-
PDF
- Split View
-
Views
-
Cite
Cite
Antonio Miceli, Daniyar Gilmanov, Michele Murzi, Federica Marchi, Matteo Ferrarini, Alfredo G. Cerillo, Eugenio Quaini, Marco Solinas, Sergio Berti, Mattia Glauber, Minimally invasive aortic valve replacement with a sutureless valve through a right anterior mini-thoracotomy versus transcatheter aortic valve implantation in high-risk patients, European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 3, March 2016, Pages 960–965, https://doi.org/10.1093/ejcts/ezv210
Close - Share Icon Share
Abstract
The aim of this study was to compare early outcomes and mid-term survival of high-risk patients undergoing minimally invasive aortic valve replacement through right anterior mini-thoracotomy (RT) with sutureless valves versus patients undergoing transcatheter aortic valve implantation (TAVI) for severe aortic stenosis.
From October 2008 to March 2013, 269 patients with severe aortic stenosis underwent either RT with perceval S sutureless valves (n = 178 patients, 66.2%) or TAVI (n = 91, 33.8%: 44 transapical and 47 trans-femoral). Of these, 37 patients undergoing RT with the perceval S valve were matched to a TAVI group by the propensity score.
Baseline characteristics were similar in both groups (mean age 79 ± 6 years) and the median logistic EuroSCORE was 14% (range 9–20%). In the matched group, the in-hospital mortality rate was 8.1% (n = 3) in the TAVI group and 0% in the RT group (P = 0.25). The incidence rate of stroke was 5.4% (n = 2) versus 0% in the TAVI and RT groups (P = 0.3). In the TAVI group, 37.8% (n = 14) had mild paravalvular leakage (PVL) and 27% (n = 10) had moderate PVL, whereas 2.7% (n = 1) had mild PVL in the RT group (P < 0.001). One- and 2-year survival rates were 91.6 vs 78.6% and 91.6 vs 66.2% in patients undergoing RT with the perceval S sutureless valve compared with those undergoing TAVI, respectively (P = 0.1).
Minimally invasive aortic valve replacement with perceval S sutureless valves through an RT is associated with a trend of better early outcomes and mid-term survival compared with TAVI.
INTRODUCTION
Aortic valve stenosis (AS) is the most common valvular heart disease in the elderly population and if untreated, it is associated with poor outcomes [1]. In patients with an acceptable operative risk profile, aortic valve replacement (AVR) represents the gold standard treatment for AS with excellent mortality, morbidity and long-term survival [2, 3]. Nevertheless, 30–40% of patients with severe AS are denied the surgical procedure owing to high surgical risk for advanced age and multiple comorbidities [4]. In this setting, transcatheter aortic valve implantation (TAVI) offers an alternative treatment option in high-risk patients, having demonstrated to be superior to medical therapy in non-operable patients and non-inferior to surgical AVR [5–6]. However, controversies still exist regarding its effect on postoperative outcomes when compared with conventional surgery. A recent meta-analysis that included 3465 patients with severe AS found no significant differences between TAVI and conventional AVR in terms of mortality, stroke and myocardial infarction. Conversely, subanalysis of randomized, controlled trials showed a higher incidence of neurological events, vascular complications, permanent pacemaker implantation and aortic regurgitation in patients undergoing TAVI [7].
In recent years, minimally invasive AVR has gained consensus among surgeons and compared with conventional surgery, it reduces postoperative mortality and morbidity, especially in high-risk patients [8, 9]. Among minimally invasive AVR techniques, right anterior minithoracomy (RT) has shown to be a safe and reproducible approach associated with a low rate of postoperative complications and good mid-term survival [10, 11]. On the other hand, a drawback of RT is the longer operative times, which might have a negative impact on very old and fragile patients [12]. In this setting, sutureless aortic valve has been considered an additional therapeutic option for the treatment of high-risk patients, as it reduces procedural times and facilitates the minimally invasive approach [13–15]. Recently, we have reported excellent surgical results with RT using the perceval S sutureless (Sorin, Salluggia, Italy) valve, raising the hypothesis that this technique might be considered the ‘real alternative’ to the TAVI procedure in high-risk operable patients [15]. Therefore, the aim of this study was to compare early outcomes and mid-term survival in high-risk patients undergoing minimally invasive AVR through RT with the perceval S sutureless valve versus patients undergoing TAVI.
