-
PDF
- Split View
-
Views
-
Cite
Cite
Ilir Hysi, Eric Kipnis, Pierre Fayoux, Marie-Christine Copin, Christophe Zawadzki, Ramadan Jashari, Thomas Hubert, Alexandre Ung, Philippe Ramon, Brigitte Jude, Alain Wurtz, Successful orthotopic transplantation of short tracheal segments without immunosuppressive therapy, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 2, February 2015, Pages e54–e61, https://doi.org/10.1093/ejcts/ezu444
Close - Share Icon Share
Results of tracheal transplantation have been disappointing due to of ischaemia and rejection. It has been experimentally demonstrated that results of tracheal autograft/allograft transplantation were correlated with both graft length and revascularization method. Recently, we demonstrated that heterotopic epithelium-denuded-cryopreserved tracheal allograft (TA) displayed satisfactory immune tolerance. We aimed at evaluating the results of such allografts in orthotopic transplantation according to graft length and prior heterotopic or single-stage orthotopic revascularization in a rabbit model.
Twenty New Zealand rabbits were used. Six females served as donors. Tracheal mucosa was mechanically peeled off and then the TAs were cryopreserved. Male recipients were divided into three groups receiving: (i) long TA segment with prior heterotopic revascularization (10–12 tracheal rings, n = 3); (ii) average TA segment with single-stage orthotopic revascularization (6–8 tracheal rings, n = 4); (iii) short TA segment with single-stage orthotopic revascularization (4–5 tracheal rings, n = 7). No immunosuppressive therapy was administered. Grafts were assessed bronchoscopically and upon death or sacrifice by macroscopic evaluation, histology and immunohistochemical staining for apoptosis.
Four animals were sacrificed from Day 33 to Day 220. The survival time of other recipients was 0–47 days (mean 19.6 ± 16.7 days). Aside from three animals that died from complications, all TA segments had satisfactory stiffness, were well vascularized, showed varying levels of neoangiogenesis and inflammatory infiltration devoid of lymphocytes, and showed evidence of only low levels of apoptosis. Varying degrees of fibroblastic proliferation originating from the lamina propria were observed in the lumen of all TAs and evolved over time into collagenized fibrosis in animals surviving over 45 days. Likewise, cartilage tracheal rings exhibited central calcification deposits, which started on Day 16 and increased over time. Epithelial regeneration was constantly observed. Intense fibroblastic proliferation led to stenosis in all animals from Groups (i) and (ii) but only one of seven animals from Group (iii).
Our results suggest that short segments of epithelium-denuded-cryopreserved TA may be reliable for tracheal transplantation in the rabbit model without problems related to graft stiffness or immune rejection. Before considering clinical applications, investigations should be conducted in larger mammals.
INTRODUCTION
The search for the ‘holy grail’ of tracheal substitutes has been going on for decades. Despite recent major advances [1–3], we have yet to find such an ideal tracheal substitute for circumferential replacements. One of the first grafts considered in this field, and the most intuitive, was the tracheal allograft (TA) harvested from dead donors. Following an initial successful report, albeit with short-term follow-up (9 weeks) [4], TAs have been regarded as failures due to necrosis, stenosis or graft malacia [5] imputed to varying degrees of ischaemia and rejection [6]. Indeed, TA has no identifiable vascular pedicle, which impedes direct revascularization and prevention of immune rejection requires an immunosuppressive treatment formally contraindicated in malignancies, thus restricting clinical applications to benign tracheal pathologies. Regarding the vascular issue, our previous findings in a rabbit model [7–9], as prior results of Delaere et al. [10], showed the feasibility of indirect revascularization of tracheal substitutes or airway segments with the lateral thoracic fascial flap. Recently, Delaere et al. have transposed experimental indirect revascularization by using a forearm fascial flap to revascularize the graft prior to a free flap procedure in humans [2, 11]. Otherwise, it has been experimentally demonstrated that (1) the success/failure of tracheal autotransplantation (thus with no rejection problems) was directly correlated with the graft length and (2) prior heterotopic revascularization is useful in promoting neoangiogenesis into long tracheal segments, while single-stage orthotopic revascularization was sufficient for average/short tracheal segments [12–14]. Similarly, the rabbits undergoing an allograft tracheal transplantation showed evidence of neoangiogenesis either into long tracheal segments after prior heterotopic revascularization [10] or into average/short tracheal segments after single-stage orthotopic revascularization [15, 16].
We addressed the issue of TA rejection in a recent study in the rabbit [9], which showed that the epithelium-denuded-cryopreserved TA implanted in heterotopy displayed satisfactory immune tolerance despite the absence of immunosuppressive drugs. In our current study, in the same rabbit model, we aimed at further evaluating the outcomes, mechanical properties, morphology, histology and immune tolerance of such grafts as reliable substitutes for orthotopic, rather than heterotopic tracheal transplantation according to graft length and prior heterotopic or single-stage orthotopic revascularization.
