-
PDF
- Split View
-
Views
-
Cite
Cite
Francesco Onorati, Fausto Biancari, Marisa De Feo, Giovanni Mariscalco, Antonio Messina, Giuseppe Santarpino, Francesco Santini, Cesare Beghi, Giannantonio Nappi, Giovanni Troise, Theodor Fischlein, Giancarlo Passerone, Juni Heikkinen, Giuseppe Faggian, Mid-term results of aortic valve surgery in redo scenarios in the current practice: results from the multicentre European RECORD (REdo Cardiac Operation Research Database) initiative, European Journal of Cardio-Thoracic Surgery, Volume 47, Issue 2, February 2015, Pages 269–280, https://doi.org/10.1093/ejcts/ezu116
Close - Share Icon Share
Abstract
Although commonly reported as single-centre experiences, redo aortic valve replacement (RAVR) has overall acceptable results. Nevertheless, trans-catheter aortic valve replacement has recently questioned the efficacy of RAVR.
Early-to-mid-term results and determinants of mortality in 711 cases of RAVR from seven European institutions were assessed in the entire population and in selected high-risk subgroups [elderly >75 years, urgent/emergent procedures, preoperative New York Heart Association (NYHA) functional Class IV and endocarditis].
Hospital mortality was 5.1%, major re-entry cardiovascular complications (MRCVCs) 4.9%, low cardiac output syndrome (LCOS) 15.3%, stroke 6.6%, acute respiratory failure (ARF) 10.6%, acute renal insufficiency (ARI) 19.3% and need for continuous renal replacement therapy (CRRT) 7.2%, transfusions 66.9% and for permanent pacemaker (PMK) 12.7%. Mid-term survival, freedom from acute heart failure (AHF), reinterventions, stroke and thrombo-embolisms were 77.2 ± 2.7, 84.4 ± 2.6, 97.2 ± 0.8, 97.2 ± 0.9 and 96.3 ± 1.2%, respectively; 87.5% of patients were in NYHA functional Class I–II. Preoperative left ventricular ejection fraction of <30% [odds ratio (OR) 8.7, 95% confidence interval (CI) 2.1–35.6], MRCVCs (OR 20.9, 95% CI 5.6–78.3), cardiopulmonary bypass time (OR 1.1, 95% CI 1.0–1.1), perioperative LCOS (OR 17.2, 95% CI 5.1–57.4) and ARI (OR 5.1, 95% CI 1.5–18.1) predicted hospital death. Endocarditis (OR 7.5, 95% CI 2.9–19.1), preoperative NYHA functional Class IV (OR 4.7, 95% CI 1.0–24.0), combined RAVR + mitral surgery (OR 5.1, 95% CI 1.5–17.3) and AHF at follow-up (OR 2.8, 95% CI 1.3–6.0) predicted late death at the Cox proportional hazard regression model. Elderly >75 years had similar hospital mortality (P = 0.06) and major morbidity, except for a higher need for PMK (P = 0.03), as well as comparable mid-term survival (P = 0.89), freedom from AHF (P = 0.81), reinterventions (P = 0.63), stroke (P = 0.21) and thrombo-embolisms (P = 0.09). Urgent/emergent indication resulted in higher hospital death, LCOS, transfusions, MRCVCs, intra-aortic balloon pumping (IABP), stroke, prolonged (>48 h) ventilation, pneumonia, ARI, CRRT, lower mid-term survival and freedom from AHF (P ≤ 0.03). Preoperative NYHA functional Class IV correlated with higher LCOS, IABP, prolonged ventilation, pneumonia, ARF, ARI, CRRT and MRCVCs and lower mid-term survival, freedom from AHF, reinterventions and stroke (P ≤ 0.02). Endocarditis demonstrated higher hospital mortality, MRCVCs, LCOS, IABP, stroke, ARF, prolonged intubation, pneumonia, ARI, CRRT, transfusions and PMK and lower mid-term survival and freedom from AHF and reinterventions (P ≤ 0.04).
RAVR achieves overall satisfactory results. Baseline risk factors and perioperative complications strongly affect outcomes and mandate improvements in perioperative management. New emerging strategies might be considered in selected high-risk cases.
INTRODUCTION
The exponential growth of the geriatric population during the last decades has led to a corresponding increase in the number of patients referred to cardiac surgery for redo procedures [1, 2]. In particular, the referral of patients with aortic valve disease after previous cardiac operations needing aortic valve replacement (AVR) (redo aortic valve replacement, RAVR) has critically grown [1, 2]. RAVR is a technically demanding procedure when compared with primary AVR, because of the scarred surgical field, the risk of iatrogenic injury to cardiovascular structures, the longer cross-clamping and cardiopulmonary bypass (CPB) times and the higher risks of bleeding, transfusions and transfusion-related morbidity. The increased risk of reoperations has recently lead to a widespread popularization of transcatheter aortic valve implantation (TAVI) in previously operated patients with a new onset of native aortic valve disease and of valve-in-valve TAVI in case of bioprosthetic malfunction, vis-à-vis the traditional replacement procedures [3, 4].
The extreme variability in surgical mortality reported after RAVR over the years appears mainly related to differences in patient risk profile, surgical skill and related hospitals referral. Moreover, the single-centre design of the majority of published studies and the lack of long-term data further contribute to confounding outcome results [1, 2, 5, 6]. Indeed, to the best of our knowledge, the literature lacks multicentre experiences with mid-to-long-term outcome data.
The aim of this study was to analyse in-hospital and mid-term results of a consecutive series of patients undergoing RAVR at seven different European centres during the last 10 years. Furthermore, we analysed subgroups of patients traditionally considered at high surgical risk, in order to identify those potentially better served with alternative procedures.
MATERIALS AND METHODS
This is an analysis of 711 consecutive patients operated on from 2003 to 2013 in a total of seven centres in Italy, Germany and Finland and contributing to the multicentre REdo Cardiac Operation Research Database (RECORD). Baseline and operative characteristics of patients are summarized in Tables 1 and 2. The only inclusion criterion for this study was any surgical AVR performed in patients with a history of prior cardiac surgery. However, the ‘isolated RAVR’ subgroup identified those patients receiving only an AVR—in the context of a redo cardiac procedure—as the index procedure (i.e. either as a ‘first’ or as a ‘repeated’ procedure); furthermore, the ‘repeat-AVR’ subgroup identified only those patients receiving at least a repeated AVR procedure for a failed previous AVR. We excluded patients who underwent TAVI because of the absence of late outcome data. Subsets of patients with acute endocarditis, elderly patients (>75 years), urgent/emergent procedures and preoperative NYHA functional Class IV were considered at high operative risk according to previously published studies [1, 2, 5–9] and were the subjects of sensitivity analysis.
| Variable . | Mean ± SD or n (%) . |
|---|---|
| Contributing centres | |
| Verona | 108 (15.2) |
| Oulu | 47 (6.6) |
| Naples | 87 (12.2) |
| Nuremberg | 206 (29.0) |
| Brescia | 124 (17.4) |
| Varese | 95 (13.4) |
| Genoa | 44 (6.2) |
| Age (years) | 68.2 ± 13.5 |
| Height (cm) | 167.5 ± 9.9 |
| Weight (kg) | 74.9 ± 14.7 |
| Body mass index | 26.9 ± 9.5 |
| EuroSCORE (additive) | 10.0 ± 3.4 |
| Age (by range, years) | |
| ≤25 | 4 (0.6) |
| 26–35 | 12 (1.7) |
| 36–45 | 47 (6.6) |
| 46–55 | 61 (8.6) |
| 56–65 | 134 (18.8) |
| 66–75 | 210 (29.5) |
| >75 | 243 (34.2) |
| Female gender | 249 (35.0) |
| Systemic hypertension | 367 (51.7) |
| Dyslipidaemia | 309 (43.5) |
| Diabetes mellitus | 165 (23.2) |
| Current smoker | 124 (20.2) |
| Peripheral vasculopathy | 74 (10.4) |
| Chronic obstructive pulmonary disease | 109 (15.3) |
| Chronic steroid therapy | 22 (3.1) |
| Pulmonary hypertension | 92 (12.9) |
| Chronic renal insufficiency | 120 (16.9) |
| Cerebrovascular disease | 89 (12.5) |
| Previous stroke | 79 (11.1) |
| Previous acute myocardial infarction | 86 (12.1) |
| Acute arrhythmias on admission | 148 (20.8) |
| Endocarditic aetiology | 154 (21.7) |
| Recurrent endocarditis | 72 (10.1) |
| Left ventricular ejection fraction (%; Simpson's method) | |
| >50 | 429 (60.3) |
| 30–50 | 241 (33.9) |
| <30 | 41 (5.8) |
| Canadian Cardiovascular Society admission | |
| I | 530 (74.5) |
| II | 61 (8.6) |
| III | 59 (8.3) |
| IV | 61 (8.6) |
| Preoperative NYHA functional Class | |
| I | 28 (3.9) |
| II | 202 (28.5) |
| III | 372 (52.3) |
| IV | 109 (15.3) |
| Urgent/emergent priority | 192 (27.0) |
| Previous CABG | 232 (32.6) |
| Previous valve surgery | 453 (63.7) |
| Previous AVR | 324 (71.5) |
| Previous mitral surgery | 126 (27.8) |
| Mitral valve repair | 88 (69.8) |
| Mitral valve replacement | 38 (30.2) |
| Other valve procedures | 3 (0.7) |
| Previous aortic surgery | 46 (6.5) |
| Variable . | Mean ± SD or n (%) . |
|---|---|
| Contributing centres | |
| Verona | 108 (15.2) |
| Oulu | 47 (6.6) |
| Naples | 87 (12.2) |
| Nuremberg | 206 (29.0) |
| Brescia | 124 (17.4) |
| Varese | 95 (13.4) |
| Genoa | 44 (6.2) |
| Age (years) | 68.2 ± 13.5 |
| Height (cm) | 167.5 ± 9.9 |
| Weight (kg) | 74.9 ± 14.7 |
| Body mass index | 26.9 ± 9.5 |
| EuroSCORE (additive) | 10.0 ± 3.4 |
| Age (by range, years) | |
| ≤25 | 4 (0.6) |
| 26–35 | 12 (1.7) |
| 36–45 | 47 (6.6) |
| 46–55 | 61 (8.6) |
| 56–65 | 134 (18.8) |
| 66–75 | 210 (29.5) |
| >75 | 243 (34.2) |
| Female gender | 249 (35.0) |
| Systemic hypertension | 367 (51.7) |
| Dyslipidaemia | 309 (43.5) |
| Diabetes mellitus | 165 (23.2) |
| Current smoker | 124 (20.2) |
| Peripheral vasculopathy | 74 (10.4) |
| Chronic obstructive pulmonary disease | 109 (15.3) |
| Chronic steroid therapy | 22 (3.1) |
| Pulmonary hypertension | 92 (12.9) |
| Chronic renal insufficiency | 120 (16.9) |
| Cerebrovascular disease | 89 (12.5) |
| Previous stroke | 79 (11.1) |
| Previous acute myocardial infarction | 86 (12.1) |
| Acute arrhythmias on admission | 148 (20.8) |
| Endocarditic aetiology | 154 (21.7) |
| Recurrent endocarditis | 72 (10.1) |
| Left ventricular ejection fraction (%; Simpson's method) | |
| >50 | 429 (60.3) |
| 30–50 | 241 (33.9) |
| <30 | 41 (5.8) |
| Canadian Cardiovascular Society admission | |
| I | 530 (74.5) |
| II | 61 (8.6) |
| III | 59 (8.3) |
| IV | 61 (8.6) |
| Preoperative NYHA functional Class | |
| I | 28 (3.9) |
| II | 202 (28.5) |
| III | 372 (52.3) |
| IV | 109 (15.3) |
| Urgent/emergent priority | 192 (27.0) |
| Previous CABG | 232 (32.6) |
| Previous valve surgery | 453 (63.7) |
| Previous AVR | 324 (71.5) |
| Previous mitral surgery | 126 (27.8) |
| Mitral valve repair | 88 (69.8) |
| Mitral valve replacement | 38 (30.2) |
| Other valve procedures | 3 (0.7) |
| Previous aortic surgery | 46 (6.5) |
AVR: aortic valve replacement; CABG: coronary artery bypass grafting; NYHA: New York Heart Association.
