-
PDF
- Split View
-
Views
-
Cite
Cite
Anna Lautamäki, Tuomas Kiviniemi, Fausto Biancari, Juhani Airaksinen, Tatu Juvonen, Jarmo Gunn, Outcome after coronary artery bypass grafting and percutaneous coronary intervention in patients with stage 3b–5 chronic kidney disease, European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 3, March 2016, Pages 926–930, https://doi.org/10.1093/ejcts/ezv233
Close - Share Icon Share
Abstract
Patients with chronic kidney disease (CKD) are generally considered to be at an increased risk for cardiovascular events and cardiac mortality. The prognostic significance of severe renal impairment in patients undergoing coronary revascularization remains mainly unknown because these patients have been excluded from randomized clinical trials. The aim of the present study was to compare the outcome after percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG) in patients with an estimated glomerular filtration rate (eGFR) of <45 ml/min/m 2 .
This retrospective study includes 110 patients who underwent PCI and 148 patients who underwent isolated CABG between 2007 and 2010. All patients had stage 3b–5 CKD (eGFR <45 ml/min/m 2 ).
The median follow-up time was 25 (interquartile range 30) months. At 30 days and 3 years, postoperative de novo dialysis was required in 3.4 and 16.2% of CABG patients and in 0 and 6.6% ( P = 0.10) of PCI patients. PCI was associated with similar mortality at 30 days (PCI 10.0% and CABG 12.2%, P = 0.068). At 3 years, PCI was associated with a significantly higher risk of mortality (50.4 vs 32.9, adjusted analysis: HR 1.77, 95% CI 1.13–2.77), repeat revascularization (20.3 vs 0.8%, too few for adjusted analysis) and major adverse cardiac and cerebrovascular events (57.8 vs 34.3%, HR 2.19, 95% CI 1.41–3.40). These findings were supported by propensity score-matched analysis.
Patients with moderate to severe CKD have a high rate of mortality and morbidity after either PCI or CABG. The fear of postoperative dialysis rates after CABG appears overemphasized since less than 5% of patients needed dialysis in the early postoperative period. This study provides evidence that this high-risk subset of patients should also be revascularized according to general recommendations. When feasible, CABG could be associated with better survival and freedom from cardiovascular events than PCI.
INTRODUCTION
Chronic kidney disease (CKD) is a well-known risk factor for cardiovascular mortality and morbidity. Decreased estimated glomerular filtration rate (eGFR) is associated with an increasing risk of adverse outcomes and predicts postprocedural morbidity and mortality in the short- and long-term follow-up either after percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) [ 1 , 2 ]. Recent reports show that stage 3b CKD (eGFR <45 ml/min/1.73 m 2 ) is associated with the sharpest rise in the overall risk profile [ 3 , 4 ].
Little is known about the risk of adverse events in this patient subset undergoing coronary revascularization. When comparing PCI with CABG, procedural invasiveness and the associated short-term complications such as postoperative de novo dialysis need to be weighed against rates of long-term events of death, myocardial infarction (MI), stroke and repeat revascularization. Both PCI and CABG procedures may entail kidney failure in the short-term follow-up [ 5 , 6 ]. However, no randomized trials comparing CABG with PCI in patients with severe renal impairment or dialysis exist. In the previous randomized clinical trials comparing PCI with CABG in patients with left main and/or multivessel disease, the number of patients with moderate to severe renal impairment has been very low and many of the trials fail to report eGFR levels [ 7–11 ]. In the SYNTAX trial, creatinine levels exceeding 200 µmol/l were detected in only 0.8% of the whole trial population, and eGFR levels were not reported [ 12 ]. Overall, long-term follow-up data exceeding 12 months are scarce. A previous meta-analysis gathered 28 observational retrospective studies from the period 1991–2012, assessed the prognosis of patients on dialysis and showed that short-term mortality was higher after CABG, but long-term mortality was better compared with patients treated with PCI [ 13 ]. Nineteen of the studies included in the meta-analysis were conducted prior to 2006; major development has, however, occurred in revascularization methods and we sought to investigate the risk of postoperative de novo dialysis as well as adverse outcomes after CABG and PCI in patients with symptomatic disease and moderate to severe renal failure.
MATERIALS AND METHODS
This study is part of a wider protocol in the process of evaluating long-term follow-up of patients undergoing PCI and CABG [ 14–16 ]. The present retrospective study comprises consecutive patients with stage 3b–5 CKD, i.e. eGFR <45 ml/min/1.73 m 2 calculated by the MDRD formula, who underwent isolated myocardial revascularization procedures for coronary artery disease between 2007 and 2010 in Western Finland. The study population included 110 patients who underwent PCI and stenting at Turku University Hospital and 148 patients who underwent isolated CABG in Finland at Turku University Hospital and Oulu University Hospital. Patients' records were reviewed up to December 2012 for baseline characteristics, procedural/operative data and later adverse events. All patients resided within the catchment area of the two hospitals allowing for complete follow-up of adverse events as treatment of these conditions is centralized. Data on date and mode of death were retrieved from the Finnish national registry, Statistics Finland; therefore, we consider that there were none lost to follow-up in this series. Ethics Committees of Turku University Hospital and Oulu University Hospitals approved the study protocol.
The main outcome endpoints were major adverse cardiac and cerebrovascular events (MACCEs) and all-cause mortality. MACCE is defined as a composite of all-cause mortality, MI, stroke or repeat revascularization. Secondary endpoints included cardiac mortality; repeat revascularization; stroke as adjudicated by a neurologist and confirmed by imaging; acute MI as defined by persistent ischaemic type chest pain with a rise of biochemical markers of myocardial necrosis to at least twice the upper normal limit and commencement of permanent dialysis (Figs 1 and 2 ).