MATERIALS AND METHODS
Our protocol (# CEEA 09212) was approved by the regional ethical board on experimental use of animals (Comité d'éthique en expérimentation animale Nord-Pas-de-Calais). The study was conducted in accordance with the experimental animal use guidelines of the French Ministry of Agriculture, food-processing industry and forest (Ministère de l'Agriculture, de l'agroalimentaire et de la forêt) that regulates animal research in France.
Animals
The study used 20 syngenetic adult New Zealand (NZ) white rabbits, weighing 3170–4575 g [C.E.G.A.V (SSC) La Passerie Saint-Mars-D'Egrenne, France]. Animals were housed in individual cages at the University Hospital Department of Experimental Research of our institution.
Harvest and cryopreservation of tracheal allografts
Six female rabbits served as donors. Following premedication with an intramuscular injection of ketamine (50 mg/kg) and xylazine (2.5 mg/kg), they were sacrificed using an intracardiac injection of embutramide, mebezonium and tetracaine (T61; Intervet, Beaucouzé, France). The trachea was exposed through a cervical incision, and then harvested from the cricoid cartilage to the carina. The rabbit trachea shares an average of 30 cartilage rings and in vivo measures ∼6 cm in length [9]. The epithelium of all specimens was mechanically peeled off the underlying lamina propria, as previously described [9], in order to obtain epithelium-denuded TAs. They were transferred to the European Homograft Bank in cold (4–8°C) Hank's medium 199 containing antimicrobial agents (vancomycin, lincomycin and polymyxin B) and were incubated in this solution for 48 h. The cryopreservation process has already been detailed elsewhere [9]. Briefly, TAs were cryopreserved after immersion in a cryoprotecting medium (10% dimethyl sulphoxide—DMSO—in Hank's solution 199) before storage in vapour liquid nitrogen p (−150 to −187°). The lengths of storage ranged from 7 to 95 days. Before use, TAs were thawed in a warm water bath at 37–40° for 10 min, and then washing out of DMSO was performed using cold isotonic sterile saline (+4°).
Anaesthesia of recipient animals
The anaesthesia protocol for the 14 recipient male NZ rabbits has already been described [8, 9]. Briefly, following induction with an intramuscular injection of ketamine (50 mg/kg) and xylazine (2.5 mg/kg), it was based on isoflurane (2%) and oxygen (60%) inhalation through mask ventilation.
Operative technique
In accordance with previous works showing that the success/failure of tracheal graft revascularization was strongly correlated with the length of the transplanted segments [12, 13], male NZ rabbit recipients were divided into three groups with seven animals initially scheduled in each of them: animals receiving, (i) a long TA segment (10–12 tracheal rings: 35% of the rabbit trachea's length), (ii) an average TA segment (6–8 tracheal rings: 25%); (iii) a short TA segment (4–5 tracheal rings: 15%). After division in segments, the distal part of all TAs was retained for histological examination in order to verify the efficacy of epithelial denudation. In Group (i), tracheal transplantation was performed after an initial step of heterotopic revascularization through thoracic lateral fascial flap procedure during 14 days, with a silicone tube placed within the graft to maintain satisfactory patency and morphology [9]. Heterotopic revascularization and tracheal transplantation were conducted according to our usual techniques [8]. Briefly, after a lateral thoracic skin incision, the lateral thoracic fascia was elevated as a pedicle using the lateral thoracic vessels. The edge of the fascial flap was rolled around the TA segment and implanted under the skin. Following the 14-day revascularization period, the tracheal transplantation was then performed. Following lateral thoracic re-entry, the TA segment and its surrounding fascia were elevated and rotated subcutaneously to the neck region through a cervical midline incision. After adequate resection of the native trachea, the TA segment was interposed and sutured by end-to-end anastomoses. In the last two groups, tracheal transplantation was performed similarly, save that a free TA segment (not revascularized prior to transplant) was interposed and sutured, and then buttressed with the strap muscles to ensure neoangiogenesis (single-stage orthotopic revascularization) [15, 16]. In either method, tracheal anastomoses were carried out with interrupted 6/0 non-absorbable Prolene sutures (ETHICON France, Issy les Moulineaux, France) (see Supplementary Data), in order to visualize suture lines during both postoperative bronchoscopic examination and macroscopic analysis of specimens. In all groups, during both resection and reconstruction steps, cross-field intubation of the distal trachea was unnecessary, as isoflurane anaesthesia allowed spontaneous breathing [8].
Follow-up
Postoperatively, all 14 recipient rabbits were observed for ∼3 h before being returned to their individual cages. They received standard feed and water ad lib. Analgesics (buprenorphine 0.05 mg/kg) were administrated twice a day on postoperative Day 1. Antibioprophylaxis (enrofloxacin 10 mg/kg) was administrated postoperatively over 5 days. No immunosuppressive therapy was administered. Animals were followed up until death or sacrificed from Day 33 to Day 220.
Rigid bronchoscopy
Animals were followed up with rigid bronchoscopies. This was done under general anaesthesia and spontaneous ventilation. Bronchoscopies were systematic examinations looking for signs of epithelial regeneration, neoangiogenesis and lumen patency (Video 1); or on-demand interventional procedures such as aspiration of secretions or dilatation of airway stenosis (Video 2). Systematic examinations were performed weekly during the first 6 weeks, and then semi-monthly.