MATERIALS AND METHODS
The local Ethical Committee approved the study and individual consent was waived. This is a retrospective, observational, cohort study of prospectively collected data from 810 consecutive patients who underwent isolated AVR at our institution between October 2008 and March 2013. The data collection form is entered in a local database and includes three sections that are filled consecutively by cardiac surgeons, anaesthetists and perfusionists involved in the care of the patients. Exclusion criteria were patients undergoing full sternotomy (n = 195) or ministernotomy (n = 168). Other exclusion criteria were: active infective endocarditis (n = 6), the presence of a porcelain aorta (n = 3), bicuspid aortic valve (n = 26), patients undergoing RT with other valves than the perceval S (n = 142) valve and those in critical preoperative state (n = 1) defined as any one or more of the following: ventricular arrhythmia, cardiac massage or aborted sudden death, ventilation before arrival in the anaesthetic room, acute renal failure and inotropic support. The final sample contained detailed clinical information about 269 patients, of whom 178 (66.2%) underwent AVR with the perceval S valve through RT and 91 (33.8%) received TAVI (Sapien; Edwards Lifescience, Inc., Irvine, CA, USA) through either a transapical (n = 44, 48.3%) or trans-femoral approach (n = 47, 51.6%). The decision to perform TAVI or RT with the Perceval S valve was based mainly on a careful evaluation of each patient's preoperative characteristics, the logistic EuroSCORE and clinical observation. Patients considered at higher risk for conventional surgery were discussed in the Heart Team at the multidisciplinary meeting for consideration of TAVI. Preoperative risk factors were defined according to the logistic EuroSCORE. Postoperative outcomes were defined according to the Valve Academic Research Consortium II definitions [16]. Before discharge, all patients underwent transthoracic echocardiography. The grade of paravalvular leakage (PVL) with aortic regurgitation was determined from the colour Doppler imaging findings and classified into four grades: trivial, 1 of 4; mild, 2 of 4; moderate, 3 of 4; and severe, 4 of 4. All patients were seen 8–12 weeks postoperatively and, thereafter, were contacted for follow-up every year up to March 2013. The median follow-up period was 13 (interquartile range 7–25) months and data were 97% complete.
Preoperative planning and surgical techniques
Preoperative planning and surgical techniques have been described previously and are summarized here [11, 18]. In brief, all patients planned for surgery underwent a 64-slice computed tomographic scan (Toshiba Aquilon; Toshiba Medical System, Tokyo, Japan) to evaluate the anatomical relationship among intercostal spaces, the ascending aorta and aortic valve. Minimally invasive AVR via RT was performed through a skin incision of 5–7 cm placed at the level of the second intercostal space without rib resection. Direct aortic cannulation was performed using a flexible cannula (Easyflow, Sorin) and venous drainage was achieved using a BioMedicus Multistage (Medtronic, Inc., Minneapolis, MN, USA) inserted through the femoral vein into the right atrium with the Seldinger technique and under transoesophageal echocardiographic guidance. A left ventricular vent was placed through the right superior pulmonary vein and patients were cooled to 34°C. The ascending aorta was clamped with the aortic Glauber clamp (Cardiomedical GmbH, Langenhagen, Germany, distributed by Sorin, Salluggia, Italy) and antegrade cardioplegic solution was instilled into the aortic root or selectively into the coronary ostia using warm blood cardioplegia. Transverse aortotomy was performed 2 cm higher than conventional aortotomy. The diseased valve was removed, the aortic annulus thoroughly decalcified and sized with an ad hoc sizer. Three guiding 4/0 Prolene sutures were placed at the nadir point of each valve sinus. The perceval S valve was collapsed and connected to the guiding sutures through the bottom holes placed on the inflow ring of the valve. Once the valve is parachuted into the aortic root, the valve is deployed and expanded with warm saline solution with a balloon. Afterwards, the guiding sutures are removed and the valve is checked in its correct position. Patients planned for TAVI underwent full work-up, including lung functional test, transthoracic and transoesophageal echocardiography, enhanced computed tomographic scanning for an accurate assessment of the aortic annulus, aorta and peripheral vessels for the selection of the TAVI procedure. All procedures were performed under general anaesthesia and transoesophageal echocardiographic guidance in the catheterization laboratory or the operating room depending on whether a trans-femoral or transapical approach was employed, respectively. The transapical approach was performed through a small intercostal incision over the left ventricular apex.
The SAPIEN valve was crimped onto a balloon catheter and advanced across the native aortic valve. After rapid ventricular pacing, the valve was delivered to the site of the native stenotic valve. Immediately after TAVI, aortography was performed to assess the location and degree of aortic regurgitation and patency of the coronary arteries. Patients in both groups underwent clinical and echocardiographic assessment at hospital discharge. Paravalvular leakage was classified into four grades: trivial (1/4), mild (2/4), moderate (3/4) and severe (4/4).
Statistical analysis
Continuous data were expressed as mean ± standard deviation, and categorical data as percentages. The Kolmogorov–Smirnov test was used to check for normality of data in the two groups before further analysis. Differences between the two groups were compared with the use of a χ2 test for categorical variables and independent sample t-test or Wilcoxon rank-sum tests, as appropriate, for continuous variables. To reduce the effect of selection bias and potential confounding, we develop a propensity score analysis. The propensity for the RT approach associated with the sutureless perceval S valve was built using a non-parsimonious multiple logistic regression analysis. All the variables listed in Table 1 and year of surgery were included in the analysis. The 5→1 digit matching was used to identify matched patients in a 1:1 manner without replacement. Specifically, we matched each patient who underwent RT with the Perceval S valve to one who received TAVI who had a propensity score that was identical to five digits. If this could not be done, we then proceeded to the next highest digit match (4-, 3-, 2- and 1-digit) to make the best matches, in a hierarchical sequence until no more matches could be made. After the propensity score match was performed, differences between the two groups were assessed with the paired t-test or the Wilcoxon signed-rank test for continuous variables, and McNemars's test or the marginal homogeneity test for categorical variables. Standardized differences (d) have been estimated to evaluate the balance between variables before and after matching. Results are reported as percentages. Survival curves were compared with a stratified log-rank test for the matched pairs and effect size is reported as the hazard ratio and 95% confidence intervals. All reported P-values are two-sided, and P-values of <0.05 were considered to indicate statistical significance. All statistical analysis was performed with SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA).