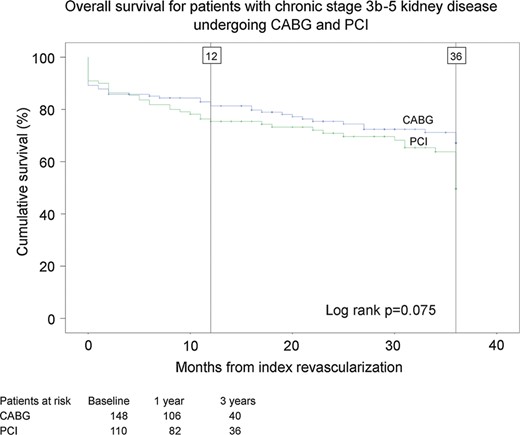
Estimates of overall cumulative survival of patients with stage 3b–5 chronic kidney disease after coronary artery bypass grafting (CABG) surgery and percutaneous coronary intervention (PCI).
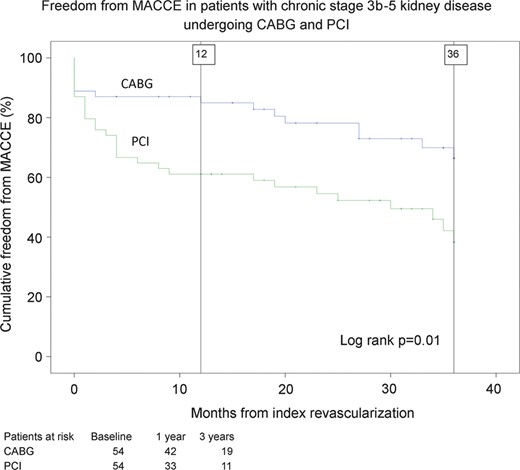
Estimates of cumulative freedom from major adverse cardiac and cerebrovascular events (MACCEs, including death, stroke, myocardial infarction and repeat revascularization) for propensity matched pairs of patients with stage 3b–5 chronic kidney disease after coronary artery bypass grafting (CABG) surgery and percutaneous coronary intervention (PCI).
Statistical analysis
Statistical analysis was performed with IBM SPSS Statistics version 22.0 for Macintosh (IBM Corp., Released 2013, Armonk, NY, USA) and R version 2.15.3. Continuous variables are reported as the mean ± standard deviation. Chi-square test, Fisher's exact test and the Mann–Whitney test were used as appropriate. Kaplan–Meier test analysis with the log-rank method was used to compare groups. Multivariable analysis was performed employing the Cox regression method with backward selection by including clinically relevant variables with P < 0.10 in univariate analysis. The treatment groups differed with respect to certain pretreatment covariates. Therefore, a propensity score for assignment to PCI was calculated by logistic regression analysis by including clinically meaningful variables with P < 0.20 on univariate analysis [eGFR, history of cardiac surgery, recent acute myocardial infarction (AMI), left main stenosis, urgency, gender, age and preoperative dialysis]. One-to-one propensity score matching between the study groups was done with a calibre width of 0.2 of the standard deviation of the logit of the propensity score. P -values of <0.050 were considered statistically significant.
RESULTS
Baseline characteristics of the study groups are detailed in Table 1 . Patients who underwent CABG more often had a history of MI within 90 days and more often had left main stenosis. Patients who underwent PCI more often had a history of previous cardiac surgery and more often had emergency or urgent procedures.
Baseline and procedural characteristics of patients with stage 3b–5 chronic kidney disease who underwent coronary revascularization
| Variables . | Overall series . | P -value . | Propensity score-matched pairs . | Standard mean difference . | ||
|---|---|---|---|---|---|---|
| CABG ( n = 148) . | PCI ( n = 110) . | CABG ( n = 54) . | PCI ( n = 54) . | |||
| Female gender | 61 (41.2%) | 48 (43.6%) | 0.70 | 24 (44.4%) | 25 (46.3%) | 0.049 |
| Age | 70.7 ± 9.9 | 73.1 ± 9.9 | 0.051 | 72.0 ± 8.5 | 72.5 ± 10.4 | 0.25 |
| eGFR (ml/min/m 2 ) | 32.2 ± 12.0 | 33.2 ± 11.2 | 0.50 | 32.2 ± 10.9 | 30.8 ± 11.2 | 0.089 |
| eGFR <30 ml/min/m 2 | 46 (31.1%) | 32 (29.1%) | 0.73 | 17 (31.5%) | 22 (40.7%) | – |
| Dialysis | 24 (16.2%) | 13 (11.8%) | 0.32 | 6 (11.1%) | 9 (16.7%) | 0.14 |
| Kidney transplant | 5 (3.4%) | 4 (3.6%) | 0.91 | 1 (1.9%) | 2 (3.7%) | 0.12 |
| Atrial fibrillation | 30 (20.3%) | 21 (19.1%) | 0.81 | 11 (20.4%) | 10 (8.5%) | −0.03 |
| Diabetes | 66 (44.6%) | 58 (52.7%) | 0.20 | 24 (44.4%) | 30 (55.6%) | 0.24 |
| Hypertension | 112 (75.7%) | 93 (84.5%) | 0.081 | 37 (68.5%) | 46 (85.2%) | 0.38 |
| Prior stroke | 16 (10.8%) | 15 (13.6%) | 0.45 | 5 (9.3%) | 7 (13.0%) | 0.22 |
| Extracardiac arteriopathy | 27 (18.2%) | 19 (17.3%) | 0.84 | 8 (14.8%) | 10 (18.5%) | 0.093 |