Video 1: Bronchoscopic examination on Day 34 (Rabbit 14, sacrificed on Day 220) revealing patent central airway, satisfactory neoangiogenesis and airway epithelium lining of the graft.
Video 2: Interventional bronchoscopy: dilatation of a graft stenosis with forceps (Rabbit 10, sacrificed on Day 33).
Macroscopic assessment
Macroscopic analysis consisted in the evaluation of graft appearance and length. Graft stiffness was assessed manually by compressing the graft with forceps as previously described [17] but no formal biomechanical measurements were performed, the adequate physical strain test device (Zwick/Roell, version Z0.5 TS, Barcelona, Spain) being unsuitable for small sample studies.
Histological examination
Specimens were embedded in paraffin, cut into 3-µm slides and stained with haematoxylin–eosin–saffron. Histological examination focused on lymphocyte infiltration, cartilage viability and cartilage calcification graded as follows: +: mild, central deposits; ++: moderate, 10–50% of the cartilage surface; +++: intense, superior to 50% of the cartilage surface [8]. Findings were compared with the morphology of epithelium-denuded-cryopreserved TA segment in a control rabbit.
Detection of apoptosis
Immunohistochemical staining for apoptosis enabled us to analyse the immunological rejection of TAs [18]. Immunohistochemistry technique for apoptosis has been detailed elsewhere [9]. Briefly, the number of apoptotic cells in cartilage and lamina propria/pericartilaginous tissue was semi-quantitatively assessed with a murine monoclonal antibody (APOSTAIN), which is specific for condensed chromatin of apoptotic cells, a hallmark of apoptosis.
RESULTS
Taking into account the results detailed below (early deaths and occurrence of graft stenosis of long and average TA segments), the number of rabbits undergoing tracheal transplantation was reduced from 7 to 3 in Group (i) and to 4 in Group (ii), respectively (Table 1) in compliance with the 3R rule for animal experimental research (Replacement, Refinement, Reduction of animals in research). Four animals were sacrificed from Day 33 to Day 220. The survival time of other recipients was 0–47 days (mean 19.6 ± 16.7 days). Although presented with all the results in Table 1, the results from animals presenting unexpected death in less than 24 h (Animal 4), or acute suppurative complications (Animals 8 and 9) were not included in the analysis and are discussed with the limits of the study.
Clinical and pathological findings in 14 male rabbits undergoing tracheal transplantation with epithelium-denuded-cryopreserved tracheal allografts
| Rabbit . | Tracheal rings (group) . | Bronchoscopy (n) Systematic/interventional . | Death day (cause) Sacrifice day . | Neoangiogenesis . | Cartilage ischaemia/calcification . | Lamina propria fibrosis . | Epithelium regeneration . | Inflammation Non-specific/lymphocytic . | Apoptosis lamina propria and PCT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 (long, HRV) | −/− | 3 (stenosis) | +++ | −/− | Minimal | +++/− | a | |
| 2 | 10 (long, HRV) | −/− | 8 (stenosis) | + | −/+ | Cellular | Metaplastic | ++/− | a |
| 3 | 12 (long, HRV) | −/− | 9 (stenosis) | + | −/++ | Cellular | − | +++/− | a |
| 4 | 8 (average) | −/− | 0 (unintended) | − | −/− | − | − | −/− | ++ |
| 5 | 6 (average) | 1/1 | 16 (stenosis) | +++ | 30%/+ | Cellular | Metaplastic | +++/− | Minimal |
| 6 | 7 (average) | −/2 | 26 (stenosis) | + | 100%/+++ | Cellular | Metaplastic | −/− | + |
| 7 | 6 (average) | 2/1 | 48 (stenosis) | + | 80%/+++ | Collagenized | Metaplastic | +/− | Minimal |
| 8 | 5 (short) | 1/1 | 19 (suppuration) | +++ | 90%/+++ | Cellular | − | +++/− | + |