| Variables . | RT (n = 178) . | TAVI (n = 91) . | d . | P . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 76.1 ± 6.7 | 79.5 ± 6.4 | 42.97 | <0.001 |
| Female [patients, n (%)] | 118 (66.3) | 46 (50.5) | 26.05 | 0.018 |
| COPD [patients, n (%)] | 32 (18) | 31 (34.1) | 29.31 | 0.005 |
| Hypertension [patients, n (%)] | 151 (84.8) | 78 (85.7) | 2.03 | 0.99 |
| Diabetes mellitus [patients, n (%)] | 43 (24.2) | 21 (23.1) | −2.07 | 0.96 |
| NYHA III–IV functional class [patients, n (%)] | 52 (29.2) | 58 (63.7) | 59.40 | <0.001 |
| Ejection fraction (mean ± SD) | 57.7 ± 9 | 50 ± 10.4 | −63.40 | <0.001 |
| Extracardiac vasculopathy [patients, n (%)] | 32 (18) | 33 (36.3) | 32.95 | 0.002 |
| Previous cardiac surgery [patients, n (%)] | 3 (1.7) | 26 (28.6) | 58.02 | <0.001 |
| Serum creatinine (mg/dl ± SD) | 1.1 ± 0.4 | 1.2 ± 0.9 | 15.39 | 0.14 |
| Pulmonary hypertension [patients, n (%)] | 22 (12.4) | 15 (16.5) | 9.37 | 0.46 |
| Logistic EuroSCORE | ||||
| Median, IQR | 7.9 (5–12.3) | 16 (12–31) | 77.62 | <0.0001 |
| Mean ± SD | 10.2 ± 7.8 | 22.4 ± 14.7 | ||
| Variables . | RT (n = 178) . | TAVI (n = 91) . | d . | P . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 76.1 ± 6.7 | 79.5 ± 6.4 | 42.97 | <0.001 |
| Female [patients, n (%)] | 118 (66.3) | 46 (50.5) | 26.05 | 0.018 |
| COPD [patients, n (%)] | 32 (18) | 31 (34.1) | 29.31 | 0.005 |
| Hypertension [patients, n (%)] | 151 (84.8) | 78 (85.7) | 2.03 | 0.99 |
| Diabetes mellitus [patients, n (%)] | 43 (24.2) | 21 (23.1) | −2.07 | 0.96 |
| NYHA III–IV functional class [patients, n (%)] | 52 (29.2) | 58 (63.7) | 59.40 | <0.001 |
| Ejection fraction (mean ± SD) | 57.7 ± 9 | 50 ± 10.4 | −63.40 | <0.001 |
| Extracardiac vasculopathy [patients, n (%)] | 32 (18) | 33 (36.3) | 32.95 | 0.002 |
| Previous cardiac surgery [patients, n (%)] | 3 (1.7) | 26 (28.6) | 58.02 | <0.001 |
| Serum creatinine (mg/dl ± SD) | 1.1 ± 0.4 | 1.2 ± 0.9 | 15.39 | 0.14 |
| Pulmonary hypertension [patients, n (%)] | 22 (12.4) | 15 (16.5) | 9.37 | 0.46 |
| Logistic EuroSCORE | ||||
| Median, IQR | 7.9 (5–12.3) | 16 (12–31) | 77.62 | <0.0001 |
| Mean ± SD | 10.2 ± 7.8 | 22.4 ± 14.7 | ||
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; IQR: interquartile range; SD: standard deviation; d: standardized differences; TAVI: transcatheter aortic valve implantation; RT: right anterior minithoracomy.
