-
PDF
- Split View
-
Views
-
Cite
Cite
Bilal H. Kirmani, Sukumaran Binukrishnan, John R. Gosney, D. Mark Pullan, Left ventricular apical masses: distinguishing benign tumours from apical thrombi, European Journal of Cardio-Thoracic Surgery, Volume 49, Issue 2, February 2016, Pages 701–703, https://doi.org/10.1093/ejcts/ezv098
Close - Share Icon Share
Differential diagnoses for cardiac left ventricular apical masses presenting following acute myocardial infarction include thrombi and cardiac tumours. We present two such cases and the multidisciplinary assessment that is required to assist with diagnosis.
INTRODUCTION
A left ventricular (LV) thrombus is common following myocardial infarction, with an incidence of between 7 and 46% [1]. Peak incidence is within 2 days of the infarct and their presence is an independent predictor of mortality. In the absence of significant embolic phenomena, medical management with anticoagulation is usually considered sufficient, with surgery indicated for strokes or other circulatory emboli refractory to heparin therapy [2]. Ventricular thrombi are most commonly diagnosed in the first instance by echocardiography, but this modality may be inconclusive in up to 46% of studies. Cardiac magnetic resonance (MR) imaging, in contrast, has a sensitivity of 88% and a specificity of 99% for LV thrombus [1].
Primary tumours of the heart are rare, with an incidence of between 1.7 and 190 per 100 000 at unselected necropsy, the majority of which are benign [3]. Cardiac myxomata account for approximately half of these, occurring most frequently in the atria (75% left atrium and 20% right atrium), and rarely in the ventricles (3–4% on either side). Other benign primary cardiac tumours include papillary fibroelastomas, fibromas and lipomas.
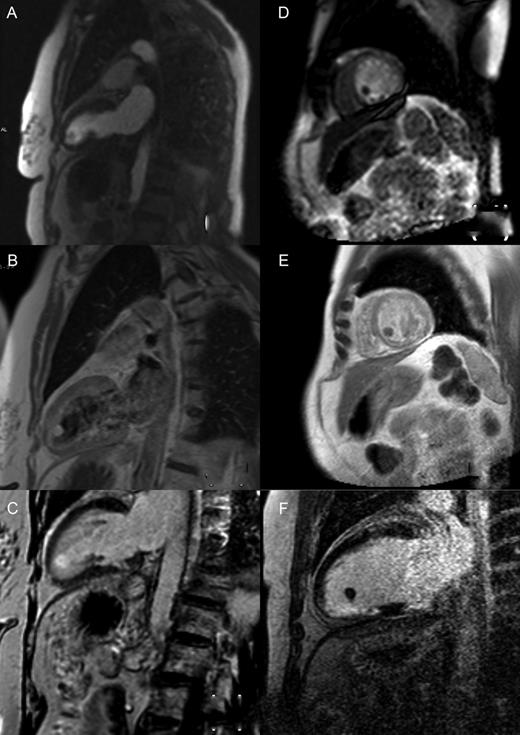
Cardiac magnetic resonance imaging demonstrating perfusion, T1 images post-gadolinium and delayed enhancement of a left ventricular apical fibroelastoma (A–C) and thrombus (D–F), respectively.
CASE PRESENTATIONS
Case 1
A 72-year old lady with type 2 diabetes mellitus, hypertension, hypercholesterolaemia and a 40 pack-year history of smoking presented with new onset central chest pain and shortness of breath. Clinical examination was unremarkable and electrocardiogram showed normal sinus rhythm with T-wave inversion in aVL only. Her Troponin I came back at 3.68 ng/ml (normal <1.5 ng/ml). Her chest pain resolved spontaneously and she resumed full exercise capacity. An echocardiogram was performed that demonstrated a smooth, well-circumscribed mass in the left ventricle with a broad base attached to the apical ventricular septum. Magnetic resonance (MR) imaging further delineated this as a likely LV myxoma. On T1-weighted images, it showed the same uptake as myocardium, with slightly higher signal on T2-weighted images. There was evidence of both perfusion and delayed enhancement on post-gadolinium sequences (Fig. 1A–C). Coronary angiogram showed no evidence of coronary artery stenoses and no contrast enhancement of the tumour.
