-
PDF
- Split View
-
Views
-
Cite
Cite
Wei-Han Chou, Nai-Hsin Chi, Yi-Chia Wang, Chi-Hsiang Huang, Metastatic breast cancer with right ventricular erosion, European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 3, March 2016, Pages 1006–1007, https://doi.org/10.1093/ejcts/ezv158
Close - Share Icon Share
Abstract
Cancer that has metastasized to the heart and pericardium has a dismal outcome. Individualized treatment to preserve the quality of life and reduce surgical mortality is important. We describe a 57-year old woman who had a recurrence of breast cancer 23 years after the initial complete treatment. Cardiac metastasis with poor anterior chest wall healing led to right ventricular rupture, which caused hypovolaemic shock. The right ventricular wall defect was repaired with a percutaneous patch and a myocutaneous flap without cardiopulmonary bypass. The patient was discharged home after intensive wound care. Our patient shows that even with complete initial treatment, clinicians should be alert for the recurrence of breast cancer.
INTRODUCTION
Breast cancer rarely metastasizes to the heart and pericardium, and this usually has a poor outcome. The clinical presentation is diverse, ranging from no symptoms, to dyspnoea, arrhythmias and congestive heart failure. Effective treatment of advanced disease is limited [ 1 ]. We report a breast cancer patient with cardiac metastasis, who developed hypovolaemic shock following ventricular rupture. Percutaneous patch repair with a myocutaneous flap cover stopped the bleeding and stabilized her condition.
CASE
A 57-year old woman underwent a modified radical mastectomy for stage II left breast cancer in 1983. She was followed up regularly, and there was no recurrence for 20 years. In 2006, she noticed a chest wall tumour, and imaging showed bilateral lung metastases. A biopsy of the chest wall tumour showed oestrogen receptor-positive mucinous adenocarcinoma. The patient underwent chemotherapy and radiation therapy for 1 year. However, the anterior sternal wound worsened after the radiation therapy. She required wound debridement surgery several times with split-thickness skin grafts, but was lost to the follow-up for 2 years after the wound surgery.
At that time, she presented to the emergency department in shock due to massive bleeding from the anterior chest wall wound (Fig. 1 A). Emergency angiography showed no obvious bleeding from the right or left internal mammary artery. Contrast computed tomography showed necrosis from the anterior chest wall to the anterior mediastinum, encasing and destroying the sternum. There was a fistula over the right ventricular free wall, with suspected free wall rupture (Fig. 2 A). Emergency surgery was arranged. Her anterior chest wall had a 15 × 15 cm wound covered by extensive necrotic tissues. We could palpate the heart beat through the wound. A perioperative transoesophageal echocardiogram (TOE) showed a 1.15 × 0.6 cm fistula over the right ventricular infundibulum (Fig. 2 B), connecting the right ventricular free wall and anterior chest wall. The right ventricular wall defect was repaired with a bovine pericardial patch without cardiopulmonary bypass (Fig. 1 B). The anterior chest wall wound was covered with a transverse rectus abdominis myocutaneous flap. Surgical wound cultures grew Pseudomonas aeruginosa , Stenotrophomonas maltophilia and coagulase-negative Staphylococcus . She was hospitalized for roughly 2 months due to the wound infection and pneumonia. After intensive wound care and debridement, she was discharged home, and is now being followed as an outpatient (Fig. 1 C).
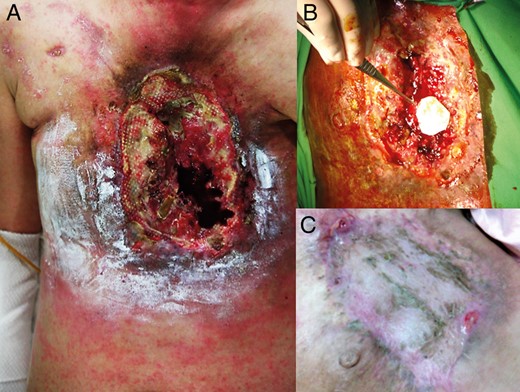
( A ) The large anterior chest wall wound with necrosis and destruction of the sternum and anterior mediastinum. ( B ) The right ventricular wall defect was repaired with a bovine pericardial patch without cardiopulmonary bypass. ( C ) The anterior chest wall healed after covering it with a myocutaneous flap and intensive wound care.
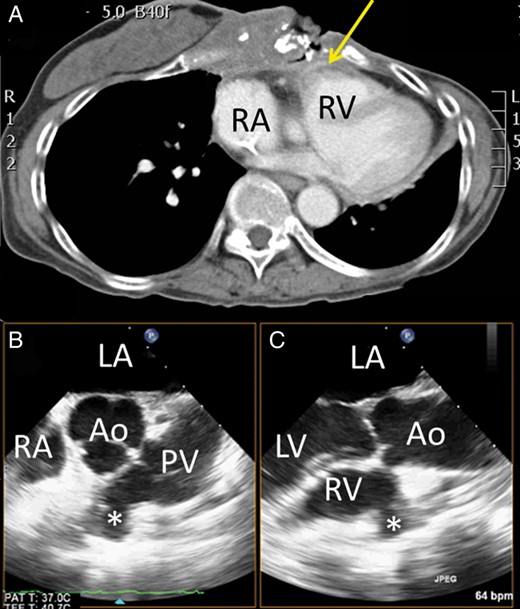
( A ) Computed tomography showed a fistula (arrow) at the anterior right ventricular outflow tract with the halo sign, which suggested recent bleeding. ( B ) Transoesophageal echocardiography (TOE) showed a fistula (asterisk) at the right ventricular outflow tract in the biplane view. LA: left atrium; RA: right atrium; Ao: aorta; PV: pulmonary valve; LV: left ventricle.
DISCUSSION
Metastatic cancer involving the heart is considered uncommon, although the reported incidence rate was 9% in a postmortem study [ 2 ]. The most common cardiac metastasis is pericardial metastasis, followed by epicardial and myocardial metastasis. Owing to its location, breast cancer can give rise to pericardial metastasis via lymphatic spread or direct invasion. Symptoms from metastatic cardiac tumours vary with the site and extent of invasion. A pericardial effusion is the most common symptom of cardiac metastasis [ 2 ]. Cardiac rupture due to metastatic breast cancer is rare and could be catastrophic. Echocardiography is the tool used most commonly to detect cardiac metastases.
Breast cancer is the second leading cause of death in female cancers, and the number of breast cancer patients has increased significantly since 1970. The thorax and soft tissues are common sites of metastasis, whereas cardiac involvement has not been seen. With the improvements in imaging techniques and autopsy information [ 3 ], we now know that asymptomatic cardiac metastasis can occur. The incidence rate of cardiac metastasis in breast cancer patients is ∼15.5% [ 2 ]. Our patient showed that even with complete initial treatment, clinicians should be alert for recurrence.
For metastatic tumours, palliative treatment for symptom relief remains the main treatment option [ 4 ]. After diagnosing cardiac metastasis, the mean survival time is ∼5.5 months [ 4 ]. Intensive treatment can decrease a patient's quality of life, and the costs and benefits should be carefully evaluated. Surgery is considered in exceptional patients who potentially have a long life expectancy, such as with tumour-related chamber or valve obstruction. The mortality is high and the treatment is individualized [ 1 ]. Our patient underwent a quick repair for haemodynamic stabilization and was discharged home with an optimal quality of life.
Conflict of interest: none declared.
REFERENCES