| Variables . | RT (n = 178) . | TAVI (n = 91) . | d . | P . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 76.1 ± 6.7 | 79.5 ± 6.4 | 42.97 | <0.001 |
| Female [patients, n (%)] | 118 (66.3) | 46 (50.5) | 26.05 | 0.018 |
| COPD [patients, n (%)] | 32 (18) | 31 (34.1) | 29.31 | 0.005 |
| Hypertension [patients, n (%)] | 151 (84.8) | 78 (85.7) | 2.03 | 0.99 |
| Diabetes mellitus [patients, n (%)] | 43 (24.2) | 21 (23.1) | −2.07 | 0.96 |
| NYHA III–IV functional class [patients, n (%)] | 52 (29.2) | 58 (63.7) | 59.40 | <0.001 |
| Ejection fraction (mean ± SD) | 57.7 ± 9 | 50 ± 10.4 | −63.40 | <0.001 |
| Extracardiac vasculopathy [patients, n (%)] | 32 (18) | 33 (36.3) | 32.95 | 0.002 |
| Previous cardiac surgery [patients, n (%)] | 3 (1.7) | 26 (28.6) | 58.02 | <0.001 |
| Serum creatinine (mg/dl ± SD) | 1.1 ± 0.4 | 1.2 ± 0.9 | 15.39 | 0.14 |
| Pulmonary hypertension [patients, n (%)] | 22 (12.4) | 15 (16.5) | 9.37 | 0.46 |
| Logistic EuroSCORE | ||||
| Median, IQR | 7.9 (5–12.3) | 16 (12–31) | 77.62 | <0.0001 |
| Mean ± SD | 10.2 ± 7.8 | 22.4 ± 14.7 | ||
| Variables . | RT (n = 178) . | TAVI (n = 91) . | d . | P . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 76.1 ± 6.7 | 79.5 ± 6.4 | 42.97 | <0.001 |
| Female [patients, n (%)] | 118 (66.3) | 46 (50.5) | 26.05 | 0.018 |
| COPD [patients, n (%)] | 32 (18) | 31 (34.1) | 29.31 | 0.005 |
| Hypertension [patients, n (%)] | 151 (84.8) | 78 (85.7) | 2.03 | 0.99 |
| Diabetes mellitus [patients, n (%)] | 43 (24.2) | 21 (23.1) | −2.07 | 0.96 |
| NYHA III–IV functional class [patients, n (%)] | 52 (29.2) | 58 (63.7) | 59.40 | <0.001 |
| Ejection fraction (mean ± SD) | 57.7 ± 9 | 50 ± 10.4 | −63.40 | <0.001 |
| Extracardiac vasculopathy [patients, n (%)] | 32 (18) | 33 (36.3) | 32.95 | 0.002 |
| Previous cardiac surgery [patients, n (%)] | 3 (1.7) | 26 (28.6) | 58.02 | <0.001 |
| Serum creatinine (mg/dl ± SD) | 1.1 ± 0.4 | 1.2 ± 0.9 | 15.39 | 0.14 |
| Pulmonary hypertension [patients, n (%)] | 22 (12.4) | 15 (16.5) | 9.37 | 0.46 |
| Logistic EuroSCORE | ||||
| Median, IQR | 7.9 (5–12.3) | 16 (12–31) | 77.62 | <0.0001 |
| Mean ± SD | 10.2 ± 7.8 | 22.4 ± 14.7 | ||
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; IQR: interquartile range; SD: standard deviation; d: standardized differences; TAVI: transcatheter aortic valve implantation; RT: right anterior minithoracomy.
RESULTS
Among 269 patients in the study, 178 (66.2%) underwent isolated AVR via RT with the perceval S sutureless valve (RT perceval S group) and 91 (33.8%, control group) received a SAPIEN (TAVI group) through either a transapical (n = 44, 48.3%) or trans-femoral approach (n = 47, 51.6%). Baseline characteristics of the study population are presented in Table 1. Compared with the TAVI group, the patients in the RT perceval S group were younger and had a higher prevalence of female gender; these were more likely to have lower New York Heart Association (NYHA) functional class and better left ventricular ejection fraction. Moreover, RT perceval S patients were also more likely to have lower prevalence of chronic obstructive pulmonary disease, extracardiac arteriopathy and pulmonary hypertension. Finally, as a result of a higher risk profile, patients undergoing TAVI procedures had higher logistic EuroSCORE. After performing propensity score analysis for the entire population, 37 patients undergoing RT with the perceval S valve were matched to the TAVI group. In the matched cohorts, preoperative characteristics were similar in both groups (Table 2). In the matched population, the in-hospital mortality rate was 8.1% (n = 3) in the TAVI group and 0% in the RT perceval S group (P = 0.25). Two patients died for cardiogenic shock and one for severe bleeding secondary to rupture of the aortic annulus. No valve migration was observed in both groups. Two patients (5.4%) undergoing TAVI required a conversion to full sternotomy for bleeding. Other postoperative outcomes are reported in Table 3. The rates of stroke and transient ischaemic attack were 5.4% (n = 2) and 2.7% (n = 1) in TAVI group, respectively, whereas no neurological events occurred in the RT perceval S group. The length of stay was shorter in the TAVI group but this was related to the fact that the majority of patients undergoing the TF approach did not require a cardiac intensive care unit (Table 3). At discharge, the mean and peak postoperative gradients were 11.4 ± 3.7 vs 10.1 ± 3.4 mmHg (P = 0.17) and 19.2 ± 6.9 vs 19.7 ± 5.4 mmHg (P = 0.26) in the RT perceval S and TAVI groups, respectively.