| 9 | 5 (short) | −/3 | 20 (suppuration) | +++ | 100%/+++ | Cellular | Metaplastic | +++/− | + |
| 10 | 4 (short) | 1/3 | 33 (sacrifice) | + | 100%/+++ | Cellular | Differentiated | ++/− | + |
| 11 | 4 (short) | 2/2 | 39 (sacrifice) | + | 100%/+++ | Cellular | Differentiated | ++/− | + |
| 12 | 4 (short) | 5/− | 47 (stenosis) | +++ | 90%/+++ | Cellular | Metaplastic | +/− | + |
| 13 | 4 (short) | 9/1 | 101 (sacrifice) | + | 60%/++ | Collagenized | Differentiated | +/− | Minimal |
| 14 | 4 (short) | 14/3 | 220 (sacrifice) | + | 100%/+++ | Collagenized | Metaplastic | −/− | − |
| Rabbit . | Tracheal rings (group) . | Bronchoscopy (n) Systematic/interventional . | Death day (cause) Sacrifice day . | Neoangiogenesis . | Cartilage ischaemia/calcification . | Lamina propria fibrosis . | Epithelium regeneration . | Inflammation Non-specific/lymphocytic . | Apoptosis lamina propria and PCT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 (long, HRV) | −/− | 3 (stenosis) | +++ | −/− | Minimal | +++/− | a | |
| 2 | 10 (long, HRV) | −/− | 8 (stenosis) | + | −/+ | Cellular | Metaplastic | ++/− | a |
| 3 | 12 (long, HRV) | −/− | 9 (stenosis) | + | −/++ | Cellular | − | +++/− | a |
| 4 | 8 (average) | −/− | 0 (unintended) | − | −/− | − | − | −/− | ++ |
| 5 | 6 (average) | 1/1 | 16 (stenosis) | +++ | 30%/+ | Cellular | Metaplastic | +++/− | Minimal |
| 6 | 7 (average) | −/2 | 26 (stenosis) | + | 100%/+++ | Cellular | Metaplastic | −/− | + |
| 7 | 6 (average) | 2/1 | 48 (stenosis) | + | 80%/+++ | Collagenized | Metaplastic | +/− | Minimal |
| 8 | 5 (short) | 1/1 | 19 (suppuration) | +++ | 90%/+++ | Cellular | − | +++/− | + |
| 9 | 5 (short) | −/3 | 20 (suppuration) | +++ | 100%/+++ | Cellular | Metaplastic | +++/− | + |
| 10 | 4 (short) | 1/3 | 33 (sacrifice) | + | 100%/+++ | Cellular | Differentiated | ++/− | + |
| 11 | 4 (short) | 2/2 | 39 (sacrifice) | + | 100%/+++ | Cellular | Differentiated | ++/− | + |
| 12 | 4 (short) | 5/− | 47 (stenosis) | +++ | 90%/+++ | Cellular | Metaplastic | +/− | + |
| 13 | 4 (short) | 9/1 | 101 (sacrifice) | + | 60%/++ | Collagenized | Differentiated | +/− | Minimal |
| 14 | 4 (short) | 14/3 | 220 (sacrifice) | + | 100%/+++ | Collagenized | Metaplastic | −/− | − |
HRV: heterotopic revascularization prior to orthotopic transplantation; PCT: pericartilaginous tissue.
aStudy not performed.
Clinical and pathological findings in 14 male rabbits undergoing tracheal transplantation with epithelium-denuded-cryopreserved tracheal allografts
| Rabbit . | Tracheal rings (group) . | Bronchoscopy (n) Systematic/interventional . | Death day (cause) Sacrifice day . | Neoangiogenesis . | Cartilage ischaemia/calcification . | Lamina propria fibrosis . | Epithelium regeneration . | Inflammation Non-specific/lymphocytic . | Apoptosis lamina propria and PCT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 (long, HRV) | −/− | 3 (stenosis) | +++ | −/− | Minimal | +++/− | a | |
| 2 | 10 (long, HRV) | −/− | 8 (stenosis) | + | −/+ | Cellular | Metaplastic | ++/− | a |
| 3 | 12 (long, HRV) | −/− | 9 (stenosis) | + | −/++ | Cellular | − | +++/− | a |
| 4 | 8 (average) | −/− | 0 (unintended) | − | −/− | − | − | −/− | ++ |
| 5 | 6 (average) | 1/1 | 16 (stenosis) | +++ | 30%/+ | Cellular | Metaplastic | +++/− | Minimal |
| 6 | 7 (average) | −/2 | 26 (stenosis) | + | 100%/+++ | Cellular | Metaplastic | −/− | + |
| 7 | 6 (average) | 2/1 | 48 (stenosis) | + | 80%/+++ | Collagenized | Metaplastic | +/− | Minimal |
| 8 | 5 (short) | 1/1 | 19 (suppuration) | +++ | 90%/+++ | Cellular | − | +++/− | + |
| 9 | 5 (short) | −/3 | 20 (suppuration) | +++ | 100%/+++ | Cellular | Metaplastic | +++/− | + |
| 10 | 4 (short) | 1/3 | 33 (sacrifice) | + | 100%/+++ | Cellular | Differentiated | ++/− | + |