| Variable . | Mean ± SD or n (%) . |
|---|---|
| Contributing centres | |
| Verona | 108 (15.2) |
| Oulu | 47 (6.6) |
| Naples | 87 (12.2) |
| Nuremberg | 206 (29.0) |
| Brescia | 124 (17.4) |
| Varese | 95 (13.4) |
| Genoa | 44 (6.2) |
| Age (years) | 68.2 ± 13.5 |
| Height (cm) | 167.5 ± 9.9 |
| Weight (kg) | 74.9 ± 14.7 |
| Body mass index | 26.9 ± 9.5 |
| EuroSCORE (additive) | 10.0 ± 3.4 |
| Age (by range, years) | |
| ≤25 | 4 (0.6) |
| 26–35 | 12 (1.7) |
| 36–45 | 47 (6.6) |
| 46–55 | 61 (8.6) |
| 56–65 | 134 (18.8) |
| 66–75 | 210 (29.5) |
| >75 | 243 (34.2) |
| Female gender | 249 (35.0) |
| Systemic hypertension | 367 (51.7) |
| Dyslipidaemia | 309 (43.5) |
| Diabetes mellitus | 165 (23.2) |
| Current smoker | 124 (20.2) |
| Peripheral vasculopathy | 74 (10.4) |
| Chronic obstructive pulmonary disease | 109 (15.3) |
| Chronic steroid therapy | 22 (3.1) |
| Pulmonary hypertension | 92 (12.9) |
| Chronic renal insufficiency | 120 (16.9) |
| Cerebrovascular disease | 89 (12.5) |
| Previous stroke | 79 (11.1) |
| Previous acute myocardial infarction | 86 (12.1) |
| Acute arrhythmias on admission | 148 (20.8) |
| Endocarditic aetiology | 154 (21.7) |
| Recurrent endocarditis | 72 (10.1) |
| Left ventricular ejection fraction (%; Simpson's method) | |
| >50 | 429 (60.3) |
| 30–50 | 241 (33.9) |
| <30 | 41 (5.8) |
| Canadian Cardiovascular Society admission | |
| I | 530 (74.5) |
| II | 61 (8.6) |
| III | 59 (8.3) |
| IV | 61 (8.6) |
| Preoperative NYHA functional Class | |
| I | 28 (3.9) |
| II | 202 (28.5) |
| III | 372 (52.3) |
| IV | 109 (15.3) |
| Urgent/emergent priority | 192 (27.0) |
| Previous CABG | 232 (32.6) |
| Previous valve surgery | 453 (63.7) |
| Previous AVR | 324 (71.5) |
| Previous mitral surgery | 126 (27.8) |
| Mitral valve repair | 88 (69.8) |
| Mitral valve replacement | 38 (30.2) |
| Other valve procedures | 3 (0.7) |
| Previous aortic surgery | 46 (6.5) |
| Variable . | Mean ± SD or n (%) . |
|---|---|
| Contributing centres | |
| Verona | 108 (15.2) |
| Oulu | 47 (6.6) |
| Naples | 87 (12.2) |
| Nuremberg | 206 (29.0) |
| Brescia | 124 (17.4) |
| Varese | 95 (13.4) |
| Genoa | 44 (6.2) |
| Age (years) | 68.2 ± 13.5 |
| Height (cm) | 167.5 ± 9.9 |
| Weight (kg) | 74.9 ± 14.7 |
| Body mass index | 26.9 ± 9.5 |
| EuroSCORE (additive) | 10.0 ± 3.4 |
| Age (by range, years) | |
| ≤25 | 4 (0.6) |
| 26–35 | 12 (1.7) |
| 36–45 | 47 (6.6) |
| 46–55 | 61 (8.6) |
| 56–65 | 134 (18.8) |
| 66–75 | 210 (29.5) |
| >75 | 243 (34.2) |
| Female gender | 249 (35.0) |
| Systemic hypertension | 367 (51.7) |
| Dyslipidaemia | 309 (43.5) |
| Diabetes mellitus | 165 (23.2) |
| Current smoker | 124 (20.2) |
| Peripheral vasculopathy | 74 (10.4) |
| Chronic obstructive pulmonary disease | 109 (15.3) |
| Chronic steroid therapy | 22 (3.1) |
| Pulmonary hypertension | 92 (12.9) |
| Chronic renal insufficiency | 120 (16.9) |
| Cerebrovascular disease | 89 (12.5) |
| Previous stroke | 79 (11.1) |
| Previous acute myocardial infarction | 86 (12.1) |
| Acute arrhythmias on admission | 148 (20.8) |
| Endocarditic aetiology | 154 (21.7) |
| Recurrent endocarditis | 72 (10.1) |
| Left ventricular ejection fraction (%; Simpson's method) | |
| >50 | 429 (60.3) |
| 30–50 | 241 (33.9) |
| <30 | 41 (5.8) |
| Canadian Cardiovascular Society admission | |
| I | 530 (74.5) |
| II | 61 (8.6) |
| III | 59 (8.3) |
| IV | 61 (8.6) |
| Preoperative NYHA functional Class | |
| I | 28 (3.9) |
| II | 202 (28.5) |
| III | 372 (52.3) |
| IV | 109 (15.3) |
| Urgent/emergent priority | 192 (27.0) |
| Previous CABG | 232 (32.6) |
| Previous valve surgery | 453 (63.7) |
| Previous AVR | 324 (71.5) |
| Previous mitral surgery | 126 (27.8) |
| Mitral valve repair | 88 (69.8) |
| Mitral valve replacement | 38 (30.2) |
| Other valve procedures | 3 (0.7) |
| Previous aortic surgery | 46 (6.5) |
AVR: aortic valve replacement; CABG: coronary artery bypass grafting; NYHA: New York Heart Association.
| Number of reintervention | |
| 1st | 626 (88.0) |
| 2nd | 62 (8.7) |
| 3rd | 20 (2.8) |
| 4th | 3 (0.4) |
| Type of intervention | |
| RAVR | 512 (72.0) |
| RAVR + CABG | 57 (8.0) |
| RAVR + mitral surgery | 77 (10.8) |
| RAVR + other | 65 (9.1) |
| Type of prosthesis | |
| Biological | 390 (54.9) |
| Mechanical | 294 (41.4) |
| Homograft | 8 (1.1) |
| Sutureless | 19 (2.7) |
| Diameter of implanted prosthesis (mm) | |
| 17 | 7 (1.0) |
| 19 | 61 (8.5) |
| 20 | 8 (1.1) |
| 21 | 186 (26.2) |
| 22 | 29 (4.1) |
| 23 | 224 (31.5) |
| 24 | 25 (3.5) |
| 25 | 97 (13.6) |
| >25 | 74 (10.5) |
| Aortic cross-clamp time (min) | 97.6 ± 46.1 |
| Cardiopulmonary bypass time (min) | 145.7 ± 64.8 |
| Number of reintervention | |
| 1st | 626 (88.0) |
| 2nd | 62 (8.7) |
| 3rd | 20 (2.8) |
| 4th | 3 (0.4) |
| Type of intervention | |
| RAVR | 512 (72.0) |
| RAVR + CABG | 57 (8.0) |
| RAVR + mitral surgery | 77 (10.8) |
| RAVR + other | 65 (9.1) |
| Type of prosthesis | |
| Biological | 390 (54.9) |
| Mechanical | 294 (41.4) |
| Homograft | 8 (1.1) |
| Sutureless | 19 (2.7) |
| Diameter of implanted prosthesis (mm) | |
| 17 | 7 (1.0) |
| 19 | 61 (8.5) |
| 20 | 8 (1.1) |
| 21 | 186 (26.2) |
| 22 | 29 (4.1) |
| 23 | 224 (31.5) |
| 24 | 25 (3.5) |
| 25 | 97 (13.6) |
| >25 | 74 (10.5) |
| Aortic cross-clamp time (min) | 97.6 ± 46.1 |
| Cardiopulmonary bypass time (min) | 145.7 ± 64.8 |
The values are denoted as mean ± SD or n (%).