| Variables . | RT (n = 37) . | TAVI (n = 37) . | d . | P . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 79 ± 4.5 | 78.8 ± 7.4 | −2.01 | 0.92 |
| Female [patients, n (%)] | 24 (69.9) | 22 (59.5) | 4.45 | 0.81 |
| COPD [patients, n (%)] | 8 (21.6) | 11 (29.7) | 9.08 | 0.59 |
| Hypertension [patients, n (%)] | 32 (86.5) | 31 (83.8) | −6.04 | 1 |
| Diabetes mellitus [patients, n (%)] | 10 (27) | 7 (18.9) | −10.2 | 0.62 |
| NYHA III–IV functional class [patients, n (%)] | 25 (67.6) | 22 (59.5) | 3.63 | 0.58 |
| Ejection fraction (mean ± SD) | 52.6 ± 9.7 | 50.6 ± 7.8 | −9.8 | 0.5 |
| Extracardiac vasculopathy [patients, n (%)] | 11 (29.7) | 9 (24.3) | −9.9 | 0.79 |
| Previous cardiac surgery [patients, n (%)] | 3 (8.1) | 3 (8.1) | 0 | 1 |
| Serum creatinine (mg/dl ± SD) | 1.1 ± 0.5 | 1.2 ± 0.9 | 3.05 | 0.87 |
| Pulmonary hypertension [patients, n (%)] | 10 (27) | 9 (24.3) | −5.01 | 0.3 |
| Logistic EuroSCORE | ||||
| Median, IQR | 14.2 (7.3–19.7) | 14 (11.1–21.5) | −8.72 | <0.0001 |
| Mean ± SD | 16.1 ± 11 | 15.7 ± 8.5 | ||
| Variables . | RT (n = 37) . | TAVI (n = 37) . | d . | P . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 79 ± 4.5 | 78.8 ± 7.4 | −2.01 | 0.92 |
| Female [patients, n (%)] | 24 (69.9) | 22 (59.5) | 4.45 | 0.81 |
| COPD [patients, n (%)] | 8 (21.6) | 11 (29.7) | 9.08 | 0.59 |
| Hypertension [patients, n (%)] | 32 (86.5) | 31 (83.8) | −6.04 | 1 |
| Diabetes mellitus [patients, n (%)] | 10 (27) | 7 (18.9) | −10.2 | 0.62 |
| NYHA III–IV functional class [patients, n (%)] | 25 (67.6) | 22 (59.5) | 3.63 | 0.58 |
| Ejection fraction (mean ± SD) | 52.6 ± 9.7 | 50.6 ± 7.8 | −9.8 | 0.5 |
| Extracardiac vasculopathy [patients, n (%)] | 11 (29.7) | 9 (24.3) | −9.9 | 0.79 |
| Previous cardiac surgery [patients, n (%)] | 3 (8.1) | 3 (8.1) | 0 | 1 |
| Serum creatinine (mg/dl ± SD) | 1.1 ± 0.5 | 1.2 ± 0.9 | 3.05 | 0.87 |
| Pulmonary hypertension [patients, n (%)] | 10 (27) | 9 (24.3) | −5.01 | 0.3 |
| Logistic EuroSCORE | ||||
| Median, IQR | 14.2 (7.3–19.7) | 14 (11.1–21.5) | −8.72 | <0.0001 |
| Mean ± SD | 16.1 ± 11 | 15.7 ± 8.5 | ||
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; IQR: interquartile range; SD: standard deviation; d: standardized differences; TAVI: transcatheter aortic valve implantation; RT: right anterior minithoracomy.
| Variables . | RT (n = 37) . | TAVI (n = 37) . | d . | P . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 79 ± 4.5 | 78.8 ± 7.4 | −2.01 | 0.92 |
| Female [patients, n (%)] | 24 (69.9) | 22 (59.5) | 4.45 | 0.81 |
| COPD [patients, n (%)] | 8 (21.6) | 11 (29.7) | 9.08 | 0.59 |
| Hypertension [patients, n (%)] | 32 (86.5) | 31 (83.8) | −6.04 | 1 |
| Diabetes mellitus [patients, n (%)] | 10 (27) | 7 (18.9) | −10.2 | 0.62 |
| NYHA III–IV functional class [patients, n (%)] | 25 (67.6) | 22 (59.5) | 3.63 | 0.58 |
| Ejection fraction (mean ± SD) | 52.6 ± 9.7 | 50.6 ± 7.8 | −9.8 | 0.5 |
| Extracardiac vasculopathy [patients, n (%)] | 11 (29.7) | 9 (24.3) | −9.9 | 0.79 |
| Previous cardiac surgery [patients, n (%)] | 3 (8.1) | 3 (8.1) | 0 | 1 |
| Serum creatinine (mg/dl ± SD) | 1.1 ± 0.5 | 1.2 ± 0.9 | 3.05 | 0.87 |
| Pulmonary hypertension [patients, n (%)] | 10 (27) | 9 (24.3) | −5.01 | 0.3 |
| Logistic EuroSCORE | ||||
| Median, IQR | 14.2 (7.3–19.7) | 14 (11.1–21.5) | −8.72 | <0.0001 |
| Mean ± SD | 16.1 ± 11 | 15.7 ± 8.5 | ||
| Variables . | RT (n = 37) . | TAVI (n = 37) . | d . | P . |
|---|---|---|---|---|
| Age (years, mean ± SD) | 79 ± 4.5 | 78.8 ± 7.4 | −2.01 | 0.92 |
| Female [patients, n (%)] | 24 (69.9) | 22 (59.5) | 4.45 | 0.81 |
| COPD [patients, n (%)] | 8 (21.6) | 11 (29.7) | 9.08 | 0.59 |
| Hypertension [patients, n (%)] | 32 (86.5) | 31 (83.8) | −6.04 | 1 |
| Diabetes mellitus [patients, n (%)] | 10 (27) | 7 (18.9) | −10.2 | 0.62 |
| NYHA III–IV functional class [patients, n (%)] | 25 (67.6) | 22 (59.5) | 3.63 | 0.58 |
| Ejection fraction (mean ± SD) | 52.6 ± 9.7 | 50.6 ± 7.8 | −9.8 | 0.5 |
| Extracardiac vasculopathy [patients, n (%)] | 11 (29.7) | 9 (24.3) | −9.9 | 0.79 |
| Previous cardiac surgery [patients, n (%)] | 3 (8.1) | 3 (8.1) | 0 | 1 |
| Serum creatinine (mg/dl ± SD) | 1.1 ± 0.5 | 1.2 ± 0.9 | 3.05 | 0.87 |
| Pulmonary hypertension [patients, n (%)] | 10 (27) | 9 (24.3) | −5.01 | 0.3 |
| Logistic EuroSCORE | ||||
| Median, IQR | 14.2 (7.3–19.7) | 14 (11.1–21.5) | −8.72 | <0.0001 |
| Mean ± SD | 16.1 ± 11 | 15.7 ± 8.5 | ||
COPD: chronic obstructive pulmonary disease; NYHA: New York Heart Association; IQR: interquartile range; SD: standard deviation; d: standardized differences; TAVI: transcatheter aortic valve implantation; RT: right anterior minithoracomy.