| 11 | 4 (short) | 2/2 | 39 (sacrifice) | + | 100%/+++ | Cellular | Differentiated | ++/− | + |
| 12 | 4 (short) | 5/− | 47 (stenosis) | +++ | 90%/+++ | Cellular | Metaplastic | +/− | + |
| 13 | 4 (short) | 9/1 | 101 (sacrifice) | + | 60%/++ | Collagenized | Differentiated | +/− | Minimal |
| 14 | 4 (short) | 14/3 | 220 (sacrifice) | + | 100%/+++ | Collagenized | Metaplastic | −/− | − |
| Rabbit . | Tracheal rings (group) . | Bronchoscopy (n) Systematic/interventional . | Death day (cause) Sacrifice day . | Neoangiogenesis . | Cartilage ischaemia/calcification . | Lamina propria fibrosis . | Epithelium regeneration . | Inflammation Non-specific/lymphocytic . | Apoptosis lamina propria and PCT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 12 (long, HRV) | −/− | 3 (stenosis) | +++ | −/− | Minimal | +++/− | a | |
| 2 | 10 (long, HRV) | −/− | 8 (stenosis) | + | −/+ | Cellular | Metaplastic | ++/− | a |
| 3 | 12 (long, HRV) | −/− | 9 (stenosis) | + | −/++ | Cellular | − | +++/− | a |
| 4 | 8 (average) | −/− | 0 (unintended) | − | −/− | − | − | −/− | ++ |
| 5 | 6 (average) | 1/1 | 16 (stenosis) | +++ | 30%/+ | Cellular | Metaplastic | +++/− | Minimal |
| 6 | 7 (average) | −/2 | 26 (stenosis) | + | 100%/+++ | Cellular | Metaplastic | −/− | + |
| 7 | 6 (average) | 2/1 | 48 (stenosis) | + | 80%/+++ | Collagenized | Metaplastic | +/− | Minimal |
| 8 | 5 (short) | 1/1 | 19 (suppuration) | +++ | 90%/+++ | Cellular | − | +++/− | + |
| 9 | 5 (short) | −/3 | 20 (suppuration) | +++ | 100%/+++ | Cellular | Metaplastic | +++/− | + |
| 10 | 4 (short) | 1/3 | 33 (sacrifice) | + | 100%/+++ | Cellular | Differentiated | ++/− | + |
| 11 | 4 (short) | 2/2 | 39 (sacrifice) | + | 100%/+++ | Cellular | Differentiated | ++/− | + |
| 12 | 4 (short) | 5/− | 47 (stenosis) | +++ | 90%/+++ | Cellular | Metaplastic | +/− | + |
| 13 | 4 (short) | 9/1 | 101 (sacrifice) | + | 60%/++ | Collagenized | Differentiated | +/− | Minimal |
| 14 | 4 (short) | 14/3 | 220 (sacrifice) | + | 100%/+++ | Collagenized | Metaplastic | −/− | − |
HRV: heterotopic revascularization prior to orthotopic transplantation; PCT: pericartilaginous tissue.
aStudy not performed.
Macroscopic assessment
All TA segments had satisfactory stiffness regardless of length and/or prior heterotopic revascularization. Likewise, all TA segments showed well-vascularized grafts.
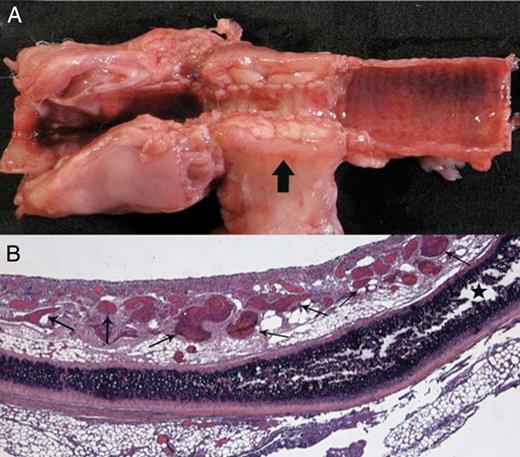
Transplantation of a long tracheal allograft segment after prior heterotopic revascularization (Rabbit 1). (A) Macroscopic aspect on Day 3: longitudinal section of the central airway showing stenosis of the fascial flap-wrapped graft, and circumferential thickening of the fascia (arrow). (B) Histological examination of the graft showing numerous neovessels in the lamina propria (small arrows), and a viable cartilage ring (star) (haematoxylin–eosin–saffron stain ×25).
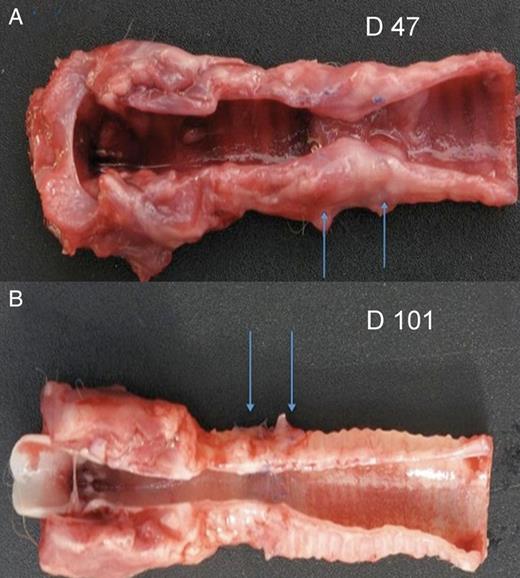
Tracheal transplantation of TA segments after single-stage orthotopic revascularization. (A) Macroscopic aspect on Day 47: longitudinal section of the central airway reveals severe stenosis of the graft (arrows), albeit with a well-epithelialized airway lining (Rabbit 12); and (B) a graft (arrows) well incorporated into the native trachea with satisfying airway patency and well-epithelialized airway lining (Rabbit 13, sacrificed on Day 101).
Aside from two animals having died from suppurative complications (Rabbits 8 and 9), and the sole animal with short TA length evolving towards late stenosis (Rabbit 12), the other animals with short TA length were sacrificed as planned (Rabbits 10, 11, 13 and 14) and exhibited good patency (Fig. 2B).