RAVR: redo aortic valve replacement.
| Number of reintervention | |
| 1st | 626 (88.0) |
| 2nd | 62 (8.7) |
| 3rd | 20 (2.8) |
| 4th | 3 (0.4) |
| Type of intervention | |
| RAVR | 512 (72.0) |
| RAVR + CABG | 57 (8.0) |
| RAVR + mitral surgery | 77 (10.8) |
| RAVR + other | 65 (9.1) |
| Type of prosthesis | |
| Biological | 390 (54.9) |
| Mechanical | 294 (41.4) |
| Homograft | 8 (1.1) |
| Sutureless | 19 (2.7) |
| Diameter of implanted prosthesis (mm) | |
| 17 | 7 (1.0) |
| 19 | 61 (8.5) |
| 20 | 8 (1.1) |
| 21 | 186 (26.2) |
| 22 | 29 (4.1) |
| 23 | 224 (31.5) |
| 24 | 25 (3.5) |
| 25 | 97 (13.6) |
| >25 | 74 (10.5) |
| Aortic cross-clamp time (min) | 97.6 ± 46.1 |
| Cardiopulmonary bypass time (min) | 145.7 ± 64.8 |
| Number of reintervention | |
| 1st | 626 (88.0) |
| 2nd | 62 (8.7) |
| 3rd | 20 (2.8) |
| 4th | 3 (0.4) |
| Type of intervention | |
| RAVR | 512 (72.0) |
| RAVR + CABG | 57 (8.0) |
| RAVR + mitral surgery | 77 (10.8) |
| RAVR + other | 65 (9.1) |
| Type of prosthesis | |
| Biological | 390 (54.9) |
| Mechanical | 294 (41.4) |
| Homograft | 8 (1.1) |
| Sutureless | 19 (2.7) |
| Diameter of implanted prosthesis (mm) | |
| 17 | 7 (1.0) |
| 19 | 61 (8.5) |
| 20 | 8 (1.1) |
| 21 | 186 (26.2) |
| 22 | 29 (4.1) |
| 23 | 224 (31.5) |
| 24 | 25 (3.5) |
| 25 | 97 (13.6) |
| >25 | 74 (10.5) |
| Aortic cross-clamp time (min) | 97.6 ± 46.1 |
| Cardiopulmonary bypass time (min) | 145.7 ± 64.8 |
The values are denoted as mean ± SD or n (%).
RAVR: redo aortic valve replacement.
The choice of analysing data from 2003—thus excluding earlier patients—was taken in order to avoid potential biases related to differences in perioperative management and care, as well as to have a picture of ‘current’ RAVR practice. All data related to hospitalization for the index procedure were retrieved from Institutional databases and hospital charts. Data on hospital course were available for all patients. Follow-up events were collected from rehabilitation' clinic charts, outpatient clinics at the individual institutions, phone contacts with referral cardiologists or general practitioners, linking with regional Social Security Death and Events Master files where available, or—in the absence of recent data—by direct phone contact with patients. Only 2 patients were lost during follow-up, which was therefore 99.7% completed. Institutional review board/Ethical Committee approved the study, but individual patient consent was waived due to the retrospective, observational nature of the study.
Surgery
The choice of a mechanical or a biological prosthesis was based on single institutional policies and on patient preference after adequate informed consent. Anaesthesia, surgery and CPB were similarly based on individual institution' standardized protocols. Preoperative chest computed tomography (CT) was not performed in all patients, based on individual institutional policies. More in detail, 402 (56.5%) patients underwent preoperative CT scan. Surgical access consisted in a median full resternotomy in all but 13 (1.8%) patients, where a ‘J-shaped’ mini-sternotomy was employed. Peripheral cannulation was chosen in 199 (28.0%) patients of the entire cohort, because surgical re-entry via median resternotomy was deemed high risk. Postoperative care was similarly left to individual institution's standardized protocols.
Outcome endpoints
Primary endpoint was ‘hospital mortality’, defined as all-cause mortality during the index hospitalization (including rehabilitation hospital stay if discharged to rehabilitation clinic) or during the first 30 postoperative days (if discharged home), and ‘late mortality’, defined as all-cause mortality occurring during follow-up. Cardiovascular mortality was defined as any fatal cardiovascular event. Secondary outcome endpoints were hospital complications and follow-up freedom from acute heart failure, reoperation, stroke and thrombo-embolisms [6, 10].
The following hospital complications were collected: (i) major cardiovascular re-entry complications, defined as any severe and/or life-threatening (i.e. requiring reanimation and/or immediate changing of the surgical plan and/or massive transfusions >4 red packed cells) injury of major vessels or cardiac structures that occurred during surgical re-entry; (ii) revision for bleeding, defined as any reoperation during the index hospitalization, due to postoperative bleeding; (iii) need for permanent pacemaker (PMK) implantation; (iv) low cardiac output syndrome (LCOS), defined as haemodynamic instability for >1 h during the ICU stay, with signs of peripheral hypo-perfusion, despite inotropic support and adequate correction of preload, afterload and all electrolyte and blood gas abnormalities [6]; (v) need for intraoperative/postoperative intra-aortic balloon pumping (IABP); (vi) acute myocardial infarction, defined as Type 5 acute myocardial infarction (AMI) according to the current guidelines [11]; (vii) prolonged intubation, defined as the need for prolonged (>48 h) mechanical ventilation; (viii) acute respiratory failure (ARF), defined as prolonged intubation and/or respiratory insufficiency after extubation with the need for reintubation or the need for non-invasive ventilation lasting >48 h [6]; (ix) pneumonia, defined as evidence of bacterial growth in the lung with at least one positive bronchoalveolar fluid lavage culture, together with new alveolar infiltrates at chest roentgenogram, irrespective of the presence of fever or leucocytosis, or as evidence of new alveolar infiltrates with leucocytosis and purulent sputum, confirmed by CT scan and/or by consultation of an independent infectivologist or pneumologist [6]; (x) stroke, defined as for current guidelines [6, 10, 12]: briefly, it was defined as perioperative cerebrovascular accident, whose symptoms lasted >24 h with or without residual disability, confirmed at CT or magnetic resonance imaging; in case of no evidence of stroke at neuro-imaging, the diagnosis of stroke was made by consultant neurologists [13]; (xi) acute renal insufficiency (ARI), defined as a >50% increase over the preoperative serum creatinine value [6, 12, 13]; (xii) need for continuous renal replacement therapy (CRRT); (xiii) blood transfusions defined as any transfusion of red blood cells, platelets and/or Octaplas/fresh frozen plasma [13]; (xiv) deep sternal wound infection, defined as any bacterial/fungal infection involving the sternum [6] and (xv) ‘early complicated postoperative course’, defined by the occurrence during hospitalization of at least one of the above-mentioned major perioperative complications.
Late events were: (i) acute heart failure (AHF), defined as any episode of acute cardiac decompensation requiring hospitalization and/or optimization of medical therapy [6, 9, 10]; (ii) reintervention, defined as any reoperation on the aortic valve prosthesis implanted at the time of the indexed RAVR [6, 9, 10]; (iii) stroke, as defined above and (iv) thrombo-embolisms, defined according to the current guidelines [6, 9, 10].
Finally, when baseline characteristics were considered, urgent/emergent operations were defined as operative procedures for life-threatening conditions, performed within 24 h of hospital admission.
Statistical analysis
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as absolute numbers and percentages. Data were checked for normality before statistical analysis. Normally distributed continuous variables were compared using the unpaired t-test, whereas the Mann–Whitney U-test was used for not-normally distributed variables. Categorical variables were analysed using either the χ2 test or Fisher's exact test as appropriate. Multivariate analysis was performed to identify determinants of hospital mortality in the entire population and in the ‘repeat-AVR’ subgroup, employing stepwise logistic regression with backward selection. Only variables with a P-value of <0.1 at univariate analysis were included in the regression models. Hosmer–Lemeshow's test was used to assess the regression models fit. The area under the receiver operating characteristic (ROC) curve was used to represent the discriminatory ability of the regression models. Models were expressed in terms of adjusted odds ratio (OR), 95% confidence interval (CI) and P-value.