| Congestive heart failure | 15 (16.3%) | 28 (25.5%) | 0.11 | 4 (11.1%) | 20 (37.0%) | 0.58 |
| Pulmonary disease | 22 (14.9%) | 13 (11.8%) | 0.48 | 11 (20.4%) | 6 (11.1%) | −0.25 |
| Prior PCI | 24 (16.2%) | 21 (19.1%) | 0.55 | 14 (25.9%) | 12 (22.2%) | −0.093 |
| Prior cardiac surgery | 3 (2.0%) | 21 (19.1%) | <0.001 | 3 (5.6%) | 7 (13.0%) | 0.19 |
| AMI <90 days | 70 (47.3%) | 22 (20.0%) | <0.001 | 20 (37.0%) | 21 (38.9%) | 0.046 |
| LVEF | ||||||
| >50% | 95 (64.2%) | 83 (75.5%) | 0.20 | 40 (74.1%) | 38 (70.4%) | 0.067 |
| 30–50% | 45 (30.4%) | 23 (20.9%) | 0.087 | 13 (24.1%) | 13 (24.1%) | 0 |
| <30% | 8 (5.4%) | 4 (3.6%) | 0.51 | 1 (1.9%) | 3 (5.6%) | 0.18 |
| Urgent/emergency procedure | 84 (56.7%) | 77 (70.0%) | 0.030 | 30 (55.6%) | 29 (53.7%) | −0.14 |
| Left main stenosis | 63 (42.6%) | 14 (12.7%) | <0.001 | 9 (16.7%) | 9 (16.7%) | 0 |
| Off-pump CABG | 69 (46.6%) | – | – | 34 (63.0%) | – | – |
| LIMA graft | 132 (89.2%) | – | – | 47 (87.0%) | – | – |
| Drug-eluting stent | – | 53 (48.2%) | – | – | 26 (48.1%) | – |
| Variables . | Overall series . | P -value . | Propensity score-matched pairs . | Standard mean difference . | ||
|---|---|---|---|---|---|---|
| CABG ( n = 148) . | PCI ( n = 110) . | CABG ( n = 54) . | PCI ( n = 54) . | |||
| Female gender | 61 (41.2%) | 48 (43.6%) | 0.70 | 24 (44.4%) | 25 (46.3%) | 0.049 |
| Age | 70.7 ± 9.9 | 73.1 ± 9.9 | 0.051 | 72.0 ± 8.5 | 72.5 ± 10.4 | 0.25 |
| eGFR (ml/min/m 2 ) | 32.2 ± 12.0 | 33.2 ± 11.2 | 0.50 | 32.2 ± 10.9 | 30.8 ± 11.2 | 0.089 |
| eGFR <30 ml/min/m 2 | 46 (31.1%) | 32 (29.1%) | 0.73 | 17 (31.5%) | 22 (40.7%) | – |
| Dialysis | 24 (16.2%) | 13 (11.8%) | 0.32 | 6 (11.1%) | 9 (16.7%) | 0.14 |
| Kidney transplant | 5 (3.4%) | 4 (3.6%) | 0.91 | 1 (1.9%) | 2 (3.7%) | 0.12 |
| Atrial fibrillation | 30 (20.3%) | 21 (19.1%) | 0.81 | 11 (20.4%) | 10 (8.5%) | −0.03 |
| Diabetes | 66 (44.6%) | 58 (52.7%) | 0.20 | 24 (44.4%) | 30 (55.6%) | 0.24 |
| Hypertension | 112 (75.7%) | 93 (84.5%) | 0.081 | 37 (68.5%) | 46 (85.2%) | 0.38 |
| Prior stroke | 16 (10.8%) | 15 (13.6%) | 0.45 | 5 (9.3%) | 7 (13.0%) | 0.22 |
| Extracardiac arteriopathy | 27 (18.2%) | 19 (17.3%) | 0.84 | 8 (14.8%) | 10 (18.5%) | 0.093 |
| Congestive heart failure | 15 (16.3%) | 28 (25.5%) | 0.11 | 4 (11.1%) | 20 (37.0%) | 0.58 |
| Pulmonary disease | 22 (14.9%) | 13 (11.8%) | 0.48 | 11 (20.4%) | 6 (11.1%) | −0.25 |
| Prior PCI | 24 (16.2%) | 21 (19.1%) | 0.55 | 14 (25.9%) | 12 (22.2%) | −0.093 |
| Prior cardiac surgery | 3 (2.0%) | 21 (19.1%) | <0.001 | 3 (5.6%) | 7 (13.0%) | 0.19 |
| AMI <90 days | 70 (47.3%) | 22 (20.0%) | <0.001 | 20 (37.0%) | 21 (38.9%) | 0.046 |
| LVEF | ||||||
| >50% | 95 (64.2%) | 83 (75.5%) | 0.20 | 40 (74.1%) | 38 (70.4%) | 0.067 |
| 30–50% | 45 (30.4%) | 23 (20.9%) | 0.087 | 13 (24.1%) | 13 (24.1%) | 0 |
| <30% | 8 (5.4%) | 4 (3.6%) | 0.51 | 1 (1.9%) | 3 (5.6%) | 0.18 |
| Urgent/emergency procedure | 84 (56.7%) | 77 (70.0%) | 0.030 | 30 (55.6%) | 29 (53.7%) | −0.14 |
| Left main stenosis | 63 (42.6%) | 14 (12.7%) | <0.001 | 9 (16.7%) | 9 (16.7%) | 0 |
| Off-pump CABG | 69 (46.6%) | – | – | 34 (63.0%) | – | – |
| LIMA graft | 132 (89.2%) | – | – | 47 (87.0%) | – | – |
| Drug-eluting stent | – | 53 (48.2%) | – | – | 26 (48.1%) | – |
Continuous variables are reported as the mean and standard deviation; categorical variables are reported as counts and percentages.