Bronchoscopic follow-up
Bronchoscopies reached an average of 4 per animal (range 2–18). Thirty-five were systematic bronchoscopies and 17 were interventional ones (Table 1): 11 aspirations of secretions and/or fibrin deposits; 6 stenosis dilatations and/or treatments of granulation tissue by application of local corticosteroids (Budesonide).
All grafts observed on Days 4/6 looked off-white colour. In animals undergoing bronchoscopies on Days 10/12, animals with average TA segment transplantation showed pink areas and then neovessels demonstrating epithelial regeneration and neoangiogenesis associated with graft stenosis (20–40% of the lumen) rapidly obstructive despite prompt bronchoscopic dilatations.
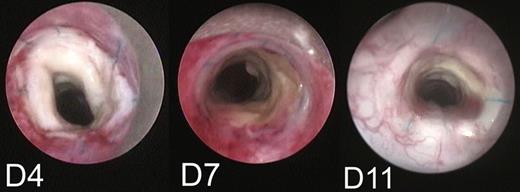
Bronchoscopic examination showing a whitish aspect of the graft lumen on Day 4. Signs of epithelial regeneration/revascularization mainly in the posterior wall of the graft appearing early on Day 7; and then, extending rapidly around the entire circumference on Day 11 (80% regeneration/revascularization).
Histopathological findings
Varying levels of neoangiogenesis involved the entire grafts, particularly the lamina propria (Fig. 1B), and were present in all types of TA, regardless of length and prior heterotopic or single-stage orthotopic revascularization.
Likewise, varying degrees of inflammatory infiltration involving the entire grafts were present in all TAs regardless of length and prior heterotopic or single-stage orthotopic revascularization, and were composed mainly of eosinophils, neutrophils and macrophages. In grafts studied after 45 days, this inflammation was attenuated. Interestingly, inflammation was always devoid of the lymphocyte cell population that is a hallmark of poor graft immune tolerance.
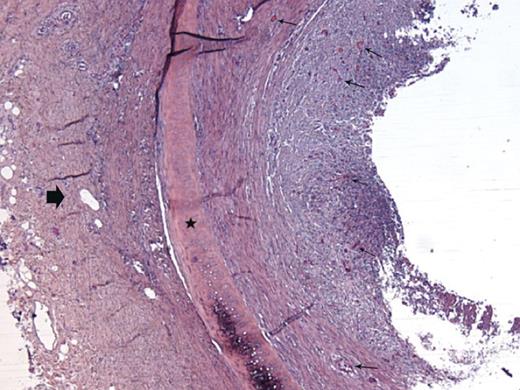
Histological examination of a fascial flap-wrapped TA segment after orthotopic transplantation on Day 9: almost complete obliteration of the graft lumen due to fibroblastic proliferation originating from the lamina propria rich in neocapillaries (small arrows); the cartilage ring is viable (star); the block arrow shows the surrounding fascia (Rabbit 3).
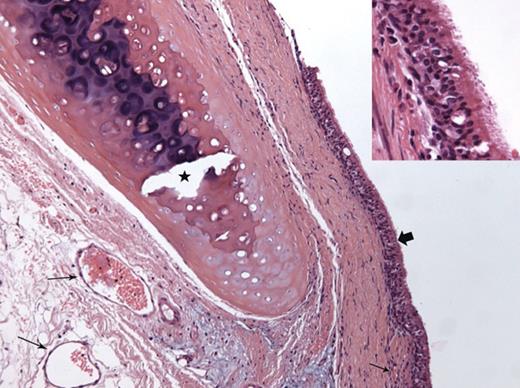
Histological examination of a TA segment after single-stage orthotopic revascularization (Rabbit 13, sacrificed on Day 101): evidence of graft revascularization (small arrows); respiratory epithelial regeneration is complete (block arrow and inset); collagenized fibrosis of the subjacent lamina propria, albeit with no thickening; the cartilage ring has been decalcified (star) (haematoxylin–eosin–saffron stain ×200; inset ×400).
Apoptosis
In Rabbit 4, which died within 24 h, moderate apoptotic events were observed in the cartilage. At the level of the lamina propria and pericartilaginous tissue, this apoptotic activity was more intense (see Supplementary Data). In the other animals, these events were only observed with a low intensity at the level of the lamina propria and pericartilaginous tissue (Table 1).
DISCUSSION
In our rabbit model, orthotopic transplantation of TAs showed that neoangiogenesis, mechanical properties and immune tolerance of TAs were satisfactory regardless of prior heterotopic or single-stage revascularization, and graft length.
Our previous disappointing experience with aortic allografts reinforced with tracheal rings [8] has led us to change our strategy in favour of cryopreserved-epithelium-denuded TAs in a well-described rabbit model that has demonstrated its relevance in several studies [7–9]. Heterotopic implantation of these cryopreserved-epithelium-denuded grafts demonstrated (1) immune tolerance, (2) absence of luminal obliteration by fibroblastic proliferation (even in the absence of an intraluminal tube) and (3) the preservation of the biomechanical rigidity due to the calcification of the cartilaginous rings [9].