ROC curve analysis was applied to determine the optimal cut-off value of CPB time for hospital mortality. The sensitivity and specificity of the cut-off value and the area under curve (AUC) of the model with 95% confidence limits were reported. Follow-up survival and freedom from events were determined with the method of Kaplan–Meier analysis. The log-rank test was performed to ascertain between-group differences. Semi-parametric Cox proportional hazard regression models were developed to identify risk factors for death and AHF at follow-up, using a P-value of >0.1 as a backward elimination criterion for stepwise regression. Sensitivity analyses were performed for subsets of patients with age >75 years, urgent/emergent procedures, preoperative NYHA functional Class IV and endocarditis. A P-value of <0.05 was considered statistically significant. Statistical analysis was performed by the SPSS program for Windows, version 13.0 (SPSS, Inc., Chicago, IL, USA).
RESULTS
Outcomes in the overall population
Mean interval from previous surgery to RAVR for the entire population was 124.5 ± 87.5 months (95% CI 118.0–130.9 months). Thirty-six patients died after surgery, resulting in 5.1% hospital mortality. Cardiovascular hospital mortality was 4.6% (33 patients). Isolated AVR in a redo context (so-called isolated RAVR) had a 4.5% hospital mortality (23 of 512 patients).
The incidence of perioperative complications were: 35 (4.9%) MRCVCs, 49 (6.9%) reoperations for bleeding, 109 (15.3%) LCOS, 20 (2.8%) AMI, 47 (6.6%) need for IABP, 47 (6.6%) strokes, 75 (10.6%) ARF, 115 (16.4%) prolonged intubations, 49 (6.9%) pneumonia, 117 (19.3%) ARI, 51 (7.2%) need for CRRT, 476 (66.9%) need for transfusions and 90 (12.7%) implantations of PMK. ‘Early complicated postoperative course’ was observed in 290 (40.7%) hospital survivors. Preoperative CT [15 MRCVCs/309 patients (4.9%) not undergoing preoperative CT scan versus 20 MRCVCs/402 patients (5.0%) undergoing preoperative CT scan; P = 1.0] and peripheral cannulation [29 MRCVCs/512 patients (5.7%) not undergoing peripheral cannulation versus 6 MRCVCs/199 patients (3.0) undergoing peripheral cannulation; P = 0.18] failed to prevent MRCVCs.
Ninety-two late deaths occurred among 675 hospital survivors (2 lost at follow-up) after a mean follow-up of 39.2 ± 31.3 months (median 31.7 months, range 1–120 months), resulting in 77.2 ± 2.7% survival. Fifty of these follow-up deaths were of cardiovascular origin, resulting in a ‘freedom from cardiovascular death’ of 88.7 ± 1.9%. AHF occurred in 58 patients at a mean follow-up of 37.7 ± 30.5 months (median 30.4 months, range 1–120 months), with a freedom from AHF of 84.4 ± 2.6%. AHF developed in 31 of 290 (10.7%) patients experiencing an early complicated postoperative course and in 27 of 383 patients having an early uncomplicated course (7.0%, P = 0.09). Thus, no association between ‘early postoperative complicated course’ and AHF emerged at follow-up. Furthermore, a semi-parametric Cox proportional hazard regression model demonstrated that urgent/emergent indication (OR 3.1, 95% CI 1.4–6.7, P < 0.01) and perioperative AMI (OR 9.9, 95% CI 1.9–51.6, P < 0.01)—but not ‘early complicated postoperative course’ (OR 0.4, 95% CI 0.2–1.2, P = 0.09)—predicted AHF at follow-up. Freedom from reinterventions on the aortic valve, from stroke and from thrombo-embolisms were 97.2 ± 0.8% (13 patients), 97.2 ± 0.9% (12 patients) and 96.3 ± 1.2% (13 patients) at mean follow-up times of 38.8 ± 30.7, 38.9 ± 30.9 and 38.8 ± 31.8 months, respectively (median months of 31.4, 31.3 and 32, respectively, and range 1–120 months for all). When NYHA functional class at the last follow-up was considered, 408 patients (60.6%) were in Class I, 181 (26.9%) in Class II, 75 (11.1%) in Class III and 9 (1.3%) in Class IV.
Determinants of hospital and follow-up mortality
Among the perioperative variables showing univariate association with hospital death (Supplementary Material, Appendix), preoperative left ventricular ejection fraction (LVEF) of <30% (OR 8.7, 95% CI 2.1–35.6, P = 0.02), MRCVCs (OR 20.9, 95% CI 5.6–78.3, P < 0.01), CPB time (OR 1.1, 95% CI 1.0–1.1, P = 0.04), perioperative LCOS (OR 17.2, 95% CI 5.1–57.4, P < 0.01) and ARI (OR 5.1, 95% CI 1.5–18.1, P = 0.01) all predicted hospital death (AUC for the final logistic regression analysis was 0.85, with a Hosmer–Lemeshow test P = 0.78). In particular, a CPB time of >165 min had a 64.8% sensitivity and a 70.6% specificity to discriminate between hospital deaths and survivors (area under the ROC was 0.70 with 95% CI 0.66–0.78, P = 0.0001).
Several factors showed univariate association with follow-up mortality (Supplementary Material, Appendix), although only endocarditis (OR 7.5, 95% CI 2.9–19.1, P < 0.01), preoperative NYHA functional Class IV (OR 4.7, 95% CI 1.0–24.0, P < 0.05), combined RAVR + mitral surgery (OR 5.1, 95% CI 1.5–17.3, P < 0.01) and AHF at follow-up (OR 2.8, 95% CI 1.3–6.0, P < 0.01) predicted follow-up death.
Results in high-risk subgroups
Elderly patients (>75 years) represented 34.2% (243 patients) of the entire population. Other age groups are reported in Table 1. Urgent/emergent indication was reported in 71 (29.2%) patients >75 years, in 27.1% (57/210) of patients within the 66- to 75-year-old range, in 26.1% (35/134) of patients within the 56- to 65-year-old range, in 23% (14/61) of patients within the 46- to 55-year-old range, in 27.7% (13/47) of patients within the 36- to 45-year-old range, in 16.7% (2/12) of patients within the 26- to 35-year-old range and in no patient of <25 years old (P = 0.77). Of elderly patients, 48 (19.8%) were referred to surgery because of endocarditis, and 46 (18.9%) were in preoperative NYHA functional Class IV. Interestingly, endocarditis was reported in 106 patients <75 years (22.6%; P = 0.39 vs elderly >75 years), and preoperative NYHA functional Class IV in 63 (13.5%; P = 0.06 vs elderly >75 years). A LVEF of >50% was reported in 147 (60.5%) patients >75 years, between 35–50% in 87 (35.8%) and <30% in 9 (3.7%). A LVEF of >50% was recorded in 282 (60.3%) patients <75 years, between 35 and 50% in 154 (32.9%) and <30% in 32 (6.8%; P = 0.21 vs elderly >75 years). Therefore, no differences were shown between elderly and younger patients in terms of major preoperative risk factors. Elderly patients had similar hospital outcomes than younger patients, with the exception of a higher need for PMK implantation (16.5 vs 10.7% in younger patients, P = 0.03—Table 3). Mid-term survival was similar between elderly and young patients (72.4 ± 6.1 vs 79.2 ± 2.8%, respectively, P = 0.89). Other long-term follow-up outcome variables were also comparable (Fig. 1).