| Variables . | RT (n = 37) . | TAVI (n = 37) . | P . |
|---|---|---|---|
| Mortality [patients, n (%)] | 0 | 3 (8.1) | 0.25 |
| Stroke [patients, n (%)] | 0 | 2 (5.4) | 0.3 |
| Conversion to sternotomy [patients, n (%)] | 0 | 2 (5.4) | 0.3 |
| Major bleeding [patients, n (%)] | 1 (2.7) | 1 (2.7) | 1 |
| Acute kidney injury [patients, n (%)] | 11 (6.2) | 4 (4.4) | 0.54 |
| Intensive care unit stay (median day, range) | 1 (1–2) | 1 (1–1) | 0.5 |
| Ward stay (median day, range) | 7 (6–8) | 4.5 (3–6) | <0.001 |
| Variables . | RT (n = 37) . | TAVI (n = 37) . | P . |
|---|---|---|---|
| Mortality [patients, n (%)] | 0 | 3 (8.1) | 0.25 |
| Stroke [patients, n (%)] | 0 | 2 (5.4) | 0.3 |
| Conversion to sternotomy [patients, n (%)] | 0 | 2 (5.4) | 0.3 |
| Major bleeding [patients, n (%)] | 1 (2.7) | 1 (2.7) | 1 |
| Acute kidney injury [patients, n (%)] | 11 (6.2) | 4 (4.4) | 0.54 |
| Intensive care unit stay (median day, range) | 1 (1–2) | 1 (1–1) | 0.5 |
| Ward stay (median day, range) | 7 (6–8) | 4.5 (3–6) | <0.001 |
TAVI: transcatheter aortic valve implantation; RT: right anterior minithoracomy.
| Variables . | RT (n = 37) . | TAVI (n = 37) . | P . |
|---|---|---|---|
| Mortality [patients, n (%)] | 0 | 3 (8.1) | 0.25 |
| Stroke [patients, n (%)] | 0 | 2 (5.4) | 0.3 |
| Conversion to sternotomy [patients, n (%)] | 0 | 2 (5.4) | 0.3 |
| Major bleeding [patients, n (%)] | 1 (2.7) | 1 (2.7) | 1 |
| Acute kidney injury [patients, n (%)] | 11 (6.2) | 4 (4.4) | 0.54 |
| Intensive care unit stay (median day, range) | 1 (1–2) | 1 (1–1) | 0.5 |
| Ward stay (median day, range) | 7 (6–8) | 4.5 (3–6) | <0.001 |
| Variables . | RT (n = 37) . | TAVI (n = 37) . | P . |
|---|---|---|---|
| Mortality [patients, n (%)] | 0 | 3 (8.1) | 0.25 |
| Stroke [patients, n (%)] | 0 | 2 (5.4) | 0.3 |
| Conversion to sternotomy [patients, n (%)] | 0 | 2 (5.4) | 0.3 |
| Major bleeding [patients, n (%)] | 1 (2.7) | 1 (2.7) | 1 |
| Acute kidney injury [patients, n (%)] | 11 (6.2) | 4 (4.4) | 0.54 |
| Intensive care unit stay (median day, range) | 1 (1–2) | 1 (1–1) | 0.5 |
| Ward stay (median day, range) | 7 (6–8) | 4.5 (3–6) | <0.001 |
TAVI: transcatheter aortic valve implantation; RT: right anterior minithoracomy.
In the TAVI group, 37.8% (n = 14) had mild PVL and 27% (n = 10) had moderate PVL, whereas 2.7% (n = 1) had mild PVL in the RT group (P < 0.001, Table 4). Although not statistical significant, 1- and 2-year survival rates were higher in patients undergoing RT with the sutureless perceval S valve compared with those undergoing TAVI: 91.6 vs 78.6% and 91.6 vs 66.2%, respectively (HR 0.7, 95% CI 0.7–49.8, P = 0.1, Fig. 1).
