testing [25].
CONCLUSION
The FET procedure with the E-vita Open graft achieves one-stage treatment of multisegmental disease of the thoracic aorta down to the mid-thoracic level. No material or concept failures have been observed during the follow-up period, making this concept a valuable option to achieve stable results. If the disease progresses further downstream, graft extension either by endovascular or surgical techniques can be easily achieved because the stented portion serves as an ideal docking zone with low rates of morbidity and mortality. We consider this concept to be our current gold standard in the treatment of multisegmental thoracic aortic disease. Any less invasive method to come will have to withstand comparison to the FET strategy.
Conflict of interest: Heinz Jakob is consultant to Jotec GmbH.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr Santer(Vienna, Austria): We have two technical questions. Number one, in chronic dissection with a small true lumen, there is always a thing about measuring the diameter of the stent-graft. So how do you choose that, or which do you choose, or do you even oversize?
The second question is, there are always concerns regarding spinal cord injury in the hybrid stent-graft. In acute dissection, how do you choose the right length of the E-vita Open Plus?
Dr Tsagakis: The true lumen diameter is calculated at the level of the pulmonary bifurcation. We measure the length of the dissected intima in the axial CT image to estimate the aortic diameter and we try to avoid any oversizing, especially in Marfan patients. In some chronic dissection we perform under sizing.
To the second question, we use an E-vita open stent graft with a length of 13 cm since 2008. We tried to evaluate the differences between the 15-cm stent-graft and 13-cm stent-graft according to the changes of the pathology distally, and no difference was found; therefore, we use the 13-cm stent graft routinely. In case of more distal re-entries we place the E-vita Open deeper. The tubular form of the E-vita Open enables this manoeuvre. The stent graft placement is controlled by angioscopy and we can treat more distal pathologies safely.
Dr Bavaria(Philadelphia, PA, USA): The first question is technical. It might have gone past me, but what do you do with the subclavian artery in these cases that you do a Zone 2 on? How do you address that?
Then the second question is, what is the percentage of cases that you use this E-vita on that are acute Type A dissections in your hospital? Is it all? If it is not all, what is the percentage that you use? In other words, it is a decision-making question.
Dr Tsagakis: We have three options for rerouting of the subclavian artery. Intrathoracally, if we have easy access to the left subclavian artery. We study the CT before the surgery and we decide.
Otherwise, we start the procedure at the left axillary artery, also in acute aortic dissection under stable haemodynamics. In this case we use an 8-mm-diameter prosthesis. After the distal anastomosis the graft is introduced intrathoracally via the intercostal space and we perform the proximal anastomosis during the reperfusion. During this period, the left arm is perfused selectively and the perfusion is controlled with a radial arterial line.
In elective cases a carotid–subclavian bypass is a further option. As to the second question, we use E-vita Open in almost 55% of acute Type A dissection.
Dr Etz(Leipzig, Germany): So my question refers to, if I remember correctly, 4 years ago, I was in a lunch symposium, and the initial results presented there had a very high percentage of paraplegia. It was up to 18%. We analysed our data in Leipzig, and we also got up to almost a 20% paraplegia rate.
The two risk factors that we identified was cross-clamp time, and you have sort of similar with CPB time, and it was temperature. We were surprised it was not length of the prosthesis.
Is there a learning curve that makes you faster, that would probably contribute to your very low and excellent paraplegia rates that you have achieved right now, or is there anything else that you do differently than maybe some other people here in the room, including us in the past?
Dr Tsagakis: Generally, we tried to perform the procedure faster and easier, and to start as soon as possible with the distal perfusion. In chronic cases, we use routinely a cerebrofluid spinal drainage for 2 or 3 days after surgery.
We tried to find out risk factors for paraplegia including temperature, distal perfusion, direction of the distal ischaemic time, and also in relation to the stent-graft, the stent-graft length and the landing zone. I cannot give an answer at the moment, but we believe that the combination of fast reperfusion distally and the use of a short graft may reduce the incidence of this very serious complication.
Dr Mascaro (Birmingham, UK): Konstantinos, on the same topic of paraplegia, I saw in one of your slides that since 2011, some of the patients, you have put a cerebrospinal fluid drain?
Dr Tsagakis: Yes, in all elective cases.
Dr Mascaro: In all elective cases?
`Dr Tsagakis: Chronic dissection and aneurysm.
Dr Mascaro: What made you change?
Dr Tsagakis: Changed the paraplegia? What do you mean?
Dr Mascaro: No, you changed from not using cerebrospinal fluid to using cerebrospinal fluid drain.
Dr Tsagakis: What should be changed, the ratio?
Dr Mascaro: No, no. In one of the slides, you showed that cerebrospinal fluid drain was being used since 2011 but not before.
Dr Tsagakis: At this time, we started to monitor the pressure with a pump. This system regulates the drainage and pressure automatically and therefore we have a continuous control. We can adjust the pressure as we want and we have a constant and controlled treatment.
Dr Martens(Hannover, Germany): In our experience, the first patients that had paraplegia using the FET technique, they had long elephant trunks. We used 15-cm grafts, and they did not have cerebrospinal fluid drainage after the operation. So we tried to do cerebrospinal fluid drainage in the acute cases, after the operation, as early as possible.
So could you comment, what do you use in the acute cases, just if you have symptoms; or do you do it routinely postoperatively?
Dr Tsagakis: Cerebrospinal fluid drainage?
Dr Martens: Yes.
Dr Tsagakis: In acute dissection we use a cerebrospinal fluid drainage only in case of symptoms.
Dr G.Belitsis (Brompton, UK): May I ask you, what are your criteria? When do you decide to do such an operation for a Type A?
Also, the second part of the same question, I guess, have you faced complications with distal re-entry points on the descending thoracic and abdominal aorta causing retrograde bleeds on the anastomosis point of the cuff? Do you consider distal re-entry points as a reason not to do this?
Dr Tsagakis: We identify the re-entries position by the angioscope.
We use the E-vita Open if case of re-entry in the distal arch and proximal descending aorta and also in cases with severe true lumen collapse, in order to improve the distal perfusion immediately and to stabilize the true lumen.
We do not use the E-vita Open in the elderly and especially in cases of old patients and severely compromised haemodynamics or other comorbidities. In this case, the treatment should be performed as fast as possible and then we do not use the E-vita Open. Also about the entries, do you mean distal or proximal entries?
Dr Belitsis: The pathology usually involves re-entry points in the descending, usually abdominal aorta and we had cases that we used the device, and retrograde bleeding from that point, the aorta started bleeding backwards. Also, we had bleeding in the anastomosis behind the subclavian. So have you faced that complication?
Dr Tsagakis: No. We do not have any complication at the level of the fixation of the stent-graft during 10 years. We have chronic dissection patients with large re-entries in the abdominal part or also in the distal descending aorta. In these cases, the false lumen may remain patent up to the mid part of the stent graft but not at the level of the proximal fixation.
Author notes
Presented at the 29th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Amsterdam, Netherlands, 3–7 October 2015.