AMI: acute myocardial infarction; CABG: coronary artery bypass grafting; eGFR: estimated glomerular filtration rate; LIMA: left internal mammary artery; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention.
Baseline and procedural characteristics of patients with stage 3b–5 chronic kidney disease who underwent coronary revascularization
| Variables . | Overall series . | P -value . | Propensity score-matched pairs . | Standard mean difference . | ||
|---|---|---|---|---|---|---|
| CABG ( n = 148) . | PCI ( n = 110) . | CABG ( n = 54) . | PCI ( n = 54) . | |||
| Female gender | 61 (41.2%) | 48 (43.6%) | 0.70 | 24 (44.4%) | 25 (46.3%) | 0.049 |
| Age | 70.7 ± 9.9 | 73.1 ± 9.9 | 0.051 | 72.0 ± 8.5 | 72.5 ± 10.4 | 0.25 |
| eGFR (ml/min/m 2 ) | 32.2 ± 12.0 | 33.2 ± 11.2 | 0.50 | 32.2 ± 10.9 | 30.8 ± 11.2 | 0.089 |
| eGFR <30 ml/min/m 2 | 46 (31.1%) | 32 (29.1%) | 0.73 | 17 (31.5%) | 22 (40.7%) | – |
| Dialysis | 24 (16.2%) | 13 (11.8%) | 0.32 | 6 (11.1%) | 9 (16.7%) | 0.14 |
| Kidney transplant | 5 (3.4%) | 4 (3.6%) | 0.91 | 1 (1.9%) | 2 (3.7%) | 0.12 |
| Atrial fibrillation | 30 (20.3%) | 21 (19.1%) | 0.81 | 11 (20.4%) | 10 (8.5%) | −0.03 |
| Diabetes | 66 (44.6%) | 58 (52.7%) | 0.20 | 24 (44.4%) | 30 (55.6%) | 0.24 |
| Hypertension | 112 (75.7%) | 93 (84.5%) | 0.081 | 37 (68.5%) | 46 (85.2%) | 0.38 |
| Prior stroke | 16 (10.8%) | 15 (13.6%) | 0.45 | 5 (9.3%) | 7 (13.0%) | 0.22 |
| Extracardiac arteriopathy | 27 (18.2%) | 19 (17.3%) | 0.84 | 8 (14.8%) | 10 (18.5%) | 0.093 |
| Congestive heart failure | 15 (16.3%) | 28 (25.5%) | 0.11 | 4 (11.1%) | 20 (37.0%) | 0.58 |
| Pulmonary disease | 22 (14.9%) | 13 (11.8%) | 0.48 | 11 (20.4%) | 6 (11.1%) | −0.25 |
| Prior PCI | 24 (16.2%) | 21 (19.1%) | 0.55 | 14 (25.9%) | 12 (22.2%) | −0.093 |
| Prior cardiac surgery | 3 (2.0%) | 21 (19.1%) | <0.001 | 3 (5.6%) | 7 (13.0%) | 0.19 |
| AMI <90 days | 70 (47.3%) | 22 (20.0%) | <0.001 | 20 (37.0%) | 21 (38.9%) | 0.046 |
| LVEF | ||||||
| >50% | 95 (64.2%) | 83 (75.5%) | 0.20 | 40 (74.1%) | 38 (70.4%) | 0.067 |
| 30–50% | 45 (30.4%) | 23 (20.9%) | 0.087 | 13 (24.1%) | 13 (24.1%) | 0 |
| <30% | 8 (5.4%) | 4 (3.6%) | 0.51 | 1 (1.9%) | 3 (5.6%) | 0.18 |
| Urgent/emergency procedure | 84 (56.7%) | 77 (70.0%) | 0.030 | 30 (55.6%) | 29 (53.7%) | −0.14 |
| Left main stenosis | 63 (42.6%) | 14 (12.7%) | <0.001 | 9 (16.7%) | 9 (16.7%) | 0 |
| Off-pump CABG | 69 (46.6%) | – | – | 34 (63.0%) | – | – |
| LIMA graft | 132 (89.2%) | – | – | 47 (87.0%) | – | – |
| Drug-eluting stent | – | 53 (48.2%) | – | – | 26 (48.1%) | – |
| Variables . | Overall series . | P -value . | Propensity score-matched pairs . | Standard mean difference . | ||
|---|---|---|---|---|---|---|
| CABG ( n = 148) . | PCI ( n = 110) . | CABG ( n = 54) . | PCI ( n = 54) . | |||
| Female gender | 61 (41.2%) | 48 (43.6%) | 0.70 | 24 (44.4%) | 25 (46.3%) | 0.049 |
| Age | 70.7 ± 9.9 | 73.1 ± 9.9 | 0.051 | 72.0 ± 8.5 | 72.5 ± 10.4 | 0.25 |
| eGFR (ml/min/m 2 ) | 32.2 ± 12.0 | 33.2 ± 11.2 | 0.50 | 32.2 ± 10.9 | 30.8 ± 11.2 | 0.089 |
| eGFR <30 ml/min/m 2 | 46 (31.1%) | 32 (29.1%) | 0.73 | 17 (31.5%) | 22 (40.7%) | – |
| Dialysis | 24 (16.2%) | 13 (11.8%) | 0.32 | 6 (11.1%) | 9 (16.7%) | 0.14 |
| Kidney transplant | 5 (3.4%) | 4 (3.6%) | 0.91 | 1 (1.9%) | 2 (3.7%) | 0.12 |
| Atrial fibrillation | 30 (20.3%) | 21 (19.1%) | 0.81 | 11 (20.4%) | 10 (8.5%) | −0.03 |
| Diabetes | 66 (44.6%) | 58 (52.7%) | 0.20 | 24 (44.4%) | 30 (55.6%) | 0.24 |
| Hypertension | 112 (75.7%) | 93 (84.5%) | 0.081 | 37 (68.5%) | 46 (85.2%) | 0.38 |
| Prior stroke | 16 (10.8%) | 15 (13.6%) | 0.45 | 5 (9.3%) | 7 (13.0%) | 0.22 |
| Extracardiac arteriopathy | 27 (18.2%) | 19 (17.3%) | 0.84 | 8 (14.8%) | 10 (18.5%) | 0.093 |