Haemodynamic results, rate of pacemaker implantation and paravalvular leakage in the matched group
| Variables . | RT (n = 37) . | TAVI (n = 37) . | P . |
|---|---|---|---|
| Peak gradient (mmHg ± SD) | 19.2 ± 6.9 | 19.7 ± 5.4 | 0.26 |
| Mean gradient (mmHg ± SD) | 11.4 ± 3.7 | 10.1 ± 3.4 | 0.17 |
| AV block requiring PMK, n (%) | 2 (5.4) | 0 | 0.5 |
| Paravalvular leakage, n (%) | 2 (5.4) | 30 (81.1) | <0.001 |
| Trivial | 1 (2.7) | 6 (12.2) | |
| Mild | 1 (2.7) | 14 (37.8) | |
| Moderate | 0 | 10 (27) | |
| Severe | 0 | 0 |
| Variables . | RT (n = 37) . | TAVI (n = 37) . | P . |
|---|---|---|---|
| Peak gradient (mmHg ± SD) | 19.2 ± 6.9 | 19.7 ± 5.4 | 0.26 |
| Mean gradient (mmHg ± SD) | 11.4 ± 3.7 | 10.1 ± 3.4 | 0.17 |
| AV block requiring PMK, n (%) | 2 (5.4) | 0 | 0.5 |
| Paravalvular leakage, n (%) | 2 (5.4) | 30 (81.1) | <0.001 |
| Trivial | 1 (2.7) | 6 (12.2) | |
| Mild | 1 (2.7) | 14 (37.8) | |
| Moderate | 0 | 10 (27) | |
| Severe | 0 | 0 |
AV: atrioventricular; PMK: pacemaker; TAVI: transcatheter aortic valve implantation; RT: right anterior minithoracomy; SD: standard deviation.
Haemodynamic results, rate of pacemaker implantation and paravalvular leakage in the matched group
| Variables . | RT (n = 37) . | TAVI (n = 37) . | P . |
|---|---|---|---|
| Peak gradient (mmHg ± SD) | 19.2 ± 6.9 | 19.7 ± 5.4 | 0.26 |
| Mean gradient (mmHg ± SD) | 11.4 ± 3.7 | 10.1 ± 3.4 | 0.17 |
| AV block requiring PMK, n (%) | 2 (5.4) | 0 | 0.5 |
| Paravalvular leakage, n (%) | 2 (5.4) | 30 (81.1) | <0.001 |
| Trivial | 1 (2.7) | 6 (12.2) | |
| Mild | 1 (2.7) | 14 (37.8) | |
| Moderate | 0 | 10 (27) | |
| Severe | 0 | 0 |
| Variables . | RT (n = 37) . | TAVI (n = 37) . | P . |
|---|---|---|---|
| Peak gradient (mmHg ± SD) | 19.2 ± 6.9 | 19.7 ± 5.4 | 0.26 |
| Mean gradient (mmHg ± SD) | 11.4 ± 3.7 | 10.1 ± 3.4 | 0.17 |
| AV block requiring PMK, n (%) | 2 (5.4) | 0 | 0.5 |
| Paravalvular leakage, n (%) | 2 (5.4) | 30 (81.1) | <0.001 |
| Trivial | 1 (2.7) | 6 (12.2) | |
| Mild | 1 (2.7) | 14 (37.8) | |
| Moderate | 0 | 10 (27) | |
| Severe | 0 | 0 |
AV: atrioventricular; PMK: pacemaker; TAVI: transcatheter aortic valve implantation; RT: right anterior minithoracomy; SD: standard deviation.
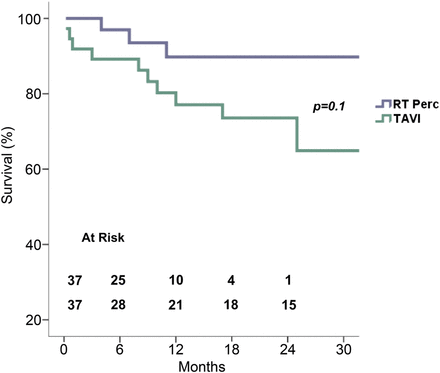
Survival between two matched groups. TAVI: transcatheter aortic valve implantation; RT: right anterior minithoracomy.
More information of the study analysis is reported in Supplementary material.
DISCUSSION
To the best of our knowledge, this is the first study that evaluates postoperative outcomes between patients undergoing AVR with sutureless valves through RT and TAVI.