| Variable . | n (%) . | P-value . | n (%) . | P-value . | n (%) . | P-value . | n (%) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age >75 years (243 patients) . | Age ≤75 years (468 patients) . | NYHA IV (109 patients) . | NYHA I–III (602 patients) . | Urgent/emergent (192 patients) . | Elective (519 patients) . | Endocarditis (154 patients) . | No endocarditis (557 patients) . | |||||
| Mortality | 7 (2.9) | 29 (6.2) | 0.06 | 9 (8.3) | 27 (4.5) | 0.09 | 20 (10.4) | 16 (3.1) | <0.01 | 15 (9.7) | 21 (3.8) | <0.01 |
| MRCVCs | 10 (4.1) | 25 (5.3) | 0.47 | 12 (11.0) | 23 (3.8) | <0.01 | 23 (12.0) | 12 (2.3) | <0.01 | 15 (9.7) | 20 (3.6) | <0.01 |
| Revision for bleeding | 17 (7.0) | 32 (6.8) | 0.93 | 11 (10.1) | 38 (6.3) | 0.15 | 15 (7.8) | 34 (6.6) | 0.56 | 14 (9.1) | 35 (6.3) | 0.22 |
| Permanent PMK | 40 (16.5) | 50 (10.7) | 0.03 | 14 (13.0) | 76 (12.6) | 0.92 | 29 (15.1) | 61 (11.8) | 0.24 | 31 (20.1) | 59 (10.6) | <0.01 |
| Acute myocardial infarction | 4 (1.6) | 16 (3.4) | 0.17 | 4 (3.7) | 16 (2.7) | 0.54 | 7 (3.7) | 13 (2.5) | 0.41 | 4 (2.6) | 16 (2.9) | 0.86 |
| Low cardiac output syndrome | 40 (16.5) | 69 (14.7) | 0.55 | 30 (27.5) | 79 (13.1) | <0.01 | 45 (23.4) | 64 (12.3) | <0.01 | 40 (26.0) | 69 (12.4) | <0.01 |
| Perioperative IABP | 14 (5.8) | 33 (7.1) | 0.51 | 15 (13.8) | 32 (5.3) | <0.01 | 19 (9.9) | 28 (5.4) | 0.03 | 17 (11.0) | 30 (5.4) | 0.01 |
| Prolonged intubation (>48 h) | 43 (18.0) | 72 (15.6) | 0.41 | 33 (30.6) | 82 (13.8) | <0.01 | 43 (22.5) | 72 (14.1) | <0.01 | 41 (27.3) | 74 (13.4) | <0.01 |
| Acute respiratory failure | 32 (13.6) | 43 (9.2) | 0.10 | 20 (18.5) | 55 (9.2) | <0.01 | 25 (13.1) | 50 (9.7) | 0.19 | 23 (15.2) | 52 (9.3) | 0.04 |
| Pneumonia | 17 (7.0) | 32 (6.9) | 0.94 | 19 (17.4) | 30 (5.0) | <0.01 | 23 (12.0) | 26 (5.0) | <0.01 | 20 (13.0) | 29 (5.2) | <0.01 |
| Stroke | 16 (6.6) | 31 (6.7) | 0.98 | 11 (10.2) | 36 (6.0) | 0.11 | 23 (12.0) | 24 (4.6) | <0.01 | 18 (11.9) | 29 (5.2) | <0.01 |
| Acute renal insufficiency | 53 (21.8) | 84 (17.9) | 0.22 | 30 (27.5) | 107 (17.8) | 0.02 | 51 (26.6) | 86 (16.6) | <0.01 | 49 (31.8) | 88 (15.8) | <0.01 |
| CRRT | 19 (7.8) | 32 (6.8) | 0.63 | 16 (14.7) | 35 (5.8) | <0.01 | 21 (10.9) | 30 (5.8) | 0.02 | 19 (12.3) | 32 (5.7) | <0.01 |
| DSWI | 4 (1.6) | 6 (1.3) | 0.69 | 3 (2.8) | 7 (1.2) | 0.19 | 4 (2.1) | 6 (1.2) | 0.35 | 2 (1.3) | 8 (1.4) | 0.89 |
| Transfusions | 156 (64.2) | 320 (68.4) | 0.26 | 76 (69.7) | 400 (66.4) | 0.50 | 156 (81.3) | 320 (61.7) | <0.01 | 125 (81.2) | 351 (63.0) | <0.01 |
| Variable . | n (%) . | P-value . | n (%) . | P-value . | n (%) . | P-value . | n (%) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age >75 years (243 patients) . | Age ≤75 years (468 patients) . | NYHA IV (109 patients) . | NYHA I–III (602 patients) . | Urgent/emergent (192 patients) . | Elective (519 patients) . | Endocarditis (154 patients) . | No endocarditis (557 patients) . | |||||
| Mortality | 7 (2.9) | 29 (6.2) | 0.06 | 9 (8.3) | 27 (4.5) | 0.09 | 20 (10.4) | 16 (3.1) | <0.01 | 15 (9.7) | 21 (3.8) | <0.01 |
| MRCVCs | 10 (4.1) | 25 (5.3) | 0.47 | 12 (11.0) | 23 (3.8) | <0.01 | 23 (12.0) | 12 (2.3) | <0.01 | 15 (9.7) | 20 (3.6) | <0.01 |
| Revision for bleeding | 17 (7.0) | 32 (6.8) | 0.93 | 11 (10.1) | 38 (6.3) | 0.15 | 15 (7.8) | 34 (6.6) | 0.56 | 14 (9.1) | 35 (6.3) | 0.22 |
| Permanent PMK | 40 (16.5) | 50 (10.7) | 0.03 | 14 (13.0) | 76 (12.6) | 0.92 | 29 (15.1) | 61 (11.8) | 0.24 | 31 (20.1) | 59 (10.6) | <0.01 |
| Acute myocardial infarction | 4 (1.6) | 16 (3.4) | 0.17 | 4 (3.7) | 16 (2.7) | 0.54 | 7 (3.7) | 13 (2.5) | 0.41 | 4 (2.6) | 16 (2.9) | 0.86 |
| Low cardiac output syndrome | 40 (16.5) | 69 (14.7) | 0.55 | 30 (27.5) | 79 (13.1) | <0.01 | 45 (23.4) | 64 (12.3) | <0.01 | 40 (26.0) | 69 (12.4) | <0.01 |
| Perioperative IABP | 14 (5.8) | 33 (7.1) | 0.51 | 15 (13.8) | 32 (5.3) | <0.01 | 19 (9.9) | 28 (5.4) | 0.03 | 17 (11.0) | 30 (5.4) | 0.01 |
| Prolonged intubation (>48 h) | 43 (18.0) | 72 (15.6) | 0.41 | 33 (30.6) | 82 (13.8) | <0.01 | 43 (22.5) | 72 (14.1) | <0.01 | 41 (27.3) | 74 (13.4) | <0.01 |
| Acute respiratory failure | 32 (13.6) | 43 (9.2) | 0.10 | 20 (18.5) | 55 (9.2) | <0.01 | 25 (13.1) | 50 (9.7) | 0.19 | 23 (15.2) | 52 (9.3) | 0.04 |
| Pneumonia | 17 (7.0) | 32 (6.9) | 0.94 | 19 (17.4) | 30 (5.0) | <0.01 | 23 (12.0) | 26 (5.0) | <0.01 | 20 (13.0) | 29 (5.2) | <0.01 |
| Stroke | 16 (6.6) | 31 (6.7) | 0.98 | 11 (10.2) | 36 (6.0) | 0.11 | 23 (12.0) | 24 (4.6) | <0.01 | 18 (11.9) | 29 (5.2) | <0.01 |
| Acute renal insufficiency | 53 (21.8) | 84 (17.9) | 0.22 | 30 (27.5) | 107 (17.8) | 0.02 | 51 (26.6) | 86 (16.6) | <0.01 | 49 (31.8) | 88 (15.8) | <0.01 |
| CRRT | 19 (7.8) | 32 (6.8) | 0.63 | 16 (14.7) | 35 (5.8) | <0.01 | 21 (10.9) | 30 (5.8) | 0.02 | 19 (12.3) | 32 (5.7) | <0.01 |
| DSWI | 4 (1.6) | 6 (1.3) | 0.69 | 3 (2.8) | 7 (1.2) | 0.19 | 4 (2.1) | 6 (1.2) | 0.35 | 2 (1.3) | 8 (1.4) | 0.89 |
| Transfusions | 156 (64.2) | 320 (68.4) | 0.26 | 76 (69.7) | 400 (66.4) | 0.50 | 156 (81.3) | 320 (61.7) | <0.01 | 125 (81.2) | 351 (63.0) | <0.01 |
CRRT: continuous renal replacement therapy; DSWI: deep sternal wound infection; IABP: intra-aortic balloon pump; MRCVCs: major re-entry cardiovascular complications; PMK: pacemaker.
| Variable . | n (%) . | P-value . | n (%) . | P-value . | n (%) . | P-value . | n (%) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age >75 years (243 patients) . | Age ≤75 years (468 patients) . | NYHA IV (109 patients) . | NYHA I–III (602 patients) . | Urgent/emergent (192 patients) . | Elective (519 patients) . | Endocarditis (154 patients) . | No endocarditis (557 patients) . | |||||
| Mortality | 7 (2.9) | 29 (6.2) | 0.06 | 9 (8.3) | 27 (4.5) | 0.09 | 20 (10.4) | 16 (3.1) | <0.01 | 15 (9.7) | 21 (3.8) | <0.01 |
| MRCVCs | 10 (4.1) | 25 (5.3) | 0.47 | 12 (11.0) | 23 (3.8) | <0.01 | 23 (12.0) | 12 (2.3) | <0.01 | 15 (9.7) | 20 (3.6) | <0.01 |
| Revision for bleeding | 17 (7.0) | 32 (6.8) | 0.93 | 11 (10.1) | 38 (6.3) | 0.15 | 15 (7.8) | 34 (6.6) | 0.56 | 14 (9.1) | 35 (6.3) | 0.22 |
| Permanent PMK | 40 (16.5) | 50 (10.7) | 0.03 | 14 (13.0) | 76 (12.6) | 0.92 | 29 (15.1) | 61 (11.8) | 0.24 | 31 (20.1) | 59 (10.6) | <0.01 |
| Acute myocardial infarction | 4 (1.6) | 16 (3.4) | 0.17 | 4 (3.7) | 16 (2.7) | 0.54 | 7 (3.7) | 13 (2.5) | 0.41 | 4 (2.6) | 16 (2.9) | 0.86 |