| Congestive heart failure | 15 (16.3%) | 28 (25.5%) | 0.11 | 4 (11.1%) | 20 (37.0%) | 0.58 |
| Pulmonary disease | 22 (14.9%) | 13 (11.8%) | 0.48 | 11 (20.4%) | 6 (11.1%) | −0.25 |
| Prior PCI | 24 (16.2%) | 21 (19.1%) | 0.55 | 14 (25.9%) | 12 (22.2%) | −0.093 |
| Prior cardiac surgery | 3 (2.0%) | 21 (19.1%) | <0.001 | 3 (5.6%) | 7 (13.0%) | 0.19 |
| AMI <90 days | 70 (47.3%) | 22 (20.0%) | <0.001 | 20 (37.0%) | 21 (38.9%) | 0.046 |
| LVEF | ||||||
| >50% | 95 (64.2%) | 83 (75.5%) | 0.20 | 40 (74.1%) | 38 (70.4%) | 0.067 |
| 30–50% | 45 (30.4%) | 23 (20.9%) | 0.087 | 13 (24.1%) | 13 (24.1%) | 0 |
| <30% | 8 (5.4%) | 4 (3.6%) | 0.51 | 1 (1.9%) | 3 (5.6%) | 0.18 |
| Urgent/emergency procedure | 84 (56.7%) | 77 (70.0%) | 0.030 | 30 (55.6%) | 29 (53.7%) | −0.14 |
| Left main stenosis | 63 (42.6%) | 14 (12.7%) | <0.001 | 9 (16.7%) | 9 (16.7%) | 0 |
| Off-pump CABG | 69 (46.6%) | – | – | 34 (63.0%) | – | – |
| LIMA graft | 132 (89.2%) | – | – | 47 (87.0%) | – | – |
| Drug-eluting stent | – | 53 (48.2%) | – | – | 26 (48.1%) | – |
Continuous variables are reported as the mean and standard deviation; categorical variables are reported as counts and percentages.
AMI: acute myocardial infarction; CABG: coronary artery bypass grafting; eGFR: estimated glomerular filtration rate; LIMA: left internal mammary artery; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention.
The median follow-up time of this study was 25 months (interquartile range 30, and 25th and 75th percentiles 11 and 41). In the overall series, there were 43 occurrences of MACCEs in the CABG group and 52 in the PCI group; in the propensity matched series, there were 15 cases of MACCEs in the CABG group and 29 in the PCI group. Immediate and late outcomes in the study groups from the overall series are summarized in Table 2 . Dialysis rates at 30 days and 3 years were similar for off-pump (69 patients) and on-pump (79 patients) CABG (30 days: 3.0 vs 3.8%, 3 years: 12.4 vs 9.5%, log-rank P = 0.797).
Outcomes of patients with stage 3b–5 chronic kidney disease after percutaneous coronary intervention and coronary artery bypass grafting
| Outcome endpoints . | Overall series . | P -value (log-rank) . | Propensity score-matched pairs . | P -value (log-rank) . | ||
|---|---|---|---|---|---|---|
| CABG ( n = 148) . | PCI ( n = 110) . | CABG ( n = 54) . | PCI ( n = 54) . | |||
| All-cause mortality | ||||||
| 30-day | 18 (12.2%) | 11 (10.0%) | 0.075 | 6 (11.1%) | 5 (9.3%) | 0.041 |
| 1-year | 25 (17.1%) | 27 (24.6%) | 8 (15.0%) | 12 (33.3%) | ||
| 3-year | 41 (32.9%) | 43 (50.4%) | 14 (30.1%) | 23 (46.7%) | ||
| Myocardial infarction | ||||||
| 1-year | 0% | 14 (13.9%) | <0.001 | 0% | 8 (20.3%) | <0.001 |
| 3-year | 1 (1.6%) | 19 (25.5%) | 0% | 12 (25.9%) | ||
| Repeat revascularization | ||||||
| 1-year | 1 (0.8%) | 9 (9.0%) | <0.001 | 0% | 3 (6.3%) | 0.036 |
| 3-year | 1 (0.8%) | 13 (20.3%) | 0% | 4 (10.2%) | ||
| Stroke rate | ||||||
| 30-day | 8 (5.4%) | 2 (1.8%) | 0.74 | 1 (1.9%) | 1 (1.9%) | 0.18 |
| 1-year | 8 (5.4%) | 3 (3.8%) | 3 (6.3%) | 1 (1.9%) | ||
| 3-year | 8 (5.4%) | 6 (8.4%) | 5 (15.0%) | 2 (4.7%) | ||
| New-onset dialysis | ||||||
| 30-day | 4 (3.4%) | 0% | 0.10 | 2 (4.7%) | 0% | 0.17 |
| 3-year | 14 (16.2%) | 4 (6.6%) | 4 (9.3%) | 1 (2.5%) | ||
| MACCE | ||||||
| 1-year | 27 (19.3%) | 35 (31.8%) | 0.004 | 8 (15.0%) | 21 (38.9%) | 0.01 |
| 3-year | 43 (34.3%) | 51 (57.0%) | 15 (32.1%) | 27 (57.8%) | ||
| Outcome endpoints . | Overall series . | P -value (log-rank) . | Propensity score-matched pairs . | P -value (log-rank) . | ||
|---|---|---|---|---|---|---|