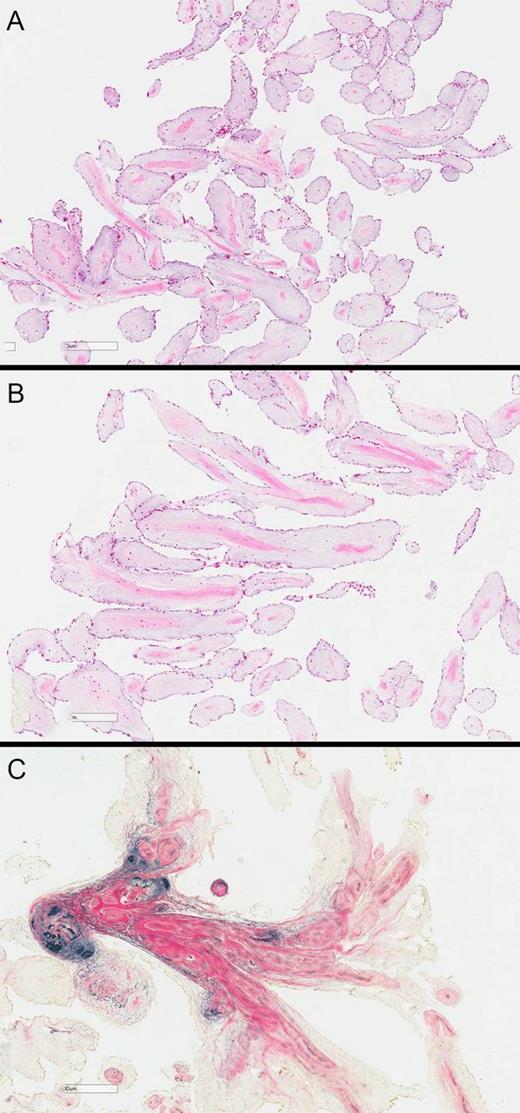
(A) Low power haematoxylin and eosin stain, showing delicate papillary fronds covered by endothelium; (B) higher power view showing condensation of connective tissue, including elastin, at the core of the papillary fronds; (C) elastin and van Gieson method (EVG) showing the elastin stained black.
Case 2
A 53-year old male smoker with a history of hypercholesterolaemia and peripheral vascular disease presented with an inferior ST-elevation myocardial infarction. Coronary angiography showed normal coronary arteries and he was treated with medical management. Six years later, he presented with atypical chest pain with old Q-waves inferiorly, but no troponin rise. An echocardiogram was performed that showed a mobile mass attached to the inferior LV wall with a pedunculated stalk. The LV ejection fraction was noted to be good. Further imaging with cardiac MR described a mobile, 1.1 cm mass with a thin stalk connecting it to the apical-inferior wall and septum. There was no perfusion and no delayed post-gadolinium enhancement within the lesion (Fig. 1D–F). In addition, the whole inferior wall demonstrated delayed enhancement consistent with previous infarct and scar tissue. A diagnosis of an LV thrombus was made and conservative management with anticoagulation and serial imaging was elected.
Due to patient non-compliance, there was a delay in starting warfarin anticoagulation and the international normalized ratio then also remained sub-therapeutic for several weeks. Once properly anticoagulated, however, the mass showed no substantial change at interval 3- and 6-month transthoracic echocardiography with possibly some reduction in pedunculation. The patient suffered no embolic phenomena, and remained keen on non-surgical management.
COMMENT
Differentiating between apical masses in the left ventricle requires clinical suspicion as well as appropriate echocardiographic and radiological investigation. Apical lesions in particular can be confounding. An algorithm for distinguishing between different types of intracardiac lesion has been proposed [5], but this relies on typical appearances and locations of tumours. Papillary fibroelastomas within a cardiac chamber rather than on a valve, such as our first case, would not have been identified by the algorithm, especially as the typical echocardiographic appearances were not seen. Emphasis must be placed, therefore, on the clinical presentation: established previous history of acute coronary syndromes associated with a regional wall abnormality should increase the suspicion of a thrombus. Normal coronary arteries with new non-specific cardiac symptoms and signs should raise a differential diagnosis of tumour. Echocardiography and cardiac MR can then be used to facilitate the diagnosis, with perfusion and post-gadolinium sequences helping to establish the vascularity of the lesion. Coronary angiography is less useful for this purpose.
Our patient cases demonstrate that the diagnosis is sometimes not clearly defined and discussion with multimodal imaging, a multidisciplinary team and the patient is necessary.
Conflict of interest: None declared.