| Low cardiac output syndrome | 40 (16.5) | 69 (14.7) | 0.55 | 30 (27.5) | 79 (13.1) | <0.01 | 45 (23.4) | 64 (12.3) | <0.01 | 40 (26.0) | 69 (12.4) | <0.01 |
| Perioperative IABP | 14 (5.8) | 33 (7.1) | 0.51 | 15 (13.8) | 32 (5.3) | <0.01 | 19 (9.9) | 28 (5.4) | 0.03 | 17 (11.0) | 30 (5.4) | 0.01 |
| Prolonged intubation (>48 h) | 43 (18.0) | 72 (15.6) | 0.41 | 33 (30.6) | 82 (13.8) | <0.01 | 43 (22.5) | 72 (14.1) | <0.01 | 41 (27.3) | 74 (13.4) | <0.01 |
| Acute respiratory failure | 32 (13.6) | 43 (9.2) | 0.10 | 20 (18.5) | 55 (9.2) | <0.01 | 25 (13.1) | 50 (9.7) | 0.19 | 23 (15.2) | 52 (9.3) | 0.04 |
| Pneumonia | 17 (7.0) | 32 (6.9) | 0.94 | 19 (17.4) | 30 (5.0) | <0.01 | 23 (12.0) | 26 (5.0) | <0.01 | 20 (13.0) | 29 (5.2) | <0.01 |
| Stroke | 16 (6.6) | 31 (6.7) | 0.98 | 11 (10.2) | 36 (6.0) | 0.11 | 23 (12.0) | 24 (4.6) | <0.01 | 18 (11.9) | 29 (5.2) | <0.01 |
| Acute renal insufficiency | 53 (21.8) | 84 (17.9) | 0.22 | 30 (27.5) | 107 (17.8) | 0.02 | 51 (26.6) | 86 (16.6) | <0.01 | 49 (31.8) | 88 (15.8) | <0.01 |
| CRRT | 19 (7.8) | 32 (6.8) | 0.63 | 16 (14.7) | 35 (5.8) | <0.01 | 21 (10.9) | 30 (5.8) | 0.02 | 19 (12.3) | 32 (5.7) | <0.01 |
| DSWI | 4 (1.6) | 6 (1.3) | 0.69 | 3 (2.8) | 7 (1.2) | 0.19 | 4 (2.1) | 6 (1.2) | 0.35 | 2 (1.3) | 8 (1.4) | 0.89 |
| Transfusions | 156 (64.2) | 320 (68.4) | 0.26 | 76 (69.7) | 400 (66.4) | 0.50 | 156 (81.3) | 320 (61.7) | <0.01 | 125 (81.2) | 351 (63.0) | <0.01 |
| Variable . | n (%) . | P-value . | n (%) . | P-value . | n (%) . | P-value . | n (%) . | P-value . | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age >75 years (243 patients) . | Age ≤75 years (468 patients) . | NYHA IV (109 patients) . | NYHA I–III (602 patients) . | Urgent/emergent (192 patients) . | Elective (519 patients) . | Endocarditis (154 patients) . | No endocarditis (557 patients) . | |||||
| Mortality | 7 (2.9) | 29 (6.2) | 0.06 | 9 (8.3) | 27 (4.5) | 0.09 | 20 (10.4) | 16 (3.1) | <0.01 | 15 (9.7) | 21 (3.8) | <0.01 |
| MRCVCs | 10 (4.1) | 25 (5.3) | 0.47 | 12 (11.0) | 23 (3.8) | <0.01 | 23 (12.0) | 12 (2.3) | <0.01 | 15 (9.7) | 20 (3.6) | <0.01 |
| Revision for bleeding | 17 (7.0) | 32 (6.8) | 0.93 | 11 (10.1) | 38 (6.3) | 0.15 | 15 (7.8) | 34 (6.6) | 0.56 | 14 (9.1) | 35 (6.3) | 0.22 |
| Permanent PMK | 40 (16.5) | 50 (10.7) | 0.03 | 14 (13.0) | 76 (12.6) | 0.92 | 29 (15.1) | 61 (11.8) | 0.24 | 31 (20.1) | 59 (10.6) | <0.01 |
| Acute myocardial infarction | 4 (1.6) | 16 (3.4) | 0.17 | 4 (3.7) | 16 (2.7) | 0.54 | 7 (3.7) | 13 (2.5) | 0.41 | 4 (2.6) | 16 (2.9) | 0.86 |
| Low cardiac output syndrome | 40 (16.5) | 69 (14.7) | 0.55 | 30 (27.5) | 79 (13.1) | <0.01 | 45 (23.4) | 64 (12.3) | <0.01 | 40 (26.0) | 69 (12.4) | <0.01 |
| Perioperative IABP | 14 (5.8) | 33 (7.1) | 0.51 | 15 (13.8) | 32 (5.3) | <0.01 | 19 (9.9) | 28 (5.4) | 0.03 | 17 (11.0) | 30 (5.4) | 0.01 |
| Prolonged intubation (>48 h) | 43 (18.0) | 72 (15.6) | 0.41 | 33 (30.6) | 82 (13.8) | <0.01 | 43 (22.5) | 72 (14.1) | <0.01 | 41 (27.3) | 74 (13.4) | <0.01 |
| Acute respiratory failure | 32 (13.6) | 43 (9.2) | 0.10 | 20 (18.5) | 55 (9.2) | <0.01 | 25 (13.1) | 50 (9.7) | 0.19 | 23 (15.2) | 52 (9.3) | 0.04 |
| Pneumonia | 17 (7.0) | 32 (6.9) | 0.94 | 19 (17.4) | 30 (5.0) | <0.01 | 23 (12.0) | 26 (5.0) | <0.01 | 20 (13.0) | 29 (5.2) | <0.01 |
| Stroke | 16 (6.6) | 31 (6.7) | 0.98 | 11 (10.2) | 36 (6.0) | 0.11 | 23 (12.0) | 24 (4.6) | <0.01 | 18 (11.9) | 29 (5.2) | <0.01 |
| Acute renal insufficiency | 53 (21.8) | 84 (17.9) | 0.22 | 30 (27.5) | 107 (17.8) | 0.02 | 51 (26.6) | 86 (16.6) | <0.01 | 49 (31.8) | 88 (15.8) | <0.01 |
| CRRT | 19 (7.8) | 32 (6.8) | 0.63 | 16 (14.7) | 35 (5.8) | <0.01 | 21 (10.9) | 30 (5.8) | 0.02 | 19 (12.3) | 32 (5.7) | <0.01 |
| DSWI | 4 (1.6) | 6 (1.3) | 0.69 | 3 (2.8) | 7 (1.2) | 0.19 | 4 (2.1) | 6 (1.2) | 0.35 | 2 (1.3) | 8 (1.4) | 0.89 |
| Transfusions | 156 (64.2) | 320 (68.4) | 0.26 | 76 (69.7) | 400 (66.4) | 0.50 | 156 (81.3) | 320 (61.7) | <0.01 | 125 (81.2) | 351 (63.0) | <0.01 |
CRRT: continuous renal replacement therapy; DSWI: deep sternal wound infection; IABP: intra-aortic balloon pump; MRCVCs: major re-entry cardiovascular complications; PMK: pacemaker.
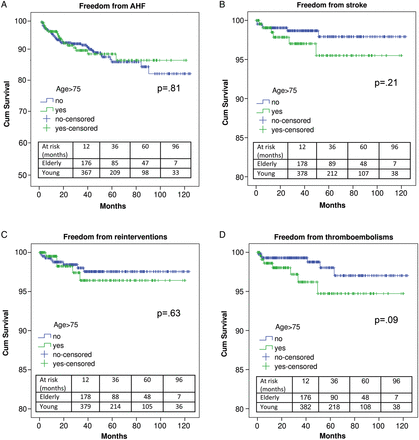
Freedom from acute heart failure (A), stroke (B), reinterventions (C) and thrombo-embolisms (D) in elderly >75 years of age and younger patients. AHF: acute heart failure.
Urgent/emergent procedures were reported in 27.0% (192 patients) of the study cohort. Patients with urgent/emergent indication reported 37.0% (71 patients) prevalence of ‘age >75 years’, 47.4% (91) of endocarditic aetiology, 19.3% (37) of recurrent endocarditis, 31.3% (60) of preoperative NYHA functional Class IV, 9.4% (18) of preoperative Canadian Cardiovascular Society Class IV, 13.5% (26) of preoperative LVEF <30% and 12.0% (23) of preoperative pulmonary hypertension, with a mean EuroSCORE of 11.6 ± 3.8. At surgery, of 121 patients undergoing ‘repeat-AVR’ with urgent/emergent indication, structural valve degeneration was found as the main mechanism of failure in 57 (47.1%) patients, paravalvular leak in 57 (47.1%) and other types of non-structural valve deterioration in 7 (5.8%). Urgent/emergent indication resulted in higher hospital death, MRCVCs, LCOS, need for IABP, stroke, prolonged ventilation, pneumonia, ARI, CRRT and transfusions (Table 3). Furthermore, a lower mid-term survival was detected in patients undergoing urgent/emergent RAVR versus elective procedures (59.6 ± 7.0 vs 84.1 ± 2.4%, respectively, P < 0.01), as well as a lower freedom from AHF (P < 0.01; Fig. 2A). On the other hand, similar rates of stroke, reinterventions and thrombo-embolisms were evidenced at follow-up (Fig. 2B–D).
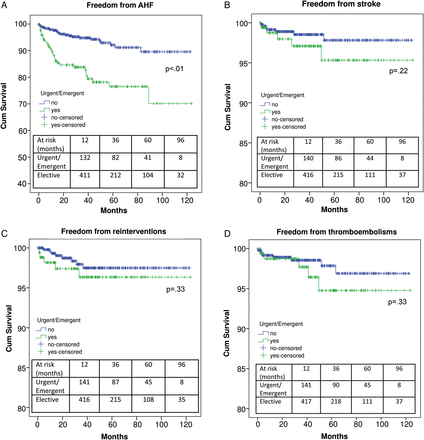
Freedom from acute heart failure (A), stroke (B), reinterventions (C) and thrombo-embolisms (D) in elective and urgent/emergent cases.
Preoperative NYHA functional Class IV was reported in 15.3% (109 patients) of the population. These patients demonstrated a higher incidence of MRCVCs, LCOS, IABP, prolonged ventilation, pneumonia, ARF, ARI and CRRT (Table 3). Furthermore, mid-term survival was lower (55.2 ± 6.9% versus preoperative NYHA functional Class ≤III: 81.2 ± 2.9, P < 0.01), as well as were freedom from AHF, stroke and reinterventions (Fig. 3A–C). Opposite to that, freedom from thrombo-embolisms was comparable regardless of NYHA functional class (Fig. 3D).