By studying such cryopreserved-epithelium-denuded grafts orthotopically rather than heterotopically, our current results show interesting findings. As known, the tracheal epithelium is one of the major components of tracheal immune rejection, but on the other hand, the same epithelium plays an important role of barrier against infection [5] and its absence is a risk factor for occurrence of an acute suppuration of the graft as observed in two of our animals. A main finding of our study is that, contrary to the results of grafts in a heterotopic position, we found that orthotopically, there is a real risk of luminal narrowing due to fibroblast proliferation originating from the lamina propria. This difference may be due to the graft environment, which was much more inflammatory orthotopically rather than heteropically, possibly because of oro-pharyngo-tracheal microbiological contamination [8]. Weidenbecher et al. also observed similar findings in a rabbit model during investigations of a neotrachea constructed with a muscle flap and a sheet of cartilage obtained by culture of chondrocytes, which were rolled around a silicone tube and implanted under the skin. After 6–10 weeks, the harvested constructs were viable and displayed stiffness and a luminal shape comparable in size with that of a native rabbit trachea. However, following orthotopic implantation, neotracheas displayed endoluminal fibroblastic proliferation leading to death of animals from 7 to 37 days [17, 19].
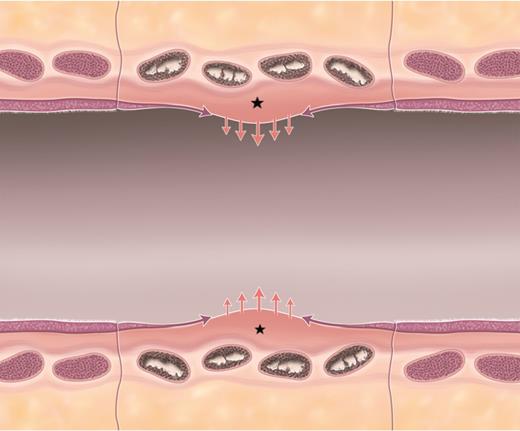
Schematic of ‘competition’ between fibroblastic proliferation and re-epithelialization. Longitudinal view of a short length free tracheal graft segment (central segment). The schematic shows transverse sections of four cartilage rings in the process of calcification. Pink arrows show the tendency of fibroblastic proliferation originating from the lamina propria (stars) trending towards stenosis in all grafts. Possibly countering or limiting this stenotic proliferation, purple arrows show the concomitant re-epithelialization of the airway surface. Thus, a hypothetical ‘competition’ between these two processes and their relative intensity and/or speed may explain the variable evolution over time of different graft lengths towards stenotic complications or moderate stenosis manageable through bronchoscopic interventions.
Similarly to heterotopic results, neither acute rejection leading to microvascular thrombosis and consecutive graft necrosis [10] nor significant chronic rejection was shown in orthotopically implanted TA segments. There was no lymphocytic infiltration, and apoptotic events were only observed at low intensities in the lamina propria/pericartilaginous tissue. Apoptosis levels seemed even lower orthotopically than heterotopically [9] suggesting even better immune tolerance, which is not surprising as the epithelial regeneration contributes to graft tolerance by the host [22]. Nonetheless, cartilaginous rings displayed progressive evanescence of chondrocytes as previously described in heterotopy [9]. Tanaka et al. hypothesized that the relative ischaemia of cartilage during the initial phase of revascularization provoked apoptosis and consecutive dystrophic cartilage calcification rather than necrosis [15].
Finally, despite this favourable immune tolerance and satisfactory strain abilities of allografts due to cartilage calcification deposits, the fibroblastic proliferation originating from the lamina propria remained a severe impediment for tracheal transplantation with long-segment of epithelium-denuded TAs (even following prior heterotopic revascularization). This difficulty might be solved by means of a temporary intraluminal silicone stent, limiting stenosis until healing of the mucosal lining and epithelial regeneration is achieved. However, the maintenance of such small silicone stent (4 mm inner diameter) in the rabbit is challenging [8]. Another option could be the administration of systemic corticosteroids.
Limitations of the study
As demonstrated by literature data in the field of tracheal replacement and transplantation [8, 10, 15, 16, 19, 23, 24] (Table 2), the rabbit is a fragile animal model, which may have been a drawback of our study. In fact, death may occur rapidly without any prodrome, which may have been the case of the unexpected death in less than 24 h of one of our animals, and the cases of animals of the first two groups, which sustained severe stenosis. Likewise, two animals were lost to suppurative complications. Thus, in accordance with experimental animal use guidelines, we reduced the number of rabbits undergoing tracheal transplantation in the first two groups resulting in a small number of studied animals. Another limitation of our study is the fact that two different methods of graft revascularization were used. Given that the lateral thoracic fascia includes a muscular layer, the heterotopic revascularization method is, however, not so different from that applied orthotopically with the strap muscles.