Our propensity-matched study demonstrated that minimally invasive AVR via RT in combination with the sutureless perceval S valve is a safe and well-tolerated procedure associated with better outcomes compared with TAVI. Although not statistically significant, rate of deaths and postoperative complications such as neurological events and conversions to sternotomy were higher in the TAVI group. Likewise, there was a trend toward a higher 1- to 2-year survival in the RT perceval S group. Finally, patients undergoing TAVI procedures had a greater incidence of postoperative PVL. In the recent years, TAVI has been considered a valid alternative to medical therapy in the treatment of high-risk non-operable patients [5]. However, controversies exist regarding its effect on postoperative mortality, morbidity and long-term outcomes when compared with conventional surgery. The investigators of the Placement of Aortic Transcatheter valves (PARTNER) trial cohort A have demonstrated similar results between the treatments of the two groups in terms of 30-day mortality and 2-year survival [6]. Furthermore, two recent meta-analyses concluded that TAVI was likely ineffective in reducing early and mid-term all-cause mortality versus surgical AVR [7, 17]. On the contrary, transcatheter procedures have been associated with an increased hazard of neurological events and PVLs, which are well-known risk factors for lower survival [6, 7, 18]. The main limitation of these studies was that surgical outcome was related to conventional surgery, consisting of full sternotomy and sutured aortic prosthetic valves. Recently, two new alternative strategies for the treatment of high-risk patients have been considered as alternative to TAVI: the surgical minimally invasive approach and the sutureless aortic valve prosthesis [9, 19–21].
Previously, we reported our experience with minimally invasive AVR with stented valves using RT and showed excellent results in terms of mortality and rate of postoperative complications when compared with different surgical techniques [10–11]. However, this approach was limited by the longer cardiopulmonary bypass and cross-clamp times, suggesting that exposure and implantation of the stented prosthetic valves were more challenging than the conventional approach. In this setting, sutureless valves have shown a consistent reduction of operative times, facilitating the minimally invasive procedures [13–15]. Recently, we described our experience with RT approach by using perceval S valves and we showed a 35–40% reduction of operative times compared with stented valves [15]. As a result, the good haemodynamic performances as well as the low rate of postoperative complications and PVLs have made this procedure a valid alternative to the new and growing TAVI technology in high-risk operable patients. Few studies have focused on clinical outcomes of sutureless aortic valves compared with transcatheter procedures in high-risk patients. In a propensity-matched multicentre study, D'Onofrio et al. did not observe significant differences in-hospital mortality and severe postoperative complications [22]. However, this study was limited by the low number of patients treated with a minimally invasive AVR approach in the sutureless group and TAVI procedures, which may not represent the usual practice in the high-risk population. Conversely, in a higher proportion of patients undergoing ministernotomy for AVR, Santarpino et al. showed better outcomes in the sutureless group, suggesting that the combination of a minimally invasive AVR associated with a sutureless valve may be the first-line treatment for high-risk patients considered to be in the grey zone between TAVI and conventional surgery [23]. Our results are similar to those of Santarpino et al., as we reported an absolute mortality risk of 8% between the two strategies and no death in the RT perceval S group. However, compared with other studies, the strength of ours is that it combines the potential effect of the RT approach with the easier implantation of the sutureless valve. Interestingly, all the above-mentioned papers reported a higher percentage of PVLs associated with TAVI procedures [6, 22, 23]. It has been shown that the presence of PVL is a predictor of lower survival [18].
Notably, in our series, 65% of TAVI patients had at least mild PVL, whereas in the RT perceval S group only 1 (2.7%) patient developed a mild leakage. Compared with TAVI, the surgical approach has the advantage of removing the calcified stenotic valve and, therefore, it may reduce the risk of neurological events and PVL. In addition, our rate of PVL is even lower than in other perceval S reports [13]. In this regard, the Sorin company recommends removal of only the bulky and eccentric calcifications; however, we believe that a complete decalcification of the aortic annulus may further decrease the risk of leakage.
Finally, an analysis of the cost-effectiveness of these procedures should be considered. A systematic review has shown that TAVI is a potentially cost-effective alternative to medical therapy for inoperable patients. In spite of this, TAVI may not be a useful economical alternative to standard AVR in high-risk but operable patients due to the similar mortality rate at 1 year and the higher proportion of postoperative complications [24]. Moreover, an economical model has shown that the perceval S valve is associated with lower costs due to shorter operative times and low rate of occurrence of complications, which leads to shorter hospital and ICU lengths of stay [25]. This study has several limitations. It was based on the retrospective analysis of our institutional, observational, prospectively collected database. The propensity score method is simply a method for reducing bias in observational studies and is based on limited available variables. Our TAVI programme started in October 2008, whereas our first perceval S implantation was performed in October 2009. The perceval S valve was systematically implanted in March 2011, becoming our first valve choice for patients undergoing RT. We recognize that the gap period between the two operative strategies may represent a bias; however, date of surgery was also included in the propensity analysis. Furthermore, the analysis performed allowed a comparison on a small number of patients. Finally, we recognize that these two techniques are not directly comparable, as the choice of the TAVI might be made taking into account other risk factors such as frailty that are not included in the logistic EuroSCORE model and in our database. In conclusion, minimally invasive AVR with sutureless perceval S valves through RT is associated with a trend towards better early outcomes and mid-term survival compared with TAVI. A prospective randomized trial with a larger sample size is required to confirm our data.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at EJCTS online.
ACKNOWLEDGEMENTS
We are very grateful to Serena Russo for her precious assistance in revising the manuscript.
Conflict of interest: Glauber, Ferrarini and Solinas have financial disclosure with Sorin in terms of proctorship and lecture fees.
REFERENCES
Author notes
Mattia Glauber and Antonio Miceli contributed equally to this manuscript.