| CABG ( n = 148) . | PCI ( n = 110) . | CABG ( n = 54) . | PCI ( n = 54) . | |||
| All-cause mortality | ||||||
| 30-day | 18 (12.2%) | 11 (10.0%) | 0.075 | 6 (11.1%) | 5 (9.3%) | 0.041 |
| 1-year | 25 (17.1%) | 27 (24.6%) | 8 (15.0%) | 12 (33.3%) | ||
| 3-year | 41 (32.9%) | 43 (50.4%) | 14 (30.1%) | 23 (46.7%) | ||
| Myocardial infarction | ||||||
| 1-year | 0% | 14 (13.9%) | <0.001 | 0% | 8 (20.3%) | <0.001 |
| 3-year | 1 (1.6%) | 19 (25.5%) | 0% | 12 (25.9%) | ||
| Repeat revascularization | ||||||
| 1-year | 1 (0.8%) | 9 (9.0%) | <0.001 | 0% | 3 (6.3%) | 0.036 |
| 3-year | 1 (0.8%) | 13 (20.3%) | 0% | 4 (10.2%) | ||
| Stroke rate | ||||||
| 30-day | 8 (5.4%) | 2 (1.8%) | 0.74 | 1 (1.9%) | 1 (1.9%) | 0.18 |
| 1-year | 8 (5.4%) | 3 (3.8%) | 3 (6.3%) | 1 (1.9%) | ||
| 3-year | 8 (5.4%) | 6 (8.4%) | 5 (15.0%) | 2 (4.7%) | ||
| New-onset dialysis | ||||||
| 30-day | 4 (3.4%) | 0% | 0.10 | 2 (4.7%) | 0% | 0.17 |
| 3-year | 14 (16.2%) | 4 (6.6%) | 4 (9.3%) | 1 (2.5%) | ||
| MACCE | ||||||
| 1-year | 27 (19.3%) | 35 (31.8%) | 0.004 | 8 (15.0%) | 21 (38.9%) | 0.01 |
| 3-year | 43 (34.3%) | 51 (57.0%) | 15 (32.1%) | 27 (57.8%) | ||
Results shown as number of events (percentages).
CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; MACCE: major adverse cardiac and cerebrovascular event.
Outcomes of patients with stage 3b–5 chronic kidney disease after percutaneous coronary intervention and coronary artery bypass grafting
| Outcome endpoints . | Overall series . | P -value (log-rank) . | Propensity score-matched pairs . | P -value (log-rank) . | ||
|---|---|---|---|---|---|---|
| CABG ( n = 148) . | PCI ( n = 110) . | CABG ( n = 54) . | PCI ( n = 54) . | |||
| All-cause mortality | ||||||
| 30-day | 18 (12.2%) | 11 (10.0%) | 0.075 | 6 (11.1%) | 5 (9.3%) | 0.041 |
| 1-year | 25 (17.1%) | 27 (24.6%) | 8 (15.0%) | 12 (33.3%) | ||
| 3-year | 41 (32.9%) | 43 (50.4%) | 14 (30.1%) | 23 (46.7%) | ||
| Myocardial infarction | ||||||
| 1-year | 0% | 14 (13.9%) | <0.001 | 0% | 8 (20.3%) | <0.001 |
| 3-year | 1 (1.6%) | 19 (25.5%) | 0% | 12 (25.9%) | ||
| Repeat revascularization | ||||||
| 1-year | 1 (0.8%) | 9 (9.0%) | <0.001 | 0% | 3 (6.3%) | 0.036 |
| 3-year | 1 (0.8%) | 13 (20.3%) | 0% | 4 (10.2%) | ||
| Stroke rate | ||||||
| 30-day | 8 (5.4%) | 2 (1.8%) | 0.74 | 1 (1.9%) | 1 (1.9%) | 0.18 |
| 1-year | 8 (5.4%) | 3 (3.8%) | 3 (6.3%) | 1 (1.9%) | ||
| 3-year | 8 (5.4%) | 6 (8.4%) | 5 (15.0%) | 2 (4.7%) | ||
| New-onset dialysis | ||||||
| 30-day | 4 (3.4%) | 0% | 0.10 | 2 (4.7%) | 0% | 0.17 |
| 3-year | 14 (16.2%) | 4 (6.6%) | 4 (9.3%) | 1 (2.5%) | ||
| MACCE | ||||||
| 1-year | 27 (19.3%) | 35 (31.8%) | 0.004 | 8 (15.0%) | 21 (38.9%) | 0.01 |
| 3-year | 43 (34.3%) | 51 (57.0%) | 15 (32.1%) | 27 (57.8%) | ||
| Outcome endpoints . | Overall series . | P -value (log-rank) . | Propensity score-matched pairs . | P -value (log-rank) . | ||
|---|---|---|---|---|---|---|
| CABG ( n = 148) . | PCI ( n = 110) . | CABG ( n = 54) . | PCI ( n = 54) . | |||
| All-cause mortality | ||||||
| 30-day | 18 (12.2%) | 11 (10.0%) | 0.075 | 6 (11.1%) | 5 (9.3%) | 0.041 |
| 1-year | 25 (17.1%) | 27 (24.6%) | 8 (15.0%) | 12 (33.3%) | ||
| 3-year | 41 (32.9%) | 43 (50.4%) | 14 (30.1%) | 23 (46.7%) | ||
| Myocardial infarction | ||||||
| 1-year | 0% | 14 (13.9%) | <0.001 | 0% | 8 (20.3%) | <0.001 |
| 3-year | 1 (1.6%) | 19 (25.5%) | 0% | 12 (25.9%) | ||
| Repeat revascularization | ||||||
| 1-year | 1 (0.8%) | 9 (9.0%) | <0.001 | 0% | 3 (6.3%) | 0.036 |
| 3-year | 1 (0.8%) | 13 (20.3%) | 0% | 4 (10.2%) | ||
| Stroke rate | ||||||
| 30-day | 8 (5.4%) | 2 (1.8%) | 0.74 | 1 (1.9%) | 1 (1.9%) | 0.18 |
| 1-year | 8 (5.4%) | 3 (3.8%) | 3 (6.3%) | 1 (1.9%) | ||