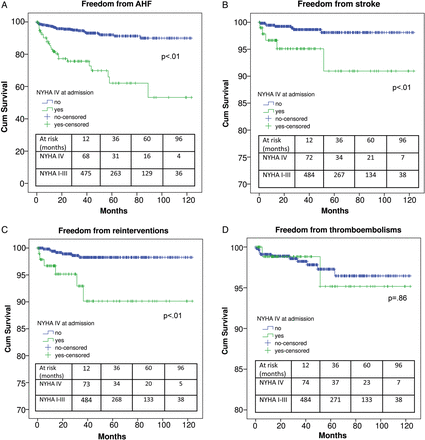
Freedom from acute heart failure (A), stroke (B), reinterventions (C) and thrombo-embolisms (D) in NYHA I–III and NYHA IV patients. NYHA: New York Heart Association.
Finally, endocarditis was shown in 21.7% (154 patients) of the study cohort. When prevalence of endocarditis was considered by ‘age-range’, it was reported in 1 (0.6%) patient within the ‘<25 years of age’ group, in 2 (1.3%) of the ‘26–35 years’ group, in 13 (8.4%) of the ‘36–45 years’ group, in 14 (9.1%) of the ‘46–55 years’ group, in 32 (20.8%) of the ‘56–65 years’ group, in 44 (28.6%) of the ‘66–75 years’ group and in 48 (31.2%) of the ‘>75 years’ group. Prevalence of recurrent endocarditis in the above-mentioned ‘age-groups’ was 1 (100%), 2 (100%), 4 (30.8%), 7 (50.0%), 15 (46.9%), 22 (50.0%) and 19 (39.6%), respectively. Mean intervals from first surgery to redo-AVR in the same ‘age-groups’ were 15, 30.0 ± 16, 83.7 ± 30.1, 58.5 ± 22.7, 64.1 ± 9.7, 45.7 ± 4.9 and 58.2 ± 6.0 months, respectively. Patients with endocarditis demonstrated a significantly higher hospital mortality, MRCVCs, LCOS, IABP, stroke, ARF, prolonged intubation, pneumonia, ARI, need for CRRT, transfusions and PMK (Table 3). Mid-term survival was lower (43.3 ± 7.0 versus non-endocarditis: 88.1 ± 2.3, P < 0.01). Similarly, freedom from AHF (Fig. 4A) and reinterventions (Fig. 4C) were significantly lower, whereas that from stroke (Fig. 4B) and thrombo-embolisms (Fig. 4D) proved to be similar.
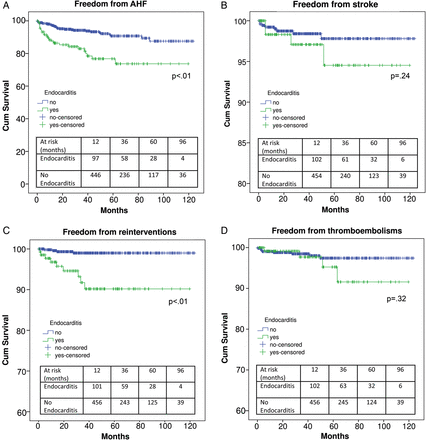
Freedom from acute heart failure (A), stroke (B), reinterventions (C) and thrombo-embolisms (D) in patients with or without endocarditis.
The significant effect—at the mean of covariates at Cox proportional hazard regression model—of ‘endocarditic aetiology’ and ‘preoperative NYHA functional Class IV’ on follow-up survival was reported in Fig. 5A and B, respectively, compared with the non-significant effects of ‘age > 75 years’ and of ‘urgent/emergent’ indication (Fig. 5C and D, respectively).

Cox proportional hazard regression model showing the significant impact on mid-term survival of endocarditic aetiology (A) and NYHA IV at surgery (B), and the not-significant effects of age >75 years (C) and urgent/emergent indication (D). OR: odds ratio; 95% CI: 95% confidence interval.
Repeat-aortic valve replacement subgroup
‘Repeat-AVR’ (i.e. reintervention on aortic valve prosthesis for failure of a previous AVR) was reported in 324 patients (45.6% of the entire population, Table 1). The mean interval from previous AVR to ‘repeat-AVR’ was 103.1 ± 78.8 months (95% CI 94.4–111.7 months). Prevalence of major preoperative risk factors were as follows: age >75 years 101 (31.2%) patients, urgent/emergent procedure 121 (37.3%), endocarditis 107 (33.0%), recurrent endocarditis 66 (20.4%), preoperative NYHA functional Class IV 69 (21.3%), preoperative LVEF <30% 24 (7.4%), LVEF 30–50% 103 (31.8%) and LVEF >50% 197 (60.8%). Mean additive EuroSCORE was 10.5 ± 3.8. Repeat-AVR was reported as a first reintervention in 262 (80.9%) patients, as a second one in 47 (14.5%), as a third one in 14 (4.3%) and as a fourth surgery in 1 (0.3%). Mean intervals from previous AVR to repeat-AVR in the above-mentioned subgroups were 110 ± 81.9, 76.7 ± 57.8, 50.4 ± 31.2 and 123 months, respectively.
At surgery, paravalvular leak was observed in 119 (36.7%) patients, structural valve degeneration in 179 (55.2%) and other types of non-structural heart valve deterioration in 26 (8.0%). Isolated repeat-AVR was reported in 217 (67.0%) patients, RAVR + CABG in 27 (8.3%), RAVR + mitral in 42 (13.0%) and RAVR + other in 38 (11.7%). When outcomes were considered, hospital mortality was 7.7% (25/324) and cardiovascular mortality 6.5% (21/324). Early complicated postoperative course was reported in 159 (49.1%) patients. The prevalence of perioperative complications and morbidity were as follows: 28 (8.6%) MRCVCs, 30 (9.3%) reoperations for bleeding, 51 (15.7%) LCOS, 7 (2.2%) AMI, 24 (7.4%) need for IABP, 23 (7.1%) strokes, 32 (9.9%) ARF, 55 (17.2%) prolonged intubations, 25 (7.7%) pneumonia, 65 (20.1%) ARI, 32 (9.9%) need for CRRT, 243 (75.0%) need for transfusions and 44 (13.6%) implantations of PMK.
Of 23 perioperative factors having a univariate association with hospital death, preoperative EuroSCORE (OR 1.4, 95% CI 1.1–1.8, P < 0.05), preoperative LVEF <30% (OR 8.2, 95% CI 1.2–70.9, P = 0.04), perioperative MRCVCs (OR 24.3, 95% CI 4.8–95.8, P < 0.01) and postoperative LCOS (OR 17.7, 95% CI 1.4–75.9, P = 0.03) independently predicted hospital mortality (AUC for the final logistic regression analysis was 0.78, with a Hosmer–Lemeshow test P = 0.23).
DISCUSSION
The first finding of this multicentre initiative is that RAVR in patients with a history of prior cardiac surgery has acceptable hospital mortality (∼5%) and respectable mid-term (and up to 10 years) results, with survival approximating 80%, freedom from AHF almost reaching 85% and freedom from stroke, reinterventions and thrombo-embolisms just a bit lower than 100%. A recent single-centre experience presenting more than 1500 patients followed over a 15-year period confirmed our findings, reporting a hospital mortality of <6%—further reduced to 4% in isolated RAVR (4.5% in our series) [5]. The same study also highlighted how the achieved results were better than those predicted by the utilized risk scores, as in our experience, where a preoperative mean EuroSCORE of 10 was calculated [5]. ‘Repeat-AVR’ reported in our study a slightly higher hospital mortality (∼7%), although still lower than predicted by EuroSCORE, and possibly related to the higher prevalence—compared with the rest of the RECORD population—of well-known risk factors such as urgent/emergent procedures, endocarditis, repeat endocarditis, preoperative NYHA functional Class IV, LVEF >30%, second or third reintervention and combined RAVR + mitral surgery as an index procedure. Indeed, these results coming from a multicentre experience encompassing different European countries during the last 10 years might also become a reliable benchmark to compare new emerging technologies.
However, hospital outcomes were undermined by a significant hospital morbidity amenable to improvements. In particular, efforts should be made to reduce major re-entry CPB, LCOS, ARI and prolonged cardiopulmonary bypass times, given their impact on early outcomes. Of these, MRCVCs and LCOS seem to play an eminent role also in determining hospital mortality in the ‘repeat-AVR’ subgroup. The importance of preoperative CT scan to correctly plan the best surgical re-entry strategy has been already documented [14]. Nevertheless, we failed to confirm such finding. Indeed, although significantly reduced by planned preventative strategies [14–16], MRCVCs still occur during cardiac reoperations [16], especially during ‘pre-pump’ dissection [16], and significantly impact on hospital outcomes and economic resource utilization [15]. We demonstrated that MRCVCs resulted in a 21-fold higher hospital mortality, thus confirming the ‘catastrophic nature’ of this complication, a finding already reported in a recent Cleveland Clinic experience [16] and also documented by the 3-fold higher hospital mortality reported by the Mayo Clinic group [17]. Similarly, postoperative LCOS and perioperative acute renal damage significantly impact on early outcomes after RAVR. The notion that LCOS represents an important risk factor for mortality in redo cardiac surgery is not new [18]. More recently, Noyez [19] demonstrated how redo cardiac surgery represents by itself an independent determinant of acute renal failure, regardless of the definition and classification of acute kidney injury. No need to say that a mixed combination of MRCVCs, major bleeding, anaemia, massive transfusions (reported in our experience to be as high as 67%) might impact on the development of LCOS and ARI, further affecting the outcomes of these patients [20]. In such perspective, improvements in CPB technologies, i.e. advance in the biocompatibility of prolonged pump times, might help to reduce transfusions in redo surgery [6, 13]. Finally, a preoperative LVEF of <30% significantly impacted on hospital mortality, with an 8.7 OR at multivariate analysis in the overall population and a not-negligible 8.2 OR in the ‘repeat-AVR subgroup’. Our data confirm those from Leontyev et al. [1] showing how severe preoperative ventricular dysfunction increased of 9-fold hospital mortality after RAVR. Indeed, the combination of LVEF <30% and postoperative LCOS—both concurring to an augmented odd for postoperative ARI—contributes to the explanation for the extremely poor prognosis of severe preoperative left ventricle dysfunction in our RAVR patients [1, 6]. These data might perhaps suggest a ‘preferential’ shift to alternative procedures in patients with redo cardiac surgery and a preoperative LVEF <30%.