| Authors (year) . | No. of rabbits . | Operative mortality . | 3-week mortality . |
|---|---|---|---|
| Delaere et al. [10] | 15 | – | 8 |
| Dodge-Khatami et al. [23] | 7 | – | 3a |
| Tanaka et al. [15] | 7 | – | 2 |
| Weidenbecher et al. [19] | 6 | – | 4 |
| Shi et al. [16] | 15 | – | 5 |
| Seguin et al. [24] | 34 | 2 | 4 |
| Wurtz et al. [8] | 10 | 1 | 3 |
| Present study | 14 | – | 7 |
| Authors (year) . | No. of rabbits . | Operative mortality . | 3-week mortality . |
|---|---|---|---|
| Delaere et al. [10] | 15 | – | 8 |
| Dodge-Khatami et al. [23] | 7 | – | 3a |
| Tanaka et al. [15] | 7 | – | 2 |
| Weidenbecher et al. [19] | 6 | – | 4 |
| Shi et al. [16] | 15 | – | 5 |
| Seguin et al. [24] | 34 | 2 | 4 |
| Wurtz et al. [8] | 10 | 1 | 3 |
| Present study | 14 | – | 7 |
aEuthanasia for acute respiratory distress.
| Authors (year) . | No. of rabbits . | Operative mortality . | 3-week mortality . |
|---|---|---|---|
| Delaere et al. [10] | 15 | – | 8 |
| Dodge-Khatami et al. [23] | 7 | – | 3a |
| Tanaka et al. [15] | 7 | – | 2 |
| Weidenbecher et al. [19] | 6 | – | 4 |
| Shi et al. [16] | 15 | – | 5 |
| Seguin et al. [24] | 34 | 2 | 4 |
| Wurtz et al. [8] | 10 | 1 | 3 |
| Present study | 14 | – | 7 |
| Authors (year) . | No. of rabbits . | Operative mortality . | 3-week mortality . |
|---|---|---|---|
| Delaere et al. [10] | 15 | – | 8 |
| Dodge-Khatami et al. [23] | 7 | – | 3a |
| Tanaka et al. [15] | 7 | – | 2 |
| Weidenbecher et al. [19] | 6 | – | 4 |
| Shi et al. [16] | 15 | – | 5 |
| Seguin et al. [24] | 34 | 2 | 4 |
| Wurtz et al. [8] | 10 | 1 | 3 |
| Present study | 14 | – | 7 |
aEuthanasia for acute respiratory distress.
Finally, systematic and interventional bronchoscopy played an important role in our results by allowing long survival in some animals undergoing short TA transplantation through secretion suctioning and treatment of moderate stenosis.
In the clinical setting, there may be potential applications of such short TA segments. In standard tracheal surgery, they might be used when the extent of the airway resection precludes reconstruction by tension-free anastomosis, or in the case of tracheal dehiscence. In both cases, single-stage orthotopic revascularization of the graft with well-vascularized tissues such as the pectoral muscle flap, thymopericardial fat flap or epiploon should be mandatory [1].
In conclusion, short segments of epithelium-denuded-cryopreserved TA may be the most reliable for tracheal transplantation in the rabbit model without problems related to graft stiffness or immune rejection. However, investigations in larger animal models (i.e. minipig or sheep) have to be conducted to develop long tracheal substitutes available for the treatment of extended tracheal tumours, benign stenoses and malacia. In the minipig, in the absence of lateral thoracic fascia, the revascularization in heterotopy of long TA segments could be achieved by means of a flap wrap in the omentum, which has the ability to reach the tracheal region. Furthermore, bronchoscopic interventions such as silicone stent placement and removal can be more easily performed in larger animals. Additionally, transplanting minipigs with gender mismatch could allow for fluorescent in situ hybridization analysis for both X and Y chromosome identification to demonstrate epithelial cell ingrowth from the recipient into the graft. Lastly, formal biomechanical measurements of grafts could be performed with the adequate physical strain test device.
In patients, anatomical studies will be necessary to define the best potential site for heterotopic revascularization. Besides the omental flap, the best candidates could be the fascia lata flap with an identifiable vascular pedicle transposable as a free flap; or the latissimus dorsi muscle, which can be used as either a free or pedicled flap [25]. Subsequent orthotopic transplantation could be achieved with the use of a temporary silicone stent to protect the graft from a potential suppuration and avoid endoluminal fibroblastic proliferation, thus facilitating epithelial cell ingrowth. This treatment plan may seem complex, but one must not forget that circumferential tracheal transplantation is a challenging problem that surgeons have not yet solved in the past 60 years.
SUPPLEMENTARY MATERIAL
ACKNOWLEDGEMENTS
We are grateful to Arnold Dive, Martin Fourdrinier and Michel Pottier for their contributions to this work.
Funding
This work was supported by the Région Nord-Pas-de-Calais, Fond Régional à l'Innovation (FRI OSEO).
Conflict of interest: none declared.
REFERENCES
- apoptosis
- ischemia
- inflammation
- epithelium
- fibrosis
- cartilage
- constriction, pathologic
- cryopreservation
- immune tolerance
- lymphocytes
- neovascularization, pathologic
- oryctolagus cuniculus
- rejection (psychology)
- tissue transplants
- therapeutic immunosuppression
- trachea
- transplantation
- revascularization
- tracheal ring
- allografting
- fibroblast proliferation
- lamina propria
- calcification
- donors