| 3-year | 8 (5.4%) | 6 (8.4%) | 5 (15.0%) | 2 (4.7%) | ||
| New-onset dialysis | ||||||
| 30-day | 4 (3.4%) | 0% | 0.10 | 2 (4.7%) | 0% | 0.17 |
| 3-year | 14 (16.2%) | 4 (6.6%) | 4 (9.3%) | 1 (2.5%) | ||
| MACCE | ||||||
| 1-year | 27 (19.3%) | 35 (31.8%) | 0.004 | 8 (15.0%) | 21 (38.9%) | 0.01 |
| 3-year | 43 (34.3%) | 51 (57.0%) | 15 (32.1%) | 27 (57.8%) | ||
Results shown as number of events (percentages).
CABG: coronary artery bypass grafting; PCI: percutaneous coronary intervention; MACCE: major adverse cardiac and cerebrovascular event.
Unadjusted all-cause mortality tended to be higher after PCI compared with CABG (at 3 years, 50.4 vs 32.9%, P = 0.075), and the difference was statistically significant on multivariate analysis (HR 1.77, 95% CI 1.13–2.77) adjusted for age, diabetes, hypertension, urgency, LVEF under 50%, history of cardiac surgery, number of treated vessels and left main stenosis. In the subgroup of patients with severe renal failure, i.e. eGFR <30 ml/min/m 2 , the all-cause mortality was significantly higher after PCI compared with CABG (at 3 years, 61.5 vs 39.9%, log-rank test, P = 0.043). PCI was also associated with a higher risk of cardiac mortality compared with CABG (at 3 years, 37.9 vs 21.8%, adjusted analysis, HR 1.83, 95% CI 1.04–3.21), MACCE (at 3 years, 57.0 vs 34.3%, adjusted analysis, HR 2.19, 95% CI 1.41–3.40) and repeat revascularization (at 3 years, 20.3 vs 0.8%, not enough cases for adjusted analysis).
Independent predictors of MACCEs in addition to PCI after revascularization were age (per year HR 1.03, 95% CI 1.01–1.05), LVEF under 50% (HR 1.62, 95% CI 1.05–2.51) and recent AMI (HR 1.84, 95% CI 1.15–2.94) in a Cox regression analysis including age, treatment group, diabetes mellitus, hypertension, elective operation, LVEF under 50%, history of cardiac surgery, number of treated vessels and left main stenosis. Predictors of MACCEs in the Cox proportional hazards model adjusted by propensity score were increasing age (HR 1.03, 95% CI 1.01–1.05), recent AMI (HR 1.84, 95% CI 1.15–2.94) and PCI (HR 2.19, 95% CI 1.05–2.51).
After estimation of a propensity score for treatment method (goodness of fit with Hosmer–Lemeshow test P = 0.870), 54 propensity score-matched pairs with well-balanced baseline characteristics were obtained for analysis (Table 1 ). Among these propensity score-matched pairs, the risk of dialysis at 30 days and 3 years was 4.7 and 9.3% after CABG and 0 and 2.5% after PCI ( P = 0.17), respectively. Patients who underwent PCI patients had a significantly higher risk of mortality (at 3 years, 46.7 vs 30.1%, P = 0.041), MI (at 3 years, 25.9 vs 0%, P < 0.0001), repeat revascularization (at 3 years, 10.2 vs 0%, P = 0.036) and MACCE (at 3 years 57.8 vs 32.1%, P = 0.01) than those who underwent CABG.
DISCUSSION
Patients with CKD have a worse prognosis than those with normal kidney function after coronary revascularization [ 14 , 15 ]. Data comparing patients with moderate to severe renal impairment undergoing PCI or CABG, however, are scarce. This retrospective study provided data on the rate of postoperative de novo dialysis, MACCEs and the long-term all-cause and cardiac mortality in this frail patient population. At 30 days, de novo dialysis was required in 3.4 vs 0% of patients after CABG and PCI, respectively, and the difference in the risk of late dialysis was not statistically different. On the other hand, survival and freedom from MACCEs appeared better in patients after CABG compared with PCI, which is in concordance with the overall results from recent randomized controlled trials comparing these two revascularization methods [ 7–11 ].