Another two preoperative factors significantly impacted on survival in this experience, although preferentially in the long-term. In detail, endocarditis proved to be the strongest predictor of follow-up mortality, whereas preoperative NYHA functional Class IV had a 4.7 OR. Similarly, Leontyev et al. [1] underscored preoperative endocarditis and preoperative NYHA functional Class IV as the only predictors of follow-up mortality after RAVR. Romano et al. [21] reported a 24% hospital mortality and a 10-year survival in prosthetic endocarditis as low as 33%. A recent single-centre German analysis evidenced how prosthetic endocarditis carries a significantly worse baseline risk profile and how it implicates a more technically demanding operation, thus resulting in a higher LCOS, need for IABP, renal failure, hospital mortality and a very low 1-year (52%) and 10-year (31%) survival, together with a 5-year freedom from recurrences of only 80% [7]. These data were already outlined in our previous single-centre experience [6], and they are further confirmed in this multicentre report. Undoubtedly, these results call for improvements in perioperative care, adjuvant medical therapy (first of all antibiotics) and care in selecting surgical timing.
Preoperative NYHA functional Class IV at surgery was demonstrated as another important preoperative factor affecting mid-to-long-term outcomes, confirming Leontyev et al. [1, 7] and Zapolanski et al. observations [22]. Furthermore, Fukunaga et al. [8] recently reported that redo valve surgery in preoperative NYHA functional Class III or IV significantly affect early mortality and long-term survival. Interestingly enough, we reported a significant morbidity early after surgery in preoperative NYHA functional Class IV patients and a worse mid-term outcome compared with patients in lower NYHA functional classes and those belonging to the other ‘high-risk’ subgroups. All these data suggest that preoperative NYHA functional Class IV should be considered a ‘proxy-variable’ and a risk factor of utmost importance in redo scenarios, mandating aggressive medical therapy to achieve adequate cardiac re-compensation before surgery [8].
It is noteworthy that we demonstrate a comparable outcome—either in the short- or in the long-term follow-up—between elderly and young patients. Furthermore, ‘age >75 years’ was not a risk factor for hospital mortality either in the overall population of RECORD or in the ‘repeat-AVR’ subgroup, and it was not a predictor of both mortality and AHF at follow-up in the studied population. These findings should be carefully considered by the Heart Team in any decision-making process, given that advanced age at reoperation is often unjustifiably considered a ‘preferential’ indication for TAVI [3, 4]. Despite several studies have shown excellent results in the elderly population after primary cardiac surgery, only few papers have investigated the role of redo valvular surgery in aged patients [6, 9, 23]. Furthermore, most of these studies were single-center experiences on case-mixed population of patients, and often dated-back to past decades [6, 9, 23]. Indeed, the Toronto group recently published the results of redo surgery in 112 elderly patients, demonstrating excellent hospital and mid-term survival, with patients often outliving the lifespan of the implanted prostheses and with results superior to those expected with projections in the absence of reinterventions [9]. To the best of our knowledge, this is the first multicentre concurrent experience on different European populations, demonstrating that outcomes of RAVR in the elderly are excellent and comparable with those of younger patients since hospitalization to mid-term follow-up (with the only exception of a higher need for permanent PMK, possibly related to the age-dependent conduction disturbances). These data further confirm the excellent impact of surgery on the prognosis of elderly patients with aortic valve disease, who should not be considered more a high-risk category at RAVR [9, 23].
Finally, patients with urgent/emergent indication demonstrated a poor hospital and follow-up outcomes. These data contradict our previous single-centre experience demonstrating good hospital outcomes and respectable results at follow-up in patients after an urgent/emergent RAVR [6]. Our multicentre study reported poor results already during hospitalization in patients operated on with urgent/emergent priority, despite priority was not reported, on the other hand, as a predictor for hospital or follow-up mortality. This apparent incongruity can be explained by the high prevalence of preoperative NYHA functional Class IV patients and endocarditic aetiology (both being the ‘real’ predictors of mortality, and constituting 31 and 47%, respectively, of patients admitted with urgent/emergent priority) in this cohort of patients. These data confirm the experiences of Tang et al. [24] and Luciani et al. [25], both reporting urgent/emergent surgery as a predictor of hospital and 3-year mortality, respectively. Again, these data—similar to those of patients with poor LVEF—characterize a peculiar patient profile, which might perhaps be better served with transcatheter approaches.
In conclusion, good early and mid-term results are nowadays achieved with RAVR in patients after previous cardiac surgery, provided that there is no endocarditic aetiology and patients are not operated on in preoperative NYHA functional Class IV or with severely depressed left ventricles. Although based on a retrospective analysis of multicentre experiences, our results also suggest that major efforts should be made in the near future to improve surgical and medical therapy of prosthetic endocarditis and to avoid catastrophic complications such as major cardiovascular injuries at re-entry.
Limitations of the study
The main limitation of the study is related to the retrospective nature of the registry. Another limitation is the absence of standardized protocols of surgery and medical care among the different institutions. However, the above-mentioned limitations stem from the observational nature of the study, which on the other hand represents—as for any observational registry—a ‘real world’ picture of RAVR in ‘all-comers’ to surgery at different European latitudes. Indeed, the literature lacks multicentre RAVR experiences. The very high percentage of completeness of follow-up, up to 10 years after surgery, is another strength of the study, giving important results potentially useful to serve as a benchmark for new technologies.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr S. Thelin(Uppsala, Sweden): I think that this is a very interesting paper, and there are a lot of results that could be discussed. But time is restricted, so I will stick to my two questions. First, one comment. I think the title is not truly appropriate because only a minority of the patients were followed for 10 years. Another title could be ‘medium-term results’ or something like that.
My two questions are, first, what is your definition for index mortality, and does that differ from 30-day mortality? Secondly, MRCVC, I mean major cardiovascular re-entry complication, is a fearful and preventable complication, and your results showed that this appears in about 5% of the cases. You also say that some of the patients had CT scan performed preoperatively and in some cases you used peripheral cannulation. Do you know whether these measures in any way prevented this fearful complication?
Dr Onorati: About the issue of mortality, we decided to collect all-cause mortality, either during hospitalization or during follow-up, and not cardiac-related mortality, which was defined as any mortality during hospitalization or during the first 30 days afterwards.
Regarding major re-entry cardiovascular complications, we know there are literature data reporting that the preoperative CT scan is able to reduce these complications. However, not all participating centres have the same policy. Therefore, we decided to look at that. Interestingly, we did not find differences in the incidence of these events between the individual institutional experiences. I mean, institutions without preoperative CT scan don't report a higher incidence of reintervention complications at re-entry compared to the others that do this kind of preoperative exam, opposite to what has already been reported in the literature.
Dr Thelin: And peripheral cannulation, was that preventable?
Dr Onorati: We don't investigate this topic in detail. Again, we have different experiences in different centres. As a general rule of thumb, when we have a potential high-risk re-entry, we prefer to cannulate peripheral femoral vessels. But again, this factor also was not reported as an independent predictor of either the avoidance or the favouring of major re-entry cardiovascular complications.
So we have looked deeper at the predictors of major re-entry cardiovascular complications, and despite the fact that the database did not collect some potential risk factors, we reported that just preoperative severe pulmonary hypertension and NYHA IV, not peripheral cannulation, were predictors of this complication. Therefore it seems that peripheral cannulation did not independently avoid this complication.
Dr M. Zembala(Zabrze, Poland): A very practical question. Based on your experience, which patients should be considered for TAVI rather than surgical redo? It's something that we need to know from this presentation.
Dr Onorati: We have an ongoing paper about this issue. In particular, we have found, with a statistical analysis called Classification Tree Analysis, that patients with a combination of left ventricular ejection fraction less than 30% and NYHA IV are those patients having a worse hospital and long-term outcome. Maybe this category of patients, in our opinion, can be preferentially shifted to TAVI regardless of frailty, EuroSCORE II, and STS score.
Author notes
Presented at the 27th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 5–9 October 2013.
- heart failure, acute
- left ventricular ejection fraction
- cardiopulmonary bypass
- endocarditis
- intra-aortic balloon pumping
- cardiac surgery procedures
- cerebrovascular accident
- aortic valve replacement
- surgical complications
- renal failure, acute
- respiratory failure, acute
- blood transfusion
- follow-up
- objective (goal)
- hospital mortality
- intubation
- perioperative care
- pneumonia
- preoperative care
- renal replacement therapy
- surgical procedures, operative
- heart
- morbidity
- mortality
- surgery specialty
- embolism
- catheters
- pacemaker, permanent
- low cardiac output syndrome
- older adult
- aortic valve surgery
- new york heart association classification
- cardiac complications