The study groups were heterogeneous with regard to the localization and severity of the coronary lesion. Patients with multivessel disease more often underwent CABG than PCI. A higher frequency of left main stenosis among these patients alludes to more extensive CAD preoperatively [ 14 , 16 ]. At present, PCI is mainly performed as a treatment of choice for AMI or, at the other end of the spectrum, in patients with stable, single-vessel coronary artery disease. On the contrary, patients undergoing CABG more frequently have multivessel disease and associated co-morbidities [ 5 ]. Therefore, a direct comparison of PCI and CABG is difficult to perform. Bearing this limitation in mind, differences in baseline characteristics were adjusted using propensity score matching. The main conclusions are, however, drawn from the overall series as the limited size of the propensity score-matched population rather implies a sensitivity analysis and corroboration of results.
Patients undergoing CABG more often required early postoperative dialysis than PCI patients. This was to be expected since cardiac surgery and the use of cardiopulmonary bypass expose patients to renal dysfunction [ 6 ]. Nonetheless, the fear of higher risk of dialysis after CABG compared with PCI appears overemphasized based on the lack of a significant difference in dialysis rates at late follow-up. Moreover, in the propensity score-matched groups, only ∼5% of patients needed dialysis shortly after operation and 9.3% at 3 years, whereas the numbers after PCI were 0 and 2.5%, respectively. An increased rate of de novo dialysis was offset by a better survival and freedom from repeat revascularization, which was markedly higher in the CABG arm compared with the PCI arm at 3 years (Table 2 ). It has been debated whether off-pump bypass can be useful in reducing the risk of acute renal failure in this patient population and is a matter of ongoing investigation. In this study, 69 (46.6%) patients in the CABG group underwent off-pump surgery with a postoperative dialysis rate similar to on-pump patients. The limited number of patients in the present study, however, does not allow for a specific subgroup analysis on this subject. There are several studies, suggesting that off-pump coronary bypass might decrease the risk of immediate postoperative renal failure, but evidence on long-term rates of renal failure are conflicting and data specifically on patients with preoperative CKD are lacking [ 16 , 17 ]. Previous reports on rates of post-revascularization dialysis have been similar to this study: 0.2–1.2% after CABG in general and 2.1% for patients with moderate (eGFR 30–60) and 14.5% for patients with severe (eGFR under 30) renal failure [ 18 , 19 ]; for PCI, the rates for postprocedural dialysis have been reported at below 1% in general populations [ 20 , 21 ], but data on dialysis rates in patients with moderate to severe renal failure are lacking.
Cardiovascular events are the most common cause of death in patients with renal failure. Reasons for this interaction include the greater prevalence of cardiovascular risk factors, such as diabetes mellitus and hypertension. Moreover, factors associated with renal impairment such as uraemic toxins, inflammation, hyperparathyroidism, elevated calcium–phosphate product, fluid overload and anaemia contribute to the severity and extent of the coronary atherosclerosis as well as the higher adverse event rates in this high-risk population. Adverse events may also be related to conditions other than epicardial coronary artery disease, such as metabolic derangements, uraemic cardiomyopathy and microvascular disease. Nonetheless, the presence of significant coronary artery stenosis has been reported to worsen the prognosis dramatically [ 22 ].
It is known that the CKD is intrinsically a major risk factor for MACCEs after coronary revascularization [ 23 , 24 ]. The rate of freedom from MACCEs after 2 years was 78.2% in the CABG group and the corresponding rate in the PCI group was 54.5%. At the 3-year follow-up, the rates of freedom from MACCEs were 65.6 and 43.0%, respectively. The long-term mortality is in accordance with previous results from patients with CKD eligible for surgical treatment, although short-term mortality was low in the present study; in general, CABG confers better long-term results offset by a higher short-term mortality [ 13 , 14 ]. The present study provides evidence that the overall survival was better after CABG even in patients with severe renal failure (eGFR under 30 ml/min/m 2 ). These findings validate the rationale for treating CKD patients with coronary disease as presented in the European Society of Cardiology and European Association for Cardio-Thoracic Surgery 2014 guidelines on myocardial revascularization, i.e. patients tend to fare better after CABG, while keeping in mind that frail and weak patients might be better treated with PCI [ 25 ].
There are a number of limitations in this present study and findings should be viewed in the light of these. The first limitation is the retrospective nature of this two-centre study. Secondly, the small patient population may also introduce a bias. Moreover, it is worth noting that, in the first place, risk factors were distributed unevenly between the study groups. Patients undergoing PCI were older and more often had a history of cardiac surgery. Patients undergoing CABG had more diffuse coronary artery disease and recent MI. This major heterogeneity between study groups is explained by clinical selection. Patients who are too frail to undergo surgery or “too healthy” to be considered for CABG undergo PCI, leading to an overrepresentation of single-vessel treatment in the PCI group. Most patients in the PCI group were treated for acute coronary syndromes. In this scenario, the stent was placed only in the culprit lesion and milder untreated stenoses might lead to symptoms later with a renewed need for revascularizations after PCI. It is also conceivable that some of the patients' characteristics cannot be accounted to affect the outcome. Therefore, the comparability of these study groups is remarkably difficult.
In conclusion, coronary artery disease patients with CKD stage 3b or higher represent a high-risk population for complications and cardiovascular mortality. However, the immediate and late results in patients with moderate to severe renal failure suggest that they should not be denied surgical revascularization based on kidney function alone. The present data indicate that, when feasible, CABG could be associated with better survival and freedom from cardiovascular events than PCI.
Funding
This study was funded by the Finnish Foundation for Cardiovascular Research, Helsinki, Finland (Juhani Airaksinen and Tuomas Kiviniemi).
Conflict of interest: Juhani Airaksinen receives advisory fees from Bayer and Boston Scientific and lecture fees from Cardiome and Boehringer Ingelheim. Tuomas Kiviniemi receives lecture fees from Bayer and AstraZeneca.
REFERENCES




